Prognostic Value of the Immunohistochemical Expression of Serine and Arginine-Rich Splicing Factor 1 (SRSF1) in Uveal Melanoma: A Clinico-Pathological and Immunohistochemical Study on a Series of 85 Cases
Abstract
:1. Introduction
2. Materials and Methods
2.1. MVD Count and Immunohistochemical Analyses
2.2. Statistical Analysis
3. Results
3.1. Clinico-Pathologic Features of UMs
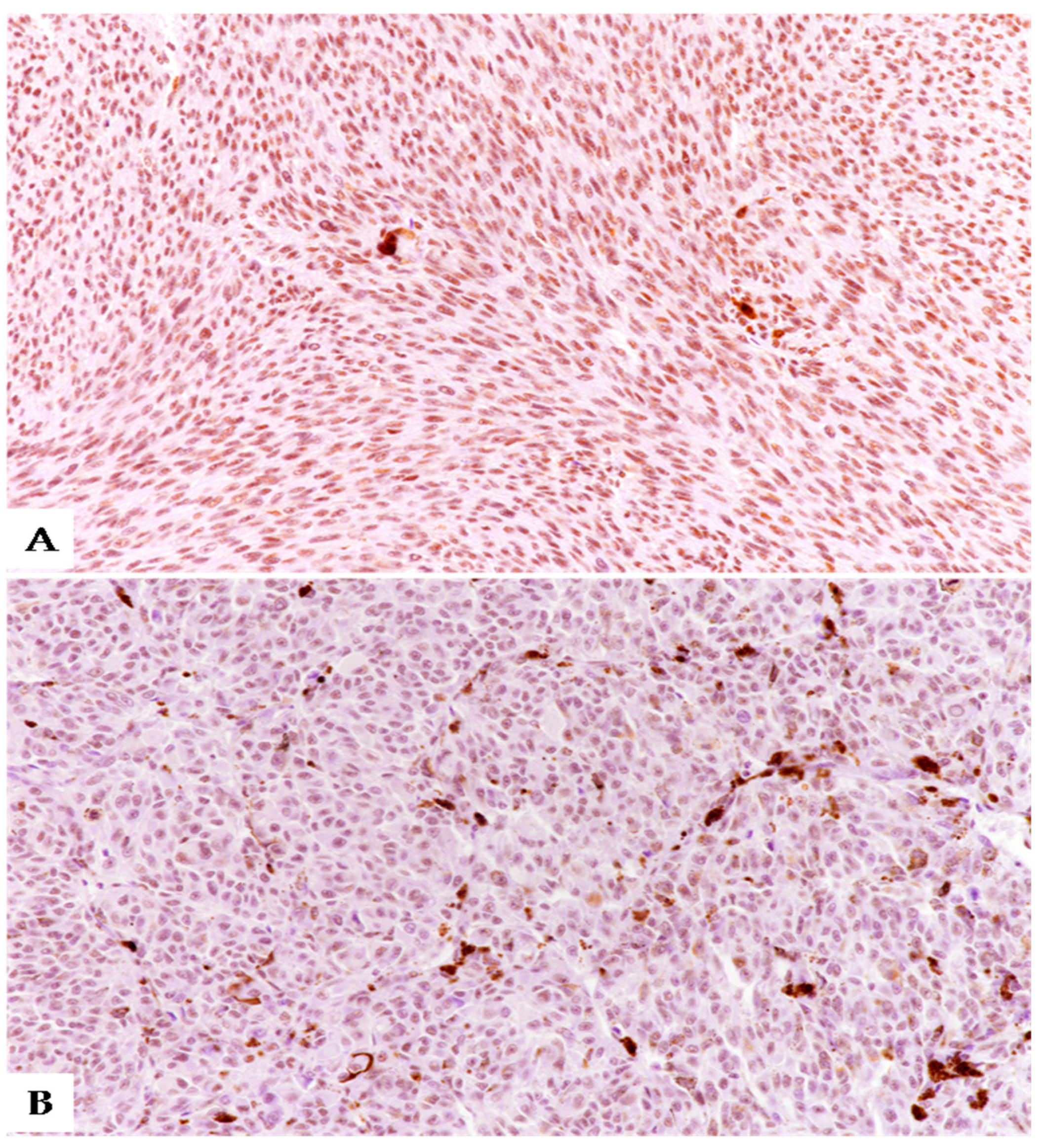
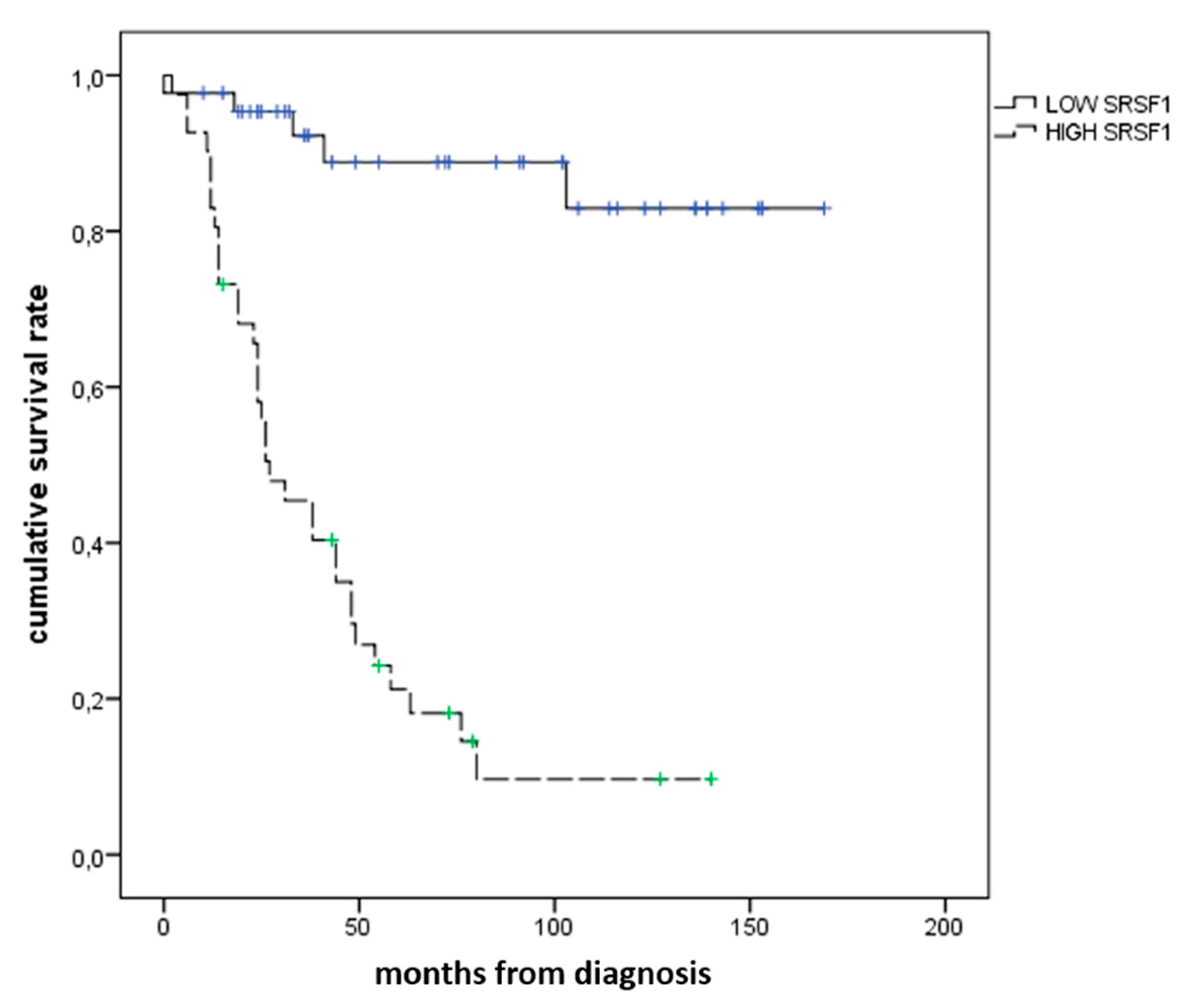
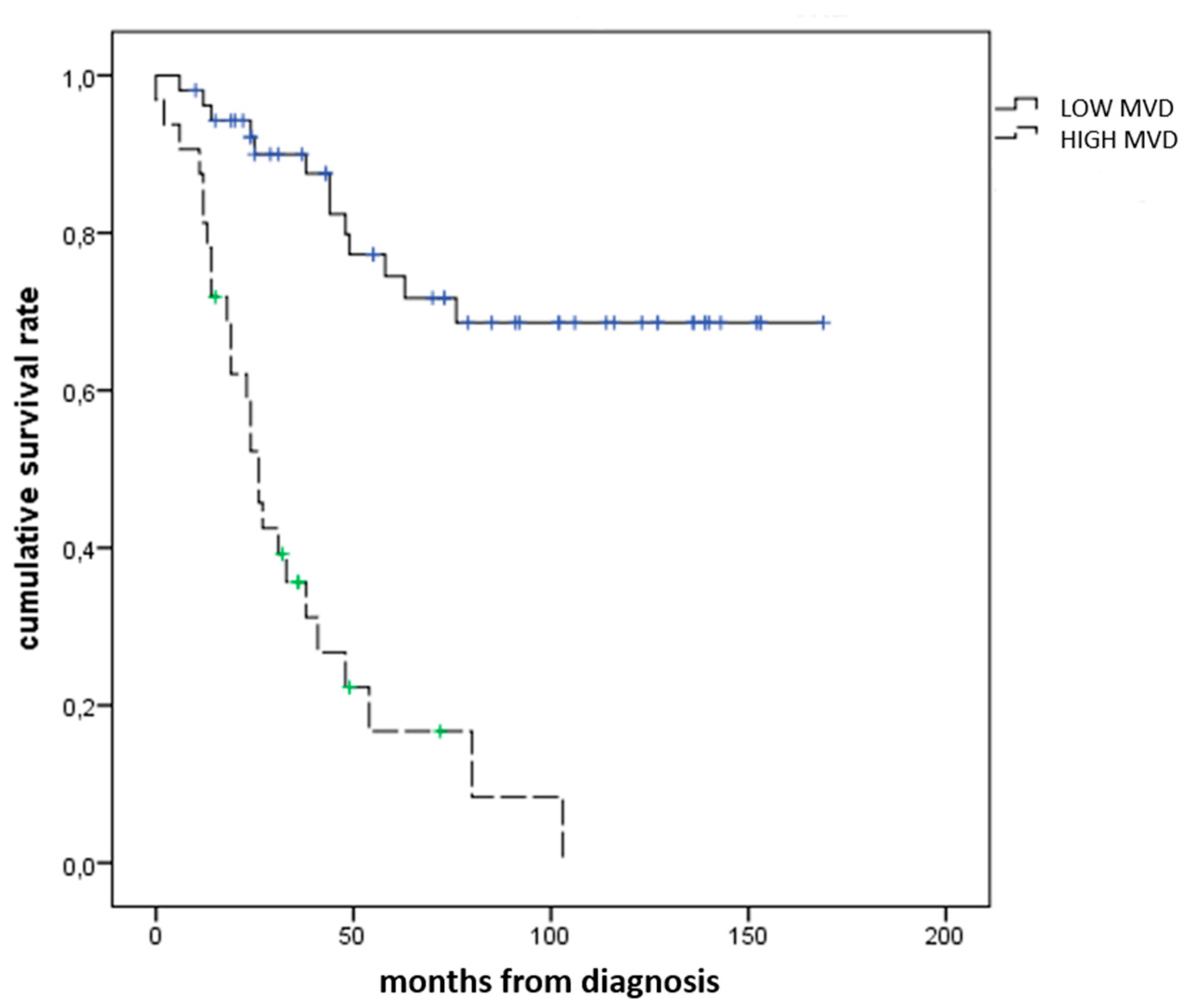
3.2. Immunohistochemical Expression of SRSF1 and MVD Count in UMs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Foti, P.V.; Travali, M.; Farina, R.; Palmucci, S.; Spatola, C.; Raffaele, L.; Salamone, V.; Caltabiano, R.; Broggi, G.; Puzzo, L.; et al. Diagnostic methods and therapeutic options of uveal melanoma with emphasis on MR imaging—Part I: MR imaging with pathologic correlation and technical considerations. Insights Imaging 2021, 12, 1–27. [Google Scholar] [CrossRef]
- Foti, P.V.; Travali, M.; Farina, R.; Palmucci, S.; Spatola, C.; Liardo, R.L.E.; Milazzotto, R.; Raffaele, L.; Salamone, V.; Caltabiano, R.; et al. Diagnostic methods and therapeutic options of uveal melanoma with emphasis on MR imaging—Part II: Treatment indications and complications. Insights Imaging 2021, 12, 1–24. [Google Scholar] [CrossRef]
- Millodot, M.; Hendler, K.; Pe’Er, J. Iris melanoma: A case report and review. Ophthalmic Physiol. Opt. 2006, 26, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Fallico, M.; Raciti, G.; Longo, A.; Reibaldi, M.; Bonfiglio, V.; Russo, A.; Caltabiano, R.; Gattuso, G.; Falzone, L.; Avitabile, T. Current molecular and clinical insights into uveal melanoma (Review). Int. J. Oncol. 2021, 58, 10. [Google Scholar] [CrossRef]
- Broggi, G.; Musumeci, G.; Puzzo, L.; Russo, A.; Reibaldi, M.; Ragusa, M.; Longo, A.; Caltabiano, R. Immunohistochemical Expression of ABCB5 as a Potential Prognostic Factor in Uveal Melanoma. Appl. Sci. 2019, 9, 1316. [Google Scholar] [CrossRef] [Green Version]
- Kaliki, S.; Shields, C.L. Uveal melanoma: Relatively rare but deadly cancer. Eye 2016, 31, 241–257. [Google Scholar] [CrossRef] [Green Version]
- Kaliki, S.; Shields, C.L.; Shields, J.A. Uveal melanoma: Estimating prognosis. Indian J. Ophthalmol. 2015, 63, 93–102. [Google Scholar] [CrossRef]
- Foti, P.; Inì, C.; Travali, M.; Farina, R.; Palmucci, S.; Spatola, C.; Liardo, R.; Milazzotto, R.; Raffaele, L.; Salamone, V.; et al. MR Imaging–Pathologic Correlation of Uveal Melanomas Undergoing Secondary Enucleation after Proton Beam Radiotherapy. Appl. Sci. 2021, 11, 4310. [Google Scholar] [CrossRef]
