Design and Validation of a Virtual Chemical Laboratory—An Example of Natural Science in Elementary Education
Abstract
:Featured Application
Abstract
1. Introduction
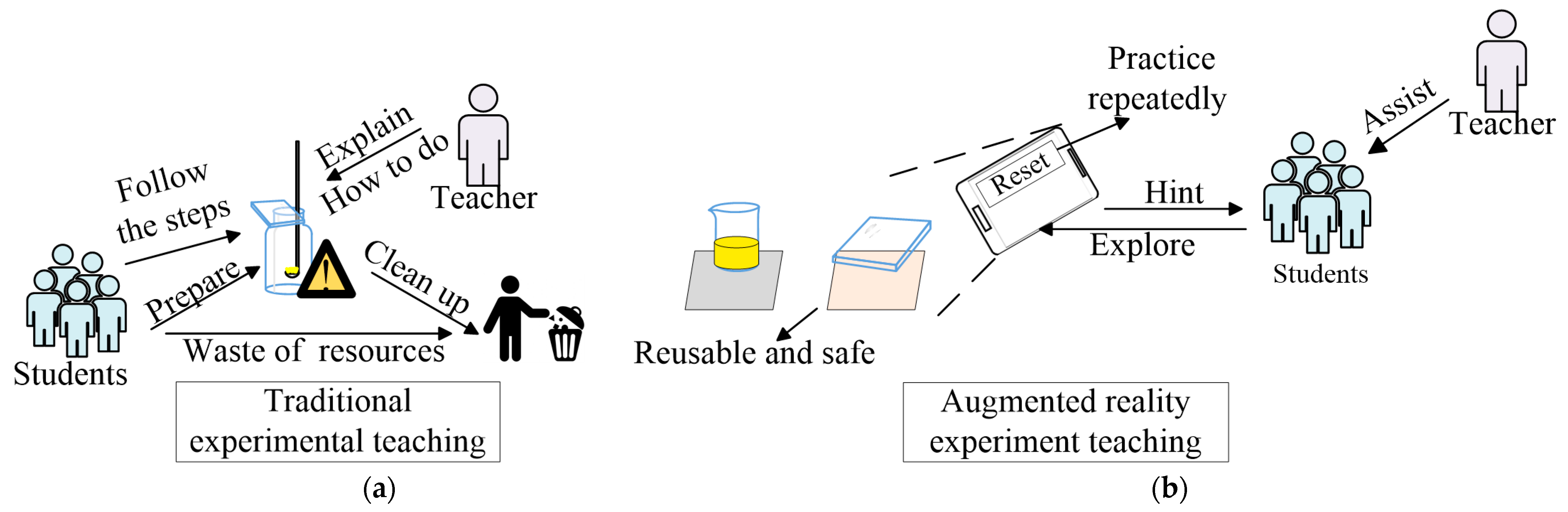
- With respect to equipment, the proposed teaching method lowers the cost of equipment needed for chemistry experimental teaching through smartphones and cards.
- With respect to environmental protection, the proposed teaching method implements the idea of green chemistry experiments, reducing the use of consumable materials and production of waste.
- With respect to safety, the proposed teaching method avoids dangers that may happen due to erroneous operation in the process of chemistry experiments.
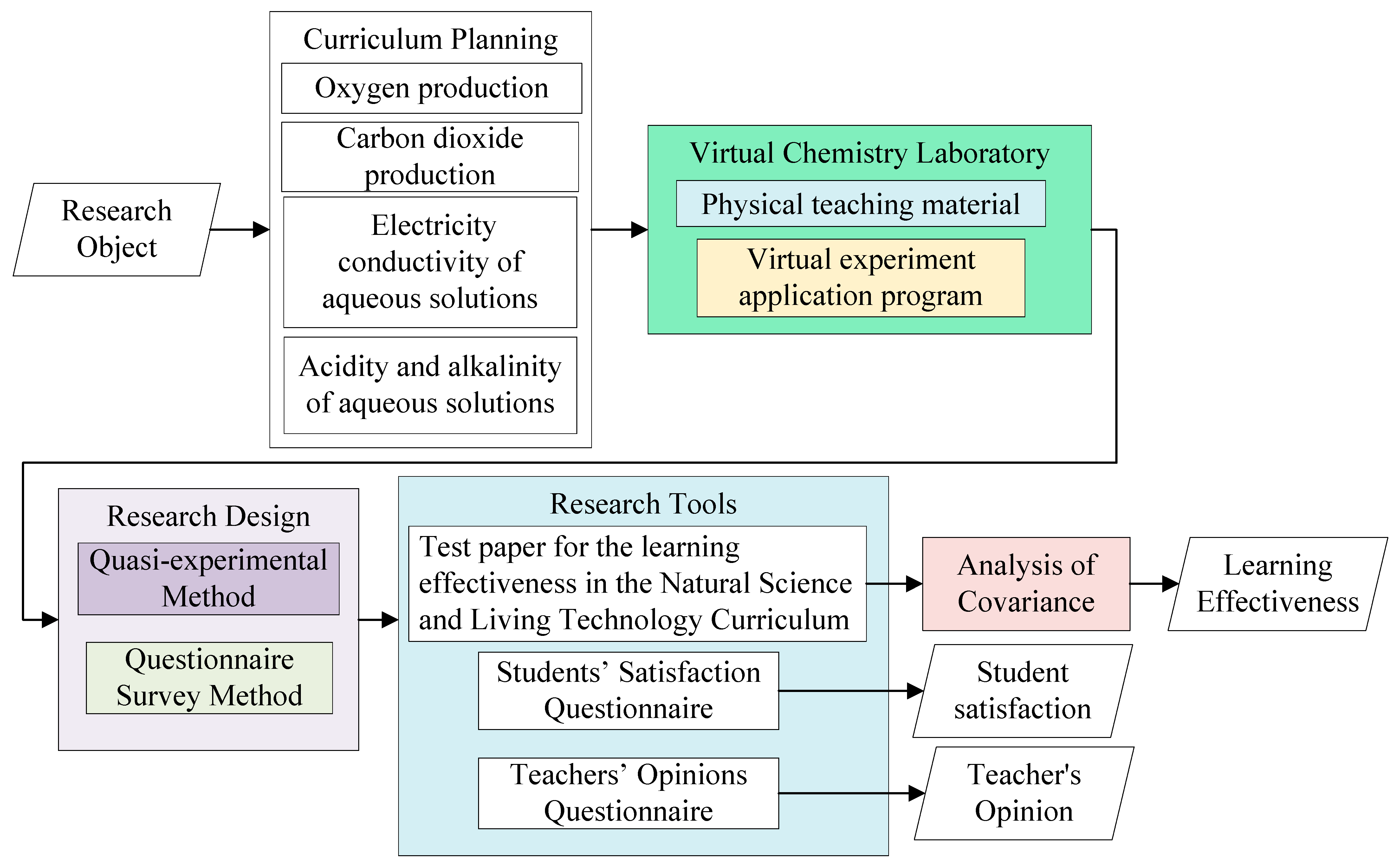
2. Research Method
2.1. Research Subjects
2.2. Curriculum Planning
2.2.1. Learning Objectives
- To design virtual experiments with safety concerns
- To design virtual experiments involving limited waste of excessive materials
- To cultivate thinking and problem-solving abilities from experiments
- To foster interest in scientific inquiry from experiments
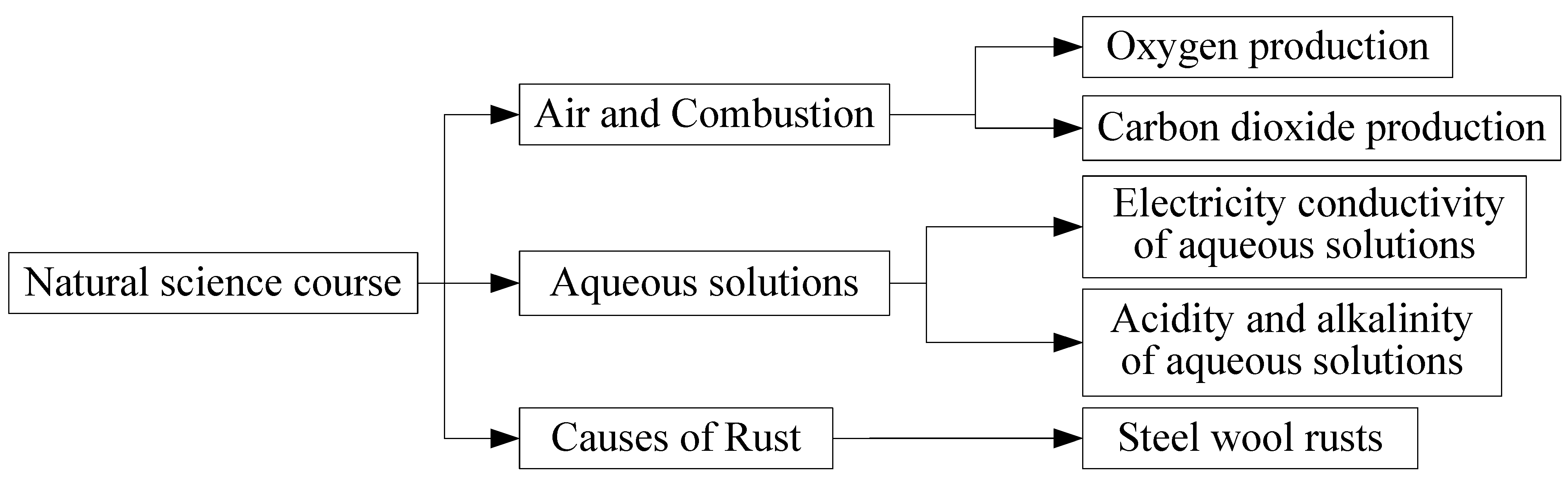
2.2.2. Learning Contents
2.3. Research Design
