The Role of Wetland Plants on Wastewater Treatment and Electricity Generation in Constructed Wetland Coupled with Microbial Fuel Cell
Abstract
:1. Introduction
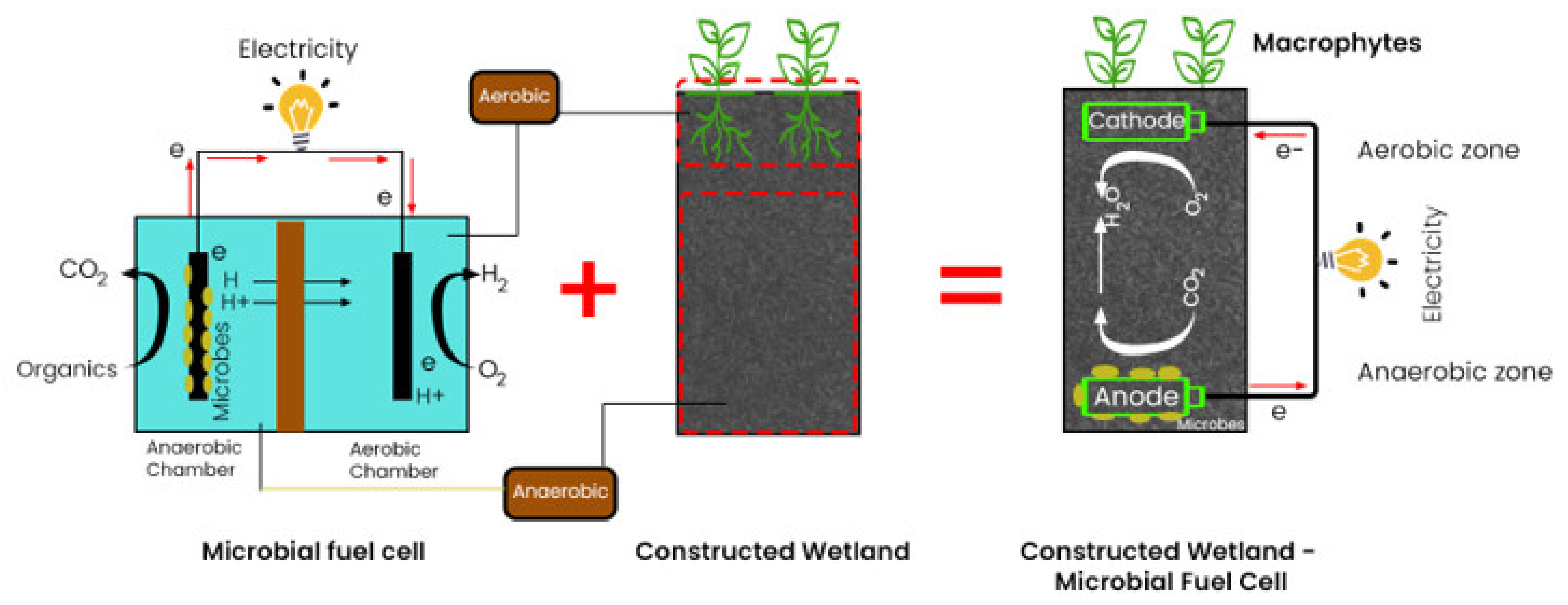
2. Configuration of CWMFC
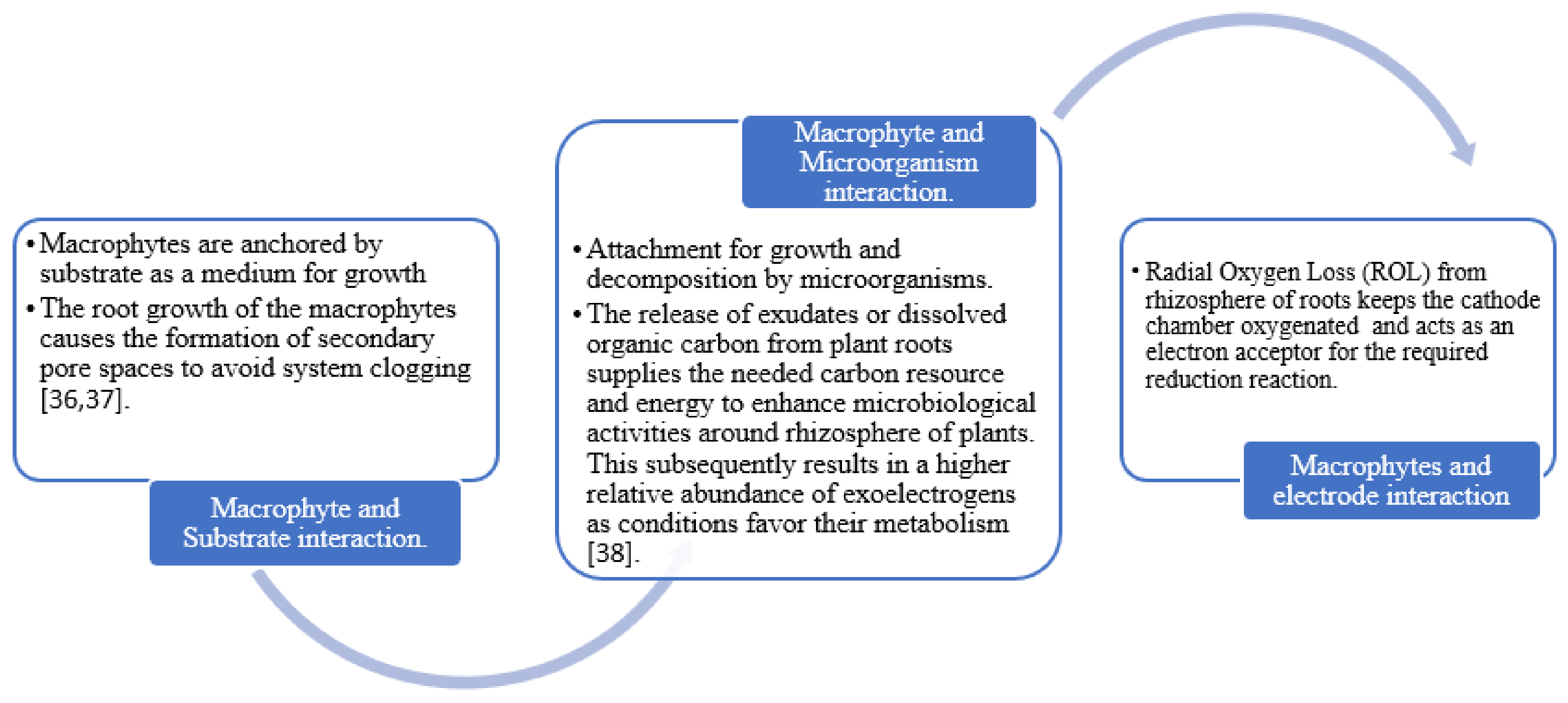
3. The Effect of Aquatic Macrophytes on Microbial Organisms
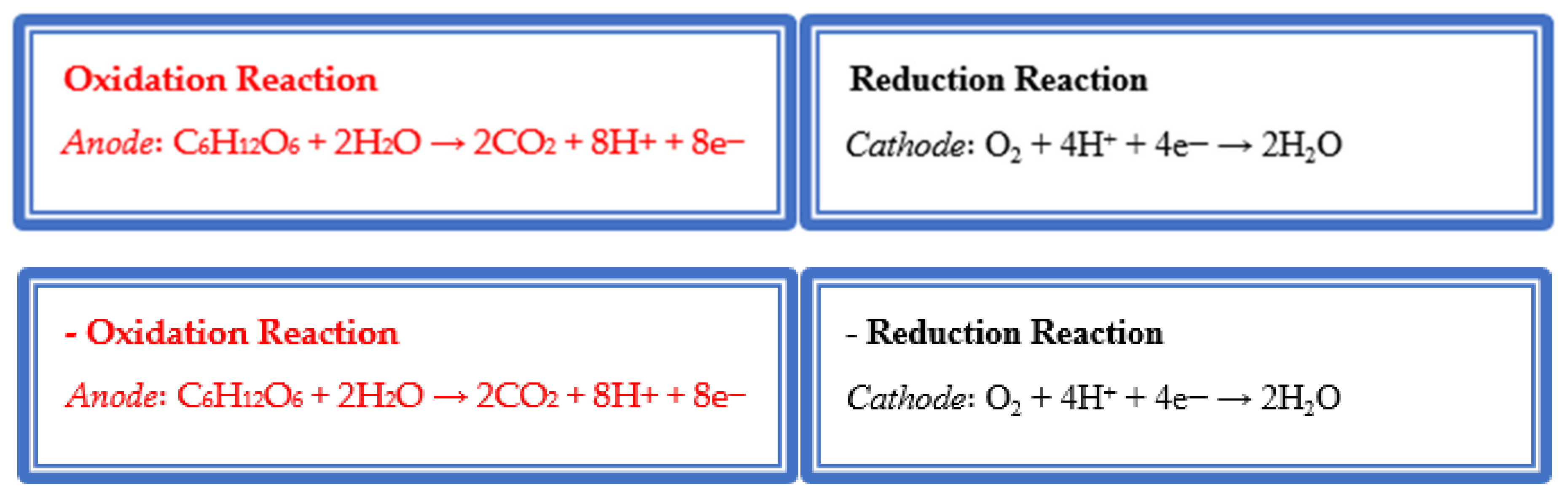
