Hydrates for Cold Storage: Formation Characteristics, Stability, and Promoters
Abstract
:1. Introduction
2. Materials and Methods
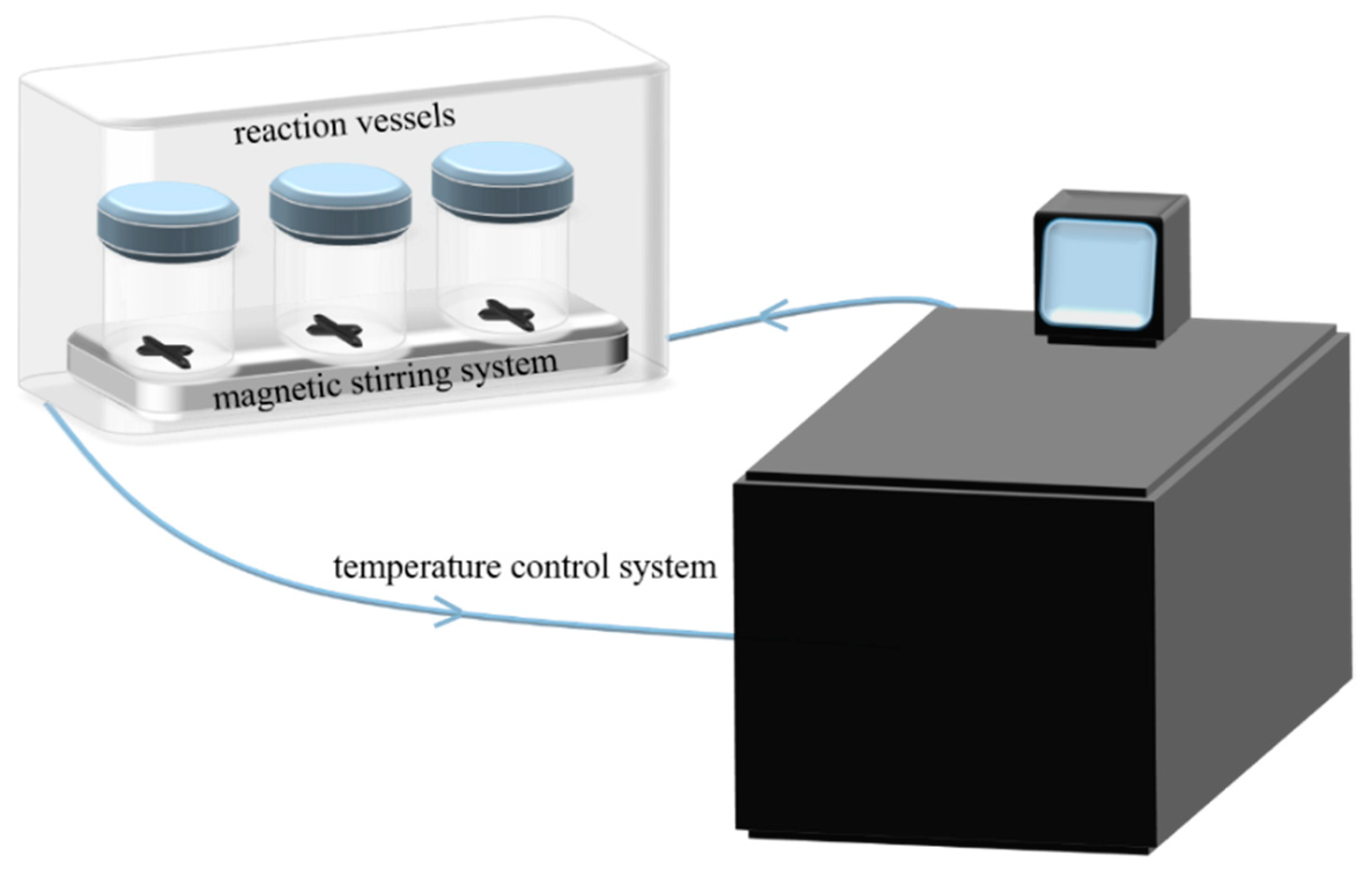
2.1. Experimental Apparatus
2.2. Materials
2.3. Methods and Procedures