2.3.1. Quasi-Experimental Method
2.3.2. Questionnaire Survey Method
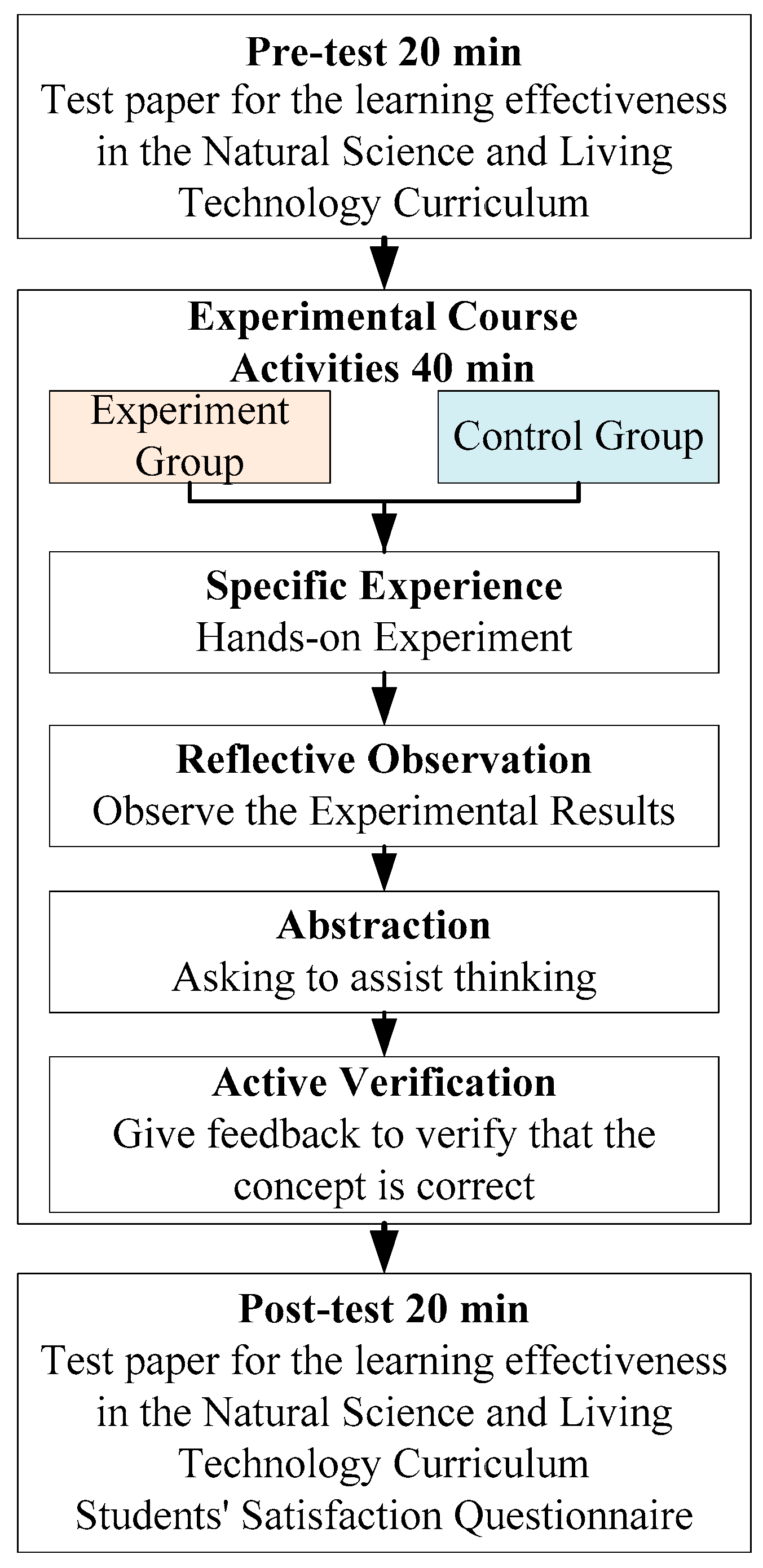
2.4. Experimental Procedure
2.5. Research Tools
2.5.1. Test Paper for the Learning Effectiveness in the Natural Science and Living Technology Curriculum
2.5.2. Student Satisfaction Questionnaire
2.5.3. Teacher Opinions Questionnaire
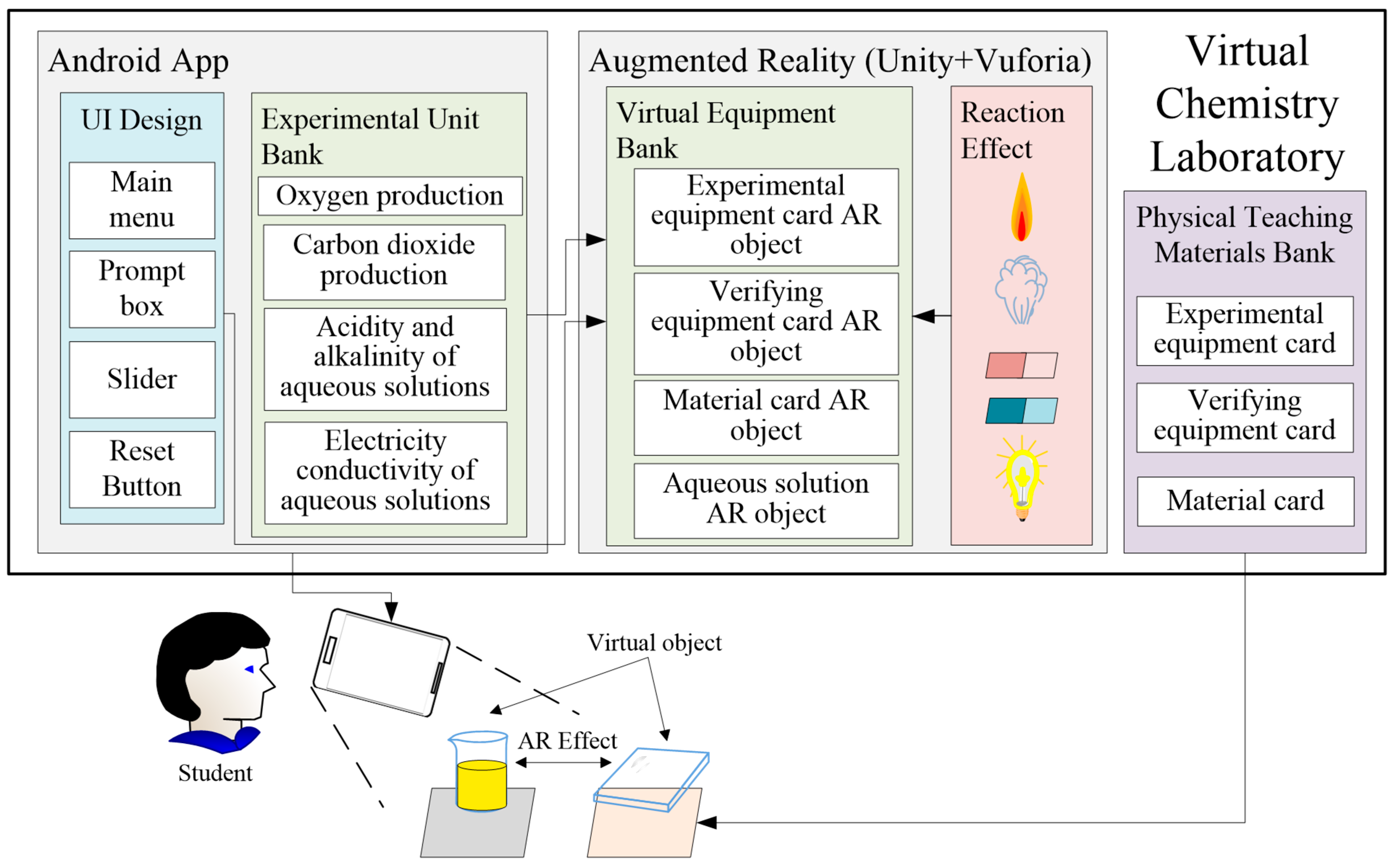
3. The Proposed Design of Virtual Chemical Laboratory
3.1. The Proposed Virtual Chemistry Laboratory
3.1.1. Android App
3.1.2. Augmented Reality (AR)
3.1.3. Physical Teaching Materials Bank
3.2. Operation Procedures
- Step 1: Prepare the equipment cards needed for the experiment.
- Step 2: Add the aqueous solutions needed for this experiment; in this step, warning signs would appear to remind the students not to add excessive solution volumes.
- Step 3: Use the materials cards to add the materials for producing oxygen; in this experiment, enoki mushrooms were used.
- Step 4: Cover the wide-mouth bottle with glass plate equipment and wait for oxygen to be produced.
- Step 5: Use the verifying equipment card of incense sticks to test the comburent phenomenon of oxygen. The more oxygen produced, the more obvious the comburent phenomenon of the incense sticks.