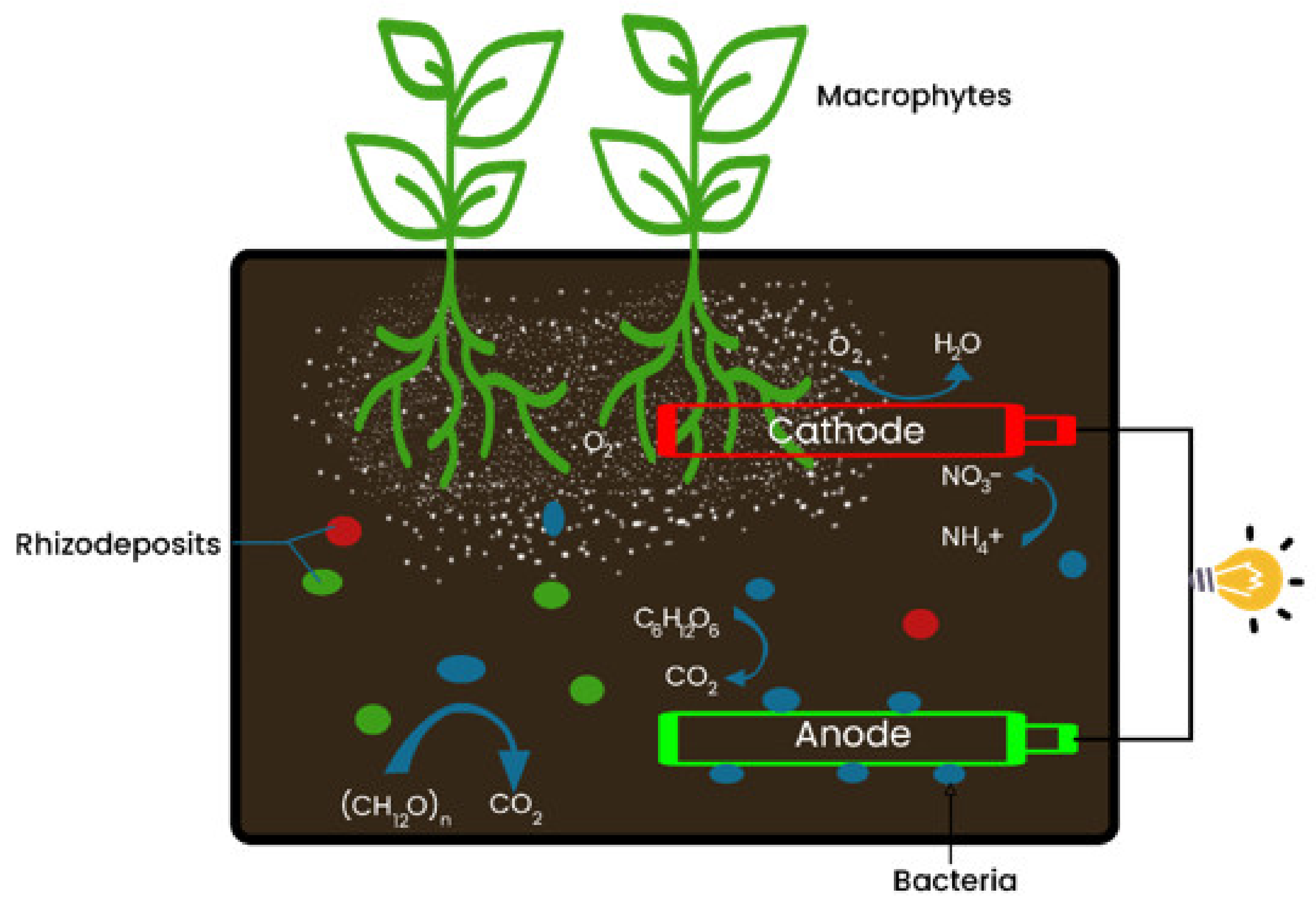
4. The Role of Macrophyte in Bioelectricity Generation
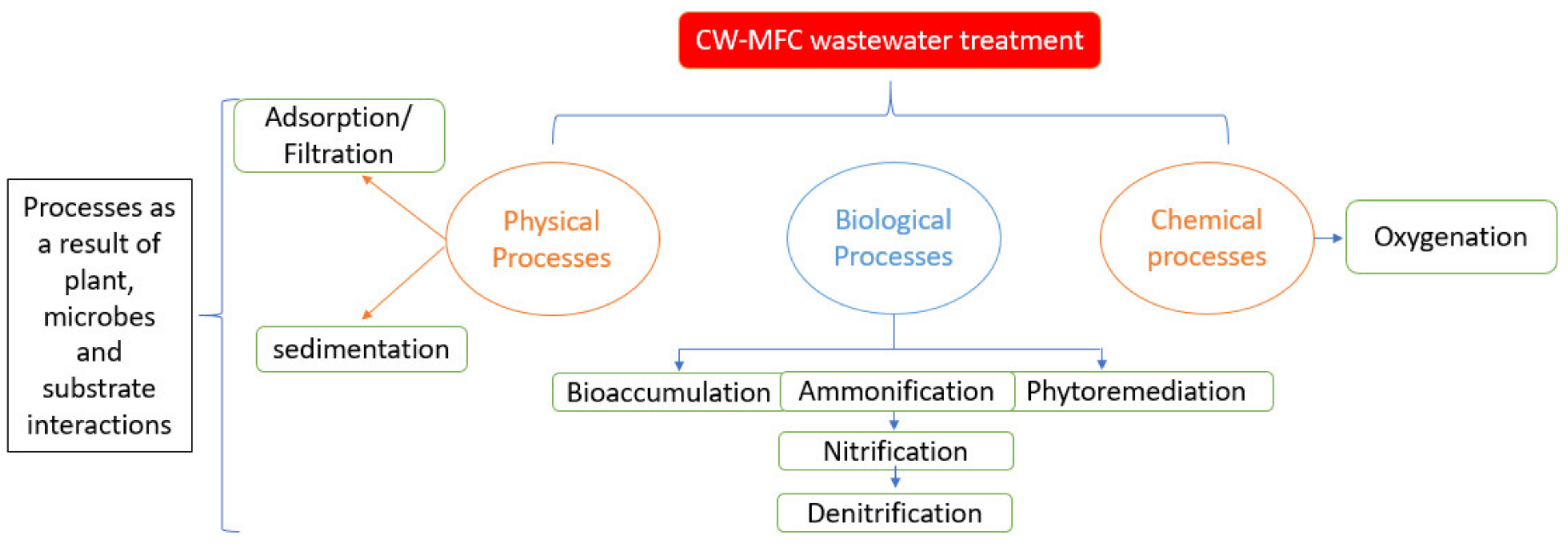
5. Role of Macrophyte in CW-MFC Contaminant Removal
6. Future Perspective
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, H.M.; Pathak, A.K.; Chopra, K.; Tyagi, V.V.; Anand, S.; Kothari, R. Microbial fuel cells: A sustainable solution for bioelectricity generation and wastewater treatment. Biofuels 2019, 10, 11–31. [Google Scholar] [CrossRef]
- Glover, H.; Guz, E.; Hanewall, C.; Hollander, A.; Kocian, A. Alternative Financing of Water and Wastewater Infrastructure in Rural Communities; United States Department of Agriculture, Rural Development: Washington, DC, USA; Maxwell School of Syracuse University: Syracuse, NY, USA, 2005.