- Milam, R.W.; Batson, S.A.; Breazzano, M.P.; Ayala-Peacock, D.N.; Daniels, A.B. Modern and Novel Radiotherapy Approaches for the Treatment of Uveal Melanoma. Int. Ophthalmol. Clin. 2017, 57, 11–27. [Google Scholar] [CrossRef]
- Broggi, G.; Salvatorelli, L. Bio-Pathological Markers in the Diagnosis and Therapy of Cancer. Cancers 2020, 12, 3113. [Google Scholar] [CrossRef]
- Falzone, L.; Romano, G.L.; Salemi, R.; Bucolo, C.; Tomasello, B.; Lupo, G.; Anfuso, C.D.; Spandidos, D.; Libra, M.; Candido, S. Prognostic significance of deregulated microRNAs in uveal melanomas. Mol. Med. Rep. 2019, 19, 2599–2610. [Google Scholar] [CrossRef] [Green Version]
- Stålhammar, G.; Grossniklaus, H. Intratumor Heterogeneity in Uveal Melanoma BAP-1 Expression. Cancers 2021, 13, 1143. [Google Scholar] [CrossRef]
- Stålhammar, G.; See, T.R.O.; Phillips, S.; Seregard, S.; Grossniklaus, H.E. Digital Image Analysis of BAP-1 Accurately Predicts Uveal Melanoma Metastasis. Transl. Vis. Sci. Technol. 2019, 8, 11. [Google Scholar] [CrossRef] [Green Version]
- Broggi, G.; Russo, A.; Reibaldi, M.; Russo, D.; Varricchio, S.; Bonfiglio, V.; Spatola, C.; Barbagallo, C.; Foti, P.V.; Avitabile, T.; et al. Histopathology and Genetic Biomarkers of Choroidal Melanoma. Appl. Sci. 2020, 10, 8081. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, R.; Li, X.; Yu, L.; Hua, D.; Sun, C.; Shi, C.; Luo, W.; Rao, C.; Jiang, Z.; et al. Splicing factor SRSF1 promotes gliomagenesis via oncogenic splice-switching of MYO1B. J. Clin. Investig. 2019, 129, 676–693. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Li, X.; Yu, L.; Wang, R.; Hua, D.; Shi, C.; Sun, C.; Luo, W.; Rao, C.; Jiang, Z.; et al. The RNA-binding protein SRSF1 is a key cell cycle regulator via stabilizing NEAT1 in glioma. Int. J. Biochem. Cell Biol. 2019, 113, 75–86. [Google Scholar] [CrossRef]
- Barbagallo, D.; Caponnetto, A.; Cirnigliaro, M.; Brex, D.; Barbagallo, C.; D’Angeli, F.; Morrone, A.; Caltabiano, R.; Barbagallo, G.M.; Ragusa, M.; et al. CircSMARCA5 Inhibits Migration of Glioblastoma Multiforme Cells by Regulating a Molecular Axis Involving Splicing Factors SRSF1/SRSF3/PTB. Int. J. Mol. Sci. 2018, 19, 480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbagallo, D.; Caponnetto, A.; Brex, D.; Mirabella, F.; Barbagallo, C.; Lauretta, G.; Morrone, A.; Certo, F.; Broggi, G.; Caltabiano, R.; et al. CircSMARCA5 Regulates VEGFA mRNA Splicing and Angiogenesis in Glioblastoma Multiforme Through the Binding of SRSF1. Cancers 2019, 11, 194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbagallo, D.; Caponnetto, A.; Barbagallo, C.; Battaglia, R.; Mirabella, F.; Brex, D.; Stella, M.; Broggi, G.; Altieri, R.; Certo, F.; et al. The GAUGAA Motif Is Responsible for the Binding between circSMARCA5 and SRSF1 and Related Downstream Effects on Glioblastoma Multiforme Cell Migration and Angiogenic Potential. Int. J. Mol. Sci. 2021, 22, 1678. [Google Scholar] [CrossRef]
