Abstract
Breast cancer is still considered a high-incidence disease, and numerous are the research efforts for the development of new useful and effective therapies. Among anticancer drugs, carbazole compounds are largely studied for their anticancer properties and their ability to interfere with specific targets, such as microtubule components. The latter are involved in vital cellular functions, and the perturbation of their dynamics leads to cell cycle arrest and subsequent apoptosis. In this context, we report the anticancer activity of a series of carbazole analogues 1–8. Among them, 2-nitrocarbazole 1 exhibited the best cytotoxic profile, showing good anticancer activity against two breast cancer cell lines, namely MCF-7 and MDA-MB-231, with IC50 values of 7 ± 1.0 and 11.6 ± 0.8 μM, respectively. Furthermore, compound 1 did not interfere with the growth of the normal cell line MCF-10A, contrarily to Ellipticine, a well-known carbazole derivative used as a reference molecule. Finally, in vitro immunofluorescence analysis and in silico studies allowed us to demonstrate the ability of compound 1 to interfere with tubulin organization, similarly to vinblastine: a feature that results in triggering MCF-7 cell death by apoptosis, as demonstrated using a TUNEL assay.
1. Introduction
Despite many research efforts, breast cancer incidence is constantly growing and remains a serious health emergency [1]. Indeed, breast cancer represents, to date, the main cancer-related cause of disease for women, and its diagnosis and mortality frequencies have risen worldwide in recent years [2]. Among the estimated 19.3 million new cancer cases worldwide in 2020, 11.7% represents female breast cancer, correlated with a mortality of 6.9% [3].
Clinically, breast cancers are classified according to specific subtypes defined by their histopathological features, and their expression of hormone receptors and growth factors, such as estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2). ER-positive breast cancer is increasing in incidence, while the triple negative causes concern about its aggressiveness and ability to give rise to metastases [4,5].
The use of cytotoxic chemotherapy in breast cancer has made significant progress in recent years due to the use of drugs able to interfere with the numerous biological pathways involved in cancer cell growth. However, the numerous side effects related to current therapies often overshadow their benefits. This spurred the need for research and development of new potent anticancer agents. Currently, medical attention has primarily focused on naturally occurring molecules with anticancer properties. Among them, the carbazole scaffold represents an important structural motif of many natural and/or synthetic pharmacologically active compounds [6]. They have been found in a large variety of organisms, including bacteria, fungi, plants, and animals and represent an important class of heterocycles, which exhibits innumerable biological activities [7]. Ellipticine is an alkaloid considered the first lead compound with anticancer activity belonging to the carbazole class [8]. It was first obtained in 1959 from the leaves of Ochrosia elliptica (Apocynacae), while now, it is prepared by entirely synthetic procedures [9]. Considering the biological importance of this molecule together with its demonstrated high toxicity [10], many Ellipticine derivatives with antioxidant, anticancer, anti-inflammatory, antibacterial, antiviral, and antidiabetic properties have been synthesized in recent decades [7,11,12,13,14,15,16]. Numerous in vitro studies, supported by docking simulations, demonstrated that some carbazole derivatives and analogues significantly disrupt the microtubule network, arresting the cell cycle and inducing cell apoptosis [17,18,19,20].
Considering these exciting data [17,18,19,20], the goal of this work was to evaluate the anticancer activity of a series of carbazole derivatives (1–8, Figure 1) against two human breast cancer cell lines, namely ER(+) MCF-7 cells and triple-negative MDA-MB-231 cells. The obtained data showed that nitrocarbazoles 1–3 exhibited the best anticancer activity on both the breast cancer cell lines used. However, 3-nitrocarbazole 2 and 2,3-dinitrocarbazole 3 showed strong cytotoxicity on the normal MCF-10A cell line, while 2-nitrocarbazole 1 did not interfere with the growth of the same cell line. Cytotoxicity of compounds on normal cells may be influenced by the position of the nitro group(s) on the aromatic ring. These interesting results pushed us to further understand the mechanism of action of the most active and safe nitrocarbazole 1 in depth. Immunofluorescence analysis demonstrated that compound 1 perturbs microtubule networks, inducing disorganization of the tubulin filaments and their accumulation around cell nuclei. The disruption of microtubules dynamics led to cancer cell death by apoptosis. The in vitro results, confirmed by in silico studies, suggest that 2-nitrocarbazole 1 represents an interesting tool in cancer treatment as a microtubule-targeting agent. These results are an important starting point in medicinal chemistry for the development of targeted therapy able to reduce the numerous toxic effects typically associated with traditional therapeutic approaches.
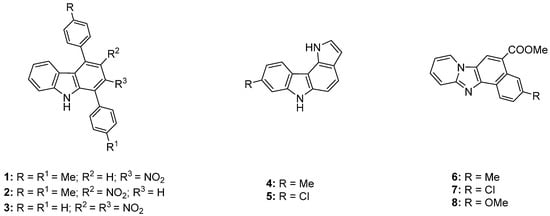
Figure 1.
Molecular structures of compounds 1–8.
2. Materials and Methods
2.1. Chemistry
The synthesis and characterization of compounds 1–3 [21], 4 and 5 [22], and 6–8 [23] has already been reported; details are provided in Scheme 1 below.