4. Results
4.1. Learning Effectiveness
4.1.1. Test of Homogeneity of Within-Group Regression Coefficient
4.1.2. Comparison of the Differences in Learning Outcomes
4.2. Survey on Students’ Satisfaction
4.3. Survey on Teachers’ Opinions
5. Conclusions and Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Treagust, D.; Nieswandt, M.; Duit, R. Sources of students difficulties in learning Chemistry. Educ. Quím. 2000, 11, 228–235. [Google Scholar] [CrossRef]
- Sirhan, G. Learning Difficulties in Chemistry: An Overview. J. Turk. Sci. Educ. 2007, 4, 2–20. [Google Scholar]
- Taber, K. Chemical misconceptions: Prevention, diagnosis and cure. J. Chem. Educ. 2003, 80, 491. [Google Scholar]
- Zoller, U. Students’ misunderstandings and misconceptions in college freshman chemistry (general and organic). J. Res. Sci. Teach. 1990, 27, 1053–1065. [Google Scholar] [CrossRef]
- Chen, H.Z. A Study of Elementary Student’s Cognition and Misconceptions on Aqueous Solution; National Taipei University of Education: Taipei City, Taiwan, 2001. [Google Scholar]
- Du, Y.H. Text analysis of “Air” unit in elementary school nature textbook. Sci. Educ. Mon. 2008, 313, 2–8. [Google Scholar]
