New Materials and Effects in Molecular Nanomagnets
Abstract
1. Introduction
2. Single-Ion Anisotropies
3. Magnetic Interactions via a Ligand
4. Single-Molecule Magnets
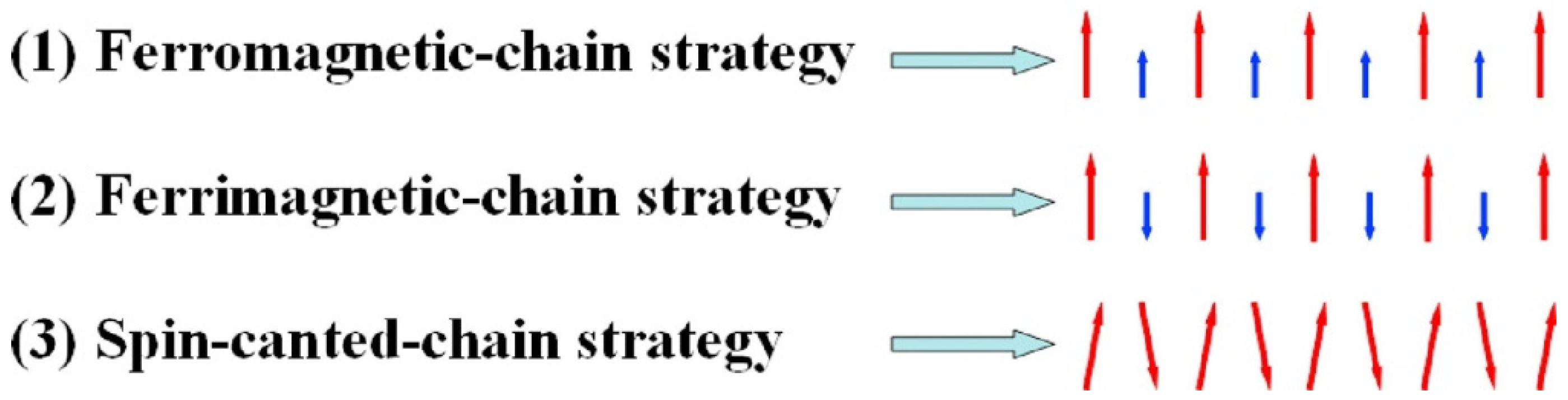
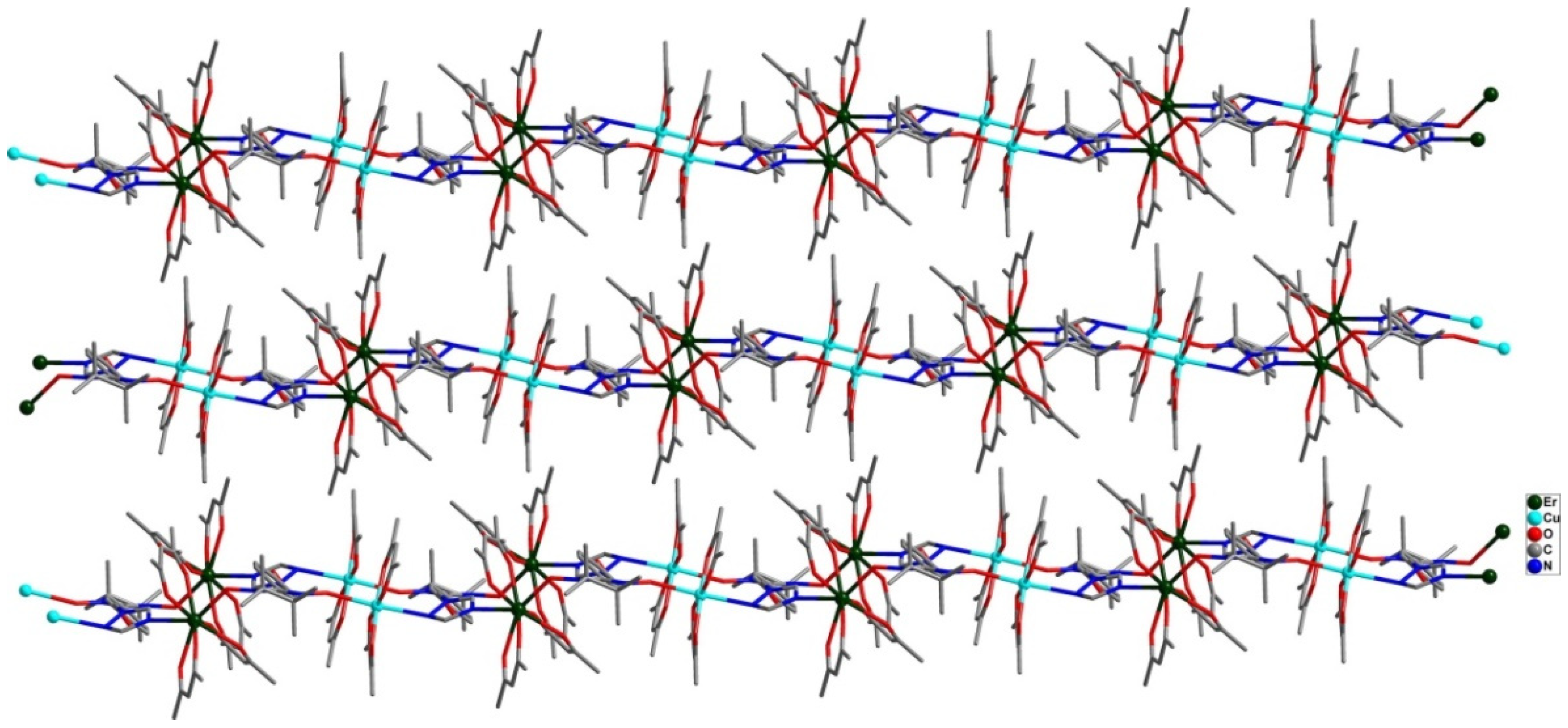
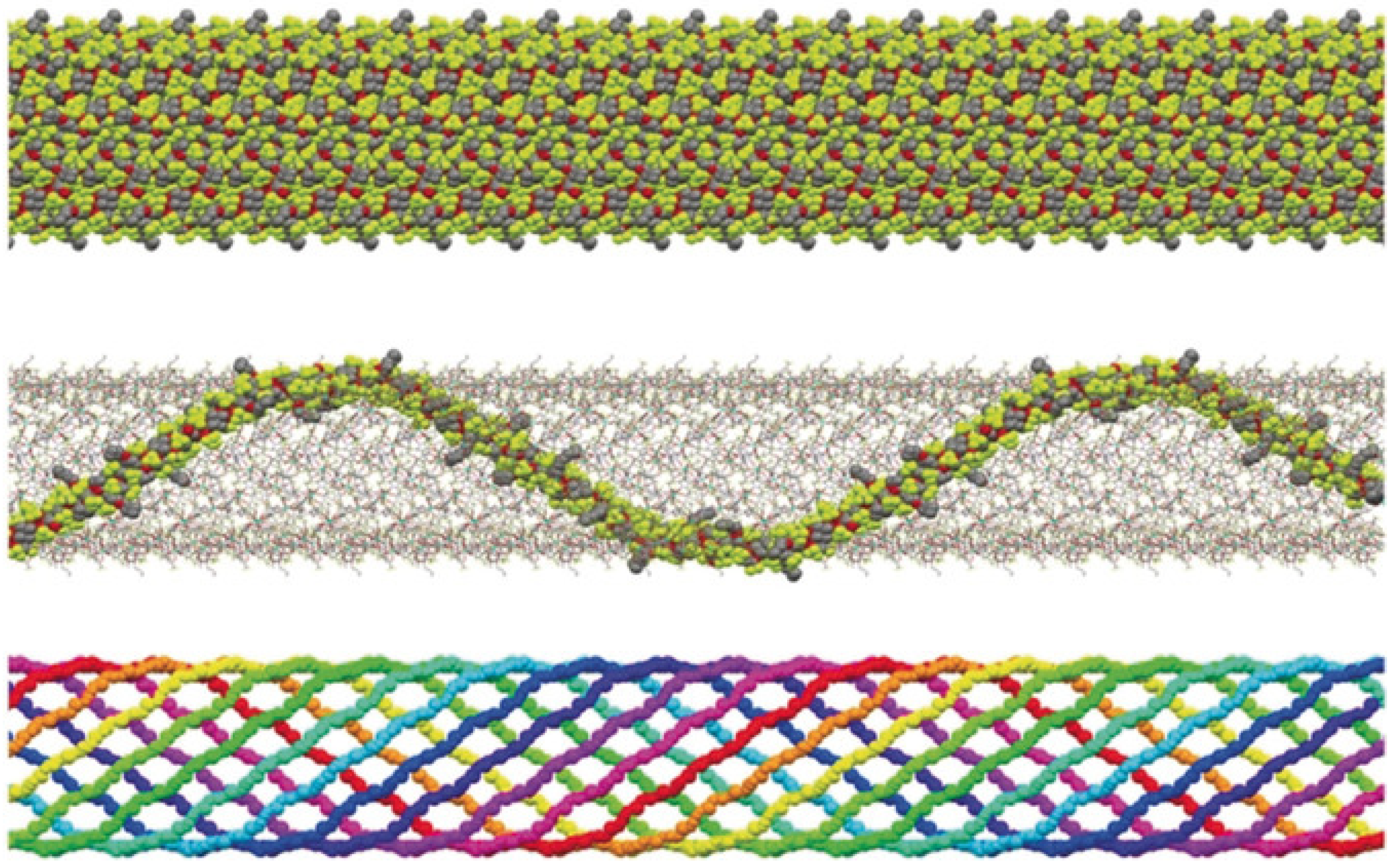
5. Single-Chain Magnets
6. Theoretical Aspects and Methods
7. Potential Challenges in the Application of Molecular Nanomagnets
8. Recent Trends in Molecular Nanomagnets
9. Reviews on Special Sub-Topics
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ding, J.; Adeyeye, A.O. Ni80Fe20/Ni binary nanomagnets for logic applications. Appl. Phys. Lett. 2012, 101, 103117. [Google Scholar] [CrossRef]
- McGuigan, M.; Davenport, J.W.; Glimm, J. Computational approach to finite size and shape effects in iron nanomagnets. J. Magn. Magn. Mater. 2008, 320, 190–196. [Google Scholar] [CrossRef]
- Sharma, N.; van Mourik, R.A.; Yin, Y.; Koopmans, B.; Parkin, S.S.P. Focused-electron-beam-induced-deposited cobalt nanopillars for nanomagnetic logic. Nanotechnology 2016, 27, 165301. [Google Scholar] [CrossRef] [PubMed]
- Morais, P.C.; Lima, E.C.D.; Rabelo, D.; Reis, A.C.; Pelegrini, F. Magnetic resonance of magnetite nanoparticles dispersed in mesoporous copolymer matrix. IEEE Trans. Magn. 2000, 36, 3038–3040. [Google Scholar] [CrossRef]
- Chatterjee, B.K.; Ghosh, C.K.; Chattopadhyay, K.K. Temperature depencence of magnetization and anisotropy in uniaxial NiFe2O4 nanomagnets: Deviations from the Callen-Callen power law. J. Appl. Phys. 2014, 116, 153904. [Google Scholar] [CrossRef]
- Ehrmann, A.; Blachowicz, T. Vortex and double-vortex nucleation during magnetization reversal in Fe nanodots of different dimensions. J. Magn. Magn. Mater. 2019, 475, 727–733. [Google Scholar] [CrossRef]
- Ehrmann, A.; Blachowicz, T. Systematic study of magnetization reversal in square Fe nanodots of varying dimensions in different orientations. Hyperfine Interact. 2018, 239, 8. [Google Scholar] [CrossRef]
- Parkin, S.S.P.; Hayashi, M.; Thomas, L. Magnetic domain-wall racetrack memory. Science 2008, 320, 190–194. [Google Scholar] [CrossRef]
- Blachowicz, T.; Ehrmann, A. Magnetization reversal in bent nanofibers of different cross-sections. J. Appl. Phys. 2018, 124, 152112. [Google Scholar] [CrossRef]
- Kumar, D.; Jin, T.L.; Risi, S.A.; Sbiaa, R.; Lew, W.S.; Piramanayagam, S.N. Domain wall motion control for racetrack memory applications. IEEE Trans. Magn. 2019, 55, 2300708. [Google Scholar] [CrossRef]
- Keller, L.; Al Mamoori, M.K.I.; Pieper, J.; Gspan, C.; Stockem, I.; Schröder, I.; Barth, S.; Winkler, R.; Plank, H.; Pohlit, M.; et al. Direct-write of free-form building blocks for artificial magnetic 3D lattices. Sci. Rep. 2018, 8, 6160. [Google Scholar] [CrossRef]
- Kern, P.; Döpke, C.; Blachowicz, T.; Steblinski, P.; Ehrmann, A. Magnetization reversal in ferromagnetic Fibonacci nano-spirals. J. Magn. Magn. Mater. 2019, 484, 37–41. [Google Scholar] [CrossRef]
- Al Mamoori, M.; Schröder, C.; Keller, L.; Huth, M.; Müller, J. First-order reversal curves (FORCs) of nano-engineered 3D Co-Fe structures. AIP Adv. 2020, 10, 015319. [Google Scholar] [CrossRef]
- Nogués, J.; Schuller, I.K. Exchange Bias. J. Magn. Magn. Mater. 1999, 192, 203–232. [Google Scholar] [CrossRef]
- Schneider, V.; Reinhold, A.; Kreibig, U.; Weirich, T.; Güntherodt, G.; Beschoten, B.; Tillmanns, A.; Krenn, H.; Rumpf, K.; Granitzer, P. Structural and magnetic properties of Ni/NiOxide and Co/CoOxide core/shell nanoparticles and their possible use for ferrofluids. Z. Phys. Chem. 2006, 220, 173–187. [Google Scholar] [CrossRef]
- Tillmanns, A.; Blachowicz, T.; Fraune, M.; Güntherodt, G.; Schuller, I.K. Anomalous magnetization reversal mechanism in unbiased Fe/FeF2 investigated by means of the magneto-optic Kerr effect. J. Magn. Magn. Mater. 2009, 321, 2932–2935. [Google Scholar] [CrossRef]
- Nogués, J.; Sort, J.; Langlais, V.; Skumryev, V.; Surinach, S.; Munoz, J.S.; Baró, M.D. Exchange bias in nanostructures. Phys. Rep. 2005, 422, 65–117. [Google Scholar] [CrossRef]
- Christou, G.; Gatteschi, D.; Hendrickson, D.N.; Sessoli, R. Single-molecule magnets. MRS Bulletin 2000, 25, 66–71. [Google Scholar] [CrossRef]
- Bogani, L.; Wernsdorfer, W. Molecular spintronic using single-molecule magnets. Nat. Mater. 2008, 7, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Sessoli, R.; Powell, A.K. Strategies towards single molecule magnets based on lanthanide ions. Coord. Chem. Rev. 2009, 253, 2328–2341. [Google Scholar] [CrossRef]
- Coulon, C.; Miyasaka, H.; Clérac, R. Single-chain magnets: Theoretical approach and experimental systems. Struct. Bond. 2006, 122, 163–206. [Google Scholar] [CrossRef]
- Sun, H.-L.; Wang, Z.-M.; Gao, S. Strategies towards single-chain magnets. Coord. Chem. Rev. 2010, 254, 1081–1100. [Google Scholar] [CrossRef]
- Böhme, M.; Plass, W. How to link theory and experiment for single-chain magnets beyond the Ising model: Magnetic properties modeled from ab initio calculations of molecular fragments. Chem. Sci. 2019, 10, 9189–9202. [Google Scholar] [CrossRef]
- Wu, B.-Y.; Yang, C.-I.; Nakano, M.; Lee, G.H. Ferromagnetic interaction and slow magnetic relaxation in a Co3 cluster-based three-dimensional framework. Dalton Trans. 2014, 43, 47–50. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yi, X.H.; Calvez, G.; Daiguebonne, C.; Guillou, O.; Bernot, K. Rational organization of lanthanide-based SMM dimers into three-dimensional networks. Inorg. Chem. 2015, 54, 5213–5219. [Google Scholar] [CrossRef]
