Bioprinting of a Zonal-Specific Cell Density Scaffold: A Biomimetic Approach for Cartilage Tissue Engineering
Abstract
:1. Introduction
2. Materials and Methods
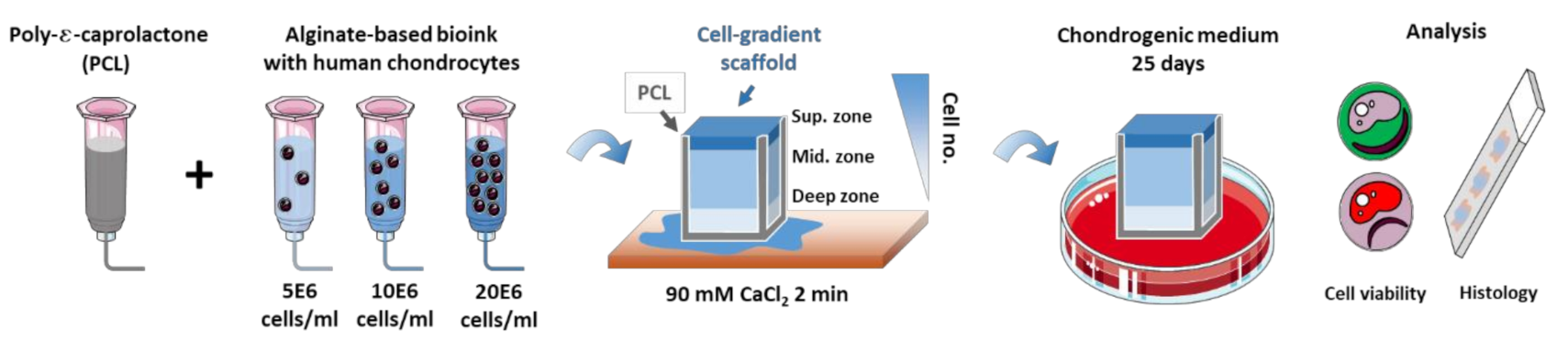
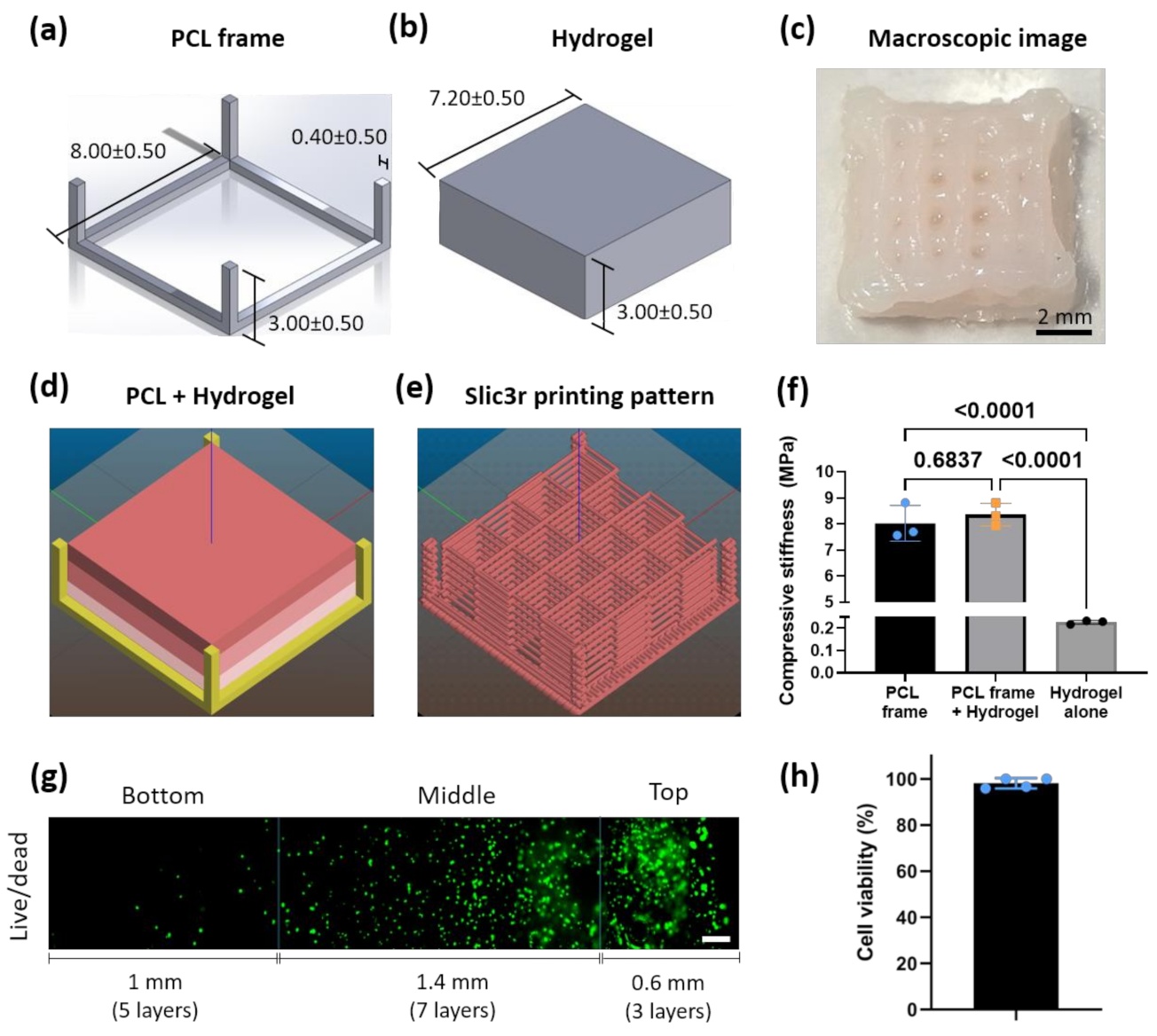
2.1. Scaffold Design
2.2. Bioink Formulation
2.3. Bioprinting
2.4. In Vitro Culture
2.5. Mechanical Characterization
2.6. Live/Dead Assay
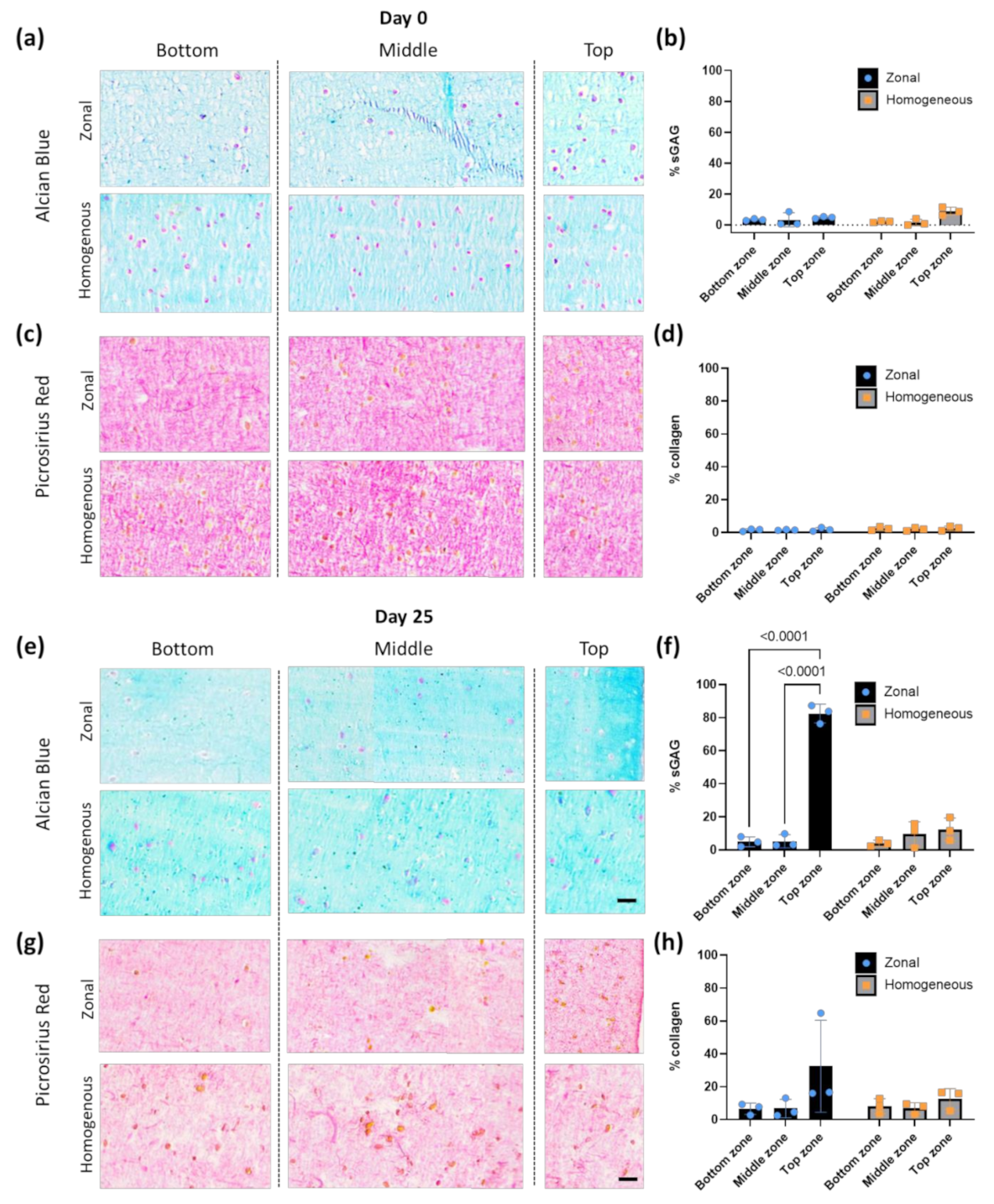
2.7. Histology Staining
2.8. Image Analysis
2.9. Statistical Analysis
3. Results
3.1. PCL-Reinforced Alginate Scaffolds with Different Cell Density Zones Can Be Successfully Fabricated as Single Units Using Bioprinting
3.2. Cell Density Can Be Maintained in the Different Zones Overtime In Vitro
3.3. Bioprinted Zonal Scaffold with Different Cell Densities Leads to a Gradient Cell-Derived ECM Deposition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Myers, K.R.; Sgaglione, N.A.; Grande, D.A. Trends in biological joint resurfacing. Bone Jt. Res. 2013, 2, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Brittberg, M.; Lindahl, A.; Nilsson, A.; Ohlsson, C.; Isaksson, O.; Peterson, L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N. Engl. J. Med. 1994, 331, 889–895. [Google Scholar] [CrossRef]