3. Results and Discussion
3.1. Characteristics of Hydrate Formation
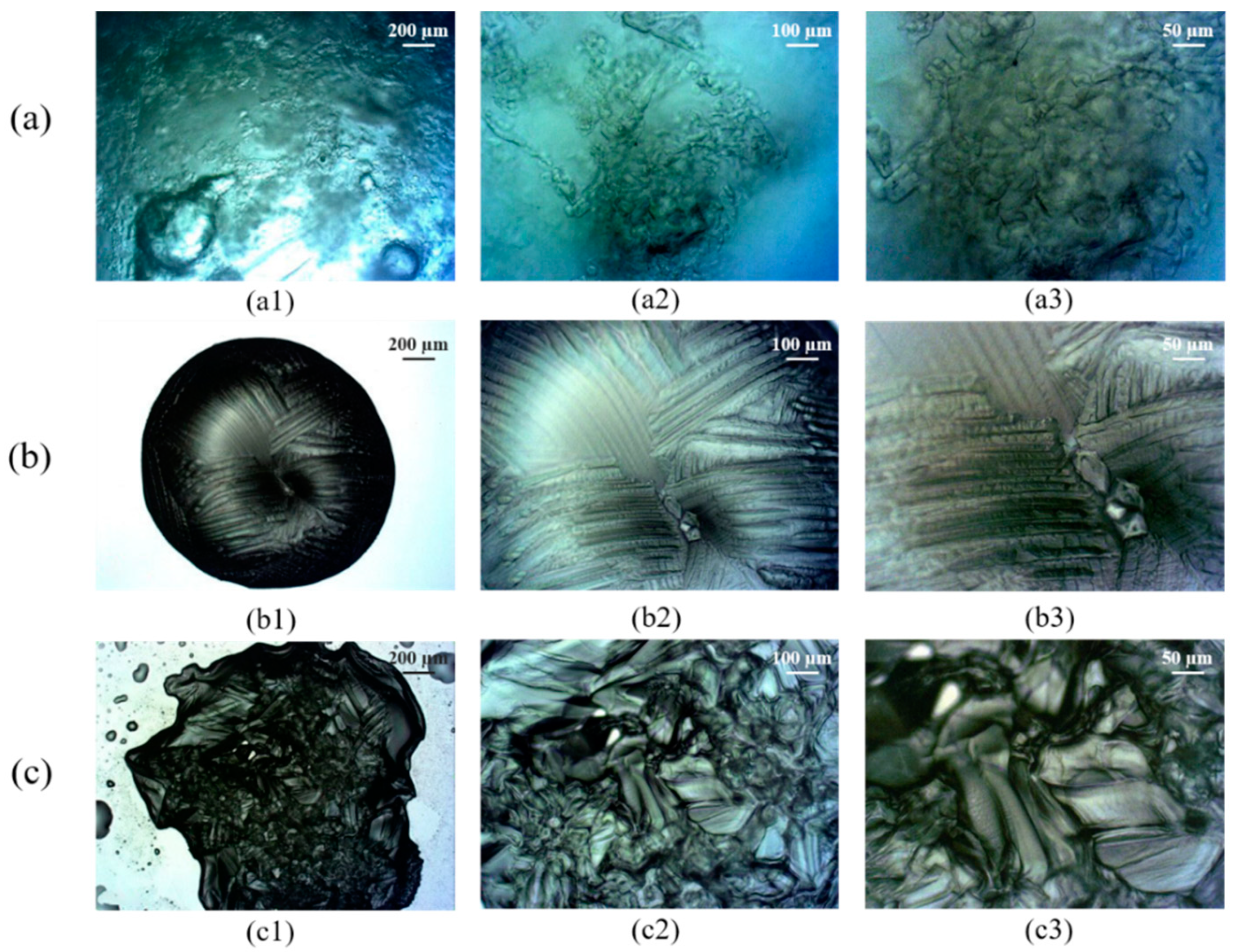
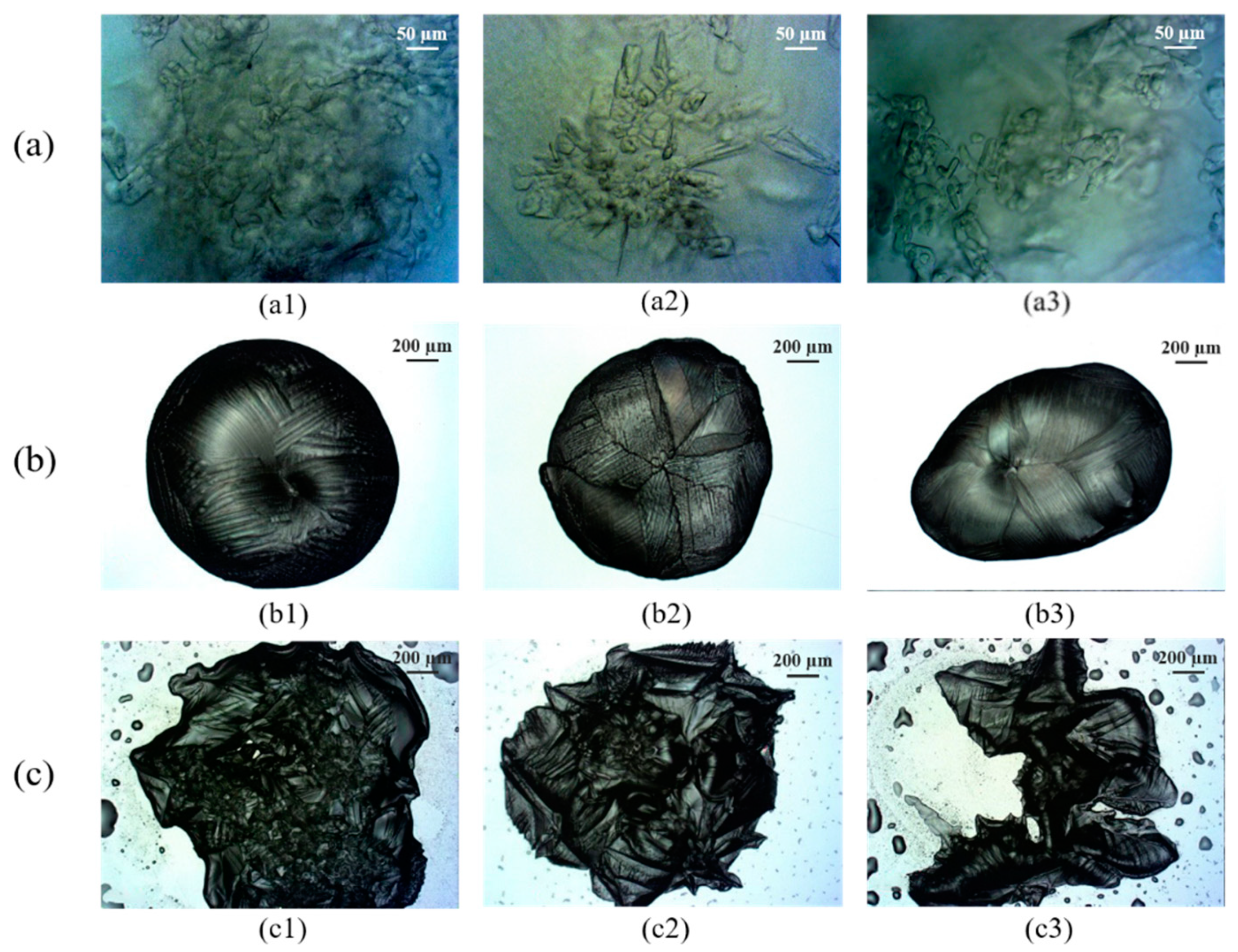
3.2. Crystal Morphology of Hydrates
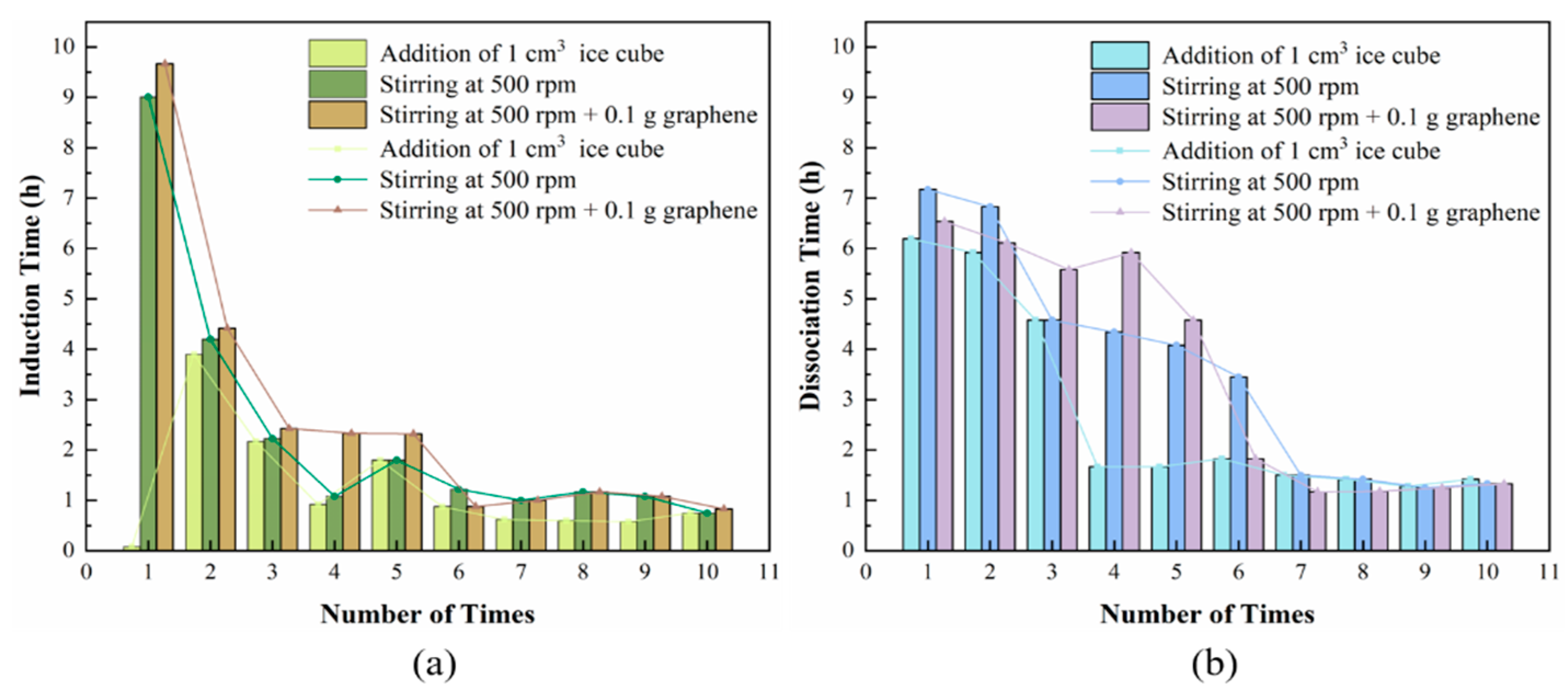
3.3. Stability of TBAB/TBAC Hydrate in Cyclic Formation
4. Conclusions
- (1)
- The R141b and TBAB/TBAC solutions did not form hydrates within 48 h when no induction or promotion measures were included. By contrast, hydrate crystals were formed from the TME solution after 3 h of reaction.