- Rusek, M.; Chroustova, K.; Bilek, M.; Skrehot, P.A.; Hon, Z. Conditions for experimental activities at elementary and high schools from chemistry teachers’ point of view. Chemistry-Didactics-Ecology-Metrology 2020, 25. [Google Scholar] [CrossRef]
- Wieman, C. Comparative cognitive task analyses of experimental science and instructional laboratory courses. Phys. Teach. 2015, 53, 349–351. [Google Scholar] [CrossRef] [Green Version]
- Hofstein, A.; Lunetta, V.N. The role of the laboratory in science teaching: Neglected aspects of research. Rev. Educ. Res. 1982, 52, 201–217. [Google Scholar] [CrossRef]
- Leva, A. A hands-on experimental laboratory for undergraduate courses in automatics control. IEEE Trans. Educ. 2003, 46, 263–272. [Google Scholar] [CrossRef]
- Walters, A.U.; Lawrence, W.; Jalsa, N.K. Chemical laboratory safety awareness, attitudes and practices of tertiary students. Saf. Sci. 2017, 96, 161–171. [Google Scholar] [CrossRef]
- Taichung City Government Information Bureau. Elementary School Children from Xinyi State Were Injured in the Laboratory. 2001. Available online: https://www.taichung.gov.tw/8868/8872/9962/914297/post (accessed on 24 October 2021).
