Laser Synthesized Graphene and Its Applications
Abstract
:1. Introduction
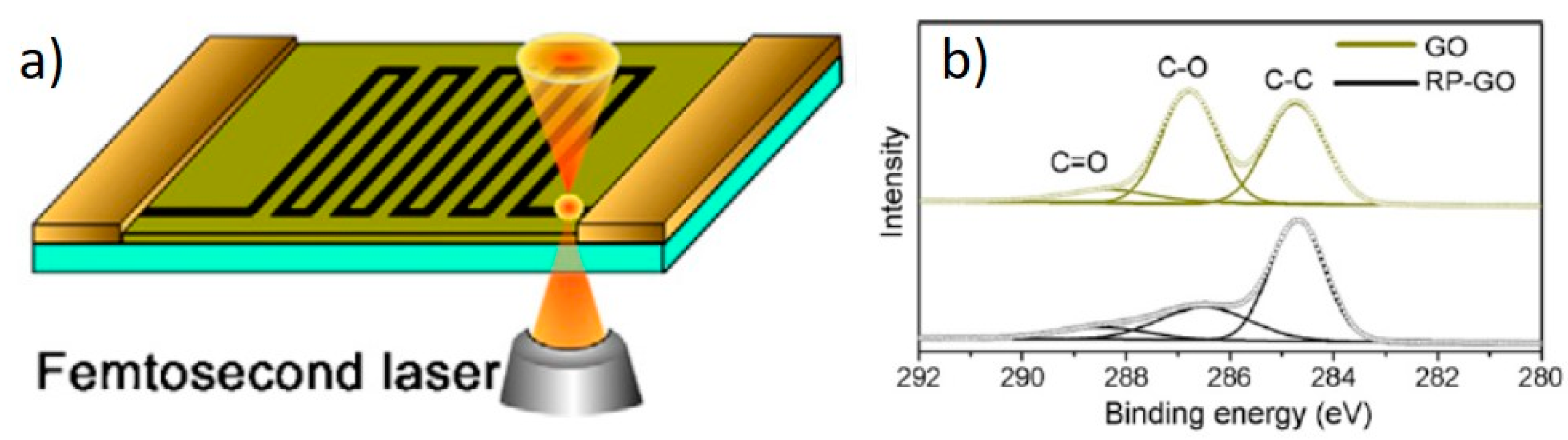
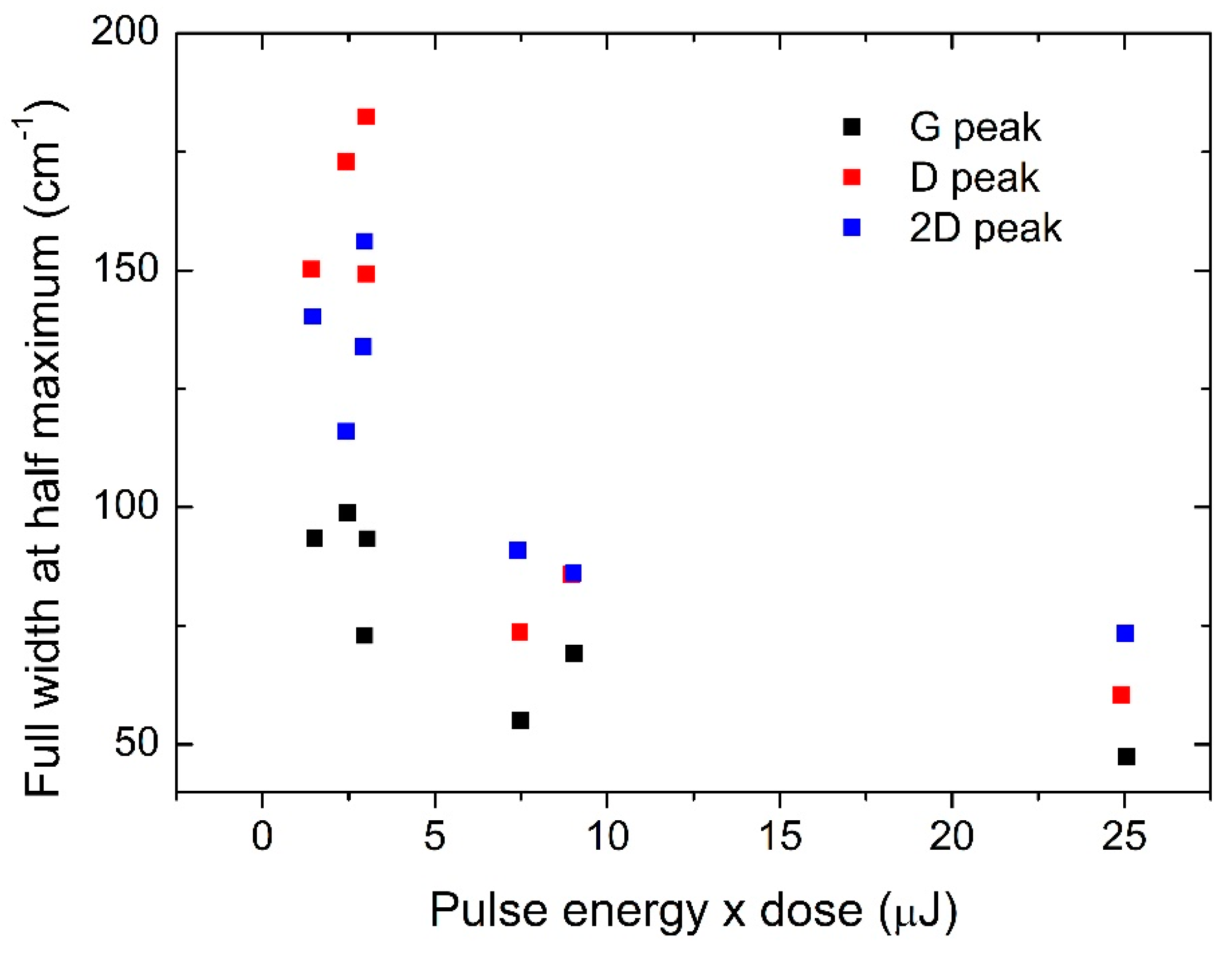