- (2)
- When a 1 cm3 ice cube was added to the reactor, the induction time for hydrate formation decreased, and the formation rate increased. When 500 rpm magnetic stirring was used, although the rate of hydrate formation from the TBAB/TBAC mixture was improved, the formation rate was significantly lower than that achieved with the addition of ice. When magnetic stirring at 500 rpm was combined with 0.1 g graphene, the induction time increased slightly. Nevertheless, because of nanostructures’ characteristics and the high thermal conductivity of graphene, it could provide a large specific surface area for the contact between the TBAB/TBAC mixture and water molecules while eliminating the heat from the system, which may promote the formation of hydrates and significantly increases the formation rate.
- (3)
- Microscopic examination was conducted to obtain the morphological characteristics of the hydrate crystals produced. The R141b hydrate crystals were mainly granular or branched and occurred as local clusters, which were easily separated by free water. The TME hydrate crystals presented an overall ellipsoid shape and a vein-like surface with large crystal grains, which may not be conducive to mass transfer. Many nucleation sites were obtained when TBAB/TBAC hydrates were formed and the crystal structure was dense, which may improve the formation rate.
- (4)
- The time for the formation of hydrates from the TBAB/TBAC mixture during the formation cycles gradually decreased with the increasing numbers of cycles and finally stabilized. When the formation process stabilized, the hydrate dissociation process also stabilized. This phenomenon could indicate the potential of these hydrates for application in cold storage owing to their good durability and short process time for heat absorption and release.
Author Contributions
Funding
Conflicts of Interest
References
- Cheng, C.; Wang, F.; Tian, Y.; Wu, X.; Zheng, J.; Zhang, J.; Li, L.; Yang, P.; Zhao, J. Review and Prospects of Hydrate Cold Storage Technology. Renew. Sustain. Energy Rev. 2020, 117, 109492. [Google Scholar] [CrossRef]
- Chen, J.; Xie, Y.; Yang, Y.; Sun, J.; Xu, Z. Alternative Refrigerant Hydrate for Cold Storage Air Conditioners. Build. Energy Effic. 2020, 48, 9–14. [Google Scholar]
- Fan, S.; Xie, Y.; Guo, K.; Liang, D.; Gu, J. Advance of Gas Hydrate Cool Storage Technology Used in Air Conditioning System. J. Chem. Ind. Eng. China 2003, 54, 131–135. [Google Scholar]
- Fang, G. Cold Storage Air Conditioning Engineering Practical New Technology; Posts & Telecom Press: Beijing, China, 2000. [Google Scholar]
- Li, H.; Du, Y.; Bao, Y. A View on Natural Gas Peak-Shaving in China from the Perspective of Power Demand Side Management. Nat. Gas. Ind. 2011, 31, 107–109. [Google Scholar]
- Wang, X.; Dennis, M.; Hou, L. Clathrate Hydrate Technology for Cold Storage in Air Conditioning Systems. Renew. Sustain. Energy Rev. 2014, 36, 34–51. [Google Scholar] [CrossRef]
- Hasnain, S.M. Review on Sustainable Thermal Energy Storage Technologies, Part II: Cool Thermal Storage. Energy Convers. Manag. 1998, 39, 1139–1153. [Google Scholar] [CrossRef]
- Li, G.; Hwang, Y.; Radermacher, R. Review of Cold Storage Materials for Air Conditioning Application. Int. J. Refrig. 2012, 35, 2053–2077. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, L.; Wang, J.; Zhao, J.; Dong, H.; Yang, M.; Liu, Y.; Song, Y. Enhanced CH4 Recovery and CO2 Storage Via Thermal Stimulation in The CH4 /CO2 Replacement of Methane Hydrate. Chem. Eng. J. 2017, 308, 40–49. [Google Scholar] [CrossRef]
