Spatiotemporal Protein Expression Profiles of QSOX1 in the Murine Uterus, Placenta, and Embryo during Pregnancy
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Tissue Collection
2.2. QSOX1 Antiserum
2.3. Western Blot Analysis
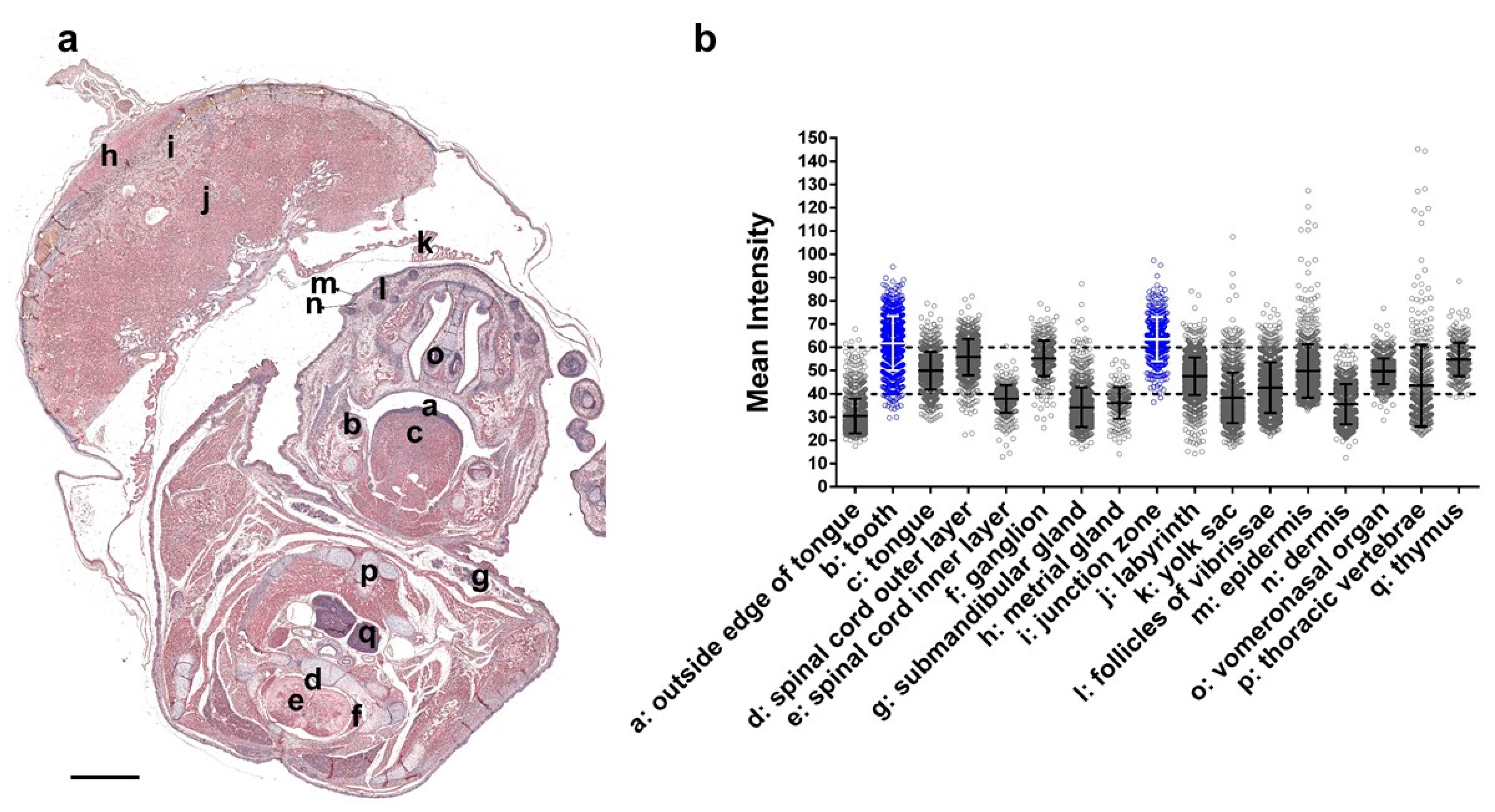
2.4. Immunohistochemical Staining and Quantification
2.5. Statistical Analysis
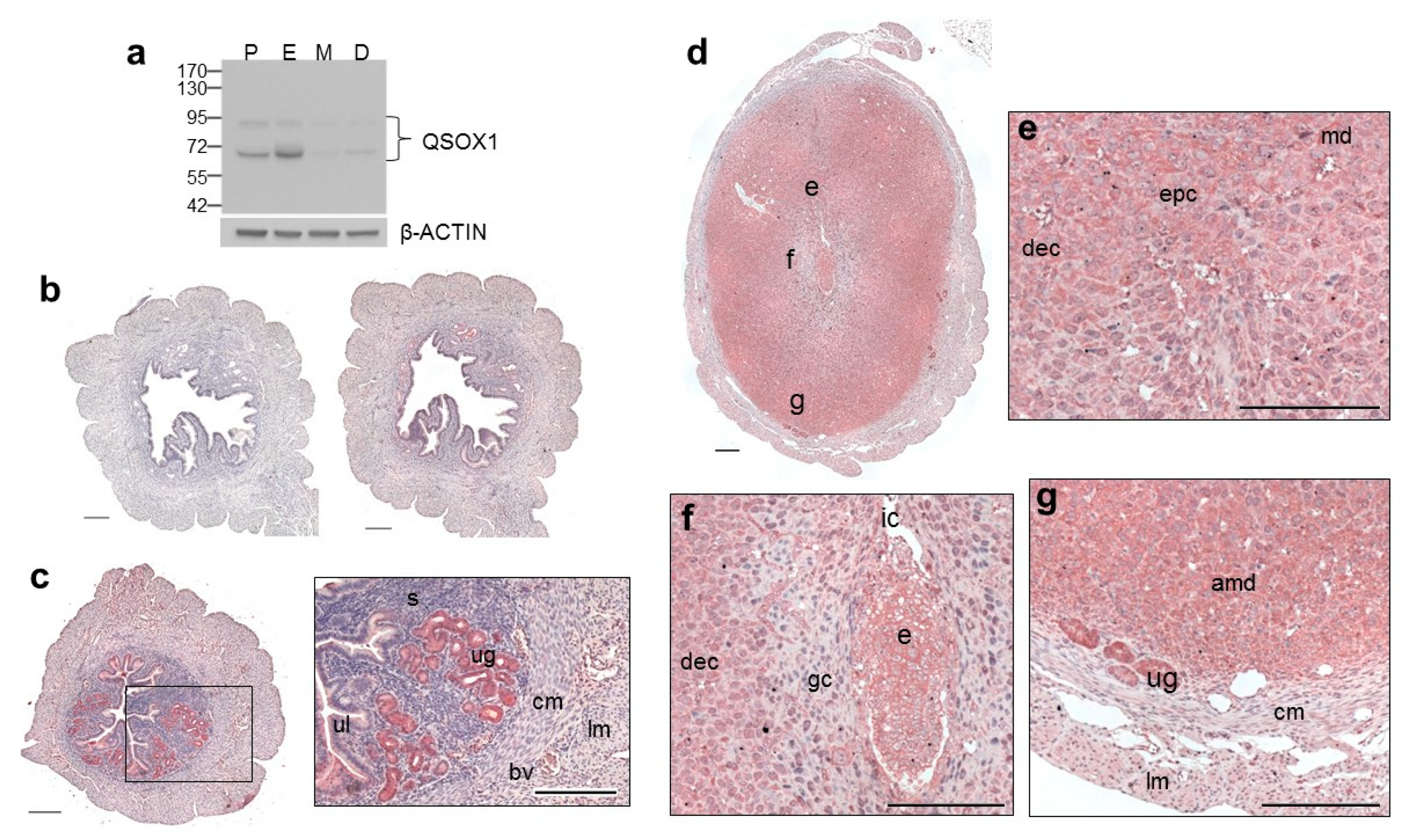
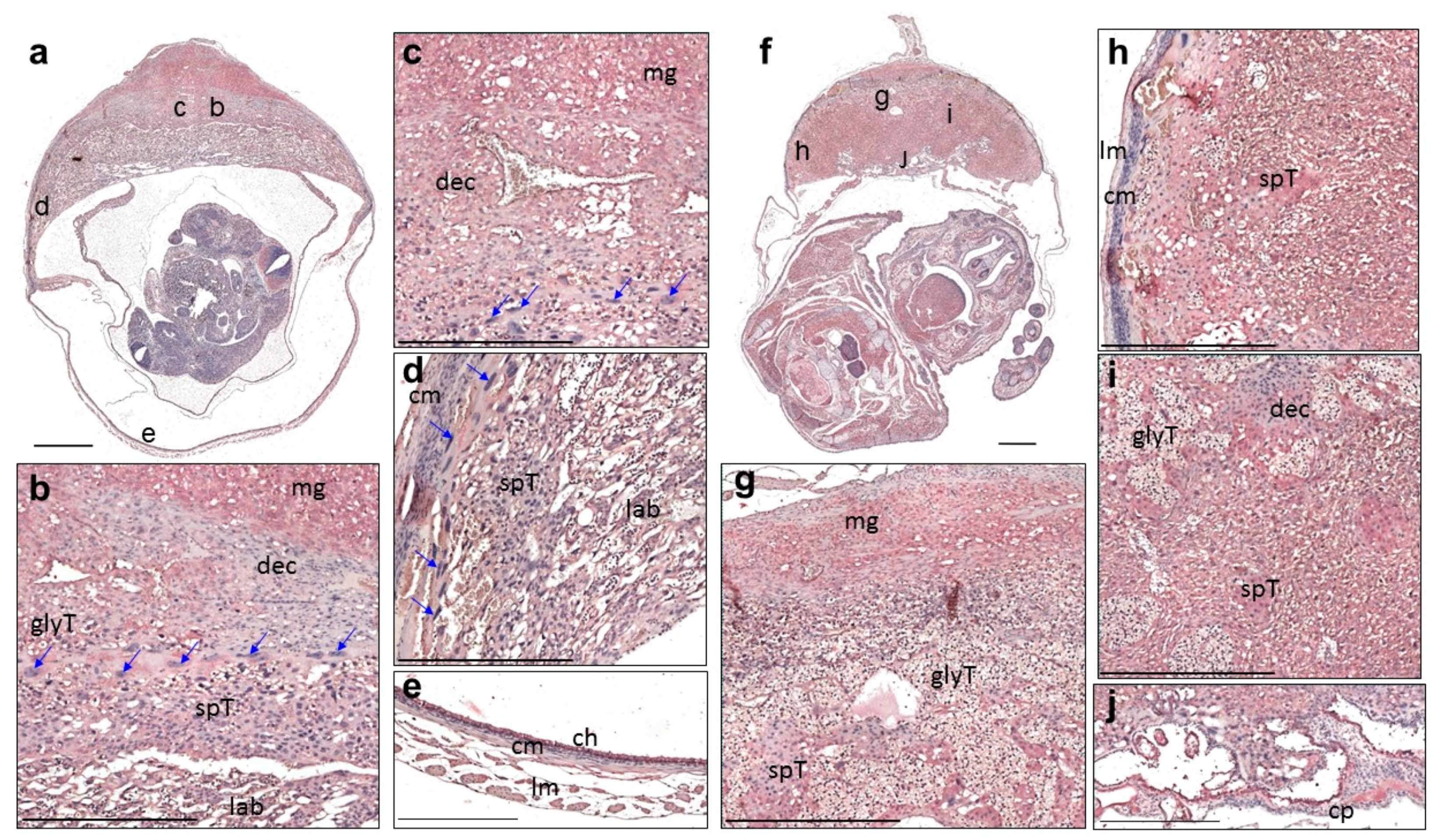
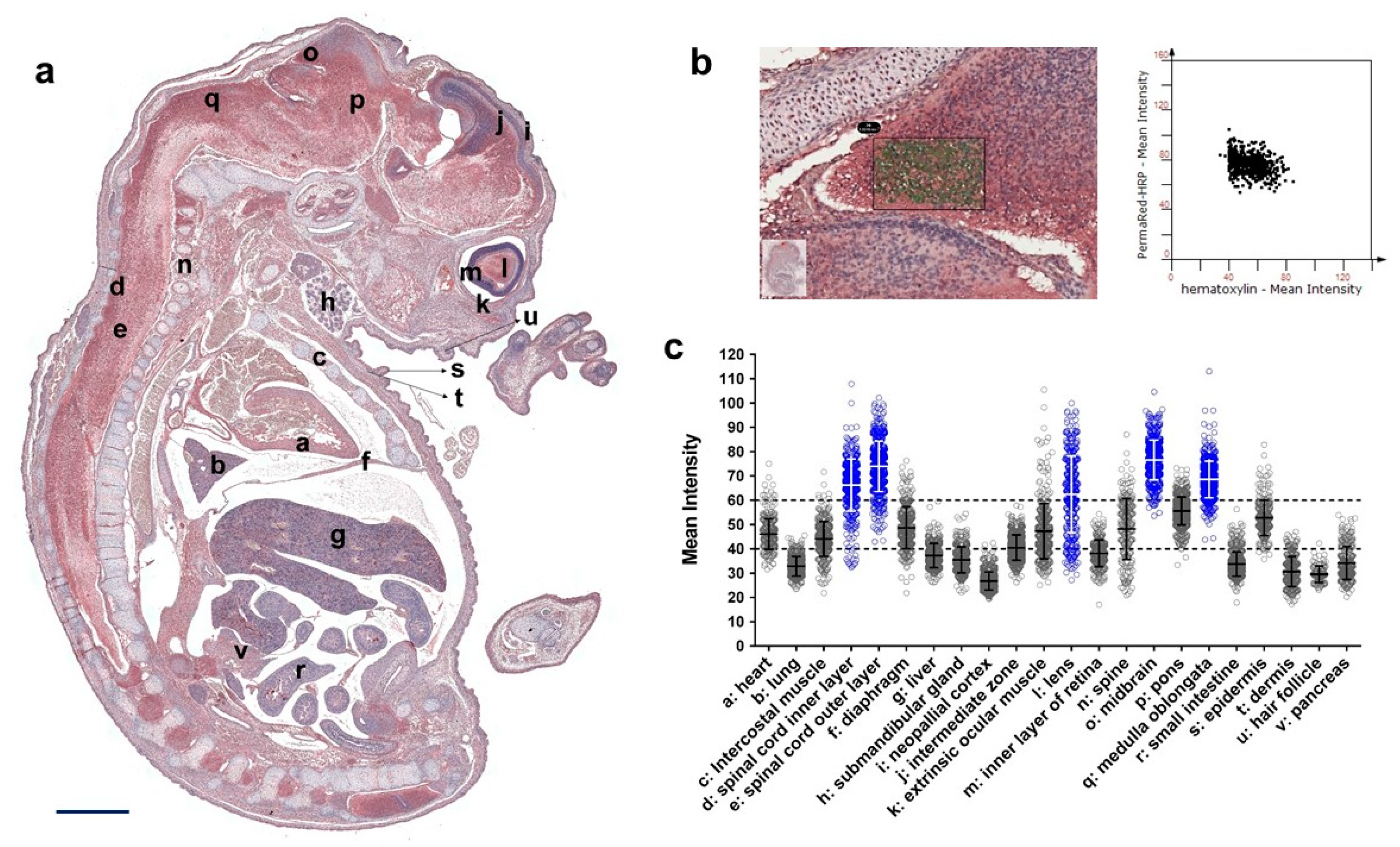
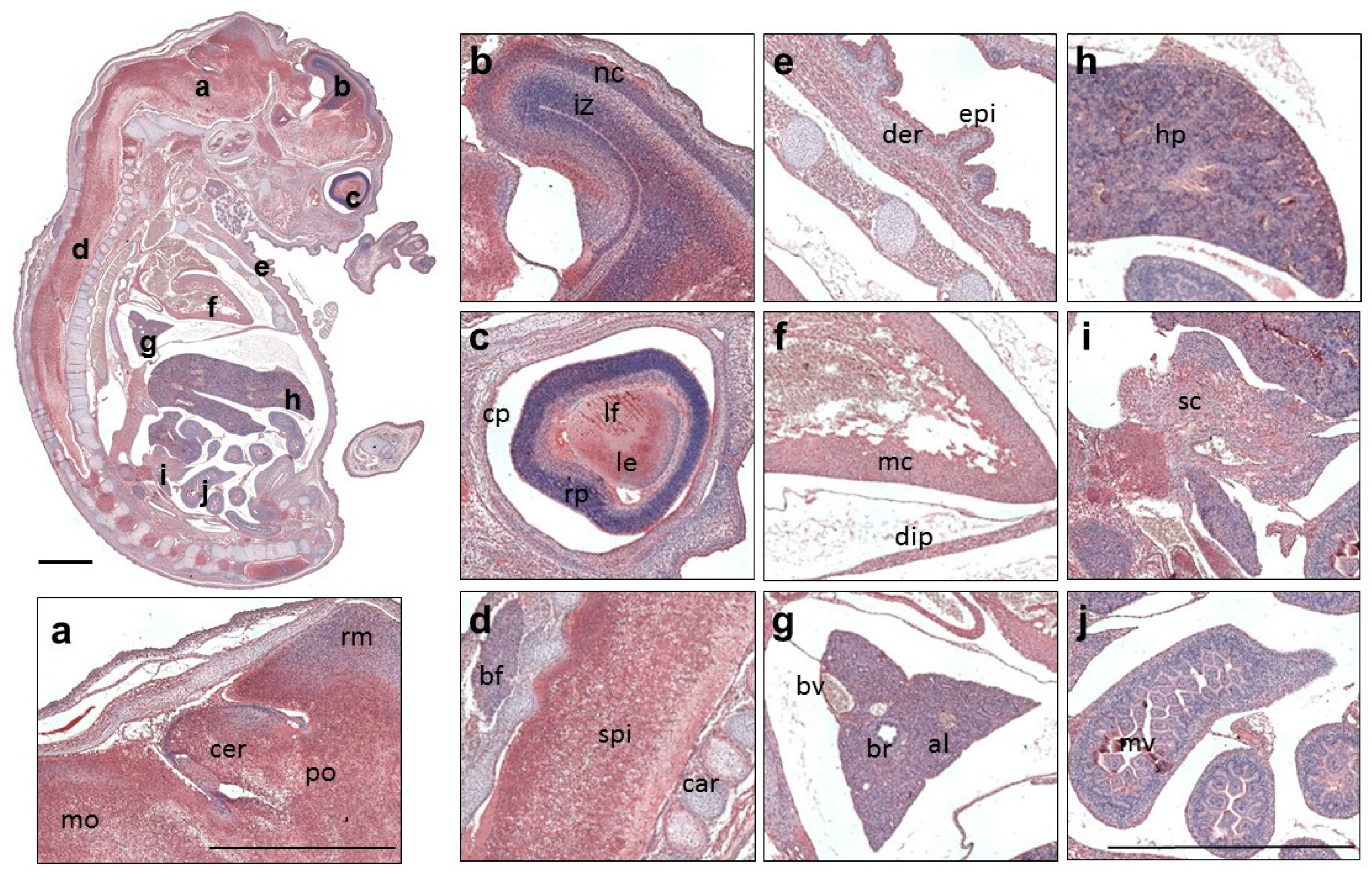
3. Results
3.1. Expression of QSOX1 in the Non-Pregnant and Early Pregnant Uterus
3.2. Expression of QSOX1 in the Pregnant Uterus and Placenta at 9.5 to 17.5 dpc
3.3. Expression Profile of QSOX1 in the Mouse Embryo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ostrowski, M.; Kistler, W.; Williams-Ashman, H. A flavoprotein responsible for the intense sulfhydryl oxidase activity of rat seminal vesicle secretion. Biochem. Biophys. Res. Commun. 1979, 87, 171–176. [Google Scholar] [CrossRef]
- Hoober, K.L.; Glynn, N.M.; Burnside, J.; Coppock, D.L.; Thorpe, C. Homology between Egg White Sulfhydryl Oxidase and Quiescin Q6 Defines a New Class of Flavin-linked Sulfhydryl Oxidases. J. Biol. Chem. 1999, 274, 31759–31762. [Google Scholar] [CrossRef][Green Version]