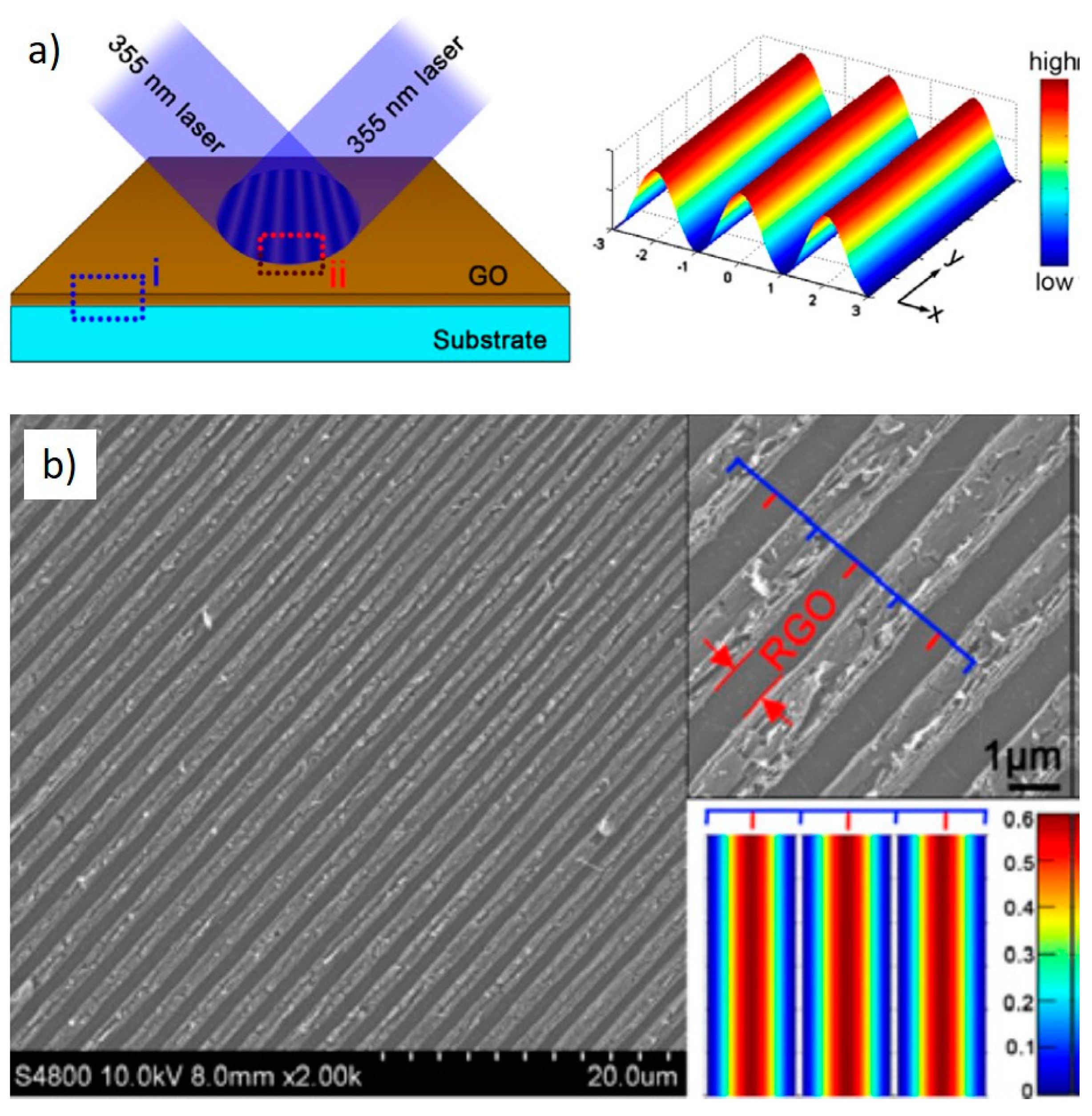
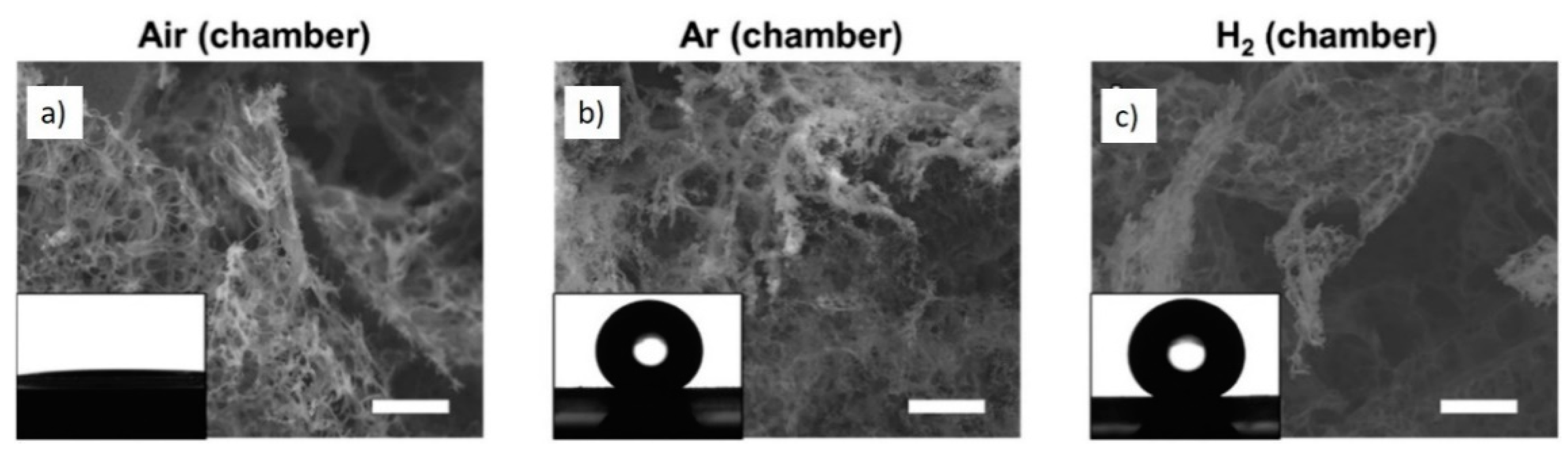