- Ahern, B.; Parvizi, J.; Boston, R.; Schaer, T. Preclinical animal models in single site cartilage defect testing: A systematic review. Osteoarthr. Cartil. 2009, 17, 705–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the global burden of disease study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef] [Green Version]
- Roseti, L.; Grigolo, B. Host environment: Scaffolds and signaling (tissue engineering) articular cartilage regeneration: Cells, scaffolds, and growth factors. In Bio-Orthopaedics: A New Approach; Springer: Berlin/Heidelberg, Germany, 2017; pp. 87–103. ISBN 9783662541814. [Google Scholar]
- Kreuz, P.; Steinwachs, M.; Erggelet, C.; Krause, S.; Konrad, G.; Uhl, M.; Südkamp, N. Results after microfracture of full-thickness chondral defects in different compartments in the knee. Osteoarthr. Cartil. 2006, 14, 1119–1125. [Google Scholar] [CrossRef] [Green Version]
- Ansari, S.; Khorshidi, S.; Karkhaneh, A. Engineering of gradient osteochondral tissue: From nature to lab. Acta Biomater. 2019, 87, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Huber, M.; Trattnig, S.; Lintner, F. Anatomy, biochemistry, and physiology of articular cartilage. Investig. Radiol. 2000, 35, 573–580. [Google Scholar] [CrossRef]
- Bracaglia, L.G.; Smith, B.T.; Watson, E.; Arumugasaamy, N.; Mikos, A.G.; Fisher, J.P. 3D printing for the design and fabrication of polymer-based gradient scaffolds. Acta Biomater. 