- Sun, L.; Wang, T.; Dong, B.; Li, M.; Yang, L.; Dong, H.; Zhang, L.; Zhao, J.; Song, Y. Pressure Oscillation Controlled CH4/CO2 Replacement in Methane Hydrates: CH4 Recovery, CO2 Storage, and Their Characteristics. Chem. Eng. J. 2021, 425, 129709. [Google Scholar] [CrossRef]
- Dong, H.; Zhang, L.; Ling, Z.; Zhao, J.; Song, Y. The Controlling Factors and Ion Exclusion Mechanism of Hydrate-Based Pollutant Removal. ACS Sustain. Chem. Eng. 2019, 7, 7932–7940. [Google Scholar] [CrossRef]
- Li, F.; Chen, Z.; Dong, H.; Shi, C.; Wang, B.; Yang, L.; Ling, Z. Promotion Effect of Graphite on Cyclopentane Hydrate Based Desalination. Desalination 2018, 445, 197–203. [Google Scholar] [CrossRef]
- Strobel, T.A.; Hester, K.C.; Koh, C.A.; Sum, A.K.; Sloan, E.D. Properties of The Clathrates of Hydrogen and Developments in Their Applicability for Hydrogen Storage. Chem. Phys. Lett. 2009, 478, 97–109. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, F.; Lipiński, W. Carbon Dioxide Hydrates for Cold Thermal Energy Storage: A review. Sol. Energy 2020, 211, 11–30. [Google Scholar] [CrossRef]
- Ternes, M.P. Studies on the R-12 Gas Hydrate Formation Process for Heat Pump Cool Storage Applications. In Proceedings of the DOE Physical and Chemical Energy Storage Annual Contractors Review Meeting, Arlington, VA, USA, 12–14 September 1983. [Google Scholar]
- Wang, K.; Magnus, E.; Hwang, Y.; Radermacher, R. Review of Secondary Loop Refrigeration Systems. Int. J. Refrig. 2010, 33, 212–234. [Google Scholar] [CrossRef]
- Lee, D.; Lee, Y.; Choi, W.; Lee, S.; Seo, Y. Accurate Measurement of Phase Equilibria and Dissociation Enthalpies of HFC-134a Hydrates in The Presence of NaCl for Potential Application in Desalination. Korean J. Chem. Eng. 2016, 33, 1425–1430. [Google Scholar] [CrossRef]
- Hong, H.J.; Ko, C.H.; Song, M.H.; Lee, S.; Seong, K. Effect of Ultrasonic Waves on Dissociation Kinetics of Tetrafluoroethane (CH2FCF3) Hydrate. J. Ind. Eng. Chem. 2016, 41, 183–189. [Google Scholar] [CrossRef]
- Liang, K.; Yuan, Z.; Wang, L.; Tan, Y.; Rui, S.; Dong, B.; Yang, S.; Liu, R. Analysis of Crystal Structure and Crystallization Characteristics of R141b Gas Hydrate. J. Chem. Eng. Chin. Univ. 2017, 31, 1293–1300. [Google Scholar]
- Suzuki, H.; Wadab, N.; Komod, Y.; Usui, H.; Ujiie, S. Drag Reduction Characteristics of Trimethylolethane Hydrate Slurries Treated with Surfactants. Int. J. Refrig. 2009, 32, 931–937. [Google Scholar] [CrossRef]
- Belandria, V.; Mohammadi, A.H.; Richon, D. Compositional Analysis of the Gas Phase for the CO2 + N2 + Tetra-n-butylammonium Bromide Aqueous Solution Systems under Hydrate Stability Conditions. Chem. Eng. Sci. 2012, 84, 40–47. [Google Scholar] [CrossRef]
- Yang, M.; Song, Y.; Liu, W.; Zhao, J.; Ruan, X.; Jiang, L.; Li, Q. Effects of Additive Mixtures (THF/SDS) on Carbon Dioxide Hydrate Formation and Dissociation in Porous Media. Chem. Eng. Sci. 2013, 90, 69–76. [Google Scholar] [CrossRef]
- Youssef, Z.; Hanu, L.; Kappels, T.; Delahaye, A.; Fournaison, L.; Zambrana, C.; Pollerberg, C. Experimental Study of Single CO2 and Mixed CO2 + TBAB Hydrate Formation and Dissociation in Oil-in-Water Emulsion. Int. J. Refrig. 2014, 46, 207–218. [Google Scholar] [CrossRef]
- Oshima, M.; Kida, M.; Jin, Y.; Nagao, J. Dissociation Behaviour of (Tetra-n-butylammonium bromide +Tetra-nbutylammonium chloride) Mixed Semiclathrate Hydrate Systems. J. Chem. Thermodyn. 2015, 90, 277–281. [Google Scholar] [CrossRef]
- Li, J.; Liang, D.; Guo, K.; Wang, R. Experiments on fast nucleation and growth of HCFC141b gas hydrate in static water columns. Int. J. Refrig. 2004, 27, 932–939. [Google Scholar] [CrossRef]
- Liang, D.; Guo, K.; Fan, S.; Wang, R. Measurement of Melting Heat of HCFC-141b Gas Hydrate with DSC. J. Eng. Thermophys.-Rus. 2002, 23, 47–49. [Google Scholar]