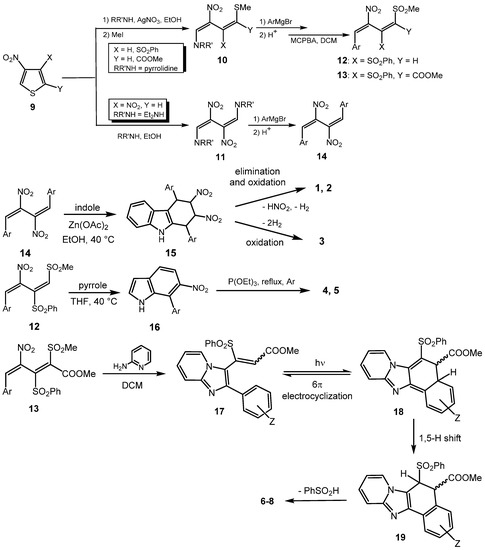
Scheme 1.
Synthetic protocol of compounds 1–8. From nitrothiophene 9, via a ring-opening–ring-closing (benzannulation) procedure.
2.2. Biology
2.2.1. Cell Culture
The three cell lines employed in this work (MCF-7, MDA-MB-231, and MCF-10A) were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA) and cultured as already described [15].
2.2.2. MTT Assay
The in vitro anticancer activity of all of the studied compounds were detected using the MTT (Sigma) assay [24,25]. In brief, cells were seeded in a 48-well plate, then starved in serum-free medium, and incubated with the target compounds dissolved in DMSO at six differing concentrations (0.1, 1, 5, 10, 100, and 200 µM) for 72 h, as already described [24]. After this period of time, MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) was incubated for 2 h at 37 °C (final concentration 0.5 mg/mL). Then, the formazan crystals were dissolved in DMSO and the optical density was measured at 570 nm using a microplate reader. All of the calculations were performed in triplicate, and the results were represented as the percent (%) of basal. The IC50 values were calculated using curve-fitting GraphPad Prism 9 software (GraphPad Software, La Jolla, CA, USA) with nonlinear regression. The values represent the mean ± standard deviation (n = 3).
2.2.3. Immunofluorescence Analysis
The cells were seeded in 48-well culture plates containing glass slides, then serum-deprived for 24 h, and incubated with the most active compound for 24 h (concentration equal to its IC50 value). Then, the methanol-fixed cells were incubated with the primary antibody (mouse anti-β-tubulin, Santa Cruz Biotechnology, Dallas, TX, USA) and then the secondary antibody (Alexa Fluor® 568 conjugate goat-anti-mouse, Thermo Fisher Scientific, MA, USA), as previously described [26]. Then, the Nuclei were stained using DAPI (Sigma Aldrich, Milan, Italy). Fluorescence was detected using a fluorescence microscope (Leica DM 6000, 20× magnification). LAS-X software was used to acquire and process all images. The images are representative of three independent experiments.
2.2.4. Docking Studies
The crystal structures of the quaternary assembly of human tubulin (αβαβ) in a complex with stathmin and vinblastine [27] (PDB code 5J2T) has been used as a target for our molecular docking simulations. We built the three-dimensional structures of compounds 1, 2, and 3 using the MarvinSketch program (ChemAxon ltd, Budapest, Hu), and once the atomic charges were assigned, we minimized all of them. As described in our previous work [15], we used the Autodock program v. 4.2.2 [28] to evaluate the possible binding modes of our ligands and to evaluate the binding energies of different derivatives to these proteins. We adopted a “blind docking” strategy: docking simulations of small molecules to the targets were conducted without a priori knowledge of the position of the binding site by the system. All of the simulations were performed by adopting the standard default values and by utilizing the same procedures described in several previous work by our group [29,30,31]. The figures were drawn using the program Chimera [32].
2.2.5. TUNEL Assay
The ability of the most active compound to induce cell death by apoptosis was detected using the TUNEL assay using the CFTM488A TUNEL Assay Apoptosis Detection Kit (Biotium, Hayward, CA, USA). The cells were grown on glass coverslips and then treated with the tested compound. Then, the methanol-fixed cells were incubated with the enzyme terminal deoxynucleotidyl transferase (TdT) for 2 h at 37 °C, as previously described [24]. The nuclei were stained using DAPI 0.2 mg/mL (Sigma Aldrich, Milan, Italy). Finally, the cells were observed under a fluorescence microscope (Leica DM6000; 20x magnification). The images are demonstrative of three separate experiments.
3. Results and Discussion
3.1. Chemistry
Although structurally different, compounds 1–8 share a synthetic protocol whereby a nitrobutadiene (12–14), deriving from the initial ring-opening of a suitably substituted 3-nitrothiophene (9) [33,34,35], acts as a benzannulating agent towards indole [21], pyrrole [22], or 2-aminopyridine [23] (Scheme 1).
Evaluation of the anticancer activity of compounds 1–8 follows a long-standing engagement by the Genoa research group in the synthesis of pharmacologically active nitroderivatives from the initial ring-opening of nitrothiophenes 9 [36,37,38,39,40,41,42,43,44], an effort that has resulted in a number of positive results in both in vitro and in vivo experiments. For instance, appreciable antitumor activity has been found for either some modified 13 [36,38,39,40,41,42] or some selected 14 [36,37,39,40,42]; furthermore, α-glucosidase inhibition [43] or antibacterial activity [44] has, on the one hand, exhibited in nitroheterocycles obtained from our nitrobutadiene building-blocks. On the other hand, the Cosenza research group has, in turn, recently highlighted the efficacy of a nitrocarbazole as an anti-HIV agent [11]. Coupled with the abovementioned results, the outcomes herein surely contribute to assessing the nitro goup’s significance as a valuable pharmacophore.