- Broggi, G.; Salvatorelli, L.; Barbagallo, D.; Certo, F.; Altieri, R.; Tirrò, E.; Massimino, M.; Vigneri, P.; Guadagno, E.; Maugeri, G.; et al. Diagnostic Utility of the Immunohistochemical Expression of Serine and Arginine Rich Splicing Factor 1 (SRSF1) in the Differential Diagnosis of Adult Gliomas. Cancers 2021, 13, 2086. [Google Scholar] [CrossRef]
- Stella, M.; Falzone, L.; Caponnetto, A.; Gattuso, G.; Barbagallo, C.; Battaglia, R.; Mirabella, F.; Broggi, G.; Altieri, R.; Certo, F.; et al. Serum Extracellular Vesicle-Derived circHIPK3 and circSMARCA5 Are Two Novel Diagnostic Biomarkers for Glioblastoma Multiforme. Pharmaceuticals 2021, 14, 618. [Google Scholar] [CrossRef] [PubMed]
- Anczukow, O.; Akerman, M.; Cléry, A.; Wu, J.; Shen, C.; Shirole, N.H.; Raimer, A.; Sun, S.; Jensen, M.A.; Hua, Y.; et al. SRSF1-Regulated Alternative Splicing in Breast Cancer. Mol. Cell 2015, 60, 105–117. [Google Scholar] [CrossRef] [Green Version]
- Sheng, J.; Zhao, Q.; Zhao, J.; Zhang, W.; Sun, Y.; Qin, P.; Lv, Y.; Bai, L.; Yang, Q.; Chen, L.; et al. SRSF1 modulates PTPMT1 alternative splicing to regulate lung cancer cell radioresistance. EBioMedicine 2018, 38, 113–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malakar, P.; Shilo, A.; Mogilevsky, A.; Stein, I.; Pikarsky, E.; Nevo, Y.; Benyamini, H.; Elgavish, S.; Zong, X.; Prasanth, K.V.; et al. Long Noncoding RNA MALAT1 Promotes Hepatocellular Carcinoma Development by SRSF1 Upregulation and mTOR Activation. Cancer Res. 2016, 77, 1155–1167. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Guo, S.; Zhang, M.; Li, L.; Wang, F.; Song, B. Long non-coding RNA AGAP2-AS1 accelerates cell proliferation, migration, invasion and the EMT process in colorectal cancer via regulating the miR-4,668-3p/SRSF1 axis. J. Gene Med. 2020, 22, e3250. [Google Scholar] [CrossRef] [PubMed]
- Broggi, G.; Angelico, G.; Filetti, V.; Ledda, C.; Lombardo, C.; Vitale, E.; Rapisarda, V.; Loreto, C.; Caltabiano, R. Immunohistochemical Expression of Serine and Arginine-Rich Splicing Factor 1 (SRSF1) in Fluoro-Edenite-Induced Malignant Mesothelioma: A Preliminary Study. Int. J. Environ. Res. Public Health 2021, 18, 6249. [Google Scholar] [CrossRef]
- Broggi, G.; Giudice, A.L.; Di Mauro, M.; Asmundo, M.G.; Pricoco, E.; Piombino, E.; Caltabiano, R.; Morgia, G.; Russo, G.I. SRSF-1 and microvessel density immunohistochemical analysis by semi-automated tissue microarray in prostate cancer patients with diabetes (DIAMOND study). Prostate 2021, 81, 882–892. [Google Scholar] [CrossRef]