- Hoober, K.L.; Sheasley, S.L.; Gilbert, H.F.; Thorpe, C. Sulfhydryl Oxidase from Egg White A facile catalyst for disulfide bond formation in proteins and peptides. J. Biol. Chem. 1999, 274, 22147–22150. [Google Scholar] [CrossRef]
- Rudolf, J.; Pringle, M.A.; Bulleid, N.J. Proteolytic processing of QSOX1A ensures efficient secretion of a potent disulfide catalyst. Biochem. J. 2013, 454, 181–190. [Google Scholar] [CrossRef]
- Israel, B.A.; Jiang, L.; Gannon, S.A.; Thorpe, C. Disulfide bond generation in mammalian blood serum: Detection and purification of quiescin-sulfhydryl oxidase. Free. Radic. Biol. Med. 2014, 69, 129–135. [Google Scholar] [CrossRef][Green Version]
- Wang, T.-E.; Yeh, L.-Y.; Kuo-Kuang Lee, R.; Lu, C.-H.; Yang, T.-H.; Kuo, Y.-W.; Joshi, R.; Tsai, P.-S.; Li, S.-H. Secretory mouse quiescin sulfhydryl oxidase 1 aggregates defected human and mouse spermatozoa in vitro and in vivo. iScience 2021, 103167. [Google Scholar] [CrossRef]
- Jaje, J.; Wolcott, H.; Fadugba, O.; Cripps, D.; Yang, A.J.; Mather, I.H.; Thorpe, C. A Flavin-Dependent Sulfhydryl Oxidase in Bovine Milk. Biochemistry 2007, 46, 13031–13040. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tury, A.; Mairet-Coello, G.; Esnard-Fève, A.; Benayoun, B.; Risold, P.-Y.; Griffond, B.; Fellmann, D. Cell-specific localization of the sulphydryl oxidase QSOX in rat peripheral tissues. Cell Tissue Res. 2006, 323, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Lake, D.F.; Faigel, U.O. The Emerging Role of QSOX1 in Cancer. Antioxid. Redox Signal. 2014, 21, 485–496. [Google Scholar] [CrossRef]
- Wang, T.-E.; Li, S.-H.; Minabe, S.; Anderson, A.L.; Dun, M.D.; Maeda, K.-I.; Matsuda, F.; Chang, H.-W.; Nixon, B.; Tsai, P.-S.J. Mouse quiescin sulfhydryl oxidases exhibit distinct epididymal luminal distribution with segment-specific sperm surface associations†. Biol. Reprod. 2018. [Google Scholar] [CrossRef]
- Balu, R.; Ramachandran, S.S.; Paramasivam, S.G. Evidence for mouse sulfhydryl oxidase-assisted cross-linking of major seminal vesicle proteins. Mol. Reprod. Dev. 2019, 86, 1682–1693. [Google Scholar] [CrossRef] [PubMed]
- Musard, J.-F.; Sallot, M.; Dulieu, P.; Fraîchard, A.; Ordener, C.; Remy-Martin, J.-P.; Jouvenot, M.; Adami, P. Identification and Expression of a New Sulfhydryl Oxidase SOx-3 during the Cell Cycle and the Estrus Cycle in Uterine Cells. Biochem. Biophys. Res. Commun. 