- Dutta, D.; Lefkidis, G.; Hübner, W. Role of the static correlations on the ultrafast spin dynamics of 3d molecular nano-magnets. Phys. Scr. 2020, 95, 065805. [Google Scholar] [CrossRef]
- Takahashi, M.; Turek, P.; Nakazawa, Y.; Tamura, M.; Nozawa, K.; Shiomi, D.; Ishikawa, M.; Kinoshita, M. Discovery of a quasi-1D organic ferromagnet, p-NPNN. Phys. Rev. Lett. 1992, 69, 1290. [Google Scholar] [CrossRef]
- Mertes, K.M.; Suzuki, Y.; Sarachik, M.P.; Myasoedov, Y.; Shtrikman, H.; Zeldov, E.; Rumberger, E.M.; Hendrickson, D.N.; Christou, G. Mn12-acetate: A prototypical single molecule magnet. Solid State Commun. 2003, 127, 131–139. [Google Scholar] [CrossRef]
- Murrie, M. Cobalt(II) single-molecule magnets. Chem. Soc. Rev. 2010, 39, 1986–1995. [Google Scholar] [CrossRef]
- Cirillo, G.A.; Turvani, G.; Graziano, M. A quantum computation model for molecular nanomagnets. IEEE Trans. Nanotechnol. 2019, 18, 1027–1039. [Google Scholar] [CrossRef]
- Rinehart, J.D.; Long, J.R. Exploiting single-ion anisotropy in the design of f-element single-molecule magnets. Chem. Sci. 2011, 2, 2078–2085. [Google Scholar] [CrossRef]
- Liu, J.-L.; Chen, Y.-C.; Tong, M.-L. Symmetry strategies for high performance lanthanide-based single-molecule magnets. Chem. Soc. Rev. 2018, 47, 2431–2453. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.F.; Guo, Y.-N.; Ungur, L.; Tang, J.K.; Chibotaru, L.F. Tuning the magnetic interactions and relaxation dynamics of Dy2 single-molecule magnets. Chem. Eur. J. 2015, 21, 14099–14106. [Google Scholar] [CrossRef]
- Gupta, T.; Beg, M.F.; Rajaraman, G. Role of single-ion anisotropy and magnetic exchange interactions in suppressing zero-field tunneling in {3d-4f} single molecule magnets. Inorg. Chem. 2016, 55, 11201–11215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, Y.-Q.; Zhang, P.; Zhao, L.; Guo, M.; Tang, J.K. Single-molecule magnet behavior enhanced by synergic effect of single-ion anisotropy and magnetic interactions. Inorg. Chem. 2017, 56, 7882–7889. [Google Scholar] [CrossRef]
- Guo, Y.-N.; Xu, G.-F.; Guo, Y.; Tang, J.K. Relaxation dynamics of dysprosium(III) single molecule magnets. Dalton Trans. 2011, 40, 9953–9963. [Google Scholar] [CrossRef]
- Slater, J.C. Average energy of states of given multiplicities in atoms. Phys. Rev. 1968, 165, 655. [Google Scholar] [CrossRef]
- Kahn, O.; Briat, B. Exchange interaction in polynuclear complexes. Part 1. Principles, model and application to the binuclear complexes of chromium. J. Chem. Soc. Faraday Trans. 2 Mol. Chem. Phys. 1976, 72, 268–281. [Google Scholar] [CrossRef]
- Domanov, O.; Weschke, E.; Saito, T.; Peterlik, H.; Pichler, T.; Eisterer, M.; Shiozawa, H. Exchange coupling in a frustrated trimetric molecular magnet reversed by a 1D nano-confinement. Nanoscale 2019, 11, 10615–10621. [Google Scholar] [CrossRef]
- Anderson, P.W.; Hasegawa, H. Considerations on double exchange. Phys. Rev. 1955, 100, 675. [Google Scholar] [CrossRef]
- Borras-Almenar, J.J.; Clemente-Juan, J.M.; Coronado, E.; Mirovitskii, V.Y.; Palii, A.V.; Tsukerblat, B.S. Double exchange in orbitally degenerate mixed valence clusters: Magnetic anisotropy, vibronic effects. In Vibronic Interactions: Jahn-Teller Effect in Crystals and Molecules; Kaplan, M.D., Zimmerman, G.O., Eds.; Springer: Dordrecht, The Netherlands, 2001; Volume 39, pp. 111–122. [Google Scholar]
- Aubin, S.M.J.; Wemple, M.W.; Adams, D.M.; Tsai, H.-L.; Christou, G.; Hendrickson, D.N. Distorted MnIVMnIII3 Cubane complexes as single-molecule magnets. J. Am. Chem. Soc. 1996, 118, 7746–7754. [Google Scholar] [CrossRef]
- Sessoli, R.; Gatteschi, D.; Caneschi, A.; Novak, M.A. Magnetic bistability in a metal-ion cluster. Nature 1993, 365, 141–143. [Google Scholar] [CrossRef]
- Aubin, S.M.J.; Sun, Z.M.; Pardi, L.; Krzystek, J.; Folting, K.; Brunel, L.-C.; Rheingold, A.L.; Christou, G.; Hendrickson, D.N. Reduced anionic Mn12 molecules with half-integer ground states as single-molecule magnets. Inorg. Chem. 1999, 38, 5329–5340. [Google Scholar] [CrossRef]
- Gatteschi, D.; Sessoli, R.; Cornia, A. Single-molecule magnets based on iron(III) oxo clusters. Chem. Commun. 2000, 2000, 725–732. [Google Scholar] [CrossRef]
- Friedman, J.R.; Sarachik, M.P.; Tejada, J.; Maciejewski, J.; Ziolo, R. Steps in the hysteresis loops of a high-spin molecule. J. Appl. Phys. 1996, 79, 6031. [Google Scholar] [CrossRef]
- Friedman, J.R.; Sarachik, M.P.; Tejada, J.; Ziolo, R. Macroscopic measurement of resonant magnetization tunneling in high-spin molecules. Phys. Rev. Lett. 1996, 76, 3830. [Google Scholar] [CrossRef] [PubMed]
- Wernsdorfer, W.; Sessoli, R.; Caneschi, A.; Gatteschi, D.; Cornia, A.; Mailly, D. Landau-Zener method to study quantum phase interference of Fe8 molecular nanomagnets. J. Appl. Phys. 2000, 87, 5481. [Google Scholar] [CrossRef]
- Sangregoria, C.; Ohm, T.; Paulsen, C.; Sessoli, R.; Gatteschi, D. Quantum Tunneling of the magnetization in an iron cluster nanomagnet. Phys. Rev. Lett. 1997, 78, 4645. [Google Scholar] [CrossRef]
- Aubin, S.M.J.; Dilley, N.R.; Pardi, L.; Krzystek, J.; Wemple, M.W.; Brunel, L.C.; Maple, M.B.; Christou, G.; Hendrickson, D.N. Resonant magnetization tunneling in the trigonal pyramidal MnIVMnIII3 complex [Mn4O3Cl(O2CCH3)3(dbm)3. J. Am. Chem. Soc. 1998, 120, 4991–5004. [Google Scholar] [CrossRef]
- Shao, D.; Wang, X.Y. Development of single-molecule magnets. Chin. J. Chem. 2020, 38, 1005–1018. [Google Scholar] [CrossRef]
- Li, M.; Wu, H.P.; Xia, Z.Q.; Ungur, L.; Liu, D.; Chibotaru, L.F.; Ke, H.S.; Chen, S.P.; Gao, S.L. An inconspicuous six-coordinate neutral DyIII single-ion magnet with remarkable magnetic anisotropy and stability. Inorg. Chem. 2020, 59, 7158–7166. [Google Scholar] [CrossRef]