3.2. Biology
3.2.1. Anticancer Activity
The anticancer activity of all the compounds against two breast cancer cell lines, namely ER(+) MCF-7 and triple-negative MDA-MB-231, were evaluated by MTT assay, and the IC50 values, derived from the experimental data, are summarized in Table 1.

Table 1.
IC50 values of compounds 1–8 and Ellipticine, expressed in µM. The means ± standard deviations are shown. The experiments were performed in triplicate.
After the breast cancer cells were incubated in the presence of compounds 1–8 for 72 h, the IC50 values indicated that some of them exhibited, in different degrees, a good anticancer activity against both cell lines.
The most promising compound was 2-nitrocarbazole 1, which exerted good anticancer activity against both breast cancer cell lines used in this assay, with IC50 values of 7.0 ± 1.0 and 11.6 ± 0.8 μM on MCF-7 and MDA-MB-231, respectively. Carbazoles 2, 3, and 5 showed much higher cytotoxicity than the other compounds on both of the cell lines screened. Indeed, the IC50 values of compounds 2, 3, and 5 were 3.4 ± 1.3, 5.4 ± 1.1, and 1.7 ± 0.6 μM against MCF-7 cells, respectively, and 12.2 ± 1.2, 14.4 ± 0.9, and 1.2 ± 1.1 μM towards MDA-MB-231 cells, respectively. Unfortunately, together with their good anticancer activity, 2, 3, and 5 also exhibited severe cytotoxicity on the normal human mammary epithelial cells MCF-10A, with IC50 values of 23.6 ± 0.7, 3.7 ± 0.6, and 27.8 ± 1.0 μM, respectively.
Instead, 1 did not interfere with the growth of the normal cell lines, showing an IC50 value higher than 200 μM on the same normal cells. Moreover, compounds 4 and 6–8 exhibited a lower, or no, anticancer activity against both breast cancer cell lines. Ellipticine, used as a reference molecule in this assay, exhibited strong anticancer activity against MCF-7 and MDA-MB-231 cells with IC50 values of 1.9 ± 0.5 and 1.3 ± 0.9 μM, respectively, together with a dramatic inhibition of normal cell growth (the IC50 on MCF-10A was 1.2 ± 0.7 μM). Concerning the structure–activity relationships, the nitrocarbazoles 1–3 resulted in the most active compounds, indicating that the presence of the nitro group could positively affect their anticancer activity. However, it seems clear that the position of the nitro group on the aromatic ring influences the cytotoxicity of the compounds on the normal cells. Indeed, if present in the 2-position (2-nitrocarbazole 1), it does not affect the growth of normal cells MCF-10A (IC50 > 200 μM), at least at the concentrations and under the conditions used, while in the 3-position, it makes compound 2 responsible for the strong cytotoxic effect on the same non-tumoral cells (IC50 = 23.6 ± 0.7 μM). Moreover, the presence of two nitro groups in the 2,3-positions on the aromatic ring gives a higher toxicity to compound 3 (IC50 = 3.7 ± 0.6 μM) than to 2.
Regarding the 8-methylpyrrolo[3,2-c]carbazole 4, it did not show an anticancer effect on the breast cancer cells used, whereas the replacement of the methyl group with a chlorine makes the 8-chloropyrrolo[3,2-c]carbazole 5 more active (IC50 = 1.7 ± 0.6 μM on MCF-7 and 1.2 ± 1.1 μM on MDA-MB-231) and highly cytotoxic on the normal cells MCF-10A (IC50 = 27.8 ± 1.0 μM). Finally, imidazopyridine 6–8 were inactive as anticancer agents on both breast cancer cell lines.
Summing up, among the tested compounds, the 2-nitrocarbazole 1 possesses the best anticancer profile, causing a growth reduction in both breast cancer cells used, even if less active than the reference molecule Ellipticine. However, contrary to the other analogues of the series and to Ellipticine, the 2-nitrocarbazole 1 did not exert any cytotoxicity against normal human mammary epithelial cells MCF-10A.
3.2.2. Induction of Cell Cytoskeleton Destabilization
Microtubule-targeting agents are widespread drugs useful in cancer treatment due to their ability to interfere with critical cellular functions, such as mitosis, cell migration, and cell signaling [45].
The efficacy of microtubule-targeting drugs has been evidenced by the use of some Vinca alkaloids and taxanes for the treatment of a large panel of human cancers [46]. Based on the mechanism of action, microtubule-targeting agents are classified into two categories: microtubule-destabilizing agents, such as the Vinca alkaloids, that inhibit the polymerization reaction; destabilizing microtubules and decreasing tubulin polymer filaments; and microtubule-stabilizing agents, such as taxanes which, contrarily, promote tubulin polymerization-stabilizing microtubules [47]. While these agents are highly effective in cancer treatment, the onset of resistance represents the principal clinical issue that limits their use [48]. Moreover, their effectiveness has been impaired by the presence of systemic toxicity and, often, the absence of bioavailability [49]. Thus, in recent years, research efforts have been focused on the development of more active and safe new compounds that could target microtubule organization [50,51,52].