- Broggi, G.; Filetti, V.; Ieni, A.; Rapisarda, V.; Ledda, C.; Vitale, E.; Varricchio, S.; Russo, D.; Lombardo, C.; Tuccari, G.; et al. MacroH2A1 Immunoexpression in Breast Cancer. Front. Oncol. 2020, 10, 1519. [Google Scholar] [CrossRef]
- Gajdzis, M.; Kaczmarek, R.; Gajdzis, P. Novel Prognostic Immunohistochemical Markers in Uveal Melanoma-Literature Review. Cancers 2021, 13, 4031. [Google Scholar] [CrossRef]
- Agarwala, S.S.; Eggermont, A.M.M.; O’Day, S.; Zager, J.S. Metastatic melanoma to the liver: A contemporary and comprehensive review of surgical, systemic, and regional therapeutic options. Cancer 2013, 120, 781–789. [Google Scholar] [CrossRef]
- Caltabiano, R.; Puzzo, L.; Barresi, V.; Ieni, A.; Loreto, C.; Musumeci, G.; Castrogiovanni, P.; Ragusa, M.; Foti, P.; Russo, A.; et al. ADAM 10 expression in primary uveal melanoma as prognostic factor for risk of metastasis. Pathol. Res. Pract. 2016, 212, 980–987. [Google Scholar] [CrossRef]
- Barbagallo, C.; Caltabiano, R.; Broggi, G.; Russo, A.; Puzzo, L.; Avitabile, T.; Longo, A.; Reibaldi, M.; Barbagallo, D.; Di Pietro, C.; et al. LncRNA LINC00518 Acts as an Oncogene in Uveal Melanoma by Regulating an RNA-Based Network. Cancers 2020, 12, 3867. [Google Scholar] [CrossRef] [PubMed]
- Salvatorelli, L.; Puzzo, L.; Russo, A.; Reibaldi, M.; Longo, A.; Ragusa, M.; Aldo, C.; Rappazzo, G.; Caltabiano, R.; Salemi, M. Immunoexpression of SPANX-C in metastatic uveal melanoma. Pathol. Res. Pract. 2019, 215, 152431. [Google Scholar] [CrossRef]
- Russo, D.; Di Crescenzo, R.M.; Broggi, G.; Merolla, F.; Martino, F.; Varricchio, S.; Ilardi, G.; Borzillo, A.; Carandente, R.; Pignatiello, S.; et al. Expression of P16INK4a in Uveal Melanoma: New Perspectives. Front. Oncol. 2020, 10, 562074. [Google Scholar] [CrossRef]
- Broggi, G.; Ieni, A.; Russo, D.; Varricchio, S.; Puzzo, L.; Russo, A.; Reibaldi, M.; Longo, A.; Tuccari, G.; Staibano, S.; et al. The Macro-Autophagy-Related Protein Beclin-1 Immunohistochemical Expression Correlates with Tumor Cell Type and Clinical Behavior of Uveal Melanoma. Front. Oncol. 2020, 10, 589849. [Google Scholar] [CrossRef]
- Luo, H.; Ma, C.; Shao, J.; Cao, J. Prognostic Implications of Novel Ten-Gene Signature in Uveal Melanoma. Front. Oncol. 2020, 10, 567512. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; He, R.; Lin, P.; Zhong, J.; Ma, J.; Yang, H.; Hu, X.; Chen, G. Profiling of prognostic alternative splicing in melanoma. Oncol. Lett. 2019, 18, 1081–1088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furney, S.; Pedersen, M.; Gentien, D.; Dumont, A.G.; Rapinat, A.; Desjardins, L.; Turajlic, S.; Piperno-Neumann, S.; De La Grange, P.; Roman-Roman, S.; et al. SF3B1 Mutations Are Associated with Alternative Splicing in Uveal Melanoma. Cancer Discov. 2013, 3, 1122–1129. [Google Scholar] [CrossRef] [Green Version]