- Chiesa, A.; Cugini, F.; Hussain, R.; Macaluso, E.; Allodi, G.; Garlatti, E.; Giansiracusa, M.; Goodwin, C.A.P.; Ortu, F.; Reta, D.; et al. Understanding magnetic relaxation in single-ion magnets with high blocking temperature. Phys. Rev. B 2020, 101, 174402. [Google Scholar] [CrossRef]
- Jin, P.B.; Zhai, Y.Q.; Yu, K.X.; Winpenny, R.E.P.; Zheng, Y.Z. Dysprosiacarboranes as organometallic single-molecule magnets. Angew. Chem. Int. Ed. 2020, 59, 9350–9354. [Google Scholar] [CrossRef]
- Spree, L.; Schlesier, C.; Kostanyan, A.; Westerström, R.; Greber, T.; Büchner, B.; Avdoshenko, S.M.; Popov, A.A. Single-Molecule Magnets DyM2N@C80 and Dy2MN@C80 (M = Sc, Lu): The Impact of Diamagnetic Metals on Dy3+ Magnetic Anisotropy, Dy⋅⋅⋅Dy Coupling, and Mixing of Molecular and Lattice Vibrations. Chem. Eur. J. 2020, 26, 2436–2449. [Google Scholar] [CrossRef] [PubMed]
- Nie, M.Z.; Xiong, J.; Zhao, C.; Meng, H.B.; Zhang, K.; Han, Y.B.; Li, J.; Wang, B.W.; Feng, L.; Wang, C.R.; et al. Luminescent single-molecule magnet of metallofullerene DyErScN@Ih-C80. Nano Res. 2019, 12, 1727–1731. [Google Scholar] [CrossRef]
- Velkos, G.; Krylov, D.S.; Kirkpatrick, K.; Spree, L.; Dubrovin, V.; Büchner, B.; Avdoshenko, S.M.; Bezmelnitsyn, V.; Davis, S.; Faust, P.; et al. High Blocking Temperature of Magnetization and Giant Coercivity in the Azafullerene Tb-2@C79N with a Single-Electron Terbium-Terbium Bond. Angew. Chem. Int. Ed. 2019, 58, 5891–5896. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Orgeta, I.F.; Herrera, J.M.; Dey, S.; Nojiri, H.; Rajaraman, G.; Colacio, E. The effect of the electronic structure and flexibility of the counteranions on magnetization relaxation in [Dy(L)2(H2O)5]3+ (L = phosphine oxide derivative) pentagonal bipyramidal SIMs. Inorg. Chem. Front. 2020, 7, 689–699. [Google Scholar] [CrossRef]
- Gould, C.A.; McClain, K.R.; Yu, J.M.; Groshens, T.J.; Furche, F.; Harvey, B.G.; Long, J.R. Synthesis and Magnetism of Neutral, Linear Metallocene Complexes of Terbium(II) and Dysprosium(II). J. Am. Chem. Soc. 2019, 141, 12967–12973. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.S.; Day, B.M.; Chen, Y.C.; Tong, M.L.; Mansikkamaki, A.; Layfield, R.A. Magnetic hysteresis up to 80 Kelvin in a dysprosium metallocene single-molecule magnet. Science 2018, 362, 1400–1403. [Google Scholar] [CrossRef]
- McClain, K.R.; Gould, C.A.; Chakarawet, K.; Teat, S.J.; Groshens, T.J.; Long, J.R.; Harvey, B.G. High-temperature magnetic blocking and magneto-structural correlations in a series of dysprosium(III) metallocenium single-molecule magnets. Chem. Sci. 2018, 9, 8492–8503. [Google Scholar] [CrossRef]
- Guo, F.-S.; Day, B.M.; Chen, Y.-C.; Tong, M.-L.; Mansikkamäki, A.; Layfield, R.A. A Dysprosium metallocene single molecule magnet functioning at the axial limit. Angew. Chem. Int. Ed. 2017, 56, 11445. [Google Scholar] [CrossRef]
- Goodwin, C.A.P.; Ortu, F.; Reta, D.; Chilton, N.F.; Mills, D.P. Molecular magnetic hysteresis at 60 Kelvin in dysprosocenium. Nature 2017, 548, 439–442. [Google Scholar] [CrossRef]
- Feltham, H.L.C.; Clérac, R.; Ungur, L.; Chibotaru, L.F.; Powell, A.K.; Brooker, S. By design: A macrocyclic 3d-4f single-molecule magnet with quantifiable zero-field slow relaxation of magnetization. Inorg. Chem. 2013, 52, 3236–3240. [Google Scholar] [CrossRef]
- Osa, S.; Kido, T.; Matsumoto, N.; Re, N.; Pochaba, A.; Mrozinski, J. A tetranuclear 3d-4f single molecule magnet: [CuIILTbIII(hfac)2]2. J. Am. Chem. Soc. 2004, 126, 420–421. [Google Scholar] [CrossRef]
- Chilton, N.F.; Langley, S.K.; Moubaraki, B.; Murray, K.S. Synthesis, structural and magnetic studies of an isostructural family of mixed 3d/4f tetranuclear “star” clusters. Chem. Commun. 2010, 46, 7787–7789. [Google Scholar] [CrossRef]
- Dey, A.; Acharya, J.; Chandrasekhar, V. Heterometallic 3d-4f complexes as single-molecule magnets. Chem. Asian J. 2019, 14, 4433–4453. [Google Scholar] [CrossRef]
- Chakraborty, A.; Goura, J.; Kalita, P.; Swain, A.; Rajaraman, G.; Chandrasekhar, V. Heterometallic 3d-4f single molecule magnets containing diamagnetic metal ions. Dalton Trans. 2018, 47, 8841–8864. [Google Scholar] [CrossRef]
- Zheng, F.-W.; Chen, H.-T.; Li, D.-J.; Han, F.-J.; Yang, L. Two types of {FeDy} heterometallic complexes containing Fe4 structure: Carboxylate derivatives effect on the structures and magnetic properties. J. Clust. Sci. 2020, 32, 461–467. [Google Scholar] [CrossRef]
- Hou, Y.L.; Yang, T.T.; Zhao, W.X.; Fan, C.J.; Yan, L.L.; Guan, X.F.; Ji, J.; Wang, W.M. Modulating SMM behaviors in phenoxo-O bridged Dy2 compounds via different β-diketonate. Inorg. Chim. Act. 2020, 507, 119595. [Google Scholar]
- Das, V.; Kaushik, R.; Hussain, F. Heterometallic 3d-4f polyoxometalates: An emerging field with structural diversity to multiple applications. Coord. Chem. Rev. 2020, 413, 213271. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Du, C.Y.; Zhao, L.H.; Zhang, X.; Wang, D.H.; Sha, J.Q.; Zhang, H.F. Two hexanuclear [Co2III-Ln4III] clusters including a [Co2III-Dy4III] single molecule magnet. Inorg. Chem. Commun. 2020, 116, 107913. [Google Scholar] [CrossRef]
- Xue, S.F.; Zhao, L.; Guo, Y.-N.; Deng, R.P.; Guo, Y.; Tang, J.K. A series of tetranuclear lanthanide complexes comprising two edge-sharing triangular units with field-induced slow magnetic relaxation for Dy4 species. Dalton Trans. 2011, 40, 8347–8352. [Google Scholar] [CrossRef]