2017, 56, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Levato, R.; Webb, W.; Otto, I.A.; Mensinga, A.; Zhang, Y.; van Rijen, M.; van Weeren, R.; Khan, I.; Malda, J. The bio in the ink: Cartilage regeneration with bioprintable hydrogels and articular cartilage-derived progenitor cells. Acta Biomater. 2017, 61, 41–53. [Google Scholar] [CrossRef] [Green Version]
- Parisi, C.; Salvatore, L.; Veschini, L.; Serra, M.P.; Hobbs, C.; Madaghiele, M.; Sannino, A.; Di Silvio, L. Biomimetic gradient scaffold of collagen–hydroxyapatite for osteochondral regeneration. J. Tissue Eng. 2020, 11, 11. [Google Scholar] [CrossRef] [Green Version]
- Liu, E.; Zhu, D.; Diaz, E.C.G.; Tong, X.; Yang, F. Gradient hydrogels for optimizing niche cues to enhance cell-based cartilage regeneration. Tissue Eng. Part A 2020, 27, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Xu, Z.; Liang, Q.; Liu, B.; Li, H.; Wu, Y.; Zhang, Y.; Lin, Z.; Wu, M.; Ruan, C.; et al. Direct 3D printing of high strength biohybrid gradient hydrogel scaffolds for efficient repair of osteochondral defect. Adv. Funct. Mater. 2018, 28, 1706644. [Google Scholar] [CrossRef]
- Chen, L.; Deng, C.; Li, J.; Yao, Q.; Chang, J.; Wang, L.; Wu, C. 3D printing of a lithium-calcium-silicate crystal bioscaffold with dual bioactivities for osteochondral interface reconstruction. Biomaterials 2019, 196, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Tong, X.; Trinh, P.; Yang, F. Mimicking cartilage tissue zonal organization by engineering tissue-scale gradient hydrogels as 3D cell niche. Tissue Eng. Part A 2018, 24, 1–10. [Google Scholar] [CrossRef]