- Liu, H.; Ramnarayanan, R.; Logan, B.E. Production of Electricity during Wastewater Treatment Using a Single Chamber Microbial Fuel Cell. Environ. Sci. Technol. 2004, 38, 2281–2285. [Google Scholar] [CrossRef]
- Gude, V.G. Energy and water autarky of wastewater treatment and power generation systems. Renew. Sustain. Energy Rev. 2015, 45, 52–68. [Google Scholar] [CrossRef]
- Virdis, B.; Freguia, S.; Rozendal, R.A.; Rabaey, K.; Yuan, Z.; Keller, J. Microbial Fuel Cells. In Treatise on Water Science; Elsevier: Amsterdam, The Netherlands, 2011; Volume 4, pp. 641–665. [Google Scholar]
- Das, D. Microbial Fuel Cell—A Bioelectrochemical System that Converts Waste to Watts; Springer: Berlin/Heidelberg, Germany, 2018; p. 20. ISBN 978-3-319-66792-8. [Google Scholar]
- Potter, M.C. Electrical effects accompanying the decomposition of organic compounds. Proc. R. Soc. B Biol. Sci. 1911, 84, 260–276. [Google Scholar] [CrossRef]
- Logan, B.E. Microbial Fuel Cells; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Vymazal, J. Constructed Wetlands for Wastewater Treatment. Water 2010, 2, 530–549. [Google Scholar] [CrossRef] [Green Version]
- Yang, E.; Chae, K.-J.; Choi, M.-J.; He, Z.; Kim, I.S. Critical review of bioelectrochemical systems integrated with membrane-based technologies for desalination, energy self-sufficiency, and high-efficiency water and wastewater treatment. Desalination 2019, 452, 40–67. [Google Scholar] [CrossRef]
- Vidal, C.C. Constructed Wetland Microbial Fuel Cells: Electricity Generation, Treatment Efficiency Improvement, COD Bioindication and Clogging Assessment. Ph.D. Thesis, Universitat Politecnica de Catalunya, Barcelona, Spain, 2017. [Google Scholar]
- Wu, H.; Zhang, J.; Ngo, H.H.; Guo, W.; Hu, Z.; Liang, S.; Fan, J.; Liu, H. A review on the sustainability of constructed wetlands for wastewater treatment: Design and operation. Bioresour. Technol. 2015, 175, 594–601. [Google Scholar] [CrossRef]
- Scholz, M.; Lee, B. Constructed wetlands: A review. Int. J. Environ. Stud. 2005, 62, 421–447. [Google Scholar] [CrossRef]
- Yadav, A.K.; Srivastava, P.; Kumar, N.; Abbassi, R.; Mishra, B.K. Constructed Wetland-Microbial Fuel Cell: An Emerging Integrated Technology for Potential Industrial Wastewater Treatment and Bio-Electricity Generation. Constr. Wetl. Ind. Wastewater Treat. 2018, 493–510. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Y.; Xu, L.; Wang, W.; Doherty, L.; Tang, C.; Ren, B.; Zhao, J. Constructed wetland integrated microbial fuel cell system: Looking back, moving forward. Water Sci. Technol. 2017, 76, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.K.; Dash, P.; Mohanty, A.; Abbassi, R.; Mishra, B.K. Performance assessment of innovative constructed wetland-microbial fuel cell for electricity production and dye removal. Ecol. Eng. 2012, 47, 126–131. [Google Scholar] [CrossRef]
- Brix, H.; Schierup, H.H. The Use of Aquatic Macrophytes in Water-Pollution Control. Ambio 1989, 28, 100–107. [Google Scholar]
- Guadarrama-Pérez, O.; Gutiérrez-Macías, T.; García-Sánchez, L.; Guadarrama-Pérez, V.H.; Estrada-Arriaga, E.B. Recent advances in constructed wetland-microbial fuel cells for simultaneous bioelectricity production and wastewater treatment: A review. Int. J. Energy Res. 2019, 43, 5106–5127. [Google Scholar] [CrossRef]
- Brisson, J.; Chazarenc, F. Maximizing pollutant removal in constructed wetlands: Should we pay more attention to macrophyte species selection? Sci. Total Environ. 2009, 407, 3923–3930. [Google Scholar] [CrossRef]
- Srivastava, P.; Abbassi, R.; Yadav, A.K.; Garaniya, V.; Asadnia, M. A review on the contribution of electron flow in electroactive wetlands: Electricity generation and enhanced wastewater treatment. Chemosphere 2020, 254, 126926. [Google Scholar] [CrossRef]
- Jingyu, H.; Miwornunyuie, N.; Ewusi-Mensah, D.; Koomson, D. Assessing the factors influencing the performance of constructed wetland–microbial fuel cell integration. Water Sci. Technol. 2020, 81, 631–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doherty, L.; Zhao, Y.; Zhao, X.; Hu, Y.; Hao, X.; Xu, L.; Liu, R. A review of a recently emerged technology: Constructed wetland–microbial fuel cells. Water Res. 2015, 85, 38–45. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, X.; Ning, X.; Yang, Q. Research progress of microbial fuel cell and constructed wetland coupling system. IOP Conf. Ser. Earth Environ. Sci. 2018, 199, 052014. [Google Scholar] [CrossRef]
- Srivastava, P.; Yadav, A.K.; Garaniya, V.; Abbassi, R. Constructed Wetland Coupled Microbial Fuel Cell Technology. In Microbial Electrochemical Technology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1021–1036. [Google Scholar]