2001, 287, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, C.; Coppock, D.L. Generating Disulfides in Multicellular Organisms: Emerging Roles for a New Flavoprotein Family. J. Biol. Chem. 2007, 282, 13929–13933. [Google Scholar] [CrossRef]
- Li, J.; Tong, C.; Xu, P.; Wang, L.; Han, T.-L.; Wen, L.; Luo, X.; Tan, B.; Zhu, F.; Gui, S.; et al. QSOX1 regulates trophoblastic apoptosis in preeclampsia through hydrogen peroxide production. J. Matern. Neonatal Med. 2018, 32, 3708–3715. [Google Scholar] [CrossRef] [PubMed]
- Portes, K.F.; Ikegami, C.M.; Getz, J.; Martins, A.P.; De Noronha, L.; Zischler, L.F.; Klassen, G.; Camargo, A.A.; Zanata, S.M.; Bevilacqua, E.; et al. Tissue distribution of quiescin Q6/sulfhydryl oxidase (QSOX) in developing mouse. J. Mol. Histol. 2007, 39, 217–225. [Google Scholar] [CrossRef]
- Bertolin, K.; Murphy, B.D. 7-Reproductive Tract Changes During the Mouse Estrous Cycle. In The Guide to Investigation of Mouse Pregnancy; Croy, B.A., Yamada, A.T., DeMayo, F.J., Adamson, Eds.; Academic Press: Boston, MA, USA, 2014; pp. 85–94. ISBN 978-0-12-394445-0. [Google Scholar]