- Sun, Y.; You, Y.; Jiang, W.; Wang, B.; Wu, Q.; Dai, K. 3D bioprinting dual-factor releasing and gradient-structured constructs ready to implant for anisotropic cartilage regeneration. Sci. Adv. 2020, 6, eaay1422. [Google Scholar] [CrossRef]
- Nowicki, M.A.; Castro, N.J.; Plesniak, M.W.; Zhang, L.G. 3D printing of novel osteochondral scaffolds with graded microstructure. Nanotechnology 2016, 27, 414001. [Google Scholar] [CrossRef]
- Jeon, O.; Alt, D.S.; Linderman, S.; Alsberg, E. Biochemical and physical signal gradients in hydrogels to control stem cell behavior. Adv. Mater. 2013, 25, 6366–6372. [Google Scholar] [CrossRef]
- Ren, X.; Wang, F.; Chen, C.; Gong, X.; Yin, L.; Yang, L. Engineering zonal cartilage through bioprinting collagen type II hydrogel constructs with biomimetic chondrocyte density gradient. BMC Musculoskelet. Disord. 2016, 17, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slic3r. Slic3r-Open Source 3D Printing Toolbox. Available online: https://slic3r.org/ (accessed on 6 July 2021).
- Hunziker, E.; Quinn, T.; Häuselmann, H.-J. Quantitative structural organization of normal adult human articular cartilage. Osteoarthr. Cartil. 2002, 10, 564–572. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org (accessed on 20 August 2019).