With the aim to understand the role of compound 1 in cytoskeleton dynamics, we carried out immunofluorescence studies using MCF-7 cells as models, since they represent the cell line on which the 2-nitrocarbazole 1 was more active. Cells treated only with the vehicle (DMSO and CTRL) showed normal organization of the microtubule network in which tubulin filaments are regularly spread into the MCF-7 cell cytoplasm (Figure 2, Panel B, CTRL). Contrarily, exposure of the same cells to vinblastine as well as to compound 1 caused microtubule disorganization (Figure 2, panels B, vinblastine and 1). Indeed, tubulin filaments become irregular and accumulate around cell nuclei (see the white arrows). These results indicate that, similar to vinblastine, 2-nitrocarbazole 1 could act as a tubulin-polymerization inhibitor.
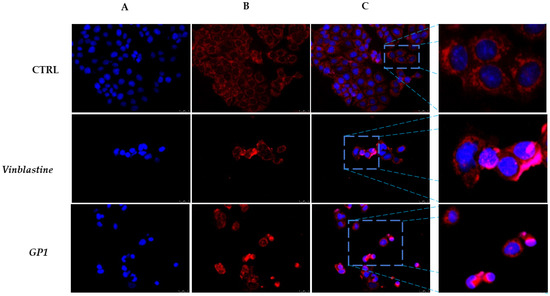
Figure 2.
Immunofluorescence studies. MCF-7 cells were treated with compound 1 and vinblastine, used as a reference molecule, (IC50 values) or with a vehicle (CTRL) for 24 h. After treatment, the cells were incubated with primary and secondary antibodies (see the Experimental section for more details) and then imaged under the inverted fluorescence microscope at 20× magnification. CTRL cells (DMSO only) showed regular organization of the cytoskeleton ((B), CTRL). MCF-7 cells treated with vinblastine and compound 1 exhibited irregular arrangement and organization, with tubulin filaments accumulated around cell nuclei (white arrows, (B), vinblastine and 1). (A) DAPI, excitation/emission wavelength 350 nm/460 nm; (B) tubulin (Alexa Fluor® 568) excitation/emission wavelength 644 nm/665 nm; and (C) a merge. A zoom-in of the overlay channels is shown on the right. Images are representative of three independent experiments.
3.2.3. Docking Studies
To study the possible binding modes of our compounds to the quaternary assembly of human tubulin and to calculate a binding energy of the complexes, we carried over molecular docking simulation runs using the crystallographic coordinates of the complex formed between tubulin α, tubulin β, and stathmin4 (PDB code 5J2T) as a protein target [27], eventually comparing our results with the binding modes of vinblastine. For all our compounds, we adopted a “blind docking” strategy: i.e., the docking of our small molecules to their targets was performed without a priori knowledge of the binding site of the ligand. This strategy was first tested and validated by repositioning vinblastine in the protein binding site with its correct binding mode, displaying a root-mean-square deviation (RMSD) of less than 0.2 Å, compared with the one determined by X-ray crystallography. This guarantees the reliability of our docking simulations. Further on, we tested our compounds and found two different binding zones: one for molecules 1 and 3, within the interface between subunits β and α in proximity to the vinblastine binding zone, and a second for 2, at the interface between subunit α and β, in proximity to the Colchicine binding site (Figure 3). Table 2 illustrates the amino acids involved in ligand interactions and the binding energies of all of the complexes formed by tubulin and our compounds.
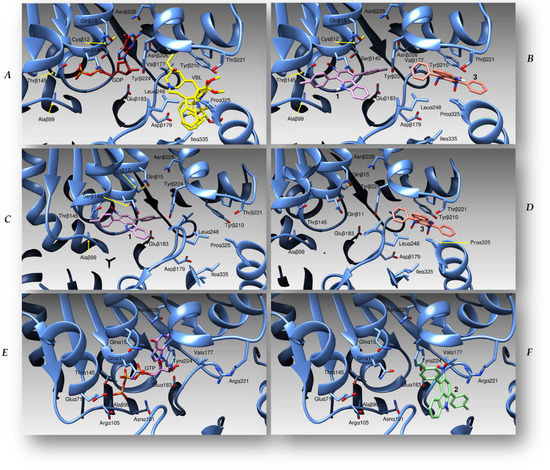
Figure 3.
(A) A cartoon representation of the tubulin (cyan ribbons) interface between subunits beta and alpha, with vinblastine (VLB, yellow sticks) and guanosyndiphosphate (GDP, red sticks) reported. (B) Binding modes of molecules 1 (purple sticks) and 3 (pink) to the beta-alpha interface of tubulin, in the same three-dimensional orientation as (A). (C,D) The position of compounds 1 (purple) and 3 (pink), respectively, within the same binding site. (E) The crystallographic structure of a guanosyntriphosphate (GTP) molecule (drawn in purple) bound to the alpha–beta interface of the protein. (F) The pose of compound 2 (green sticks) within the same binding site. We showed and labelled the residues involved in the interactions of the three molecules in the tubulin quaternary structure.

Table 2.
Energies of the complexes formed by tubulin and compounds 1, 2, and 3, and protein residues interacting with the ligands.
3.2.4. TUNEL Assay
The perturbation of microtubule dynamics leads to disruption of the mitotic spindle in dividing cells, causing cell cycle arrest and, as a last step, the induction of subsequent cell death by apoptosis [53,54]. Thus, we determined whether 2-nitrocarbazole 1 is able to trigger apoptosis using TUNEL assay, which allows the formation of DNA fragments to be detected.
When compared with the vehicle-treated cells (CTRL), a detectable level of green fluorescence, related to the formation of DNA fragments, was evident in MCF-7 cells after 24 h treatment with compound 1 at its IC50 value (Figure 4). This evidence indicates that this compound is able to induce MCF-7 cell death by triggering apoptosis, and this effect is probably linked to its ability to perturb microtubule dynamics.