- Koto, M.; Miwa, M.; Togashi, M.; Tsuji, K.; Okamoto, M.; Adachi, J. A Method for Detecting the Optimum Day for Mating during 4-day Estrous Cycle in the Rat; Measuring the Value of Electrical Impedance of Vagina. Exp. Anim. 1987, 36, 195–198. [Google Scholar] [CrossRef]
- Chern, S.-R.; Li, S.-H.; Lu, C.-H.; Chen, E.I.T. Spatiotemporal expression of the serine protease inhibitor, SERPINE2, in the mouse placenta and uterus during the estrous cycle, pregnancy, and lactation. Reprod. Biol. Endocrinol. 2010, 8, 127. [Google Scholar] [CrossRef] [PubMed]
- Mairet-Coello, G.; Tury, A.; Esnard-Feve, A.; Fellmann, D.; Risold, P.-Y.; Griffond, B. FAD-linked sulfhydryl oxidase QSOX: Topographic, cellular, and subcellular immunolocalization in adult rat central nervous system. J. Comp. Neurol. 2004, 473, 334–363. [Google Scholar] [CrossRef] [PubMed]
- Mairet-Coello, G.; Tury, A.; Fellmann, D.; Risold, P.-Y.; Griffond, B. Ontogenesis of the sulfhydryl oxidase QSOX expression in rat brain. J. Comp. Neurol. 2005, 484, 403–417. [Google Scholar] [CrossRef]
- Matsuba, S.; Suga, Y.; Ishidoh, K.; Hashimoto, Y.; Takamori, K.; Kominami, E.; Wilhelm, B.; Seitz, J.; Ogawa, H. Sulfhydryl oxidase (SOx) from mouse epidermis: Molecular cloning, nucleotide sequence, and expression of recombinant protein in the cultured cells. J. Dermatol. Sci. 2002, 30, 50–62. [Google Scholar] [CrossRef]