| Sex | Age (Years) | Location | Thickness (mm) | Largest Diameter (mm) | Cell Type | PT Stage | MFS (Months) | Follow-Up (Months) | SRSF1 | MVD (n/mm2) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IS | ES | IRS | L (<3) H (≥3) | L (<43) H (≥43) | ||||||||||
| F | 29 | ch | 14.2 | 16.2 | mixed | pT2a | 169 | 169 | 0 | 0 | 0 | L | 18 | L |
| F | 83 | ch/cb | 14.84 | 16.8 | mixed | pT2b | 123 (†) | 123 (†) | 1 | 1 | 1 | L | 24 | L |
| F | 55 | ch | 9.8 | 13.9 | spindle | pT2a | 153 | 153 | 0 | 0 | 0 | L | 21 | L |
| F | 30 | ch/cb | 12.05 | 9.2 | spindle | pT2b | 153 | 153 | 0 | 0 | 0 | L | 16 | L |
| M | 74 | ch/cb | 10.04 | 16.1 | spindle | pT2b | 152 | 152 | 1 | 1 | 1 | L | 26 | L |
| M | 64 | ch | 7.7 | 11.5 | spindle | pT1a | 143 | 143 | 0 | 0 | 0 | L | 13 | L |
| F | 36 | ch | 5.81 | 12.7 | spindle | pT1a | 140 | 140 | 2 | 2 | 4 | H | 20 | L |
| F | 59 | ch | 8.4 | 16.7 | mixed | pT2a | 139 | 139 | 0 | 0 | 0 | L | 19 | L |
| M | 36 | ch | 6.47 | 9.8 | mixed | pT1a | 139 | 139 | 1 | 2 | 2 | L | 32 | L |
| M | 84 | ch/cb | 11.9 | 14.8 | mixed | pT2b | 106 (†) | 106 (†) | 1 | 1 | 1 | L | 25 | L |
| F | 67 | ch | 10.42 | 13.2 | mixed | pT3a | 136 | 136 | 0 | 0 | 0 | L | 24 | L |
| M | 73 | ch | 9.7 | 11.3 | mixed | pT2a | 102 (†) | 102 (†) | 0 | 0 | 0 | L | 17 | L |
| F | 45 | ch | 13.7 | 10.2 | mixed | pT2a | 127 | 127 | 2 | 2 | 4 | H | 33 | L |
| M | 58 | ch | 13.1 | 14.3 | mixed | pT2a | 127 | 127 | 0 | 0 | 0 | L | 29 | L |
| M | 63 | ch | 3.3 | 11.7 | spindle | pT2a | 116 | 116 | 0 | 0 | 0 | L | 42 | L |
| M | 54 | ch | 6.32 | 10 | spindle | pT2a | 114 | 114 | 0 | 0 | 0 | L | 29 | L |
| M | 83 | ch | 10.62 | 9.4 | epit | pT3a | 72 (†) | 72 (†) | 0 | 0 | 0 | L | 44 | H |
| F | 71 | ch | 3.68 | 6.4 | epit | pT1a | 102 | 102 | 1 | 2 | 2 | L | 33 | L |
| M | 55 | ch/cb | 7.5 | 8.9 | epit | pT2b | 92 | 92 | 0 | 0 | 0 | L | 16 | L |
| M | 52 | ch | 9.2 | 12.1 | spindle | pT2b | 91 | 91 | 0 | 0 | 0 | L | 14 | L |
| M | 46 | ch | 8.76 | 11.3 | spindle | pT2a | 85 | 85 | 1 | 1 | 1 | L | 24 | L |
| F | 76 | ch | 8.02 | 10.7 | mixed | pT1a | 79 | 79 | 2 | 2 | 4 | H | 18 | L |
| F | 63 | ch | 10.3 | 13.7 | mixed | pT2a | 73 | 73 | 2 | 1 | 2 | L | 26 | L |
| F | 41 | ch | 5.85 | 10.3 | mixed | pT1a | 73 | 73 | 0 | 0 | 0 | L | 35 | L |
| F | 55 | ch | 3.2 | 7.6 | mixed | pT2a | 55 | 55 | 0 | 0 | 0 | L | 28 | L |
| M | 68 | ch/cb | 10.1 | 10.1 | epit | pT1b | 55 | 55 | 1 | 4 | 4 | H | 37 | L |
| M | 74 | ch/cb | 14.45 | 17.5 | epit | pT4b | 49 | 49 | 0 | 0 | 0 | L | 45 | H |
| M | 70 | ch/cb | 16.27 | 20.8 | spindle | pT4b | 43 | 43 | 1 | 1 | 1 | L | 31 | L |