2. Laser Synthesis of Graphene
3. Applications of Laser Synthesized Graphene
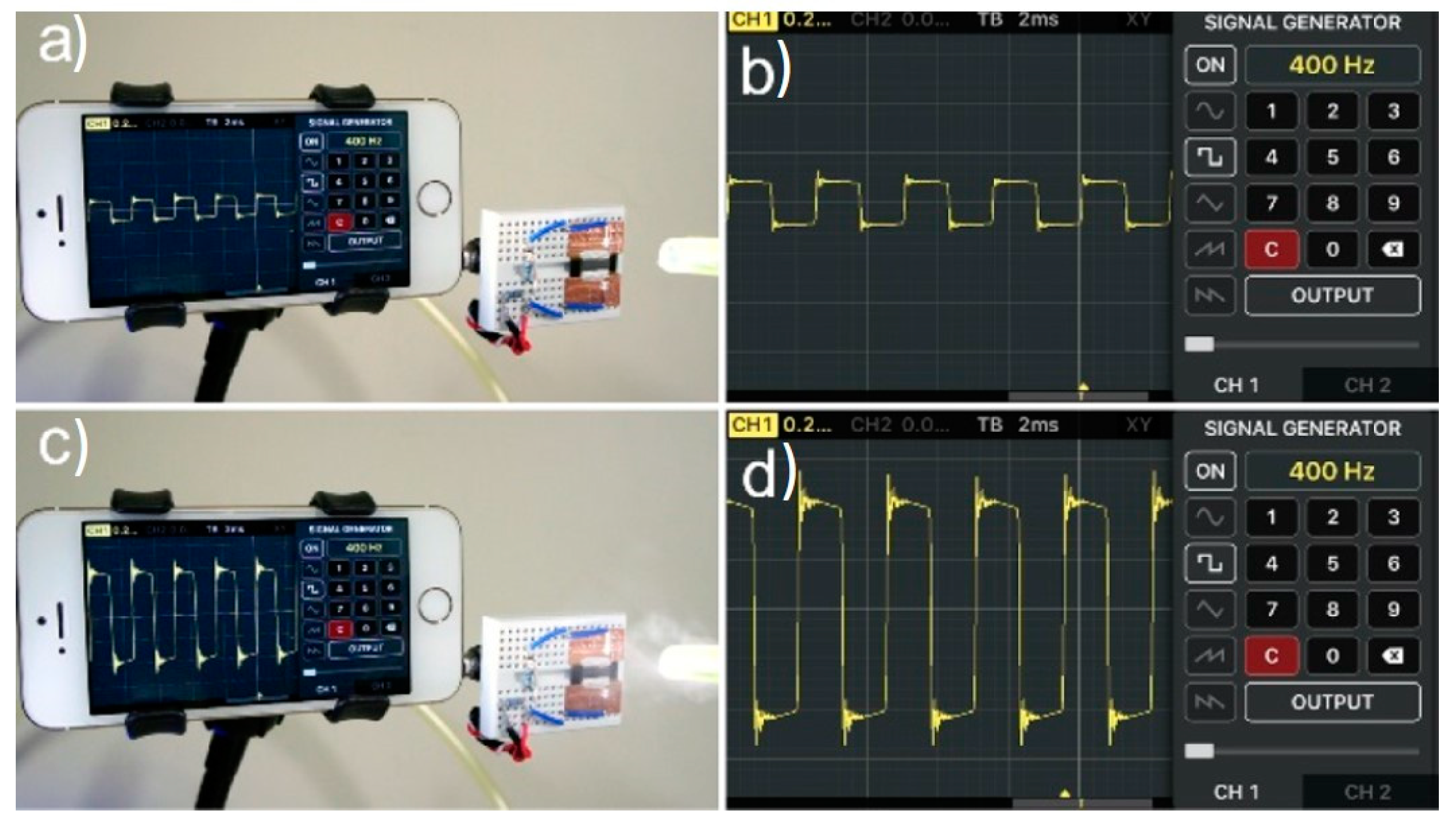
3.1. Humidity Sensing