- Jiang, G.Y. Jiangsu Teacher Experimented with Alcohol and 4 School Children Were Tragically Burned in an Explosion. 2020. Available online: https://www.hk01.com/%E5%A4%A7%E5%9C%8B%E5%B0%8F%E4%BA%8B/529052/%E6%B1%9F%E8%98%87%E6%95%99%E5%B8%AB%E5%81%9A%E5%AF%A6%E9%A9%97%E6%B7%BB%E9%85%92%E7%B2%BE%E4%B8%8D%E8%A6%8F%E7%AF%84%E7%99%BC%E7%94%9F%E7%88%86%E7%82%B8-4%E5%90%8D%E5%AD%B8%E7%AB%A5%E6%85%98%E8%A2%AB%E7%87%92%E5%82%B7 (accessed on 24 October 2021).
- Qian, Y.S.; Lin, J.L.; Lu, J.W.; Qiu, W.M.; Wang, T.Z.; Tao, J.Z.; Guo, Y.B. Research on the hazard evaluation of school dangerous laboratories. Chin-Yi J. 2000, 18, 95–104. [Google Scholar]
- Li, L.X. 2011–2016 Analysis Report on Major Accidents in Campus Laboratories. 2018. Available online: https://www.safelab.edu.tw/FileStorage/files/100-105%E5%B9%B4%E6%A0%A1%E5%9C%92%E5%AF%A6%E9%A9%97%E5%AE%A4%E9%87%8D%E5%A4%A7%E4%BA%8B%E6%95%85%E7%81%BD%E5%AE%B3%E5%88%86%E6%9E%90.pdf (accessed on 24 October 2021).
- Education Bureau. 2017/2018 Investigation Report on Laboratory Accidents in Middle School in the School Year. 2018. Available online: https://cd1.edb.hkedcity.net/cd/science/laboratory/safety/Report_of_the_Survey_on_Laboratory_Accidents_2017-18_c.pdf (accessed on 24 October 2021).
- University of Pennsylvania. Vacuum Pump Explosion in Chemistry Building. 2020. Available online: https://ehrs.upenn.edu/health-safety/lab-safety/safety-alerts-and-faqs/vacuum-pump-explosion-chemistry-building (accessed on 24 October 2021).
- Yang, M.H. The Study of Environmental Improvements for Experiment Courses of Science and Technology and Teachers’ Attitudes and Behaviors towards Environmental Protection in Experiment Courses; National Taichung University of Education: Taichung City, Taiwan, 2006. [Google Scholar]
- Nidbox. Conductivity of Fifth Grade Aqueous Solution. 2020. Available online: https://bobo2983.nidbox.com/diary/read/10018422 (accessed on 24 October 2021).
- Jiang, C.L.; Fu, Y.X. High School Vocational Green Chemistry Attenuation and Reduction Creativity Competition Achievement Report. 2019. Available online: https://chem.moe.edu.tw/green/Home/GetEFile?fid=a9a53516-cacf-489e-88a0-cb0409860b10&fn=6T6dHWcWhsm99UymxUpA2MkVWBf85qO1 (accessed on 24 October 2021).
- Zhang, C.X. Educational Psychology: Theory and Practice of Three Orientations; New Moon Education Co., Ltd.: Taipei, Taiwan, 1994. [Google Scholar]
- Kusurkar, R.A.; Ten Cate, T.J.; Vos, C.M.P.; Westers, P.; Croiset, G. How motivation affects academic performance: A structural equation modelling analysis. Adv. Health Sci. Educ. 2013, 18, 57–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chi, C.C. A reflection on the science education of Taiwan-The voice from the elites in Taiwan. Chin. J. Sci. Educ. 2007, 15, 627–646. [Google Scholar]
- Hofstein, A.; Eilks, I.; Bybee, R. Societal issues and their importance for contemporary science education—A pedagogical justification and the state-of-the-art in Israel, Germany, and the USA. Int. J. Sci. Math. Educ. 2011, 9, 1459–1483. [Google Scholar] [CrossRef]
- Schiefele, U. Interest, Learning, and Motivation. Educ. Psychol. 1991, 26, 299–363. [Google Scholar]
- Lin, B.J. A Study of Augmented Reality on Language Teaching—A Case Study of Elementary English Teaching; National Changhua University of Education: Changhua, Taiwan, 2009. [Google Scholar]
- Yen, H.H. The Effect of Experiential Digital Simulation on Elementary School Students’ Performance in Learning Electromagnetic Concepts; National Taiwan Normal University: Taipei, Taiwan, 2013. [Google Scholar]
- He, P.X. The Effects of AR-Based Learning Assistance and Instructional Strategy on Junior High Students’ Learning Performance and Motivation in Robot Programming; National Taiwan Normal University: Taipei, Taiwan, 2017. [Google Scholar]
- Lasica, I.E.; Katzis, K.; Meletiou-Mavrotheris, M.; Dimopoulos, C. Augmented reality in laboratory-based education: Could it change the way students decide about their future studies? In Proceedings of the 2017 IEEE Global Engineering Education Conference (EDUCON), Athens, Greece, 25–28 April 2017; pp. 1473–1476. [Google Scholar]
- Tee, N.Y.K.; Gan, H.S.; Li, J.; Cheong, B.H.P.; Tan, H.Y.; Liew, O.W.; Ng, T.W. Developing and Demonstrating an Augmented Reality Colorimetric Titration Tool. J. Chem. Educ. 2018, 95, 393–399. [Google Scholar] [CrossRef]
- Yang, S.; Mei, B.; Yue, X. Mobile Augmented Reality Assisted Chemical Education: Insights from Elements 4D. J. Chem. Educ. 2018, 95, 1060–1062. [Google Scholar] [CrossRef]
- Zhu, B.; Feng, M.; Lowe, H.; Kesselman, J.; Harrison, L.; Dempski, R.E. Increasing enthusiasm and enhancing learning for biochemistry-laboratory safety with an augmented-reality program. J. Chem. Educ. 2018, 95, 1747–1754. [Google Scholar] [CrossRef]
- Caluya, N.R.; Plopski, A.; Ty, J.F. Transferability of spatial maps: Augmented versus virtual reality training. In Proceedings of the 2018 IEEE Conference on Virtual Reality and 3D User Interfaces, Reutlingen, Germany, 18–22 March 2018; pp. 387–393. [Google Scholar]