- Feuersenger, J.; Prodius, D.; Mereacre, V.; Clérac, R.; Anson, C.E.; Powell, A.K. Synthesis, structure and magnetic properties of hexanuclear CoIII-LnIII clusters. Polyhedron 2013, 66, 257–263. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Ma, Y.Q.; Liu, R.; Yang, L.L.; Tian, G.G.; Shen, N. Tetranuclear complexes with {M 4O4} (M = CoII, NiII) Cubane-Like Core: Synthesis, Crystal Structure, and Magnetic Properties. Z. Anorg. Allg. Chem. 2016, 642, 546–550. [Google Scholar] [CrossRef]
- Wang, R.R.; Wang, H.L.; Wang, J.; Bai, F.F.; Ma, Y.; Li, L.C.; Wang, Q.L.; Zhao, B.; Cheng, P. The different magnetic relaxation behaviors in [Fe(CN)6]3− or [Co(CN)6]3− bridged 3d–4f heterometallic compounds. CrystEngComm 2020, 22, 2998–3004. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Y.-C.; Liu, J.; Chen, W.-B.; Huang, G.-Z.; Wu, S.-G.; Wang, J.; Liu, J.-L.; Tong, M.-L. Cyanometallate-bridged didysprosium single-molecule magnets constructed with single-ion magnet building block. Inorg. Chem. 2020, 59, 687–694. [Google Scholar] [CrossRef]
- Yin, Y.; Wang, J.H.; Zychowicz, M.; Zakrzewski, J.J.; Nakabayashi, K.; Sieklucka, B.; Chorazy, S.; Ohkoshi, S.-I. Dehydration-hydration switching of single-molecule magnet behavior and visible photoluminescence in a cyanide-bridged DyIIICoIII framework. J. Am. Chem. Soc. 2019, 141, 18211–18220. [Google Scholar]
- Zakrzewski, J.J.; Liberka, M.; Zychowicz, M.; Chorazy, S. Diverse physical functionalities of rare-earth hexacyanidometallate frameworks and their molecular analogues. Inorg. Chem. Front. 2021, 8, 452–483. [Google Scholar] [CrossRef]
- Babeshkin, K.A.; Gavrikov, A.V.; Petrosyants, S.P.; Ilyukhin, A.B.; Belova, E.V.; Efimov, N.N. Unexpected supremacy of non-dysprosium single-ion magnets within a series of isomorphic lanthanide cyanocobaltate(III) complexes. Eur. J. Inorg. Chem. 2020, 2020, 4380–4390. [Google Scholar] [CrossRef]
- Lun, H.-J.; Kong, X.-J.; Long, L.-S.; Zheng, L.-S. Trigonal bipyramidal CoIII2Dy3 cluster exhibiting single-molecule magnet behavior. Dalton Trans. 2020, 49, 2421–2425. [Google Scholar] [CrossRef]
- Zhang, J.-W.; Li, W.-H.; Wang, C.-R.; Liu, B.-Q.; Dong, Y.-P. A series of linear CoII2LnIII2 clusters derived from 3,4-dichlorobenzoate and 2,2′-bipyridine: Syntheses, structures, and properties. Inorg. Chim. Acta 2020, 502, 119343. [Google Scholar] [CrossRef]
- Xi, L.; Sun, J.; Wang, K.; Lu, J.; Jing, P.; Li, L.C. Slow magnetic relaxation in CoII–LnIII heterodinuclear complexes achieved through a functionalized nitronyl nitroxide biradical. Dalton Trans. 2020, 49, 1089–1096. [Google Scholar] [CrossRef]
- Yu, Y.Z.; Pan, X.X.; Cui, C.H.; Luo, X.M.; Li, N.F.; Mei, H.; Xu, Y. A Series Three-Dimensional Ln4Cr4 (Ln = Gd, Tb, Er) Heterometallic Cluster-Based Coordination Polymers Containing Interesting Nanotubes Exhibiting High Magnetic Entropy. Inorg. Chem. 2020, 59, 5593–5599. [Google Scholar] [CrossRef]
- Fan, X.-T.; Yang, H.; Li, D.-C.; Tian, H.-Q.; Cao, F.; Dou, J.-M. Three new heterometallic ZnII–LnIII complexes with a windmill-like framework and field-induced SMM behavior. New J. Chem. 2020, 44, 2555–2560. [Google Scholar] [CrossRef]
- Maity, S.; Mondal, A.; Konar, S.; Ghosh, A. The role of 3d–4f exchange interaction in SMM behaviour and magnetic refrigeration of carbonato bridged Cu II2Ln III2 (Ln = Dy, Tb and Gd) complexes of an unsymmetrical N2O4 donor ligand. Dalton Trans. 2019, 48, 15170–15183. [Google Scholar] [CrossRef]
- Darii, M.; Kravtsov, V.C.; Krämer, K.; Hauser, J.; Decurtins, S.; Liu, S.-X.; Affronte, M.; Baca, S.G. Aggregation of a Giant Bean-like {Mn26Dy6} Heterometallic Oxo-Hydroxo-Carboxylate Nanosized Cluster from a Hexanuclear {Mn6} Precursor. Cryst. Growth Des. 2020, 20, 33–38. [Google Scholar] [CrossRef]
- Gao, F.; Feng, X.W.; Yang, L.; Chen, X.Y. New sandwich-type lanthanide complexes based on closed-macrocyclic Schiff base and phthalocyanine molecules. Dalton Trans. 2016, 45, 7476–7482. [Google Scholar] [CrossRef]
- Li, Z.G.; Gao, F.; Xiao, Z.G.; Wu, X.Z.; Zuo, J.L.; Song, Y.L. Nonlinear optical properties and excited state dynamics of sandwich-type mixed (phthalocyaninato)(Schiff-base) triple-decker complexes: Effect of rare earth atom. Opt. Laser Technol. 2018, 103, 42–47. [Google Scholar] [CrossRef]
- Wang, H.L.; Cao, W.; Liu, T.; Duan, C.Y.; Jiang, J.H. Synthesis, Structure, and Single-Molecule Magnetic Properties of Rare-Earth Sandwich Complexes with Mixed Phthalocyanine and Schiff Base Ligands. Chem. Eur. J. 2013, 19, 2266–2270. [Google Scholar] [CrossRef]
- Suzuki, A.; Oku, T. Effects of central metal on electronic structure, magnetic properties, infrared and Raman spectra of double-decker phthalocyanine. Appl. Surf. Sci. 2016, 380, 127–134. [Google Scholar] [CrossRef]
- Sato, T.; Matsuzawa, S.; Kato, K.; Breedlove, B.K.; Yamashita, M. Relationship between the Coordination Geometry and Spin Dynamics of Dysprosium(III) Heteroleptic Triple-Decker Complexes. Magnetochemistry 2019, 5, 65. [Google Scholar] [CrossRef]
- Patrascu, A.A.; Briganti, M.; Soriano, S.; Clancea, S.; Cassaro, R.A.A.; Totti, F.; Vaz, M.G.F.; Andruh, M. SMM Behavior Tuned by an Exchange Coupling LEGO Approach for Chimeric Compounds: First 2p–3d–4f Heterotrispin Complexes with Different Metal Ions Bridged by One Aminoxyl Group. Inorg. Chem. 2019, 58, 13090–13101. [Google Scholar] [CrossRef]
- Patrascu, A.A.; Calancea, S.; Briganti, M.; Soriano, S.; Madalan, A.M.; Cassaro, R.A.A.; Caneschi, A.; Totti, F.; Vaz, M.G.F.; Andruh, M. A chimeric design of heterospin 2p–3d, 2p–4f, and 2p–3d–4f complexes using a novel family of paramagnetic dissymmetric compartmental ligands. Chem. Commun. 2017, 53, 6504–6507. [Google Scholar] [CrossRef]