- Sierra, M.A.; Esteve Núñez, A.; Salas Rodriguez, J.J. Integrating Microbial Electrochemical Systems in Constructed Wetlands, a New Paradigm for Treating Wastewater in Small Communities. Ph.D. Thesis, Universidad de Alcalá, Madrid, Spain, 2017; pp. 100–165. [Google Scholar]
- Yang, Y.; Zhao, Y.; Liu, R.; Morgan, D. Global development of various emerged substrates utilized in constructed wetlands. Bioresour. Technol. 2018, 261, 441–452. [Google Scholar] [CrossRef]
- Yan, D.; Song, X.; Weng, B.; Yu, Z.; Bi, W.; Wang, J. Bioelectricity generation from air-cathode microbial fuel cell connected to constructed wetland. Water Sci. Technol. 2018, 78, 1990–1996. [Google Scholar] [CrossRef]
- Fang, Z.; Cheng, S.; Wang, H.; Cao, X.; Li, X. Feasibility study of simultaneous azo dye decolorization and bioelectricity generation by microbial fuel cell-coupled constructed wetland: Substrate effects. RSC Adv. 2017, 7, 16542–16552. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, P.; Yadav, A.K.; Mishra, B.K. The effects of microbial fuel cell integration into constructed wetland on the performance of constructed wetland. Bioresour. Technol. 2015, 195, 223–230. [Google Scholar] [CrossRef]
- Araneda, I.; Tapia, N.F.; Allende, K.L.; Vargas, I.T. Constructed Wetland-Microbial Fuel Cells for Sustainable Greywater Treatment. Water 2018, 10, 940. [Google Scholar] [CrossRef] [Green Version]
- Kalathil, S.; Patil, S.A.; Pant, D. Microbial Fuel Cells: Electrode Materials; Elsevier Inc.: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Doherty, L.; Zhao, X.; Zhao, Y.; Wang, W. The effects of electrode spacing and flow direction on the performance of microbial fuel cell-constructed wetland. Ecol. Eng. 2015, 79, 8–14. [Google Scholar] [CrossRef]
- Doherty, L.; Zhao, Y.; Zhao, X.; Wang, W. Nutrient and organics removal from swine slurry with simultaneous electricity generation in an alum sludge-based constructed wetland incorporating microbial fuel cell technology. Chem. Eng. J. 2015, 266, 74–81. [Google Scholar] [CrossRef]
- Fang, Z.; Song, H.-L.; Cang, N.; Li, X.-N. Performance of microbial fuel cell coupled constructed wetland system for decolorization of azo dye and bioelectricity generation. Bioresour. Technol. 2013, 144, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Stottmeister, U.; Wießner, A.; Kuschk, P.; Kappelmeyer, U.; Kästner, M.; Bederski, O.; Müller, R.; Moormann, H. Effects of plants and microorganisms in constructed wetlands for wastewater treatment. Biotechnol. Adv. 2003, 22, 93–117. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Li, X.; Han, B. A labscale study on constructed wetland microbial fuel cell. Acta Sci. Circumstantiae 2017, 37, 3656–3663. [Google Scholar]
- Zhai, X.; Piwpuan, N.; Arias, C.A.; Headley, T.; Brix, H. Can root exudates from emergent wetland plants fuel denitrification in subsurface flow constructed wetland systems? Ecol. Eng. 2013, 61, 555–563. [Google Scholar] [CrossRef]
- Guang, L.; Koomson, D.A.; Jingyu, H.; Ewusi-Mensah, D.; Miwornunyuie, N.; Miwornunyuie, N. Performance of Exoelectrogenic Bacteria Used in Microbial Desalination Cell Technology. Int. J. Environ. Res. Public Health 2020, 17, 1121. [Google Scholar] [CrossRef] [Green Version]
- ElZein, Z.; Abdou, A.; ElGawad, I.A. Constructed Wetlands as a Sustainable Wastewater Treatment Method in Communities. Procedia Environ. Sci. 2016, 34, 605–617. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Sun, L.; Wan, J.; Shen, L.; Yu, Y.; Hu, L.; Zhou, Y. Performance of different macrophytes in the decontamination of and electricity generation from swine wastewater via an integrated constructed wetland-microbial fuel cell process. J. Environ. Sci. 2020, 89, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Colares, G.S.; Dell’Osbel, N.; Barbosa, C.V.; Lutterbeck, C.; Oliveira, G.A.; Rodrigues, L.R.; Bergmann, C.P.; Lopez, D.R.; Rodriguez, A.L.; Vymazal, J.; et al. Floating treatment wetlands integrated with microbial fuel cell for the treatment of urban wastewaters and bioenergy generation. Sci. Total Environ. 2021, 766, 142474. [Google Scholar] [CrossRef]
- Di, L.; Li, Y.; Nie, L.; Wang, S.; Kong, F. Influence of plant radial oxygen loss in constructed wetland combined with microbial fuel cell on nitrobenzene removal from aqueous solution. J. Hazard. Mater. 2020, 394, 122542. [Google Scholar] [CrossRef] [PubMed]