| M | 66 | ch | 9.2 | 14.1 | mixed | pT3a | 43 | 43 | 1 | 4 | 4 | H | 20 | L |
| M | 64 | ch | 9.3 | 15.2 | mixed | pT2a | 29 | 29 | 0 | 0 | 0 | L | 15 | L |
| M | 71 | ch | 13.93 | 10.2 | mixed | pT2a | 25 | 25 | 0 | 0 | 0 | L | 22 | L |
| M | 19 | ch | 9.77 | 14.8 | mixed | pT2a | 20 | 20 | 1 | 1 | 1 | L | 25 | L |
| M | 73 | ch | 15.89 | 18 | mixed | pT2a | 19 | 19 | 0 | 0 | 0 | L | 39 | L |
| F | 80 | ch | 14.61 | 14.3 | epit | pT1b | 15 | 15 | 2 | 2 | 4 | H | 18 | L |
| F | 81 | ch/cb | 8.9 | 10.7 | mixed | pT2a | 15 | 15 | 1 | 1 | 1 | L | 51 | H |
| F | 78 | ch | 12 | 12 | mixed | pT3a | 10 | 10 | 1 | 1 | 1 | L | 31 | L |
| M | 52 | ch | 12 | 12 | spindle | pT3a | 22 | 22 | 0 | 0 | 0 | L | 18 | L |
| M | 59 | ch | 16 | 16 | spindle | pT4a | 24 | 24 | 0 | 0 | 0 | L | 40 | L |
| M | 48 | ch | 5 | 9 | mixed | pT1a | 24 | 24 | 0 | 0 | 0 | L | 23 | L |
| F | 75 | ch | 5 | 10 | spindle | pT2a | 31 | 31 | 0 | 0 | 0 | L | 24 | L |
| F | 58 | ch | 8 | 21 | spindle | pT4a | 32 | 32 | 0 | 0 | 0 | L | 45 | H |
| M | 54 | ch | 8 | 13 | spindle | pT3a | 36 | 36 | 0 | 0 | 0 | L | 71 | H |
| M | 73 | ch | 12 | 14 | spindle | pT3b | 36 | 36 | 1 | 1 | 1 | L | 59 | H |
| F | 48 | ch | 12 | 16 | epit | pT3b | 37 | 37 | 1 | 1 | 1 | L | 28 | L |
| F | 70 | ch/cb | 15 | 20 | mixed | pT4b | 70 | 70 | 0 | 0 | 0 | L | 40 | L |
| F | 74 | ch | 10 | 16 | mixed | pT3a | 136 | 136 | 0 | 0 | 0 | L | 37 | L |
| Sex | Age (Years) | Location | Thickness (mm) | Largest Diameter (mm) | Cell Type | PT Stage | MFS (Months) | Follow-Up (Months) | SRSF1 | MVD (n/mm2) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IS | ES | IRS | L (<3) H (≥3) | L (<43) H (≥43) | ||||||||||
| F | 58 | ch | 6.04 | 17.8 | mixed | pT2a | 63 | 64 (†) | 2 | 3 | 6 | H | 39 | L |
| M | 69 | ch | 7.21 | 15.8 | mixed | pT2a | 54 | 81 (†) | 1 | 4 | 4 | H | 69 | H |
| F | 75 | ch/cb | 15.5 | 15.3 | mixed | pT3b | 44 | 62 (†) | 3 | 3 | 9 | H | 40 | L |
| F | 50 | ch | 7.36 | 15.6 | epit | pT2a | 41 | 111 | 0 | 0 | 0 | L | 56 | H |
| M | 62 | ch | 13.68 | 16 | mixed | pT3a | 38 | 51 (†) | 2 | 4 | 8 | H | 63 | H |
| F | 51 | ch/cb | 11.4 | 18.5 | mixed | pT3b | 38 | 92 | 2 | 2 | 4 | H | 35 | L |
| M | 71 | ch | 13.14 | 17.1 | epit | pT3a | 33 | 34 (†) | 0 | 0 | 0 | L | 58 | H |
| M | 76 | ch/cb | 11.6 | 6.5 | mixed | pT1a | 31 | 70 | 2 | 3 | 6 | H | 54 | H |
| M | 72 | ch | 10.3 | 15.4 | mixed | pT3b | 27 | 35 (†) | 3 | 2 | 6 | H | 70 | H |
| F | 85 | ch/cb | 7.3 | 14.7 | spindle | pT2d * | 26 | 49 (†) | 2 | 2 | 4 | H | 45 | H |
| M | 73 | ch | 5.73 | 11.7 | epit | pT2a | 26 | 42 (†) | 3 | 3 | 9 | H | 56 | H |
| F | 51 | ch | 9.42 | 19 | mixed | pT3a | 25 | 71 | 2 | 3 | 6 | H | 12 | L |
| F | 84 | ch | 11.7 | 17.4 | mixed | pT3a | 76 | 78 (†) | 3 | 3 | 9 | H | 28 | L |