- Feng, M.; Tong, M.L. Single ion magnets from 3d to 5f: Developments and strategies. Chem. A Eur. J. 2018, 24, 7574–7594. [Google Scholar] [CrossRef] [PubMed]
- Lunghi, A.; Totti, F.; Sessoli, R.; Sanvito, S. The role of anharmonic phonons in under-barrier spin relaxation of single molecule magnets. Nat. Commun. 2017, 8, 14620. [Google Scholar] [CrossRef]
- Lunghi, A.; Totti, F.; Sanvito, S.; Sessoli, R. Intra-molecular origin of the spin-phonon coupling in slow-relaxing molecular magnets. Chem. Sci. 2017, 8, 6051–6059. [Google Scholar] [CrossRef] [PubMed]
- Lunghi, A.; Sanvito, S. How do phonons relax molecular spins? Sci. Adv. 2019, 5, eaax7163. [Google Scholar] [CrossRef]
- Vonci, M.; Giansiracusa, M.J.; van den Heuvel, W.; Gable, R.W.; Moubaraki, B.; Murray, K.S.; Yu, D.; Mole, R.A.; Soncini, A.; Boskovic, C. Magnetic Excitations in Polyoxotungstate-Supported Lanthanoid Single-Molecule Magnets: An Inelastic Neutron Scattering and ab Initio Study. Inorg. Chem. 2017, 56, 378–394. [Google Scholar] [CrossRef]
- Gransbury, G.K.; Boulon, M.-E.; Mole, R.A.; Gable, R.W.; Moubaraki, B.; Murray, K.S.; Sorace, L.; Soncini, A.; Boskovic, C. Single-ion anisotropy and exchange coupling in cobalt(II)-radical complexes: Insights from magnetic and ab initio studies. Chem. Sci. 2019, 10, 8855–8871. [Google Scholar] [CrossRef]
- Sorensen, M.A.; Hansen, U.B.; Perfetti, M.; Pedersen, K.S.; Bartolomé, E.; Simeoni, G.G.; Mutka, H.; Rols, S.; Jeong, M.; Zivkovic, I.; et al. Chemical tunnel-splitting-engineering in a dysprosium-based molecular nanomagnet. Nat. Commun. 2018, 9, 1292. [Google Scholar] [CrossRef] [PubMed]
- Perfetti, M.; Sorensen, M.A.; Hansen, U.B.; Bamberger, H.; Lenz, S.; Hallmen, P.P.; Fennell, T.; Simeoni, G.G.; Arauzo, A.; Bartolomé, J.; et al. Magnetic anisotropy switch: Easy axis to easy plane conversion and vice versa. Adv. Funct. Mater. 2018, 28, 1801846. [Google Scholar] [CrossRef]
- Bonde, N.A.; Petersen, J.B.; Sorensen, M.A.; Nielsen, U.G.; Fak, B.; Rols, S.; Ollivier, J.; Weihe, H.; Bendix, J.; Perfetti, M. Importance of Axial Symmetry in Elucidating Lanthanide–Transition Metal Interactions. Inorg. Chem. 2020, 59, 235–243. [Google Scholar] [CrossRef]
- Pointillart, F.; Gonzales, J.F.; Montigaud, V.; Tesi, L.; Cherkasov, V.; Le Guennic, B.; Cador, O.; Ouahab, L.; Sessoli, R.; Kuropatov, V. Redox- and solvato-magnetic switching in a tetrathiafulvalene-based triad single-molecule magnet. Inorg. Chem. Front. 2020, 7, 2322–2334. [Google Scholar] [CrossRef]
- Cador, O.; Le Guennic, B.; Ouahab, L.; Pointillart, F. Decorated Tetrathiafulvalene-Based Ligands: Powerful Chemical Tools for the Design of Single-Molecule Magnets. Eur. J. Inorg. Chem. 2020, 2020, 148–164. [Google Scholar] [CrossRef]
- Yao, X.-N.; Du, J.-Z.; Zhand, Y.-Q.; Leng, X.-B.; Yang, M.-W.; Jiang, S.-D.; Wang, Z.-X.; Qouyang, Z.-W.; Deng, L.; Wang, B.-W.; et al. Two-coordinate Co(II) imido complexes as outstanding single-molecule magnets. J. Am. Chem. Soc. 2017, 139, 373–380. [Google Scholar] [CrossRef]
- Bunting, P.C.; Atanasov, M.; Damgaard-Moller, E.; Perfetti, M.; Crassee, I.; Orlita, M.; Overgaard, J.; van Slageren, J.; Neese, F.; Long, J.R. A linear cobalt(II) complex with maximal orbital angular momentum from a non-Aufbau ground state. Science 2018, 362, eaat7319. [Google Scholar] [CrossRef]
- Caneschi, A.; Gatteschi, D.; Lalioti, N.; Sangregorio, C.; Sessoli, R.; Venturi, G.; Vindigni, A.; Rettori, A.; Pini, M.G.; Novak, M.A. Cobalt(II)-Nitronyl Nitroxide Chains as Molecular Magnetic Nanowires. Angew. Chem. Int. Ed. 2001, 40, 1760–1763. [Google Scholar] [CrossRef]
- Clérac, R.; Miyasaka, H.; Yamashita, M.; Coulon, C. Evidence for Single-Chain Magnet Behavior in a MnIII−NiII Chain Designed with High Spin Magnetic Units: A Route to High Temperature Metastable Magnets. J. Am. Chem. Soc. 2002, 124, 12837–12844. [Google Scholar] [CrossRef] [PubMed]
- Lescouezec, R.; Vaissermann, J.; Ruiz-Pérez, C.; Lloret, F.; Carrasco, R.; Julve, M.; Verdaguer, M.; Dromzee, Y.; Geteschi, D.; Wernsdorfer, W. Cyanide-Bridged Iron(III)–Cobalt(II) Double Zigzag Ferromagnetic Chains: Two New Molecular Magnetic Nanowires. Angew. Chem. Int. Ed. 2003, 42, 1483–1486. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.M.; Prosvirin, A.V.; Zhao, H.H.; Mao, J.G.; Dunbar, K.R. New type of single chain magnet based on spin canting in an antiferromagnetically coupled Co(II) chain. J. Appl. Phys. 2005, 97, 10B305. [Google Scholar] [CrossRef]
- Tanaka, H.; Kajiwara, T.; Kaneko, Y.; Takaishi, S.; Yamashita, M. Synthesis, structure, and magnetic property of a new Fe(II)–Fe(III) alternating single-chain magnet constructed with a methyl-substituted bpca− ligand. Polyhedron 2007, 26, 2105–2109. [Google Scholar] [CrossRef]
- Bogani, L.; Vindigni, A.; Sessoli, R.; Gatteschi, D. Single chain magnets: Where to from here? J. Mater. Chem. 2008, 18, 4750–4758. [Google Scholar] [CrossRef]
- Zhang, W.-X.; Ishikawa, R.; Breedlove, B.; Yamashita, M. Single-chain magnets: Beyond the Glauber model. RSC Adv. 2013, 3, 3772–3798. [Google Scholar] [CrossRef]
- Nadeem, M.A.; Ng, M.C.C.; van Leusen, J.; Kogerler, P.; Stride, J.A. Magnetic phase transitions in a Ni4O4-cubane-based metal-organic framework. Chem. A Eur. J. 2020, 26, 7589–7594. [Google Scholar] [CrossRef]
- Liu, X.Q.; Feng, X.W.; Meihaus, K.R.; Meng, X.X.; Zhang, Y.; Li, L.; Liu, J.L.; Pedersen, K.S.; Keller, L.; Shi, W.; et al. Coercive fields above 6 T in two cobalt(II)–radical chain compounds. Angew. Chem. Int. Ed. 2020, 59, 10610–10618. [Google Scholar] [CrossRef]