- Guo, R.; Cui, J.; Zhao, W.; Li, S.; Hao, A. Hand-by-hand mentor: An AR based training system for piano performance. In Proceedings of the 2021 IEEE Conference on Virtual Reality and 3D User Interfaces Abstracts and Workshops (VRW), Lisbon, Portugal, 27 March–1 April 2021; pp. 436–437. [Google Scholar]
- Lupascu, A.G.; Ciupe, A.; Meza, S.; Orza, B. ARThings–enhancing the visitors’ experience in museums through collaborative AR. In Proceedings of the 2021 IEEE Conference on Virtual Reality and 3D User Interfaces Abstracts and Workshops (VRW), Lisbon, Portugal, 27 March–1 April 2021; pp. 669–670. [Google Scholar]
- Lu, T.C. A study of Apparatus in the Primary School Science Laboratory; University of Taipei: Taipei, Taiwan, 2002. [Google Scholar]
- Sheng, H.L. The Influence of Infusing Augmented Reality into Mobile Learning on Elementary School Students’ Learning Motivation and Achievement in Science and Technology; National University of Tainan: Tainan, Taiwan, 2009. [Google Scholar]
- Fang, C.M. The Research of Applying Augmented Reality to the Junior High School Instruction—Take “Nature and Life Technology Field” as an Example; National Taitung University: Taitung, Taiwan, 2011. [Google Scholar]
- Szu, J.L. The Effects of Augmented Reality on Game-Based Learning and Encoding Strategy in the Learning of Lever Principle for Elementary Students; National Taiwan Normal University: Taipei, Taiwan, 2014. [Google Scholar]
- Wu, Z.S. Effects of Teacher’s Identity and Type of Feedback on Teachers’ Learning of Innovation Diffusion Strategies from Computer Simulation; National Taiwan Normal University: Taipei, Taiwan, 2016. [Google Scholar]
| Institute | Year | Nation/Area | Grade | Factors Causing Dangerous Incidents |
|---|---|---|---|---|
| Taichung City Government Information Bureau | 2001 | Taiwan | Elementary School | Explosion caused by carbon dioxide |
| Ministry of Education | 2018 | Taiwan | High School | Fires and cut injuries |
| Education Bureau | 2018 | Hong Kong | Secondary School | Glass cuts and fire burns |
| Qianjie Elementary School | 2020 | China | Elementary School | Instantaneous explosion caused by alcohol |
| University of Pennsylvania | 2020 | USA | University | Explosion caused by volatile gases |
| Reference | Year | Characteristics |
|---|---|---|
| [26] | 2009 | Using AR to assist students in their English learning by using novel methods to stimulate learning |
| [27] | 2013 | Enabling students to understand the abstract electromagnetic force unit through the method of AR simulation |
| [28] | 2017 | Assisting students in performing robotics programming through using AR in combination with smart glasses |
| [29] | 2017 | Analyzing teaching methods using virtual laboratories, as compared to those using traditional ones, which can transform the future learning method of students |
| [30] | 2018 | Conducting experiments with the assistance of the method of AR in conjunction with physical chemistry equipment |
| [31] | 2018 | Visualizing chemical molecular models by displaying them on labelled cards to help students learn chemistry more clearly |
| [32] | 2018 | Enabling users to understand laboratory safety rules with mixed reality technology through smart glasses |
| [33] | 2018 | Comparing the effectiveness of spatial memory training between AR and virtual reality (VR) in a control-room scenario to evaluate the impact of AR and VR training on short-term and long-term memory. The results show that VR training is better than AR in short-term memory, and AR training is better than VR in long-term memory |
| [34] | 2021 | Visualizing 3D piano performance animations for both hands based on the input MIDI file, which contains the time sequences of different keys |
| [35] | 2021 | Using collaborative AR to extend the concept of the touring of painting art exhibitions towards collecting itinerary and interaction analytics |
| Group | 11-Year-Old Students |
|---|---|
| Control Group | 40 |
| Experiment Group | 41 |
| Theme | Unit Experiment | Learning Objectives | Learning Content | Safety or Environmental Protection Factor |
|---|---|---|---|---|
| Air and Combustion | Oxygen production | Learn how to produce oxygen and examine its properties | Investigate what reaction oxygen and fire will produce | Instantaneous explosion |
| Carbon dioxide production | Learn how to produce carbon dioxide and examine its properties | Investigate which materials, when mixed together, will produce carbon dioxide | Instantaneous explosion | |
| Aqueous solutions | Electricity conductivity of aqueous solutions | To know that different aqueous solutions have different electrical conductivity | Test whether different aqueous solutions can conduct electricity through battery-operated light bulbs | Waste of materials |
| Acidity and alkalinity of aqueous solutions | Understand color changes of litmus paper in aqueous solutions with different acidity and alkalinity | Distinguish the acidity and alkalinity of different aqueous solutions and test them with litmus paper | Waste of materials |