| M | 73 | ch | 9.24 | 17.7 | epit | pT2a | 103 | 112 (†) | 2 | 4 | 8 | L | 76 | H |
| F | 74 | ch | 5.7 | 12.1 | spindle | pT2a | 24 | 37 (†) | 2 | 2 | 4 | H | 45 | H |
| F | 67 | ch | 3.49 | 20 | mixed | pT4a | 24 | 31 (†) | 3 | 3 | 9 | H | 55 | H |
| M | 74 | ch | 11.35 | 10.5 | epit | pT3a | 19 | 78 | 2 | 3 | 6 | H | 69 | H |
| M | 82 | ch | 9.7 | 11 | epit | pT2a | 19 | 42 (†) | 2 | 2 | 4 | H | 78 | H |
| F | 72 | ch | 6.7 | 15.2 | epit | pT2a | 14 | 28 (†) | 3 | 3 | 9 | H | 72 | H |
| M | 76 | ch | 13.7 | 17.1 | mixed | pT2a | 14 | 101 | 2 | 4 | 8 | H | 64 | H |
| M | 79 | ch | 13.91 | 16.1 | epit | pT3b | 13 | 79 | 2 | 2 | 4 | H | 45 | H |
| F | 66 | ch/cb | 8.95 | 12.5 | mixed | pT2b | 12 | 37 (†) | 3 | 3 | 9 | H | 58 | H |
| F | 74 | ch | 8.6 | 10.2 | mixed | pT4b | 23 | 43 | 2 | 3 | 6 | H | 69 | H |
| F | 60 | ch | 8.25 | 16.5 | epit | pT2a | 11 | 37 (†) | 2 | 2 | 4 | H | 74 | H |
| F | 57 | ch/cb | 13.6 | 19 | epit | pT2b | 6 | 86 | 2 | 3 | 6 | H | 46 | H |
| M | 72 | ch/cb | 13.3 | 15.4 | mixed | pT3b | 0 | 82 | 3 | 3 | 9 | L | 58 | H |
| M | 78 | ch | 16.58 | 16.6 | epit | pT2b | 2 | 3 (†) | 2 | 3 | 6 | H | 44 | H |
| F | 60 | ch | 3.2 | 13.5 | spindle | pT3a | 44 | 44 | 2 | 2 | 4 | H | 11 | L |
| F | 66 | ch | 15 | 18 | spindle | pT2a | 48 | 48 | 3 | 3 | 9 | H | 66 | H |
| F | 50 | ch/cb | 9 | 12 | epit | pT4b | 49 | 49 | 2 | 4 | 8 | H | 24 | L |
| F | 70 | ch/cb | 23 | 23 | spindle | pT2b | 58 | 58 | 2 | 4 | 8 | H | 15 | L |
| F | 81 | ch | 15 | 18 | mixed | pT4a | 6 | 12 (†) | 3 | 3 | 9 | H | 28 | L |
| M | 60 | ch/cb | 6 | 6 | spindle | pT4d * | 12 | 17 (†) | 3 | 3 | 9 | H | 44 | H |
| F | 73 | ch/cb | 15 | 15 | epit | pT3d * | 14 | 18 (†) | 3 | 3 | 9 | H | 33 | L |
| M | 59 | ch | 12 | 11 | mixed | pT4a | 12 | 18 (†) | 3 | 3 | 9 | H | 34 | L |
| M | 68 | ch | 9 | 15 | spindle | pT3b | 18 | 24 (†) | 2 | 1 | 2 | L | 54 | H |
| M | 56 | ch | 11 | 9 | spindle | pT3a | 24 | 36 (†) | 2 | 4 | 8 | H | 25 | L |
| M | 82 | ch | 15 | 18 | mixed | pT4a | 48 | 60 (†) | 2 | 4 | 8 | H | 23 | L |
| M | 66 | ch | 3.2 | 13.5 | spindle | pT2a | 80 | 111 (†) | 2 | 2 | 4 | H | 58 | H |
| Sex m-f | Age (Years) | Location | Thickness | Largest Diameter | Cell Type | Pathological T stage | MFS (Months) | Follow-Up (Months) | SRSF1 | MVD (n/mm2) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| All (n = 85) | 44–41 | 67 (29–85) | ch 64 ch/cb 21 | 10.0 (3.2–16.3) | 14.3 (6.4–20.8) | Epit: 20 Spindle: 25 Mixed: 40 | pT1a: 15 pT1b: 4 pT2a: 44 pT2b: 16 pT2d: 1 ee pT3a: 20 pT3b: 10 pT4a: 6 pT4b: 8 pT4d: 1 | 41 (0–138) | 58 (8–138) | 2 (0–9) | 34 (11–78) |