- Alufasi, R.; Gere, J.; Chakauya, E.; Lebea, P.; Parawira, W.; Chingwaru, W. Mechanisms of pathogen removal by macrophytes in constructed wetlands. Environ. Technol. Rev. 2017, 6, 135–144. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, J.; Liu, D.; Hu, Z.; Liu, H. Enhance performance of microbial fuel cell coupled surface flow constructed wetland by using submerged plants and enclosed anodes. Chem. Eng. J. 2018, 351, 312–318. [Google Scholar] [CrossRef]
- Wang, J.; Song, X.; Wang, Y.; Bai, J.; Bai, H.; Yan, D.; Cao, Y.; Li, Y.; Yu, Z.; Dong, G. Bioelectricity generation, contaminant removal and bacterial community distribution as affected by substrate material size and aquatic macrophyte in constructed wetland-microbial fuel cell. Bioresour. Technol. 2017, 245, 372–378. [Google Scholar] [CrossRef]
- Wang, J.; Song, X.; Wang, Y.; Bai, J.; Li, M.; Dong, G.; Lin, F.; Lv, Y.; Yan, D. Bioenergy generation and rhizodegradation as affected by microbial community distribution in a coupled constructed wetland-microbial fuel cell system associated with three macrophytes. Sci. Total Environ. 2017, 607–608, 53–62. [Google Scholar] [CrossRef]
- Vymazal, J. Plants used in constructed wetlands with horizontal subsurface flow: A review. Hydrobiologia 2011, 674, 133–156. [Google Scholar] [CrossRef]
- Liu, S.; Song, H.; Li, X.; Yang, F. Power Generation Enhancement by Utilizing Plant Photosynthate in Microbial Fuel Cell Coupled Constructed Wetland System. Int. J. Photoenergy 2013, 2013, 172010. [Google Scholar] [CrossRef]
- Gude, V.G. Microbial Fuel Cells for Wastewater Treatment and Energy Generation Wastewater treatment in microbial fuel cells e an overview. J. Clean. Prod. 2018, 122, 287–307. [Google Scholar] [CrossRef]
- Clauwaert, P.; Rabaey, K.; Aelterman, P.; de Schamphelaire, L.; Pham, T.H.; Boeckx, P.; Boon, N.; Verstraete, W. Biological Denitrification in Microbial Fuel Cells. Environ. Sci. Technol. 2007, 41, 3354–3360. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, D.A.; Ghadge, A.N.; Mondal, D.; Ghangrekar, M.M. Comparison of oxygen and hypochlorite as cathodic electron acceptor in microbial fuel cells. Bioresour. Technol. 2014, 154, 330–335. [Google Scholar] [CrossRef]
- Xu, L.; Zhao, Y.; Doherty, L.; Hu, Y.; Hao, X. The integrated processes for wastewater treatment based on the principle of microbial fuel cells: A review. Crit. Rev. Environ. Sci. Technol. 2016, 46, 60–91. [Google Scholar] [CrossRef]
- Guo, L.; He, K.; Wu, S.; Sun, H.; Wang, Y.; Huang, X.; Dong, R. Optimization of high-rate TN removal in a novel constructed wetland integrated with microelectrolysis system treating high-strength digestate supernatant. J. Environ. Manag. 2016, 178, 42–51. [Google Scholar] [CrossRef]
- Oon, Y.-L.; Ong, S.-A.; Ho, L.-N.; Wong, Y.-S.; Dahalan, F.A.; Lehl, H.; Thung, W.-E.; Nordin, N. Role of macrophyte and effect of supplementary aeration in up-flow constructed wetland-microbial fuel cell for simultaneous wastewater treatment and energy recovery. Bioresour. Technol. 2017, 224, 265–275. [Google Scholar] [CrossRef]
- Saba, B.; Khan, M.; Christy, A.D.; Veno, B. Bioelectrochemistry Microbial phyto-power systems—A sustainable integration of phytoremediation and microbial fuel cells. Bioelectrochemistry 2019, 127, 1–11. [Google Scholar] [CrossRef]
- Corbella, C.; Garfí, M.; Puigagut, J. Vertical redox profiles in treatment wetlands as function of hydraulic regime and macrophytes presence: Surveying the optimal scenario for microbial fuel cell implementation. Sci. Total Environ. 2014, 470–471, 754–758. [Google Scholar] [CrossRef]
- Wang, Q.; Hu, Y.; Xie, H.; Yang, Z. Constructed Wetlands: A Review on the Role of Radial Oxygen Loss in the Rhizosphere by Macrophytes. Water 2018, 10, 678. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Huang, Y.-C.; Liang, J.-H.; Zhao, F.; Zhu, Y.-G. A novel sediment microbial fuel cell with a biocathode in the rice rhizosphere. Bioresour. Technol. 2012, 108, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Zhu, H.; Yan, B.; Xu, Y.; Shutes, B. Treatment of typical antibiotics in constructed wetlands integrated with microbial fuel cells: Roles of plant and circuit operation mode. Chemosphere 2020, 250, 126252. [Google Scholar] [CrossRef] [PubMed]
- Guadarrama-Pérez, O.; Bahena-Rabadan, K.Y.; Dehesa-Carrasco, U.; Pérez, V.H.G.; Estrada-Arriaga, E.B. Bioelectricity production using shade macrophytes in constructed wetlands-microbial fuel cells. Environ. Technol. 2020, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xu, D.; Xiao, E.; Xu, D.; Xu, P.; Zhang, X.; Zhou, Q.; He, F.; Wu, Z. Relationship between electrogenic performance and physiological change of four wetland plants in constructed wetland-microbial fuel cells during non-growing seasons. J. Environ. Sci. 2018, 70, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Greenway, M. The Role of Macrophytes in Nutrient Removal using Constructed Wetlands. Environ. Bioremediat. Technol. 2007, 331–351. [Google Scholar] [CrossRef]