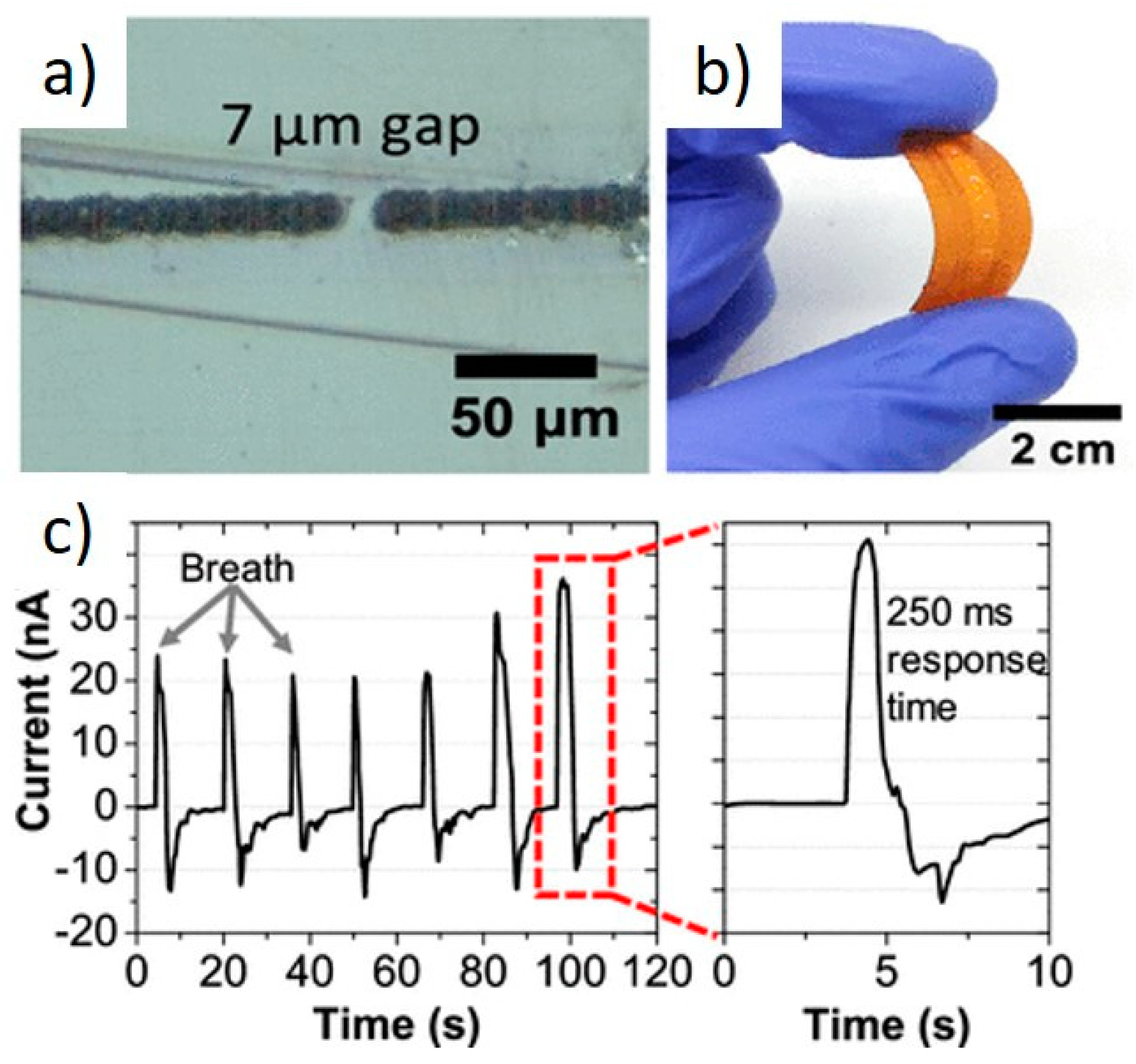
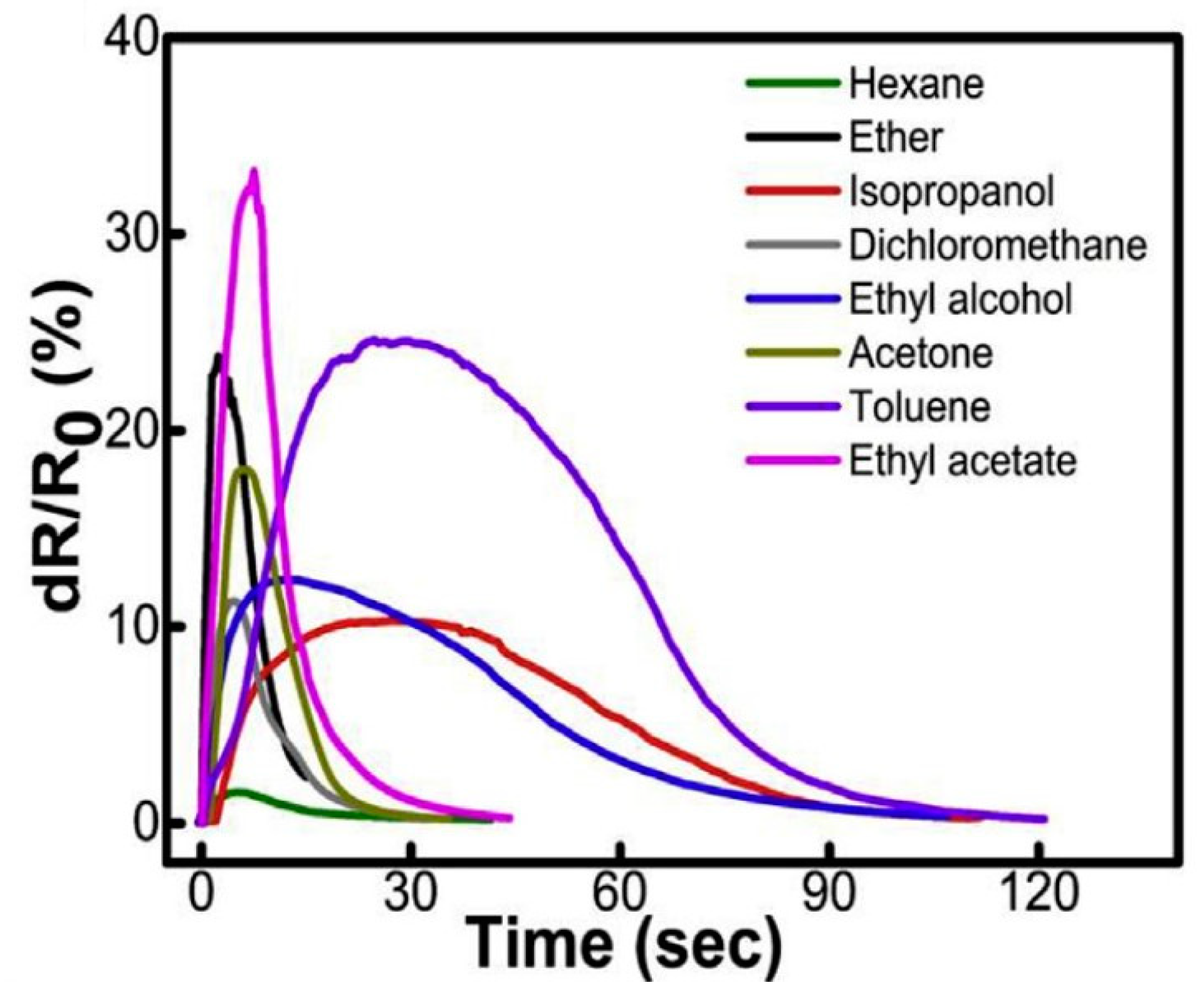