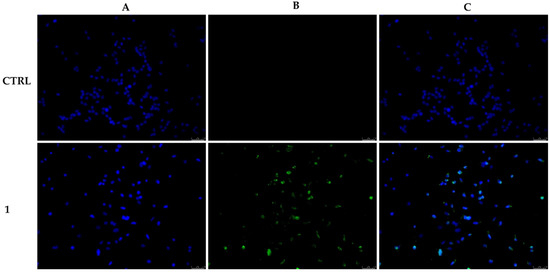
Figure 4.
MCF-7 cells were exposed to compound 1 at its IC50 value or with the vehicle DMSO (CTRL) for 24 h. Then, cells were incubated with the TdT enzyme and observed under an inverted fluorescence microscope at 20× magnification. Apoptotic cells are indicated by a clear green nuclear fluorescence in compound 1-treated cells. (A) DAPI (CTRL and 1) λex/em = 350 nm/460 nm. (B) CFTM488A (CTRL and 1) λex/em = 490 nm/515 nm. (C) A merge. Fields are representative of three separate experiments.
4. Conclusions
In this paper, we reported the evaluation of the anticancer properties of some nitrocarbazoles in in silico and in vitro studies. The lead compound showed a good cytotoxic profile, being active mostly against MCF-7 cells. Moreover, docking simulations and immunofluorescence studies suggest a role in perturbing the MCF-7 cell microtubule network and triggering cancer cell death by apoptosis.
Author Contributions
Conceptualization, G.P. and M.S.S.; methodology, D.I.; software, C.R. and M.P.; validation, D.S., formal analysis, J.C. and C.T.; investigation, J.C. and C.T.; data curation, A.B. (Alexia Barbarossa); writing—original draft preparation, J.C. and G.P.; writing—review and editing, M.S.S. and C.T.; visualization, A.B. (Alice Benzi), L.B., and M.M.; supervision, D.S. and G.P.; funding acquisition, C.T. and G.P. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support was provided by grants from Genoa University (FRA 2020).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
C.R. acknowledges the support of a grant from the Italian Ministry of Health (Ricerca Corrente 2020).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Global Burden of Disease Cancer Collaboration; Fitzmaurice, C.; Akinyemiju, T.F.; Al Lami, F.H.; Alam, T.; Alizadeh-Navaei, R.; Allen, C.; Alsharif, U.; Alvis-Guzman, N.; Amini, E.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2016: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2018, 4, 1553–1568. [Google Scholar]
- Ghoncheh, M.; Pournamdar, Z.; Salehiniya, H. Incidence and Mortality and Epidemiology of Breast Cancer in the World. Asian Pac. J. Cancer Prev. APJCP 2016, 17, 43–46. [Google Scholar] [CrossRef] [Green Version]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Bigaard, J.; Stahlberg, C.; Jensen, M.B.; Ewertz, M.; Kroman, N. Breast cancer incidence by estrogen receptor status in Denmark from 1996 to 2007. Breast Cancer Res. Treat. 2012, 136, 559–564. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Yin, Y.; Meng, Q.; Lyu, Y. The role of EMT-related lncRNA in the process of triple-negative breast cancer metastasis. Biosci. Rep. 2021, 41, BSR20203121. [Google Scholar] [CrossRef]
- Schmidt, A.W.; Reddy, K.R.; Knolker, H.J. Occurrence, biogenesis, and synthesis of biologically active carbazole alkaloids. Chem. Rev. 2012, 112, 3193–3328. [Google Scholar] [CrossRef]
- Issa, S.; Prandina, A.; Bedel, N.; Rongved, P.; Yous, S.; Le Borgne, M.; Bouaziz, Z. Carbazole scaffolds in cancer therapy: A review from 2012 to 2018. J. Enzym. Inhib. Med. Chem. 2019, 34, 1321–1346. [Google Scholar] [CrossRef] [Green Version]
- Stiborova, M.; Sejbal, J.; Borek-Dohalska, L.; Aimova, D.; Poljakova, J.; Forsterova, K.; Rupertova, M.; Wiesner, J.; Hudecek, J.; Wiessler, M.; et al. The anticancer drug ellipticine forms covalent DNA adducts, mediated by human cytochromes P450, through metabolism to 13-hydroxyellipticine and ellipticine N2-oxide. Cancer Res. 2004, 64, 8374–8380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, C.M.; McCarthy, F.O. Isolation, biological activity and synthesis of the natural product ellipticine and related pyridocarbazoles. RSC Adv. 2012, 2, 8883–8918. [Google Scholar] [CrossRef]
- Stiborova, M.; Poljakova, J.; Martinkova, E.; Borek-Dohalska, L.; Eckschlager, T.; Kizek, R.; Frei, E. Ellipticine cytotoxicity to cancer cell lines—A comparative study. Interdiscip. Toxicol. 2011, 4, 98–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caruso, A.; Ceramella, J.; Iacopetta, D.; Saturnino, C.; Mauro, M.V.; Bruno, R.; Aquaro, S.; Sinicropi, M.S. Carbazole Derivatives as Antiviral Agents: An Overview. Molecules 2019, 24, 1912. [Google Scholar] [CrossRef] [Green Version]