- Vymazal, J. Removal of nutrients in various types of constructed wetlands. Sci. Total Environ. 2007, 380, 48–65. [Google Scholar] [CrossRef]
- Wen, H.; Zhu, H.; Yan, B.; Shutes, B.; Yu, X.; Cheng, R.; Chen, X.; Wang, X. Constructed wetlands integrated with microbial fuel cells for COD and nitrogen removal affected by plant and circuit operation mode. Environ. Sci. Pollut. Res. 2021, 28, 3008–3018. [Google Scholar] [CrossRef]
- Chiranjeevi, P.; Yeruva, D.K.; Kumar, A.K.; Mohan, S.V.; Varjani, S. Plant-microbial fuel cell technology. Microb. Electrochem. Technol. 2019, 549–564. [Google Scholar] [CrossRef]
- Oodally, A.; Gulamhussein, M.; Randall, D.G. Investigating the performance of constructed wetland microbial fuel cells using three indigenous South African wetland plants. J. Water Process Eng. 2019, 32, 100930. [Google Scholar] [CrossRef]
- Zhao, Y.; Collum, S.; Phelan, M.; Goodbody, T.; Doherty, L.; Hu, Y. Preliminary investigation of constructed wetland incorporating microbial fuel cell: Batch and continuous flow trials. Chem. Eng. J. 2013, 229, 364–370. [Google Scholar] [CrossRef] [Green Version]
- Villaseñor, J.; Capilla, P.; Rodrigo, M.A.; Cañizares, P.; Fernández, F. Operation of a horizontal subsurface flow constructed wetland–microbial fuel cell treating wastewater under different organic loading rates. Water Res. 2013, 47, 6731–6738. [Google Scholar] [CrossRef]
- Fang, Z.; Song, H.-L.; Cang, N.; Li, X.-N. Electricity production from Azo dye wastewater using a microbial fuel cell coupled constructed wetland operating under different operating conditions. Biosens. Bioelectron. 2015, 68, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Oon, Y.-L.; Ong, S.-A.; Ho, L.-N.; Wong, Y.-S.; Lehl, H.; Thung, W.-E. Hybrid system up-flow constructed wetland integrated with microbial fuel cell for simultaneous wastewater treatment and electricity generation. Bioresour. Technol. 2015, 186, 270–275. [Google Scholar] [CrossRef]
- Oon, Y.-L.; Ong, S.-A.; Ho, L.-N.; Wong, Y.-S.; Dahalan, F.A.; Lehl, H.; Thung, W.-E. Synergistic effect of up-flow constructed wetland and microbial fuel cell for simultaneous wastewater treatment and energy recovery. Bioresour. Technol. 2016, 203, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Corbella, C.; Garfí, M.; Puigagut, J. Long-term assessment of best cathode position to maximise microbial fuel cell performance in horizontal subsurface flow constructed wetlands. Sci. Total Environ. 2016, 563–564, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.; Dwivedi, S.; Kumar, N.; Abbassi, R.; Garaniya, V.; Yadav, A.K. Performance assessment of aeration and radial oxygen loss assisted cathode based integrated constructed wetland-microbial fuel cell systems. Bioresour. Technol. 2017, 244, 1178–1182. [Google Scholar] [CrossRef]
- Song, H.; Zhang, S.; Long, X.; Yang, X.; Li, H.; Xiang, W. Optimization of Bioelectricity Generation in Constructed Wetland-Coupled Microbial Fuel cell Systems. Water 2017, 9, 185. [Google Scholar] [CrossRef]
- Xu, F.; Cao, F.-Q.; Kong, Q.; Zhou, L.-L.; Yuan, Q.; Zhu, Y.-J.; Wang, Q.; Du, Y.-D.; Wang, Z.-D. Electricity production and evolution of microbial community in the constructed wetland-microbial fuel cell. Chem. Eng. J. 2018, 339, 479–486. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, Y.; Tang, C.; Liu, R.; Chen, T. Dual role of macrophytes in constructed wetland-microbial fuel cells using pyrrhotite as cathode material: A comparative assessment. Chemosphere 2021, 263, 128354. [Google Scholar] [CrossRef]
- Moqsud, M.; Yoshitake, J.; Bushra, Q.; Hyodo, M.; Omine, K.; Strik, D. Compost in plant microbial fuel cell for bioelectricity generation. Waste Manag. 2015, 36, 63–69. [Google Scholar] [CrossRef]
- Saz, Ç.; Türe, C.; Türker, O.C.; Yakar, A. Effect of vegetation type on treatment performance and bioelectric production of constructed wetland modules combined with microbial fuel cell (CW-MFC) treating synthetic wastewater. Environ. Sci. Pollut. Res. 2018, 25, 8777–8792. [Google Scholar] [CrossRef]
- Vogelmann, S.; Awe, G.O.; Prevedello, J. Selection of plant species used in wastewater treatment. Water Air Soil Pollut. 2017, 9–18. Available online: https://www.researchgate.net/publication/313874379 (accessed on 18 July 2021).