- Arganda-Carreras, I.; Kaynig, V.; Rueden, C.; Eliceiri, K.; Schindelin, J.E.; Cardona, A.; Seung, H.S. Trainable weka segmentation: A machine learning tool for microscopy pixel classification. Bioinformatics 2017, 33, 2424–2426. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Faulkner-Jones, A.; Dun, A.R.; Jin, J.; Chen, P.; Xing, Y.; Yang, Z.; Li, Z.; Shu, W.; Liu, D.; et al. Rapid formation of a supramolecular polypeptide-DNA hydrogel for in situ three-dimensional multilayer bioprinting. Angew. Chem. Int. Ed. 2015, 54, 3957–3961. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tan, Y.J.; Leong, K.F.; Li, L. 3D bioprinting of highly thixotropic alginate/methylcellulose hydrogel with strong interface bonding. ACS Appl. Mater. Interfaces 2017, 9, 20086–20097. [Google Scholar] [CrossRef]
- Kolesky, D.B.; Homan, K.A.; Skylar-Scott, M.A.; Lewis, J.A. Three-dimensional bioprinting of thick vascularized tissues. Proc. Natl. Acad. Sci. USA 2016, 113, 3179–3184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, X.; Liu, L.; Ouyang, J.; Xingliang, D.; Zhang, X.; Lan, Q.; Xu, T. Coaxial 3D bioprinting of self-assembled multicellular heterogeneous tumor fibers. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Madero, C.; De León-Derby, M.D.; Samandari, M.; Ceballos-González, C.F.; Bolívar, E.; Mendoza-Buenrostro, C.C.; Holmberg, S.; Garza-Flores, N.A.; Almajhadi, M.A.; González-Gamboa, I.; et al. Using chaotic advection for facile high-throughput fabrication of ordered multilayer micro- and nanostructures: Continuous chaotic printing. Biofabrication 2020, 12, 035023. [Google Scholar] [CrossRef]
- Lee, J.-S.; Hong, J.M.; Jung, J.W.; Shim, J.-H.; Oh, J.-H.; Cho, D.-W. 3D printing of composite tissue with complex shape applied to ear regeneration. Biofabrication 2014, 6, 024103. [Google Scholar] [CrossRef] [PubMed]
- Daly, A.; Critchley, S.E.; Rencsok, E.M.; Kelly, D.J. A comparison of different bioinks for 3D bioprinting of fibrocartilage and hyaline cartilage. Biofabrication 2016, 8, 045002. [Google Scholar] [CrossRef] [PubMed]
- Castilho, M.; Hochleitner, G.; Wilson, W.; Van Rietbergen, B.; Dalton, P.D.; Groll, J.; Malda, J.; Ito, K. Mechanical behavior of a soft hydrogel reinforced with three-dimensional printed microfibre scaffolds. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Freeman, F.; Browe, D.; Nulty, J.; Von Euw, S.; Grayson, W.L.; Kelly, D.J. Biofabrication of multiscale bone extracellular matrix scaffolds for bone tissue engineering. Eur. Cells Mater. 2019, 38, 168–187. [Google Scholar] [CrossRef]
- Critchley, S.; Sheehy, E.; Cunniffe, G.; Diaz-Payno, P.; Carroll, S.F.; Jeon, O.; Alsberg, E.; Brama, P.A.; Kelly, D.J. 3D printing of fibre-reinforced cartilaginous templates for the regeneration of osteochondral defects. Acta Biomater. 2020, 113, 130–143. [Google Scholar] [CrossRef]
- Schuurman, W.; Khristov, V.; Pot, M.W.; van Weeren, P.R.; Dhert, W.J.A.; Malda, J. Bioprinting of hybrid tissue constructs with tailorable mechanical properties. Biofabrication 2011, 3, 021001. [Google Scholar] [CrossRef]
- Schipani, R.; Scheurer, S.; Florentin, R.; Critchley, S.E.; Kelly, D.J. Reinforcing interpenetrating network hydrogels with 3D printed polymer networks to engineer cartilage mimetic composites. Biofabrication 2020, 12, 035011. [Google Scholar] [CrossRef]