- Yamazaki, M.; Sasaki, C.; Kakiuchi, H.; Osano, Y.T.; Suga, H. Thermal and Structural Characterization of Trimethylolethane Trihydrate. Thermochim. Acta 2002, 387, 39–45. [Google Scholar] [CrossRef]
- Koyama, R.; Yuta, A.; Yuji, Y.; Satoshi, T.; Fuyuaki, E.; Atsushi, H.; Ohmura, R. Thermophysical Properties of Trimethylolethane (TME) Hydrate as Phase Change Material for Cooling Lithium-Ion Battery in Electric Vehicle. J. Power Sources 2019, 427, 70–76. [Google Scholar] [CrossRef]
- Oyama, H.; Shimada, W.; Ebinuma, T.; Kamata, Y.; Takeya, S.; Uchida, T.; Nagao, J.; Narita, H. Phase Diagram, Latent Heat, and Specific Heat of TBAB Semiclathrate Hydrate Crystals. Fluid Phase Equilibr. 2005, 234, 131–135. [Google Scholar] [CrossRef]
- Rodionova, T.; Komarov, V.; Villevald, G.; Aladko, L.; Karpova, T.; Manakov, A. Calorimetric and Structural Studies of Tetrabutylammonium Chloride Ionic Clathrate Hydrates. J. Phys. Chem. B 2010, 114, 11838–11846. [Google Scholar] [CrossRef] [PubMed]
- Sloan, E.D., Jr.; Koh, C.A. Clathrate Hydrates of Natural Gases, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Kvamme, B.; Coffin, R.B.; Zhao, J.; Wei, N.; Zhou, S.; Li, Q.; Saeidi, N.; Chien, Y.-C.; Dunn-Rankin, D.; Sun, W.; et al. Stages in the Dynamics of Hydrate Formation and Consequences for Design of Experiments for Hydrate Formation in Sediments. Energies 2019, 12, 3399. [Google Scholar] [CrossRef] [Green Version]
- Kvamme, B. Consistent Thermodynamic Calculations for Hydrate Properties and Hydrate Phase Transitions. J. Chem. Eng. Data 2020, 65, 2872–2893. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, W.; Lang, C.; Liu, R.; Song, Y.; Li, Y. Viscosity Investigation on Metastable Hydrate Suspension in Oil-Dominated Systems. Chem. Eng. Sci. 2021, 238, 116608. [Google Scholar] [CrossRef]
- Zhao, J.; Liang, H.; Yang, L.; Zhang, X.; Song, Y.; Sum, A.K. Growth Kinetics and Gas Diffusion in Formation of Gas Hydrates from Ice. J. Phys. Chem. C 2020, 124, 12999–13007. [Google Scholar] [CrossRef]
- Zhang, L.; Kuang, Y.; Dai, S.; Wang, J.; Zhao, J.; Song, Y. Kinetic Enhancement of Capturing and Storing Greenhouse Gas and Volatile Organic Compound: Micro-Mechanism and Micro-Structure of Hydrate Growth. Chem. Eng. J. 2020, 379, 122357. [Google Scholar] [CrossRef]
- Li, Y.; Wu, P.; Sun, X.; Liu, W.; Song, Y. Mechanical Behaviors of Hydrate-Bearing Sediment with Different Cementation Spatial Distributions at Microscales. iScience 2021, 24, 102448. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.L. Natural Gas Hydrates: Properties, Occurrence and Recovery; Butterworth: Boston, MA, USA, 1983. [Google Scholar]
- Sa, J.H.; Sum, A.K. Promoting Gas Hydrate Formation with Ice-Nucleating Additives for Hydrate-Based Applications. Appl. Energy 2019, 251, 113352. [Google Scholar] [CrossRef]
- Hwang, M.J.; Wright, D.A.; Kapur, A.; Holder, G.D. An Experimental Study of Crystallization and Crystal Growth of Methane Hydrates from Melting Ice. J. Incl. Phenom. Mol. Recognit. Chem. 1990, 8, 103–116. [Google Scholar] [CrossRef]
- Liu, Z.; Song, Y.; Liu, W.; Lang, C.; Zhao, J.; Li, Y. Formation of Methane Hydrate in Oil–Water Emulsion Governed by the Hydrophilic and Hydrophobic Properties of Non-Ionic Surfactants. Energy Fuels 2019, 33, 5777–5784. [Google Scholar] [CrossRef]
- Foroutan, S.; Mohsenzade, H.; Dashti, A.; Roosta, H. New Insights into The Evaluation of Kinetic Hydrate Inhibitors and Energy Consumption in Rocking and Stirred Cells. Energy 2021, 218, 119507. [Google Scholar] [CrossRef]
- Golombok, M.; Ineke, E.; Luzardo, J.; Yuan, Y.H.; Zitha, P. Resolving CO2 and Methane Hydrate Formation Kinetics. Environ. Chem. Lett. 2009, 7, 325–330. [Google Scholar] [CrossRef]