- Caruso, A.; Lancelot, J.C.; El-Kashef, H.; Sinicropi, M.S.; Legay, R.; Lesnard, A.; Rault, S. A rapid and versatile synthesis of novel pyrimido[5,4-b]carbazoles. Tetrahedron 2009, 65, 10400–10405. [Google Scholar] [CrossRef]
- Caruso, A.; Sinicropi, M.S.; Lancelot, J.C.; El-Kashef, H.; Saturnino, C.; Aubert, G.; Ballandonne, C.; Lesnard, A.; Cresteil, T.; Dallemagne, P.; et al. Synthesis and evaluation of cytotoxic activities of new guanidines derived from carbazoles. Bioorg. Med. Chem. Lett. 2014, 24, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Rizza, P.; Pellegrino, M.; Caruso, A.; Iacopetta, D.; Sinicropi, M.S.; Rault, S.; Lancelot, J.C.; El-Kashef, H.; Lesnard, A.; Rochais, C.; et al. 3-(Dipropylamino)-5-hydroxybenzofuro[2,3-f]quinazolin-1(2H)-one (DPA-HBFQ-1) plays an inhibitory role on breast cancer cell growth and progression. Eur. J. Med. Chem. 2016, 107, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Sinicropi, M.S.; Iacopetta, D.; Rosano, C.; Randino, R.; Caruso, A.; Saturnino, C.; Muia, N.; Ceramella, J.; Puoci, F.; Rodriquez, M.; et al. N-thioalkylcarbazoles derivatives as new anti-proliferative agents: Synthesis, characterisation and molecular mechanism evaluation. J. Enzym. Inhib. Med. Chem. 2018, 33, 434–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caruso, A.; Iacopetta, D.; Puoci, F.; Cappello, A.R.; Saturnino, C.; Sinicropi, M.S. Carbazole Derivatives: A Promising Scenario for Breast Cancer Treatment. Mini-Rev. Med. Chem. 2016, 16, 630–643. [Google Scholar] [CrossRef] [PubMed]
- Ceramella, J.; Caruso, A.; Occhiuzzi, M.A.; Iacopetta, D.; Barbarossa, A.; Rizzuti, B.; Dallemagne, P.; Rault, S.; El-Kashef, H.; Saturnino, C.; et al. Benzothienoquinazolinones as new multi-target scaffolds: Dual inhibition of human Topoisomerase I and tubulin polymerization. Eur. J. Med. Chem. 2019, 181, 111583. [Google Scholar] [CrossRef] [PubMed]
- Diaz, P.; Horne, E.; Xu, C.; Hamel, E.; Wagenbach, M.; Petrov, R.R.; Uhlenbruck, B.; Haas, B.; Hothi, P.; Wordeman, L.; et al. Modified carbazoles destabilize microtubules and kill glioblastoma multiform cells. Eur. J. Med. Chem. 2018, 159, 74–89. [Google Scholar] [CrossRef]
- Niu, F.; Liu, Y.; Jing, Z.; Han, G.; Sun, L.; Yan, L.; Zhou, L.; Wu, Y.; Xu, Y.; Hu, L.; et al. Novel carbazole sulfonamide microtubule-destabilizing agents exert potent antitumor activity against esophageal squamous cell carcinoma. Cancer Lett. 2018, 420, 60–71. [Google Scholar] [CrossRef]
- Padmaja, P.; Koteswara Rao, G.; Indrasena, A.; Subba Reddy, B.V.; Patel, N.; Shaik, A.B.; Reddy, N.; Dubey, P.K.; Bhadra, M.P. Synthesis and biological evaluation of novel pyrano[3,2-c]carbazole derivatives as anti-tumor agents inducing apoptosis via tubulin polymerization inhibition. Org. Biomol. Chem. 2015, 13, 1404–1414. [Google Scholar] [CrossRef]
- Bianchi, L.; Giorgi, G.; Maccagno, M.; Petrillo, G.; Scapolla, C.; Tavani, C. An original route to newly-functionalized indoles and carbazoles starting from the ring-opening of nitrothiophenes. Tetrahedron Lett. 2012, 53, 752–757. [Google Scholar] [CrossRef]
- Benzi, A.; Bianchi, L.; Maccagno, M.; Pagano, A.; Petrillo, G.; Tavani, C. Sequential Annulations to Interesting Novel Pyrrolo[3,2-c]carbazoles. Molecules 2019, 24, 3802. [Google Scholar] [CrossRef] [Green Version]
- Benzi, A.; Bianchi, L.; Giorgi, G.; Maccagno, M.; Petrillo, G.; Tavani, C. 2-Aryl-3-Vinyl Substituted Imidazo[1,2-a]pyridines and Fluorescent Electrocyclization Derivatives therefrom. Chemistryselect 2020, 5, 4552–4558. [Google Scholar] [CrossRef]
- Iacopetta, D.; Rosano, C.; Puoci, F.; Parisi, O.I.; Saturnino, C.; Caruso, A.; Longo, P.; Ceramella, J.; Malzert-Freon, A.; Dallemagne, P.; et al. Multifaceted properties of 1,4-dimethylcarbazoles: Focus on trimethoxybenzamide and trimethoxyphenylurea derivatives as novel human topoisomerase II inhibitors. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2017, 96, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Fazio, A.; Iacopetta, D.; La Torre, C.; Ceramella, J.; Muia, N.; Catalano, A.; Carocci, A.; Sinicropi, M.S. Finding solutions for agricultural wastes: Antioxidant and antitumor properties of pomegranate Akko peel extracts and beta-glucan recovery. Food Funct. 2018, 9, 6618–6631. [Google Scholar] [CrossRef] [PubMed]