- Yang, J.; Deng, Y.F.; Zhang, Y.Z. Two azido-bridged homospin Fe(II)/Co(II) coordination polymers featuring single-chain magnet behavior. Dalton Trans. 2020, 49, 4805–4810. [Google Scholar] [CrossRef]
- Shi, L.; Shao, D.; Wei, X.-Q.; Dunbar, K.R.; Wang, X.-Y. Enhanced single-chain magnet behavior via anisotropic exchange in a cyano-bridged MoIII–MnII chain. Angew. Chem. Int. Ed. 2020, 59, 10379–10384. [Google Scholar] [CrossRef]
- Jiang, W.-J.; Lu, H.-H.; Meng, Y.-S.; Jiao, C.-Q.; Liu, T. Dehydration-actuated single-chain magnet through charge transfer in a cyanide-bridged Fe2Co chain. Inorg. Chem. Comm. 2020, 112, 107715. [Google Scholar] [CrossRef]
- Korzeniak, T.; Nowicka, B.; Sieklucka, B. Hybrid organic-inorganic cyanide-bridged networks. In Organometallic Magnets; Chandrasekhar, V., Pointillart, F., Eds.; Topics in Organometallic Chemistry; Springer: Cham, Switzerland, 2019; Volume 64, pp. 1–34. [Google Scholar]
- Reczynski, M.; Nowicka, B.; Näther, C.; Koziel, M.; Nakabayashi, K.; Ohkoshi, S.-I.; Sieklucka, B. Dehydration-Triggered Charge Transfer and High Proton Conductivity in (H3O)[NiIII(cyclam)][MII(CN)6] (M = Ru, Os) Cyanide-Bridged Chains. Inorg. Chem. 2018, 57, 13415–13422. [Google Scholar] [CrossRef]
- Sieklucka, B.; Pinkowicz, D. (Eds.) Molecular Magnetic Materials; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2017. [Google Scholar]
- Alexandru, M.-G.; Visinescu, D.; Shova, S.; Lloret, F.; Julve, M. Cyanido-Bridged {LnIIIWV} Heterobinuclear Complexes: Synthesis and Magneto-Structural Study. Inorg. Chem. 2017, 56, 12594–12605. [Google Scholar] [CrossRef]
- Wei, R.-M.; Cao, F.; Li, J.; Yang, L.; Han, Y.; Zhang, X.-L.; Zhang, Z.C.; Wang, X.-Y.; Song, Y. Single-Chain Magnets Based on Octacyanotungstate with the Highest Energy Barriers for Cyanide Compounds. Sci. Rep. 2016, 6, 24372. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-Z.; Dolinar, B.S.; Liu, S.H.; Brown, A.J.; Zhang, X.; Wang, Z.-X.; Dunbar, K.R. Enforcing Ising-like magnetic anisotropy via trigonal distortion in the design of a W(V)–Co(II) cyanide single-chain magnet. Chem. Sci. 2018, 9, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Liang, X.H.; Zhang, Y.D.; Ouyang, Z.J.; Dong, W. A family of 3d-4f Cu-Ln ladder-like complexes: Synthesis, structures and magnetic properties. Polyhedron 2020, 180, 114435. [Google Scholar] [CrossRef]
- Rubín, J.; Badía-Romano, L.; Luis, F.; Mereacre, V.; Prodius, D.; Arauzo, A.; Bartolomé, F.; Bartolomé, J. Magnetic chains of Fe3 clusters in the {Fe3YO2} butterfly molecular compound. Dalton Trans. 2020, 49, 2979–2988. [Google Scholar] [CrossRef]
- Yang, M.; Liang, X.H.; Zhang, Y.; Ouyang, Z.J.; Dong, W. A nitronyl nitroxide and its two 1D chain Cu–Tb complexes: Synthesis, structures, and magnetic properties. RSC Adv. 2020, 10, 8490–8496. [Google Scholar] [CrossRef]
- Houard, F.; Evrard, Q.; Calvez, G.; Suffren, Y.; Daiguebonne, C.; Guillou, O.; Gendron, F.; Le Guennic, B.; Guizouarn, T.; Dorcet, V.; et al. Chiral supramolecular nanotubes of single-chain magnets. Angew. Chem. Int. Ed. 2020, 59, 780–784. [Google Scholar] [CrossRef]
- Zhang, S.-L.; Li, S.-S.; Zeng, S.-Y.; Shi, Y.; Wang, D.-Q.; Chen, L. Slow magnetic relaxation in O–Se–O bridged manganese(III) Schiff base complexes. New J. Chem. 2020, 44, 2408–2413. [Google Scholar] [CrossRef]
- Zhao, F.; Dong, Z.-P.; Liu, Z.-L.; Wang, Y.-Q. An unusual homospin CoII ferrimagnetic single-chain magnet with large hysteresis. CrystEngComm 2019, 21, 6958–6983. [Google Scholar] [CrossRef]
- Zang, J.; Zhao, Q.-N.; Yang, F.; Yin, L.; Li, M.-M.; Wang, Z.X.; Ouyang, Z.W.; Xia, Z.-C.; Hu, T.-P. A cobalt(II) chain based on pymca generated in situ from the hydrolysis of 2-cyanopyrimidine: Spin canting and magnetic relaxation. RSC Adv. 2019, 9, 31115–31121. [Google Scholar] [CrossRef]
- Wang, M.M.; Gou, X.S.; Shi, W.; Cheng, P. Single-chain magnets assembled in cobalt(ii) metal–organic frameworks. Chem. Commun. 2019, 55, 11000–11012. [Google Scholar] [CrossRef]
- Takahashi, S.; Tupitsyn, I.S.; van Tol, J.; Beedle, C.C.; Hendrickson, D.N.; Stamp, P.C.E. Decoherence in crystals of quantum molecular magnets. Nature 2011, 476, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Landau, L. Zur Theorie der Energieübertragung. Phys. Z. Sov. 1932, 2, 46–51. [Google Scholar]
- Zener, C. Non-adiabatic crossing of energy levels. Proc. R. Soc. Lond. A 1932, 137, 696–702. [Google Scholar]
- Troiani, F.; Godfrin, C.; Thiele, S.; Balestro, F.; Wernsdorfer, W.; Klyatskaya, S.; Ruben, M.; Affronte, M. Landau-Zener transition in a continously measured single-molecule spin transistor. Phys. Rev. Lett. 2017, 118, 257701. [Google Scholar] [CrossRef]
- Mirzoyan, R.; Hadt, R.G. The dynamic ligand field of a molecular qubit: Decoherence through spin-phonon coupling. Phys. Chem. Chem. Phys. 2020, 22, 11249–11265. [Google Scholar] [CrossRef] [PubMed]
- Postnikov, A.V.; Kortus, J.; Pederson, M.R. Density functional studies of molecular magnets. Phys. Status Solidi B 2006, 243, 2533–2572. [Google Scholar] [CrossRef]
- Taran, G.; Bonet, E.; Wernsdorfer, W. Decoherence measurements in crystals of molecular magnets. Phys. Rev. B 2019, 99, 180408(R). [Google Scholar] [CrossRef]
- Lunghi, A.; Sanvito, S. Electronic spin-spin decoherence contribution in molecular qubits by quantum unitary spin dynamics. J. Magn. Magn. Mater. 2019, 487, 165325. [Google Scholar] [CrossRef]
- Hu, C.; Chen, J.; Zhang, X.G. Modeling Hyperfine Coupling in Molecular Magnets. Bull. Am. Phys. Soc. 2020, 65, D12. 00007. Available online: https://meetings.aps.org/Meeting/MAR20/Session/D12.7 (accessed on 3 July 2021).