| Metastasis free (n=46) | 25–21 | 64 (19–84) | ch 36 ch/cb 10 | 9.9 (3.2–16.2) | 12.9 (6.4–21) | Epit: 7 Spindle: 16 Mixed: 23 | pT1a: 7 pT1b: 2 pT2a: 17 pT2b: 6 pT3a: 7 pT3b: 2 pT4a: 2 pT4b: 3 | 73 (10–169) | 73 (10–169) 4 deaths | 0 (0–8) | 26 (13–71) |
| Metastasis (n=39) | 19–20 | 71 (50–85) | ch 28 ch/cb 11 | 10.3 (3.2–23) | 15.4 (6–23) | Epit: 13 Spindle: 9 Mixed: 17 | pT1a: 8 pT1b: 2 pT2a: 28 pT2b: 10 pT2d: 1 ee pT3a: 13 pT3b: 8 pT4a: 4 pT4b: 5 pT4d: 1 | 25 (0–109) | 49 (1–112) 25 deaths | 6 (0–9) | 54 (11–78) |
| p (metastasis free vs. metastasis) | 0.666 ° | 0.099 * | 0.615 ° | 0.932 * | 0.009 * | 0.493 * | 0.271 * | <0.001 * | 0.031 * | <0.001 * | <0.001 * |
| SRSF1 | MVD (n/mm2) | |||
|---|---|---|---|---|
| Low (<3) | High (≥3) | Low (<43) | High (≥43) | |
| Metastasis free (n = 46) | 39 (84.8%) | 7 (15.2%) | 40 (87.0%) | 6 (13.0%) |
| Metastasis (n = 39) | 5 (12.8%) | 34 (87.2%) | 13 (33.3%) | 26 (66.7%) |
| p (Fisher’s exact test) | <0.001 | <0.001 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Broggi, G.; Falzone, L.; Fallico, M.; Russo, A.; Reibaldi, M.; Longo, A.; Avitabile, T.; De Pasquale, R.; Puzzo, L.; Foti, P.V.; et al. Prognostic Value of the Immunohistochemical Expression of Serine and Arginine-Rich Splicing Factor 1 (SRSF1) in Uveal Melanoma: A Clinico-Pathological and Immunohistochemical Study on a Series of 85 Cases. Appl. Sci. 2021, 11, 7874. https://doi.org/10.3390/app11177874
Broggi G, Falzone L, Fallico M, Russo A, Reibaldi M, Longo A, Avitabile T, De Pasquale R, Puzzo L, Foti PV, et al. Prognostic Value of the Immunohistochemical Expression of Serine and Arginine-Rich Splicing Factor 1 (SRSF1) in Uveal Melanoma: A Clinico-Pathological and Immunohistochemical Study on a Series of 85 Cases. Applied Sciences. 2021; 11(17):7874. https://doi.org/10.3390/app11177874
Chicago/Turabian StyleBroggi, Giuseppe, Luca Falzone, Matteo Fallico, Andrea Russo, Michele Reibaldi, Antonio Longo, Teresio Avitabile, Rocco De Pasquale, Lidia Puzzo, Pietro Valerio Foti, and et al. 2021. "Prognostic Value of the Immunohistochemical Expression of Serine and Arginine-Rich Splicing Factor 1 (SRSF1) in Uveal Melanoma: A Clinico-Pathological and Immunohistochemical Study on a Series of 85 Cases" Applied Sciences 11, no. 17: 7874. https://doi.org/10.3390/app11177874
APA StyleBroggi, G., Falzone, L., Fallico, M., Russo, A., Reibaldi, M., Longo, A., Avitabile, T., De Pasquale, R., Puzzo, L., Foti, P. V., Russo, D., Di Crescenzo, R. M., Libra, M., Staibano, S., & Caltabiano, R. (2021). Prognostic Value of the Immunohistochemical Expression of Serine and Arginine-Rich Splicing Factor 1 (SRSF1) in Uveal Melanoma: A Clinico-Pathological and Immunohistochemical Study on a Series of 85 Cases. Applied Sciences, 11(17), 7874. https://doi.org/10.3390/app11177874