- Garrett, Q.; Simmons, P.A.; Xu, S.; Vehige, J.; Zhao, Z.; Ehrmann, K.; Willcox, M. Carboxymethylcellulose binds to human corneal epithelial cells and is a modulator of corneal epithelial wound healing. Investig. Ophthalmol. Vis. Sci. 2007, 48, 1559–1567. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Lu, Z.; Wu, H.; Li, W.; Zheng, L.; Zhao, J. Collagen-alginate as bioink for three-dimensional (3D) cell printing based cartilage tissue engineering. Mater. Sci. Eng. C 2018, 83, 195–201. [Google Scholar] [CrossRef]
- Ávila, H.M.; Schwarz, S.; Rotter, N.; Gatenholm, P. 3D bioprinting of human chondrocyte-laden nanocellulose hydrogels for patient-specific auricular cartilage regeneration. Bioprinting 2016, 1–2, 22–35. [Google Scholar] [CrossRef]
- Bendtsen, S.T.; Quinnell, S.P.; Wei, M. Development of a novel alginate-polyvinyl alcohol-hydroxyapatite hydrogel for 3D bioprinting bone tissue engineered scaffolds. J. Biomed. Mater. Res. Part A 2017, 105, 1457–1468. [Google Scholar] [CrossRef] [PubMed]
- Markstedt, K.; Mantas, A.; Tournier, I.; Ávila, H.M.; Hägg, D.; Gatenholm, P. 3D bioprinting human chondrocytes with Nanocellulose–Alginate bioink for cartilage tissue engineering applications. Biomacromolecules 2015, 16, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- de Melo, B.A.G.; Jodat, Y.A.; Mehrotra, S.; Calabrese, M.; Kamperman, T.; Mandal, B.B.; Santana, M.H.A.; Alsberg, E.; Leijten, J.; Shin, S.R. 3D printed cartilage-like tissue constructs with spatially controlled mechanical properties. Adv. Funct. Mater. 2019, 29, 1906330. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [Green Version]
- Van Susante, J.L.C.; Buma, P.; Van Osch, G.J.; Versleyen, D.; Van Der Kraan, P.M.; Van Der Berg, W.B.; Homminga, G.N. Culture of chondrocytes in alginate and collagen carrier gels. Acta Orthop. Scand. 1995, 66, 549–556. [Google Scholar] [CrossRef] [Green Version]
- van Osch, G.J.; Berg, W.B.V.D.; Hunziker, E.B.; Häusselmann, H.J. Differential effects of IGF-1 ans TGFβ-2 on the assembly of proteoglycans in pericellular and territorial matrix by cultured bovine articular chondrocytes. Osteoarthr. Cartil. 1998, 6, 187–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malda, J.; Hoope, W.T.; Schuurman, W.; Van Osch, G.J.; Van Weeren, P.R.; Dhert, W.J. Localization of the potential zonal marker clusterin in native cartilage and in tissue-engineered constructs. Tissue Eng. Part A 2010, 16, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Pleumeekers, M.M.; Nimeskern, L.; Koevoet, W.L.M.; Kops, N.; Poublon, R.M.L.; Stok, K.S.; Van Osch, G.J.V.M. The in vitro and in vivo capacity of culture-expanded human cells from several sources encapsulated in alginate to form cartilage. Eur. Cells Mater. 2014, 27, 264–280. [Google Scholar] [CrossRef]
- Marijnissen, W.J.; van Osch, G.J.; Aigner, J.; van der Veen, S.W.; Hollander, A.P.; Verwoerd-Verhoef, H.L.; Verhaar, J. Alginate as a chondrocyte-delivery substance in combination with a non-woven scaffold for cartilage tissue engineering. Biomaterials 2002, 23, 1511–1517. [Google Scholar] [CrossRef]
- Talukdar, S.; Nguyen, Q.T.; Chen, A.C.; Sah, R.L.; Kundu, S.C. Effect of initial cell seeding density on 3D-engineered silk fibroin scaffolds for articular cartilage tissue engineering. Biomaterials 2011, 32, 8927–8937. [Google Scholar] [CrossRef] [Green Version]