- Stamp, P.C.E. Environment decoherence versus intrinsic decoherence. Philos. Trans. R. Soc. A 2012, 370, 4429–4453. [Google Scholar] [CrossRef]
- Liu, J.; Wu, B.; Fu, L.B.; Diener, R.B.; Niu, Q. Quantum step heights in hysteresis loops of molecular magnets. Phys. Rev. B 2002, 65, 224401. [Google Scholar] [CrossRef]
- Bhandary, S.; Ghosh, S.; Herper, H.; Wende, H.; Eriksson, O.; Sanyal, B. Graphene as a reversible spin manipulator of molecular magnets. Phys. Rev. Lett. 2011, 107, 257202. [Google Scholar] [CrossRef]
- Baker, M.L.; Timco, G.A.; Piligkos, S.; Mathieson, J.S.; Mutka, H.; Tuna, F.; Kozlowski, P.; Antkowiak, M.; Guidi, T.; Rath, H. A classification of spin frustration in molecular magnets from a physical study of large odd-numbered-metal, odd electron rings. Proc. Natl. Acad. Sci. USA 2012, 109, 19113–19118. [Google Scholar] [CrossRef]
- Abufager, P.N.; Robles, R.; Lorente, N. FeCoCp3 molecular magnets as spin filters. J. Phys. Chem. C 2015, 119, 12119–12129. [Google Scholar] [CrossRef]
- Vieru, V.; Ungur, L.; Cemortan, V.; Sukhanov, A.; Baniodeh, A.; Anson, C.E.; Powell, A.K.; Voronkova, V.; Chibotaru, L.F. Magnetization blocking in Fe2IIIDy2III molecular magnets: Ab initio calculations and EPR spectroscopy. Chem. Eur. J. 2018, 24, 16652–16661. [Google Scholar] [CrossRef]
- Gupta, T.; Rajaraman, G. How strongly are the magnetic anisotropy and coordination numbers correlated in lanthanide based molecular magnets? J. Chem. Sci. 2014, 126, 1569–1579. [Google Scholar] [CrossRef]
- Dey, S.; Rajaraman, G. In silico design of pseudo D5h actinide based molecular magnets: Role of covalency in magnetic anisotropy. J. Chem. Sci. 2019, 131, 124. [Google Scholar] [CrossRef]
- Vonci, M.; Giansiracusa, M.J.; Gable, R.W.; van den Heuvel, W.; Latham, K.; Moubaraki, B.; Murray, K.S.; Yu, D.H.; Mole, R.A.; Soncini, A.; et al. Ab initio calculations as a quantitative tool in the inelastic neutron scattering study of a single-molecule magnet analogue. Chem. Commun. 2016, 52, 2091–2094. [Google Scholar] [CrossRef]
- Pineda, E.M.; Chilton, N.F.; Tuna, F.; Winpenny, R.E.P.; McInnes, E.J.L. Systematic Study of a Family of Butterfly-Like {M2Ln2} Molecular Magnets (M = MgII, MnIII, CoII, NiII, and CuII.; Ln = YIII, GdIII, TbIII, DyIII, HoIII, and ErIII). Inorg. Chem. 2015, 54, 5930–5941. [Google Scholar] [CrossRef]
- Mannini, M.; Bertani, F.; Tudisco, C.; Malavolti, L.; Poggini, L.; Misztal, K.; Menozzi, D.; Motta, A.; Otero, E.; Ohresser, P.; et al. Magnetic behavior of TbPc2 single-molecule magnets chemically grafted on silicon surface. Nat. Commun. 2014, 5, 4582. [Google Scholar] [CrossRef]
- Valiev, M.; Bylaska, E.J.; Govind, N.; Kowalski, K.; Straatsma, T.P.; van Dam, H.J.J.; Wang, D.; Nieplocha, J.; Apra, E.; Windus, T.L.; et al. NWChem: A comprehensive and scalable open-source solution for large scale molecular simulations. Comput. Phys. Commun. 2010, 181, 1477–1489. [Google Scholar] [CrossRef]
- Prsa, K.; Nehrkorn, J.; Corbey, J.F.; Evans, W.J.; Demir, S.; Long, J.R.; Guidi, T.; Waldmann, O. Perspectives on neutron scattering in lanthanide-based single-molecule magnets and a case study of the Tb2(µ-N2) system. Magnetochemistry 2016, 2, 45. [Google Scholar] [CrossRef]
- Albuquerque, A.F.; Alet, F.; Corboz, P.; Dayal, P.; Feiguin, A.; Fuchs, S.; Gamper, L.; Gull, E.; Gürtler, S.; Honecker, A.; et al. The ALPS project release 1.3: Open-source software for strongly correlated systems. J. Magn. Magn. Mater. 2007, 310, 1187–1193. [Google Scholar] [CrossRef]
- Baniodeh, A.; Magnani, N.; Lan, Y.H.; Buth, G.; Anson, C.E.; Richter, J.; Affronte, M.; Schnack, J.; Powell, A.K. High spin cycles: Topping the spin recored for a single molecule verging on quantum criticality. Npj Quantum Mater. 2018, 3, 10. [Google Scholar] [CrossRef]
- CP2K Developers Group. CP2K Open Source Molecular Dynamics. Available online: http://www.cp2k.org/ (accessed on 3 July 2021).