- A Katchman, B.; Ocal, I.T.; E Cunliffe, H.; Chang, Y.-H.; Hostetter, G.; Watanabe, A.; LoBello, J.; Lake, D.F. Expression of quiescin sulfhydryl oxidase 1 is associated with a highly invasive phenotype and correlates with a poor prognosis in Luminal B breast cancer. Breast Cancer Res. 2013, 15, R28. [Google Scholar] [CrossRef]
- Katchman, B.A.; Antwi, K.; Hostetter, G.; Demeure, M.J.; Watanabe, A.; Decker, G.A.; Miller, L.J.; Von Hoff, D.D.; Lake, D.F. Quiescin Sulfhydryl Oxidase 1 Promotes Invasion of Pancreatic Tumor Cells Mediated by Matrix Metalloproteinases. Mol. Cancer Res. 2011, 9, 1621–1631. [Google Scholar] [CrossRef]
- Price, S.R. Cell adhesion and migration in the organization of spinal motor neurons. Cell Adhes. Migr. 2012, 6, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, S.; Song, Y.; Han, J.; Chai, Y.; Chen, Y. Timing of odontogenic neural crest cell migration and tooth-forming capability in mice. Dev. Dyn. 2003, 226, 713–718. [Google Scholar] [CrossRef]
- Cvekl, A.; Ashery-Padan, R. The cellular and molecular mechanisms of vertebrate lens development. Development 2014, 141, 4432–4447. [Google Scholar] [CrossRef]