- Ceramella, J.; Loizzo, M.R.; Iacopetta, D.; Bonesi, M.; Sicari, V.; Pellicano, T.M.; Saturnino, C.; Malzert-Freon, A.; Tundis, R.; Sinicropi, M.S. Anchusa azurea Mill. (Boraginaceae) aerial parts methanol extract interfering with cytoskeleton organization induces programmed cancer cells death. Food Funct. 2019, 10, 4280–4290. [Google Scholar] [CrossRef] [PubMed]
- Waight, A.B.; Bargsten, K.; Doronina, S.; Steinmetz, M.O.; Sussman, D.; Prota, A.E. Structural Basis of Microtubule Destabilization by Potent Auristatin Anti-Mitotics. PLoS ONE 2016, 11, e0160890. [Google Scholar] [CrossRef] [Green Version]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [Green Version]
- Rosano, C.; Lappano, R.; Santolla, M.F.; Ponassi, M.; Donadini, A.; Maggiolini, M. Recent advances in the rationale design of GPER ligands. Curr. Med. Chem. 2012, 19, 6199–6206. [Google Scholar] [CrossRef]
- Iacopetta, D.; Grande, F.; Caruso, A.; Mordocco, R.A.; Plutino, M.R.; Scrivano, L.; Ceramella, J.; Muia, N.; Saturnino, C.; Puoci, F.; et al. New insights for the use of quercetin analogs in cancer treatment. Future Med. Chem. 2017, 9, 2011–2028. [Google Scholar] [CrossRef]
- Cesarini, S.; Spallarossa, A.; Ranise, A.; Schenone, S.; Rosano, C.; La Colla, P.; Sanna, G.; Busonera, B.; Loddo, R. N-acylated and N,N’-diacylated imidazolidine-2-thione derivatives and N,N’-diacylated tetrahydropyrimidine-2(1H)-thione analogues: Synthesis and antiproliferative activity. Eur. J. Med. Chem. 2009, 44, 1106–1118. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera--a visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianchi, L.; Maccagno, M.; Petrillo, G.; Sancassan, F.; Spinelli, D.; Tavani, C. 2,3-Dinitro-1,3-butadienes: Versatile building-blocks from the ring-opening of 3,4-dinitrothiophene. In Targets in Heterocyclic Systems: Chemistry and Properties, 1st ed.; Attanasi, O.A., Spinelli, D., Eds.; Società Chimica Italiana: Rome, Italy, 2006; Volume 10, pp. 1–23. [Google Scholar]
- Bianchi, L.; Maccagno, M.; Petrillo, G.; Rizzato, E.; Sancassan, F.; Severi, E.; Spinelli, D.; Tavani, C.; Viale, M. Versatile nitrobutadienic building-blocks from the ring opening of 2- and 3-nitrothiophenes. In Targets in Heterocyclic Systems: Chemistry and Properties, 1st ed.; Attanasi, O.A., Spinelli, D., Eds.; Società Chimica Italiana: Rome, Italy, 2007; Volume 11, pp. 1–20. [Google Scholar]
- Petrillo, G.; Benzi, A.; Bianchi, L.; Maccagno, M.; Pagano, A.; Tavani, C.; Spinelli, D. Recent advances in the use of conjugated nitro or dinitro-1,3-butadienes as building-blocks for the synthesis of heterocycles. Tetrahedron Lett. 2020, 61, 152297. [Google Scholar] [CrossRef]
- Fenoglio, C.; Grosso, A.; Petrillo, G.; Boncompagni, E.; Aiello, C.; Cordazzo, C.; Spinelli, D.; Ognio, E.; Mariggio, M.A.; Cassano, A.; et al. A histochemical approach to the evaluation of the in vivo cytotoxicity of the nitrobutadienes (1E,3E)-1,4-bis(1-naphthyl)-2,3-dinitro-1,3-butadiene and methyl (2Z,4E)-2-methylsulfanyl-5-(1-naphthyl)-4-nitro-2,4-pentadienoate in mice liver and kidney. Anticancer Res. 2008, 28, 813–823. [Google Scholar] [PubMed]
- Fontana, A.; Viale, M.; Guernelli, S.; Gasbarri, C.; Rizzato, E.; Maccagno, M.; Petrillo, G.; Aiello, C.; Ferrini, S.; Spinelli, D. Strategies for improving the water solubility of new antitumour nitronaphthylbutadiene derivatives. Org. Biomol. Chem. 2010, 8, 5674–5681. [Google Scholar] [CrossRef] [PubMed]
- Novi, M.; Ottone, M.; Dell’Erba, C.; Barbieri, F.; Chiavarina, B.; Maccagno, M.; Viale, M. 1,4-Bis(1-naphthyl)-2,3-dinitro-1,3-butadiene a novel anticancer compound effective against tumor cell lines characterized by different mechanisms of resistance. Oncol. Rep. 2004, 12, 91–96. [Google Scholar] [CrossRef]
- Petrillo, G.; Fenoglio, C.; Ognio, E.; Aiello, C.; Spinelli, D.; Mariggio, M.A.; Maccagno, M.; Morganti, S.; Cordazzo, C.; Viale, M. Naphthylnitrobutadienes as pharmacologically active molecules: Evaluation of the in vivo antitumour activity. Investig. New Drugs 2007, 25, 535–544. [Google Scholar] [CrossRef]