- Burgess, J.A.J.; Malavolti, L.; Lanzilotto, V.; Mannini, M.; Yan, S.C.; Ninova, S.; Totti, F.; Rolf-Pissarczyk, S.; Cornia, A.; Sessoli, R.; et al. Magnetic fingerprint of individual Fe4 molecular magnets under compression by a scanning tunneling microscope. Nat. Commun. 2015, 6, 8216. [Google Scholar] [CrossRef]
- Tandon, S.; Schmitt, W.; Watson, G.W. J2suscep: Calculation of magnetic exchange coupling and temperature dependence of magnetic susceptibility. J. Open Source Softw. 2021, 6, 2838. [Google Scholar] [CrossRef]
- Smith, D.G.A.; Burns, L.A.; Simmonett, A.C.; Parrish, R.M.; Schieber, M.C.; Galvelis, R.; Kraus, P.; Kruse, H.; di Remigio, R.; Alenaizan, A.; et al. PSI4 1.4: Open-source software for high-throughput quantum chemistry. J. Chem. Phys. 2020, 152, 184108. [Google Scholar] [CrossRef] [PubMed]
- Pronk, S.; Páll, S.; Schulz, R.; Larsson, P.; Bjeklmar, P.; Apostolov, R.; Shirts, M.R.; Smith, J.C.; Kasson, P.M.; van der Spoel, D.; et al. GROMACS 4.5: A high-throughput and highly parallel opuen source molecular simulation toolkit. Bioinformatics 2013, 29, 845. [Google Scholar] [CrossRef] [PubMed]
- Veloso, S.R.S.; Andrade, R.G.D.; Castanheira, E.M.S. Review on the advancements of magnetic gels: Towards multifunctional magnetic liposome-hydrogel composites for biomedical applications. Adv. Colloid Interface Sci. 2021, 288, 102351. [Google Scholar] [CrossRef]
- Rusakov, V.; Raikher, Y. Magnetorelaxometry in the presence of a DC bias field of ferromagnetic nanoparticles bearing a viscoelastic corona. Sensors 2018, 18, 1661. [Google Scholar] [CrossRef]
- Kurlyandskaya, G.V.; Litvinova, L.S.; Safronov, A.P.; Schupletsova, V.V.; Tyukova, I.S.; Khaziakhmatova, O.G.; Slepchenko, G.B.; Yurova, K.A.; Cherempey, E.G.; Kulesh, N.A.; et al. Water-based suspensions of iron oxide nanoparticles with electrostatic or steric stabilization by chitosan: Fabrication, characterization and biocompatibility. Sensors 2017, 17, 2605. [Google Scholar] [CrossRef]
- Domingo, N.; Bellido, E.; Ruiz-Molina, D. Advances on structuring, integration and magnetic characterization of molecular nanomagnets on surfaces and devices. Chem. Soc. Rev. 2012, 41, 258–302. [Google Scholar] [CrossRef] [PubMed]
- Vaheb, Y.; Calvet, L.E.; Dia, N.; Mallah, T.; Catala, L. Assembly of molecular nanomagnets into nanogap electrodes by dielectrophoresis. J. Nanosci. Nanotechnol. 2012, 12, 8710–8714. [Google Scholar] [CrossRef] [PubMed]
- Messina, P.; Mannini, M.; Caneschi, A.; Gatteschi, D.; Sorace, L. Spin noise fluctuations from paramagnetic molecular adsorbates on surfaces. J. Appl. Phys. 2007, 101, 053916. [Google Scholar] [CrossRef]
- Fonin, M.; Voss, S.; Herr, S.; de Loubens, G.; Kent, A.D.; Burgert, M.; Groth, U.; Rüdiger, U. Influence of the ligand shell on the surface orientation of Mn12 single molecule magnets. Polyhedron 2009, 28, 1977–1981. [Google Scholar] [CrossRef]
- Jia, J.-H.; Li, Q.-W.; Chen, Y.-C.; Liu, J.-L.; Tong, M.-L. Luminescent single-molecule magnets based on lanthanides: Design strategies, recent advances and magneto-luminescent studies. Coord. Chem. Rev. 2019, 378, 365–381. [Google Scholar] [CrossRef]
- Yi, X.H.; Bernot, K.; Pointillart, F.; Poneti, G.; Calvez, G.; Daiguebonne, C.; Guillou, O.; Sessoli, R. A luminescent and sublimable DyIII-based single-molecule magnet. Chem. A Eur. J. 2012, 18, 11379–11387. [Google Scholar] [CrossRef]
- Jiménez, J.-R.; Díaz-Ortega, I.F.; Ruiz, E.; Aravena, D.; Pope, S.J.A.; Colacio, E.; Herrera, J.M. Lanthanide tetrazolate complexes combining single-molecule magnet and luminescence properties: The effect of the replacement of tetrazolate N3 by β-diketonate ligands on the anisotropy energy barrier. Chem. A Eur. J. 2016, 22, 14548–14559. [Google Scholar] [CrossRef]
- Wang, J.H.; Zakrzewski, J.J.; Heczko, M.; Zychowsicz, M.; Nakagawa, K.; Nakabayashi, K.; Sieklucka, B.; Chorazy, S.; Ohkoshi, S.-I. Proton conductive luminescent thermometer based on near-infrared emissive {YbCo2} molecular nanomagnets. J. Am. Chem. Soc. 2020, 142, 3970–3979. [Google Scholar] [CrossRef]
- Long, J. Luminescent Schiff-base lanthanide single-molecule magnets: The association between optical and magnetic properties. Front. Chem. 2019, 7, 63. [Google Scholar] [CrossRef]
- Long, J.; Ivanov, M.S.; Khomchenko, V.A.; Mamontova, E.; Thibaud, J.-M.; Rouquette, J.; Beaudhuin, M.; Granier, D.; Ferreira, R.A.; Carlos, L.D.; et al. Room temperature magnetoelectric coupling in a molecular ferroelectric ytterbium(III) complex. Science 2020, 367, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Katoh, K.; Breedlove, B.K.; Yamashita, M. Electro-conductive single-molecule magnet composed of a dysprosium(III)-phthalocyaninato double-decker complex with magnetoresistance. Angew. Chem. Int. Ed. 2021. early view. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.-H.; Meng, Y.; Chen, Y.-C.; Li, Q.-W.; Wang, B.-Y.; Leng, J.-D.; Bao, D.-H.; Jia, J.-H.; Tong, M.-L. A zigzag DyIII4 cluster exhibiting single-molecule magnet, ferroelectric and white-light emitting properties. J. Mater. Chem. C 2014, 2, 8858–8864. [Google Scholar] [CrossRef]
- Li, X.-L.; Chen, C.-L.; Gao, Y.-L.; Liu, C.-M.; Feng, X.-L.; Gui, Y.-H.; Fang, S.-M. Modulation of homochiral DyIII complexes: Single-molecule magnets with ferroelectric properties. Chem. Eur. J. 2012, 18, 14632–14637. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, P.; Mukadam, M.D.; Meena, S.S.; Mishra, S.K.; Mittal, R.; Sastry, P.U.; Mandal, B.P.; Yusuf, S.M. Room temperature ferroelectricity in one-dimensional single chain molecular magnets [{M(Δ)M(Λ)}(ox)2(phen)2]n (M = Fe and Mn). Appl. Phys. Lett. 2017, 110, 102901. [Google Scholar] [CrossRef]
- Bai, Y.-L.; Tao, J.; Wernsdorfer, W.; Sato, O.; Huang, R.-B.; Zheng, L.-S. Coexistence of magnetization relaxation and dielectric relaxation in a single-chain magnet. J. Am. Chem. Soc. 2006, 128, 16428–16429. [Google Scholar] [CrossRef]
- Slota, M.; Bogani, L. Combining molecular spintronics with electron paramagnetic resonance: The path towards single-molecule pulsed spin spectroscopy. Appl. Magn. Reson. 2020, 51, 1357–1409. [Google Scholar] [CrossRef]
- Aravena, D.; Ruiz, E. Spin dynamics in single-molecule magnets and molecular qubits. Dalton Trans. 2020, 49, 9916–9928. [Google Scholar] [CrossRef]
- Zhu, Z.H.; Li, X.-L.; Liu, S.T.; Tang, J.K. External stimuli modulate the magnetic relaxation of lanthanide single-molecule magnets. Inorg. Chem. Front. 2020, 7, 3315–3326. [Google Scholar] [CrossRef]
- Kalita, P.; Acharya, J.; Chandrasekhar, V. Mononuclear pentagonal bipyramidal Ln(III) complexes: Syntheses and magnetic properties. J. Magn. Magn. Mater. 2020, 498, 166098. [Google Scholar] [CrossRef]
- Tian, H.; Zheng, L.M. Cyclic lanthanide-based molecular clusters: Assembly and single molecule magnet behavior. Acta Chim. Sin. 2020, 78, 34–55. [Google Scholar] [CrossRef]
- Chorazy, S.; Zakrzewski, J.J.; Magott, M.; Korzeniak, T.; Nowicka, B.; Pinkowicz, D.; Podgajny, R.; Sieklucka, B. Octacyanidometallates for multifunctional molecule-based materials. Chem. Soc. Rev. 2020, 49, 5945–6001. [Google Scholar] [CrossRef]
- Guan, R.N.; Chen, M.Q.; Yang, S.F. Metal fullerene monomolecular magnet. Chin. Sci. Bull. 2020, 65, 2209–2224. [Google Scholar] [CrossRef]
- Reczynski, M.; Nakabayashi, K.; Ohkoshi, S.-I. Tuning the optical properties of magnetic materials. Eur. J. Inorg. Chem. 2020, 2020, 2669–2678. [Google Scholar] [CrossRef]
- Coronado, E. Molecular magnetism: From chemical design to spin control in molecules, materials and devices. Nat. Rev. Mater. 2020, 5, 87–104. [Google Scholar] [CrossRef]
- Hao, G.H.; Cheng, R.H.; Dowben, P.A. The emergence of the local moment molecular spin transistor. J. Phys. Condens. Matter 2020, 32, 234002. [Google Scholar] [CrossRef]
- Perlepe, P.S.; Maniaki, D.; Pilichos, E.; Katsoulakou, E.; Perlepes, S.P. Smart ligands for efficient 3d-, 3d- and 5d-metal single-molecule magnets and single-ion magnets. Inorganics 2020, 8, 39. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blachowicz, T.; Ehrmann, A. New Materials and Effects in Molecular Nanomagnets. Appl. Sci. 2021, 11, 7510. https://doi.org/10.3390/app11167510
Blachowicz T, Ehrmann A. New Materials and Effects in Molecular Nanomagnets. Applied Sciences. 2021; 11(16):7510. https://doi.org/10.3390/app11167510
Chicago/Turabian StyleBlachowicz, Tomasz, and Andrea Ehrmann. 2021. "New Materials and Effects in Molecular Nanomagnets" Applied Sciences 11, no. 16: 7510. https://doi.org/10.3390/app11167510
APA StyleBlachowicz, T., & Ehrmann, A. (2021). New Materials and Effects in Molecular Nanomagnets. Applied Sciences, 11(16), 7510. https://doi.org/10.3390/app11167510