| Card Type | Physical Teaching Material | Function Description | |||
|---|---|---|---|---|---|
| Experimental equipment card |  |  |  |  | Equipment Assembly |
| Name | Wide mouth bottle | Glass | Beaker | Dropper | |
| AR object |  |  |  |  | |
| Material card |  |  | Addition of material | ||
| Name | Enoki mushroom | Baking soda | |||
| AR object |  |  | |||
| Verifying equipment card |  |  |  |  | Displaying chemical reaction |
| Name | Incense | Candle | Litmus paper | Battery bulb group | |
| AR object |  |  |  | 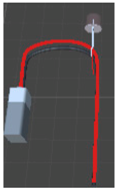 | |
| Experimental Unit | Operational Procedure | Related Chemical Reaction |
|---|---|---|
| Oxygen production | Prepare a wide-mouth bottle → Add hydrogen peroxide to the bottle → Add enoki mushrooms → Cover the bottle with a glass plate → Place incense sticks in the wide-mouth bottle to verify the comburent phenomenon | 1. The gas produced by adding too little solution is insufficient to set the incense sticks on fire 2. Under standard condition, the incense sticks will be lit and burn 3. The gas produced by adding too much solution will render the incense sticks to burn more vigorously than under standard conditions |
| Carbon dioxide production | Prepare a wide-mouth bottle → Add vinegar to the bottle → Add baking soda → Cover the bottle with a glass plate → Place a candle over the wide-mouth bottle to verify the phenomenon of inhibiting combustion | 1. The gas produced by adding too little solution is insufficient to extinguish the candle 2. Under standard conditions, the candle will go out 3. The bubble produced resulting from the reaction of adding too much solution will spill over the container, making it impossible to continue the experiment |
| Acidity and alkalinity of aqueous solutions | Prepare a beaker → Add solution to the beaker → Use a dropper to suck up the solution → Place the solution sucked up by the dropper on a piece of litmus paper → Verify the acidity and alkalinity of the solution | 1. Neutral solution → No change in the color of the litmus paper 2. Acidic solution → No change in the color of red litmus paper, the blue turns red 3. Alkaline solution → Red litmus paper turns blue; the blue remains unchanged |
| Electrical conductivity of aqueous solutions | Prepare a beaker → Add solution to the beaker → Place a set of battery-operated light bulbs in the solution → Verify whether the solution has electrical conductivity | 1. Solutions possessing conductivity → light bulbs will light up 2. Solutions possessing no electrical conductivity → light bulbs will not light up |
| Step 1 | 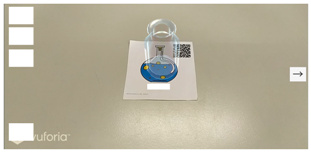  |
| Screen description | Take the experimental equipment card of the wide mouth beaker, and click the right arrow on the right side of the screen to choose the solution to be added. |
| Step 2 | 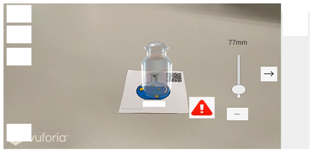 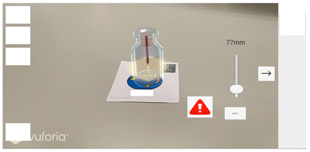 |
| Screen description | If the user adds excessive amounts of solution, there will be a warning and animation to remind her/him. |
| Step 3 | 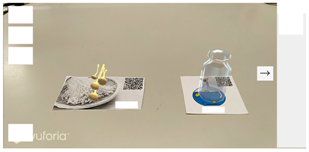  |
| Screen description | Take the enoki mushroom card and add it to the experimental equipment card of the wide mouth bottle, and then observe the phenomenon of oxygen generation. |
| Step 4 |   |
| Screen description | Take and then place the experimental equipment card of the glass plate over the experimental equipment card of the wide mouth bottle. |
| Step 5 | 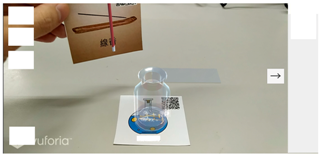  |
| Screen description | Take and place the verifying equipment card of the incense sticks in the experimental equipment card of the wide-mouth bottle, and then observe the comburent phenomenon. |
| Source of Change | Sum of Squared Deviation from the Mean | Degree of Freedom | Mean Sum of Squares | F-Test | p-Significance |
|---|---|---|---|---|---|
| Pre-test results | 6859.834 | 1 | 6859.834 | 279.385 | 0.000 |
| Group | 37.443 | 1 | 37.443 | 1.525 | 0.221 |
| Interaction effect(pre-test results × group) | 23.714 | 1 | 23.714 | 0.966 | 0.329 |
| Error | 1890.607 | 77 | 24.553 |
| Group | Source of Change | Mean | Standard Deviation | Number of People |
|---|---|---|---|---|
| Control group | Pre-test | 80.875 | 10.794 | 40 |
| Post-test | 83.000 | 11.367 | 40 | |
| Experimental group | Pre-test | 82.195 | 9.490 | 41 |
| Post-test | 86.585 | 9.901 | 41 |
| Source of Change | Type III Sum of Squares | Degree of Freedom | Mean Sum of Squares | F-Test | p-Significance |
|---|---|---|---|---|---|
| Group in the experiment | 112.046 | 1 | 112.046 | 4.565 | 0.036 * |
| Pre-test result | 7047.630 | 1 | 7047.630 | 287.159 | 0.000 |
| Error | 1914.321 | 78 | 24.543 | - | - |
| Group | Mean | Standard Error | 95% Confidence Interval | |
|---|---|---|---|---|
| Lower Limit | Upper Limit | |||
| Control group | 83.621 | 0.784 | 82.060 | 85.183 |
| Experimental group | 85.979 | 0.775 | 84.437 | 87.521 |
| No. | Item Content | Mean Value | Standard Deviation | No. of People |
|---|---|---|---|---|