- Katreddi, R.R.; Forni, P.E. Mechanisms underlying pre- and postnatal development of the vomeronasal organ. Cell. Mol. Life Sci. 2021, 78, 5069–5082. [Google Scholar] [CrossRef] [PubMed]
- El-Hashash, A.H.; Warburton, D.; Kimber, S.J. Genes and signals regulating murine trophoblast cell development. Mech. Dev. 2010, 127, 1–20. [Google Scholar] [CrossRef]
- DeSimone, D.; Mecham, R. Extracellular Matrix in Development; The Company of Biologists Ltd.: Cambridge, UK, 2013; ISBN 978-3-642-35934-7. [Google Scholar]
- Ilani, T.; Alon, A.; Grossman, I.; Horowitz, B.; Kartvelishvily, E.; Cohen, S.R.; Fass, D. A Secreted Disulfide Catalyst Controls Extracellular Matrix Composition and Function. Science 2013, 341, 74–76. [Google Scholar] [CrossRef] [PubMed]
- Borges, B.E.; Appel, M.H.; Cofré, A.R.; Prado, M.L.; Steclan, C.A.; Esnard, F.; Zanata, S.M.; Laurindo, F.R.; Nakao, L.S. The flavo-oxidase QSOX1 supports vascular smooth muscle cell migration and proliferation: Evidence for a role in neointima growth. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2015, 1852, 1334–1346. [Google Scholar] [CrossRef]