- Białowiec, A.; Albuquerque, A.; Randerson, P.F. The influence of evapotranspiration on vertical flow subsurface constructed wetland performance. Ecol. Eng. 2014, 67, 89–94. [Google Scholar] [CrossRef]
| Macrophyte | Initial COD (mg/L) | COD Removal (%) | HRT (hr) | Max. Power | Author |
|---|---|---|---|---|---|
| Canna indica | 1500 | 74.9 | 96 | 15.7 mW∙m−2 | [16] |
| Phragmites australis | 1058 | 76.5 | N. A | 9.4 mW∙m−2 | [67] |
| Ipomoea aquatica | 180 | 86 | 72 | 0.302 W∙m−3 | [34] |
| Phragmites australis | 250 | 80–100 | N. A | 0.15 mW∙m−2 | [68] |
| Ipomoea aquatica | 193–205 | 94.8 | 48 | 12.42 mW∙m−2 | [48] |
| Ipomoea aquatica | 300 | 72.5 | 72 | 0.852 W∙m−3 | [69] |
| Phragmites australis | 411–854 | 64 | N. A | 0.268 W∙m−3 | [32] |
| Typha latifolia | 314.8 | 100 | N. A | 6.12 mW∙m−2 | [70] |
| Phragmites australis | 583 | 64 | N. A | 0.276 W∙m−3 | [33] |
| Taifa latifolia | 624 | 99 | 24 | 93 mW∙m−3 | [71] |
| Phragmite australis | 323 | 60.6 | 62.4 | 131 mW∙m−2 | [72] |
| Elodea nuttallii | 643 | 97–98 | 24 | 184.75 mW∙m−3 | [54] |
| Canna indica | -- | 78.71 | 72 | 31.04 mW∙m−3 | [73] |
| Phragmites australis | 200 | 90.45 | 48 | 0.20 W∙m−3 | [74] |
| Phragmites australis | -- | 82 | 72 | 3714 mW∙m−2 | [75] |
| Macrophyte Properties | Relevance in CWMFC |
|---|---|
| rapid growth and high biomass production | For winter insulation in cold and temperate regions, and particularly for the removal of nutrients by harvesting, as nutrients are absorbed by macrophytes to build their biomass [47]. In addition, according to Yang et al. [76], species with high biomass production in CWMFC enhance the cell voltage and reduce the internal resistance of the system, which often results in higher bioenergy production. |
| good natural adaptation to the local climate | Native species should be best preferred. According to Sierra et al. [25], CWMFC plants are selected based on the region’s most common aquatic plants. Oodally et al. [66], also concluded that native species are best preferred due to their local climate adaptability. In their experimentation, the most common aquatic plants in the region showed improved performance in CWMFC than exotic species. |
| good root development | To provide a substrate for attached bacteria and oxygenation [46,47]. Additionally, the root development or maturity of the wetland plant affects oxygen release. In a sediment microbial fuel cell (SMFC) with wetland plant experiments conducted by Chen et al. [58], their investigation has shown that young roots can excrete more oxygen than mature or aging species. Similarly, Colares et al. [41] also observed that plant species with good root development produced better oxygen, which presented the highest voltage value, compared to plants with smaller poor root systems. In addition, Moqsud et al. [77] operated a series of 6-CWMFC using Oriza sativa species. In their experimentation, they observed a reduction in power production as plants attained maturation. This was mainly because the maturation of the plant affected both oxygen release and exudate production. This signifies that the maturity of the root and its development is an essential factor in wetland plant selection [58]. |
| High oxygen transfer capacity | Oxygen transfer capacity from the roots creates an aerobic environment. Due to the great diversity of flora, different species have different radial oxygen loss (ROL) [25]. |
| nutrient absorption capacity | High nutrient absorption capacity helps in the effective removal of contaminants from the system. Species with high NAC use absorbed nutrients as a resource for their metabolism and growth [48,62]. |
| adaptation and ease of propagation | The ease in obtaining seedlings, seeds, or vegetative propagules must be well considered to ensure system sustainability. |
| Good Rhizodeposition; release of carbon sources as rhizodeposits from plant roots. | Rhizodeposition supports the growth and activities of microorganisms associated with bioelectricity production [78]. |
| C4 Plants | The photosynthetic activity of plants is categorized into three phases: C3, C4, and CAM. In terms of oxygen production and CO2 fixation, plants in each category have different photosynthetic pathways. Plants in the group of C4 are those with more advanced photosynthetic activity than plants in the C3 and CAM groups. Consequently, because they have a higher conversion rate of solar energy into bioelectricity, it is suggested to integrate C4 plants [18]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, K.; Qi, J.; Zhang, F.; Miwornunyuie, N.; Amaniampong, P.S.; Koomson, D.A.; Chen, L.; Yan, Y.; Dong, Y.; Setordjie, V.E.; et al. The Role of Wetland Plants on Wastewater Treatment and Electricity Generation in Constructed Wetland Coupled with Microbial Fuel Cell. Appl. Sci. 2021, 11, 7454. https://doi.org/10.3390/app11167454
Li K, Qi J, Zhang F, Miwornunyuie N, Amaniampong PS, Koomson DA, Chen L, Yan Y, Dong Y, Setordjie VE, et al. The Role of Wetland Plants on Wastewater Treatment and Electricity Generation in Constructed Wetland Coupled with Microbial Fuel Cell. Applied Sciences. 2021; 11(16):7454. https://doi.org/10.3390/app11167454
Chicago/Turabian StyleLi, Ke, Jingyao Qi, Fuguo Zhang, Nicholas Miwornunyuie, Paulette Serwaa Amaniampong, Desmond Ato Koomson, Lei Chen, Yu Yan, Yanhong Dong, Victor Edem Setordjie, and et al. 2021. "The Role of Wetland Plants on Wastewater Treatment and Electricity Generation in Constructed Wetland Coupled with Microbial Fuel Cell" Applied Sciences 11, no. 16: 7454. https://doi.org/10.3390/app11167454
APA StyleLi, K., Qi, J., Zhang, F., Miwornunyuie, N., Amaniampong, P. S., Koomson, D. A., Chen, L., Yan, Y., Dong, Y., Setordjie, V. E., & Samwini, A. M.-n. (2021). The Role of Wetland Plants on Wastewater Treatment and Electricity Generation in Constructed Wetland Coupled with Microbial Fuel Cell. Applied Sciences, 11(16), 7454. https://doi.org/10.3390/app11167454