| 1 | I feel that identifying picture cards using smartphones is helpful. | 4.07 | 0.848 | 41 |
| 2 | I feel that it is very convenient to operate the AR-based teaching method through smartphones. | 4.12 | 0.872 | 41 |
| 3 | The steps of operating the AR-based teaching method are simple and clear. | 4.17 | 0.919 | 41 |
| 4 | I feel that the AR-based teaching method can attract my attention. | 4.32 | 0.820 | 41 |
| 5 | I like the method of learning through the AR-based teaching method. | 4.29 | 0.814 | 41 |
| 6 | I feel that operating experiments through the AR-based teaching method will enliven the curriculum. | 4.29 | 0.716 | 41 |
| 7 | I feel that operating experiments through the AR-based teaching method makes me more focused on learning. | 4.20 | 0.679 | 41 |
| 8 | I feel that operating experiments through the AR-based teaching method helps me learn the curriculum of the natural science domain more easily. | 4.17 | 0.803 | 41 |
| 9 | I feel that operating experiments through the AR-based teaching method helps me understand the process of natural science experiments more easily. | 4.17 | 0.771 | 41 |
| 10 | I hope the AR-based teaching method can also be applied to other learning domains. | 4.41 | 0.836 | 41 |
| 11 | The teaching method for air and combustion enabled me to understand whether oxygen can help support the combustion of substances. | 4.22 | 0.909 | 41 |
| 12 | The teaching method for air and combustion helped me understand how to produce oxygen and examine its properties. | 4.34 | 0.794 | 41 |
| 13 | The teaching method for air and combustion helped me learn the uses of oxygen. | 4.24 | 0.799 | 41 |
| 14 | The teaching method for air and combustion helped me understand that carbon dioxide cannot support the combustion of substances. | 4.39 | 0.737 | 41 |
| 15 | The teaching method for air and combustion helped me understand how to produce carbon dioxide and examine its properties. | 4.24 | 0.734 | 41 |
| 16 | The teaching method for air and combustion helped me learn the uses of carbon dioxide. | 4.22 | 0.759 | 41 |
| 17 | The teaching method for aqueous solutions helped me learn what litmus paper is and how to use it. | 4.34 | 0.762 | 41 |
| 18 | The teaching method for aqueous solutions enabled me to understand that we use litmus paper to test the properties of aqueous solutions. | 4.24 | 0.830 | 41 |
| 19 | The teaching method for aqueous solutions enabled me to know that some aqueous solutions possess electrical conductivity. | 4.32 | 0.722 | 41 |
| No. | Item Content | Median | No. of People |
|---|---|---|---|
| 1 | I think the AR-based teaching method is in line with the objectives of the current curriculum design. | 4 | 8 |
| 2 | I think the number of times students are actually conducting experiments is not enough. | 4 | 8 |
| 3 | I think learning through the AR-based teaching method can increase students’ concentration with respect to the curriculum. | 4 | 8 |
| 4 | I think traditional teaching materials like pictures or videos are enough to enable students to understand [natural science] knowledge. | 2 | 8 |
| 5 | I think using the AR-based teaching method can enhance students’ learning outcomes. | 4 | 8 |
| 6 | I think the AR-based teaching method is much safer compared with traditional experiments. | 4 | 8 |
| 7 | I think the AR teaching method can shorten my lesson preparation time. | 4 | 8 |
| 8 | I think the AR-based teaching method can strengthen students’ learning motivation. | 4 | 8 |
| 9 | I will incorporate the AR-based teaching method in my teaching plan. | 4 | 8 |
| 10 | I think the effects of AR-based teaching method are better than those of traditional teaching materials. | 3 | 8 |
| 11 | I am willing to use AR-based teaching method in my class. | 4 | 8 |
| 12 | I think the AR-based teaching method can present different teaching methods. | 5 | 8 |
| 13 | I think the AR-based teaching method is helpful to teachers in more effectively imparting the content of the curriculum. | 4 | 8 |
| 14 | I think the AR-based teaching method is simple and clear in operational terms. | 3.5 | 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, C.-Y.; Ho, Y.-C.; Nisar, H. Design and Validation of a Virtual Chemical Laboratory—An Example of Natural Science in Elementary Education. Appl. Sci. 2021, 11, 10070. https://doi.org/10.3390/app112110070
Tsai C-Y, Ho Y-C, Nisar H. Design and Validation of a Virtual Chemical Laboratory—An Example of Natural Science in Elementary Education. Applied Sciences. 2021; 11(21):10070. https://doi.org/10.3390/app112110070
Chicago/Turabian StyleTsai, Chi-Yi, Yu-Chen Ho, and Humaira Nisar. 2021. "Design and Validation of a Virtual Chemical Laboratory—An Example of Natural Science in Elementary Education" Applied Sciences 11, no. 21: 10070. https://doi.org/10.3390/app112110070
APA StyleTsai, C.-Y., Ho, Y.-C., & Nisar, H. (2021). Design and Validation of a Virtual Chemical Laboratory—An Example of Natural Science in Elementary Education. Applied Sciences, 11(21), 10070. https://doi.org/10.3390/app112110070