- Chern, S.-R.; Li, S.-H.; Chiu, C.-L.; Chang, H.-H.; Chen, C.-P.; Chen, E.I.T. Spatiotemporal expression of SERPINE2 in the human placenta and its role in extravillous trophoblast migration and invasion. Reprod. Biol. Endocrinol. 2011, 9, 106. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.-S.; Lee, R.K.-K.; Yang, T.-H.; Fang, H.-W.; Li, S.-H. Spatiotemporal Protein Expression Profiles of QSOX1 in the Murine Uterus, Placenta, and Embryo during Pregnancy. Appl. Sci. 2021, 11, 10151. https://doi.org/10.3390/app112110151
Lin H-S, Lee RK-K, Yang T-H, Fang H-W, Li S-H. Spatiotemporal Protein Expression Profiles of QSOX1 in the Murine Uterus, Placenta, and Embryo during Pregnancy. Applied Sciences. 2021; 11(21):10151. https://doi.org/10.3390/app112110151
Chicago/Turabian StyleLin, Hung-Shih, Robert Kuo-Kuang Lee, Tsung-Hsien Yang, Hsu-Wei Fang, and Sheng-Hsiang Li. 2021. "Spatiotemporal Protein Expression Profiles of QSOX1 in the Murine Uterus, Placenta, and Embryo during Pregnancy" Applied Sciences 11, no. 21: 10151. https://doi.org/10.3390/app112110151
APA StyleLin, H.-S., Lee, R. K.-K., Yang, T.-H., Fang, H.-W., & Li, S.-H. (2021). Spatiotemporal Protein Expression Profiles of QSOX1 in the Murine Uterus, Placenta, and Embryo during Pregnancy. Applied Sciences, 11(21), 10151. https://doi.org/10.3390/app112110151