- Mauck, R.; Wang, C.-B.; Oswald, E.; Ateshian, G.; Hung, C. The role of cell seeding density and nutrient supply for articular cartilage tissue engineering with deformational loading. Osteoarthr. Cartil. 2003, 11, 879–890. [Google Scholar] [CrossRef] [Green Version]
- Moshtagh, P.R.; Pouran, B.; Korthagen, N.M.; Zadpoor, A.A.; Weinans, H. Guidelines for an optimized indentation protocol for measurement of cartilage stiffness: The effects of spatial variation and indentation parameters. J. Biomech. 2016, 49, 3602–3607. [Google Scholar] [CrossRef] [PubMed]
- Ebenstein, D.; Kuo, A.; Rodrigo, J.J.; Reddi, A.H.; Ries, M.; Pruitt, L. A nanoindentation technique for functional evaluation of cartilage repair tissue. J. Mater. Res. 2004, 19, 273–281. [Google Scholar] [CrossRef]
- Bae, W.C.; Lewis, C.W.; Levenston, M.; Sah, R.L. Indentation testing of human articular cartilage: Effects of probe tip geometry and indentation depth on intra-tissue strain. J. Biomech. 2006, 39, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Antons, J.; Marascio, M.G.M.; Nohava, J.; Martin, R.; Applegate, L.A.; Bourban, P.E.; Pioletti, D.P. Zone-dependent mechanical properties of human articular cartilage obtained by indentation measurements. J. Mater. Sci. Mater. Med. 2018, 29, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, Z.; Zhang, P.; Liu, Y.; Lv, L.; Zhou, Y. Four-dimensional bioprinting: Current developments and applications in bone tissue engineering. Acta Biomater. 2020, 101, 26–42. [Google Scholar] [CrossRef] [PubMed]

| Title 1 | Material | Nozzle Size | Temperature | Pressure | Speed |
|---|---|---|---|---|---|
| Printhead 1 | Hydrogel (deep zone) | 0.2 mm | RT | 14 kPa | 12 mm/s |
| Printhead 2 | Hydrogel (superficial and middle zones) | 0.2 mm | RT | 14 kPa | 12 mm/s |
| Printhead 3 | PCL | 0.2 mm | 210 °C | 200 kPa | 4 mm/s (base) 0.4 mm/s (struts) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimaraki, A.; Díaz-Payno, P.J.; Minneboo, M.; Nouri-Goushki, M.; Hosseini, M.; Kops, N.; Narcisi, R.; Mirzaali, M.J.; van Osch, G.J.V.M.; Fratila-Apachitei, L.E.; et al. Bioprinting of a Zonal-Specific Cell Density Scaffold: A Biomimetic Approach for Cartilage Tissue Engineering. Appl. Sci. 2021, 11, 7821. https://doi.org/10.3390/app11177821
Dimaraki A, Díaz-Payno PJ, Minneboo M, Nouri-Goushki M, Hosseini M, Kops N, Narcisi R, Mirzaali MJ, van Osch GJVM, Fratila-Apachitei LE, et al. Bioprinting of a Zonal-Specific Cell Density Scaffold: A Biomimetic Approach for Cartilage Tissue Engineering. Applied Sciences. 2021; 11(17):7821. https://doi.org/10.3390/app11177821
Chicago/Turabian StyleDimaraki, Angeliki, Pedro J. Díaz-Payno, Michelle Minneboo, Mahdiyeh Nouri-Goushki, Maryam Hosseini, Nicole Kops, Roberto Narcisi, Mohammad J. Mirzaali, Gerjo J. V. M. van Osch, Lidy E. Fratila-Apachitei, and et al. 2021. "Bioprinting of a Zonal-Specific Cell Density Scaffold: A Biomimetic Approach for Cartilage Tissue Engineering" Applied Sciences 11, no. 17: 7821. https://doi.org/10.3390/app11177821
APA StyleDimaraki, A., Díaz-Payno, P. J., Minneboo, M., Nouri-Goushki, M., Hosseini, M., Kops, N., Narcisi, R., Mirzaali, M. J., van Osch, G. J. V. M., Fratila-Apachitei, L. E., & Zadpoor, A. A. (2021). Bioprinting of a Zonal-Specific Cell Density Scaffold: A Biomimetic Approach for Cartilage Tissue Engineering. Applied Sciences, 11(17), 7821. https://doi.org/10.3390/app11177821