- Petrillo, G.; Mariggio, M.A.; Aiello, C.; Cordazzo, C.; Fenoglio, C.; Morganti, S.; Croce, M.; Rizzato, E.; Spinelli, D.; Maccagno, M.; et al. Design, synthesis, and in vitro evaluation of new naphthylnitrobutadienes with potential antiproliferative activity: Toward a structure/activity correlation. Bioorg. Med. Chem. 2008, 16, 240–247. [Google Scholar] [CrossRef]
- Petrillo, G.; Tavani, C.; Bianchi, L.; Benzi, A.; Cavalluzzi, M.M.; Salvagno, L.; Quintieri, L.; De Palma, A.; Caputo, L.; Rosato, A.; et al. Densely Functionalized 2-Methylideneazetidines: Evaluation as Antibacterials. Molecules 2021, 26, 3891. [Google Scholar] [CrossRef]
- Tavani, C.; Bianchi, L.; De Palma, A.; Passeri, G.I.; Punzi, G.; Pierri, C.L.; Lovece, A.; Cavalluzzi, M.M.; Franchini, C.; Lentini, G.; et al. Nitro-substituted tetrahydroindolizines and homologs: Design, kinetics, and mechanism of alpha-glucosidase inhibition. Bioorg. Med. Chem. Lett. 2017, 27, 3980–3986. [Google Scholar] [CrossRef]
- Viale, M.; Petrillo, G.; Aiello, C.; Fenoglio, C.; Cordazzo, C.; Mariggio, M.A.; Cassano, A.; Prevosto, C.; Ognio, E.; Maccagno, M.; et al. Synthesis, in vitro activity and in vivo toxicity of the new 2,3-dinitrobutadiene derivative (1E,3E)-1,4-bis(2-naphthyl)-2,3-dinitro-1,3-butadiene. Pharm. Res. 2007, 56, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Viale, M.; Petrillo, G.; Maccagno, M.; Castagnola, P.; Aiello, C.; Cordazzo, C.; Mariggio, M.A.; Jadhav, S.A.; Bianchi, L.; Leto, G.; et al. Sensitivity of different resistant tumour cell lines to the two novel compounds (2Z,4E)-2-methylsulfanyl-5-(1-naphthyl)-4-nitro-2,4-pentadienoate and (1E,3E)-1,4-bis(2-naphthyl)-2,3-dinitro-1,3-butadiene. Eur. J. Pharm. 2008, 588, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Cermak, V.; Dostal, V.; Jelinek, M.; Libusova, L.; Kovar, J.; Rosel, D.; Brabek, J. Microtubule-targeting agents and their impact on cancer treatment. Eur. J. Cell Biol. 2020, 99, 151075. [Google Scholar] [CrossRef]
- Karahalil, B.; Yardim-Akaydin, S.; Nacak Baytas, S. An overview of microtubule targeting agents for cancer therapy. Arh. Hig. Rada Toksikol. 2019, 70, 160–172. [Google Scholar] [CrossRef] [Green Version]
- Tangutur, A.D.; Kumar, D.; Krishna, K.V.; Kantevari, S. Microtubule Targeting Agents as Cancer Chemotherapeutics: An Overview of Molecular Hybrids as Stabilizing and Destabilizing Agents. Curr. Top. Med. Chem. 2017, 17, 2523–2537. [Google Scholar] [CrossRef]
- Krause, W. Resistance to anti-tubulin agents: From vinca alkaloids to epothilones. Cancer Drug Resist. 2019, 2, 82–106. [Google Scholar] [CrossRef]
- Zhou, J.; Giannakakou, P. Targeting microtubules for cancer chemotherapy. Curr. Med. Chem. Anticancer Agents 2005, 5, 65–71. [Google Scholar] [CrossRef] [Green Version]
- Cong, H.; Zhao, X.; Castle, B.T.; Pomeroy, E.J.; Zhou, B.; Lee, J.; Wang, Y.; Bian, T.; Miao, Z.; Zhang, W.; et al. An Indole-Chalcone Inhibits Multidrug-Resistant Cancer Cell Growth by Targeting Microtubules. Mol. Pharm. 2018, 15, 3892–3900. [Google Scholar] [CrossRef]
- Mukhtar, E.; Adhami, V.M.; Mukhtar, H. Targeting microtubules by natural agents for cancer therapy. Mol. Cancer 2014, 13, 275–284. [Google Scholar] [CrossRef] [Green Version]
- Raja, V.J.; Lim, K.H.; Leong, C.O.; Kam, T.S.; Bradshaw, T.D. Novel antitumour indole alkaloid, Jerantinine A, evokes potent G2/M cell cycle arrest targeting microtubules. Investig. New Drugs 2014, 32, 838–850. [Google Scholar] [CrossRef] [PubMed]
- Tantak, M.P.; Malik, M.; Klingler, L.; Olson, Z.; Kumar, A.; Sadana, R.; Kumar, D. Indolyl-alpha-keto-1,3,4-oxadiazoles: Synthesis, anti-cell proliferation activity, and inhibition of tubulin polymerization. Bioorg. Med. Chem. Lett. 2021, 37, 127842. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Liu, W.; Gong, Z.; Huang, Y.; Li, Y.; Peng, Z. Design, synthesis, biological evaluation and molecular docking studies of new chalcone derivatives containing diaryl ether moiety as potential anticancer agents and tubulin polymerization inhibitors. Bioorg. Chem. 2020, 95, 103565. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).