- Karamoddin, M.; Varaminian, F.; Daraee, M. Kinetic Study on the Process of CHClF2 (R22) Hydrate Formation in The Presence of SDS Surfactant Based on Chemical Affinity. J. Nat. Gas. Sci. Eng. 2014, 19, 46–51. [Google Scholar] [CrossRef]
- Zhang, J.S.; Lo, C.; Somasundaran, P.; Lee, J.W. Competitive Adsorption between SDS and Carbonate on Tetrahydrofuran Hydrates. J. Colloid Interface Sci. 2010, 341, 286–288. [Google Scholar] [CrossRef]
- Wang, F.; Meng, H.; Guo, G.; Luo, S.; Guo, R. Methane Hydrate Formation Promoted by −SO3−-Coated Graphene Oxide Nanosheets. ACS Sustain. Chem. Eng. 2017, 5, 6597–6604. [Google Scholar] [CrossRef]
- Yan, S.; Dai, W.; Wang, S.; Rao, Y.; Zhou, S. Graphene Oxide: An Effective Promoter for CO2 Hydrate Formation. Energies 2018, 11, 1756. [Google Scholar] [CrossRef] [Green Version]
- Zhou, S.; Yu, Y.; Zhao, M.; Wang, S.; Zhang, G. Effect of Graphite Nanoparticles on Promoting CO2 Hydrate Formation. Energy. Fuels 2014, 28, 4694–4698. [Google Scholar] [CrossRef]
- Hosseini, M.; Ghozatloo, A.; Niassar, M.S. Effect of CVD Graphene on Hydrate Formation of Natural Gas. J. Nanostructure Chem. 2015, 5, 219–226. [Google Scholar] [CrossRef] [Green Version]
- Ohmura, R.; Matsuda, S.; Uchida, T.; Ebinuma, T.; Narita, H. Clathrate Hydrate Crystal Growth in Liquid Water Saturated with a Guest Substance: Observations in a Methane + Water System. Cryst. Growth Des. 2005, 5, 953–957. [Google Scholar] [CrossRef]
- Yasuda, K.; Ohmura, R. Phase Equilibrium for Clathrate Hydrates Formed with Methane, Ethane, Propane, or Carbon Dioxide at Temperatures below the Freezing Point of Water. J. Chem. Eng. Data 2008, 53, 2182–2188. [Google Scholar] [CrossRef]
- Koyanagi, S.; Ohmura, R. Crystal Growth of Ionic Semiclathrate Hydrate Formed in CO2 Gas + Tetrabutylammonium Bromide Aqueous Solution System. Cryst. Growth Des. 2013, 13, 2087–2093. [Google Scholar] [CrossRef]
- Akiba, H.; Ueno, H.; Ohmura, R. Crystal Growth of Ionic Semiclathrate Hydrate Formed at the interface between CO2 Gas and Tetra-n-butylammonium Bromide Aqueous Solution. Cryst. Growth Des. 2015, 3963–3968. [Google Scholar] [CrossRef]
- Makogon, T.Y.; Larsen, R.; Knight, C.A.; Sloan, E.D. Melt growth of tetrahydrofuran clathrate hydrate and its inhibition: Method and first results. J. Cryst. Growth. 1997, 179, 258–262. [Google Scholar] [CrossRef]
- Delahaye, A.; Fournaison, L.; Guilpart, J. Characterisation of Ice and THF Hydrate Slurry Crystal Size Distribution by Microscopic Observation Method. Int. J. Refrig. 2010, 33, 1639–1647. [Google Scholar] [CrossRef]
- Tanaka, R.; Sakemoto, R.; Ohmura, R. Crystal Growth of Clathrate Hydrates Formed at the Interface of Liquid Water and Gaseous Methane, Ethane, or Propane: Variations in Crystal Morphology. Cryst. Growth Des. 2009, 9, 2529–2536. [Google Scholar] [CrossRef]
- Peng, L.; Ning, F.L.; Wei, L.I.; Cao, P.Q.; Liu, Z.C.; Wang, D.D.; Zhang, Z.; Sun, J.X.; Zhang, L.; Jiang, G.S.; et al. Investigation on the Effect of growth temperature and contact interface on surface characteristics of THF clathrate hydrates by Atomic Force Microscopy. Sci. Sin. Phys. Mech. Astron. 2019, 49, 034612. [Google Scholar] [CrossRef]
- Kuang, Y.; Feng, Y.; Yang, L.; Song, Y.; Zhao, J. Effects of Micro-Bubbles on the Nucleation and Morphology of Gas Hydrate Crystals. Phys. Chem. Chem. Phys. 2019, 21, 23401–23407. [Google Scholar] [CrossRef]
- Ozawa, K.; Ohmura, R. Crystal Growth of Clathrate Hydrate with Methane plus Partially Water Soluble Large-Molecule Guest Compound. Cryst. Growth Des. 2019, 19, 1689–1694. [Google Scholar] [CrossRef]
- Tanasawa, I.; Takao, S. Clathrate Hydrate Slurry of Tetra-n-Butylammonium Bromide as A Cold-Storage Material. In Proceedings of the 4th International Conference for Global Health (ICGH), Yokohama, Japan, 26–28 September 2002. [Google Scholar]
- Martínez, M.C.; Dalmazzone, D.; Fürst, W.; Delahaye, A.; Fournaison, L. Thermodynamic Properties of THF + CO2 Hydrates in Relation with Refrigeration Applications. AIChE J. 2010, 54, 1088–1095. [Google Scholar] [CrossRef]

| Guest Molecule | State | Water Miscibility | Molar Mass (g/mol) | Hydration Number | Dissociation Temperature (°C) | Dissociation Heat (kJ/kg) | Dissociation Pressure (MPa) |
|---|---|---|---|---|---|---|---|
| R141b | Liquid | No | 116.94 | 17 a | 6.5 b | 330 b | 0.1 |
| TME | Powder | Yes | 120.15 | 3 c | 10.4–29.7 d | 113.6 ± 5.9–282.5 ± 4.7 d | 0.1 |
| TBAB | Powder | Yes | 322.37 | 26 or 38 e | 12.3 f | 184.8 ± 1.9 f | 0.1 |
| TBAC | Powder | Yes | 277.92 | 32.7 or 30.4 g | 15.0 f | 186.7 ± 1.9 f | 0.1 |
| TBAB/TBAC | Powder | Yes | — | — | 12.3–15.3 f | 184.8 ± 1.9–205.0 ± 4.0 | 0.1 |
| Case | Guest Working Substance | Concentration (wt%) | Subcooling (°C) | Experimental System |
|---|---|---|---|---|
| Case 1 | R141b | 27.65 | 1.5 | Pure water |
| Case 2 | R141b | 27.65 | 1.5 | Addition of 1 cm3 ice cube |
| Case 3 | TME | 36.36 | 16.8 | Pure water |
| Case 4 | TME | 36.36 | 16.8 | Addition of 1 cm3 ice cube |
| Case 5 | TBAB/TBAC | 40.00/36.50 | 10.1 | Pure water |
| Case 6 | TBAB/TBAC | 40.00/36.50 | 10.1 | Addition of 1 cm3 ice cube |
| Case 7 | TBAB/TBAC | 40.00/36.50 | 10.1 | Stirring at 500 rpm |
| Case 8 | TBAB/TBAC | 40.00/36.50 | 10.1 | Stirring at 500 rpm + 0.1 g graphene |
| Case | Whether There Is Formation | Induction Time (h) | Formation Time (h) |
|---|---|---|---|
| Case 1 | No | — | — |
| Case 2 | Yes | 0.17 | 0.58 |
| Case 3 | Yes | 3.00 | 1.17 |
| Case 4 | Yes | 0.17 | 0.33 |
| Case 5 | No | — | — |
| Case 6 | Yes | 0.08 | 0.25 |
| Case 7 | Yes | 9.01 | 0.62 |
| Case 8 | Yes | 9.67 | 0.53 |
| Number of Times | Induction Time (h) | Dissociation Time (h) | ||||
|---|---|---|---|---|---|---|
| Addition of 1 cm3 Ice Cube | Stirring at 500 rpm | Stirring at 500 rpm + 0.1 g Graphene | Addition of 1 cm3 Ice Cube | Stirring at 500 rpm | Stirring at 500 rpm + 0.1 g Graphene | |
| 1 | 0.08 | 9.01 | 9.67 | 6.19 | 7.17 | 6.54 |
| 2 | 3.90 | 4.20 | 4.42 | 5.92 | 6.83 | 6.11 |
| 3 | 2.17 | 2.23 | 2.43 | 4.58 | 4.58 | 5.58 |
| 4 | 0.92 | 1.08 | 2.33 | 1.67 | 4.34 | 5.92 |
| 5 | 1.8 | 1.80 | 2.32 | 1.67 | 4.08 | 4.58 |
| 6 | 0.88 | 1.22 | 0.88 | 1.83 | 3.45 | 1.83 |
| 7 | 0.62 | 1.00 | 1.00 | 1.50 | 1.50 | 1.17 |
| 8 | 0.60 | 1.17 | 1.17 | 1.42 | 1.42 | 1.18 |
| 9 | 0.58 | 1.08 | 1.08 | 1.28 | 1.25 | 1.25 |
| 10 | 0.75 | 0.75 | 0.83 | 1.42 | 1.33 | 1.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Han, B.; Lang, C.; Wen, M.; Fan, B.; Liu, Z. Hydrates for Cold Storage: Formation Characteristics, Stability, and Promoters. Appl. Sci. 2021, 11, 10470. https://doi.org/10.3390/app112110470
Chen H, Han B, Lang C, Wen M, Fan B, Liu Z. Hydrates for Cold Storage: Formation Characteristics, Stability, and Promoters. Applied Sciences. 2021; 11(21):10470. https://doi.org/10.3390/app112110470
Chicago/Turabian StyleChen, Huan, Bingyue Han, Chen Lang, Min Wen, Baitao Fan, and Zheyuan Liu. 2021. "Hydrates for Cold Storage: Formation Characteristics, Stability, and Promoters" Applied Sciences 11, no. 21: 10470. https://doi.org/10.3390/app112110470
APA StyleChen, H., Han, B., Lang, C., Wen, M., Fan, B., & Liu, Z. (2021). Hydrates for Cold Storage: Formation Characteristics, Stability, and Promoters. Applied Sciences, 11(21), 10470. https://doi.org/10.3390/app112110470