3.2. Gas and Liquid Sensing
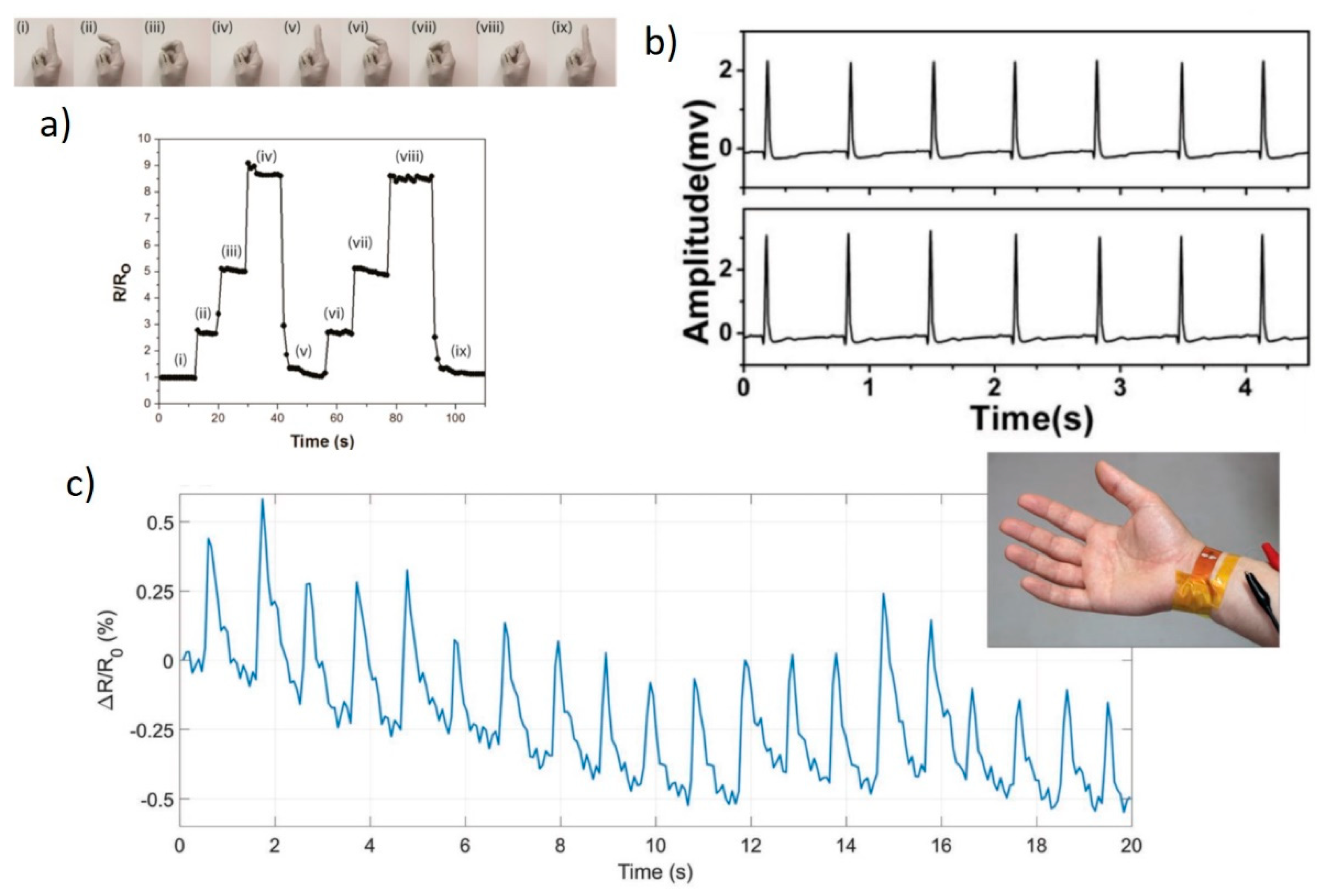
3.3. Strain Sensing
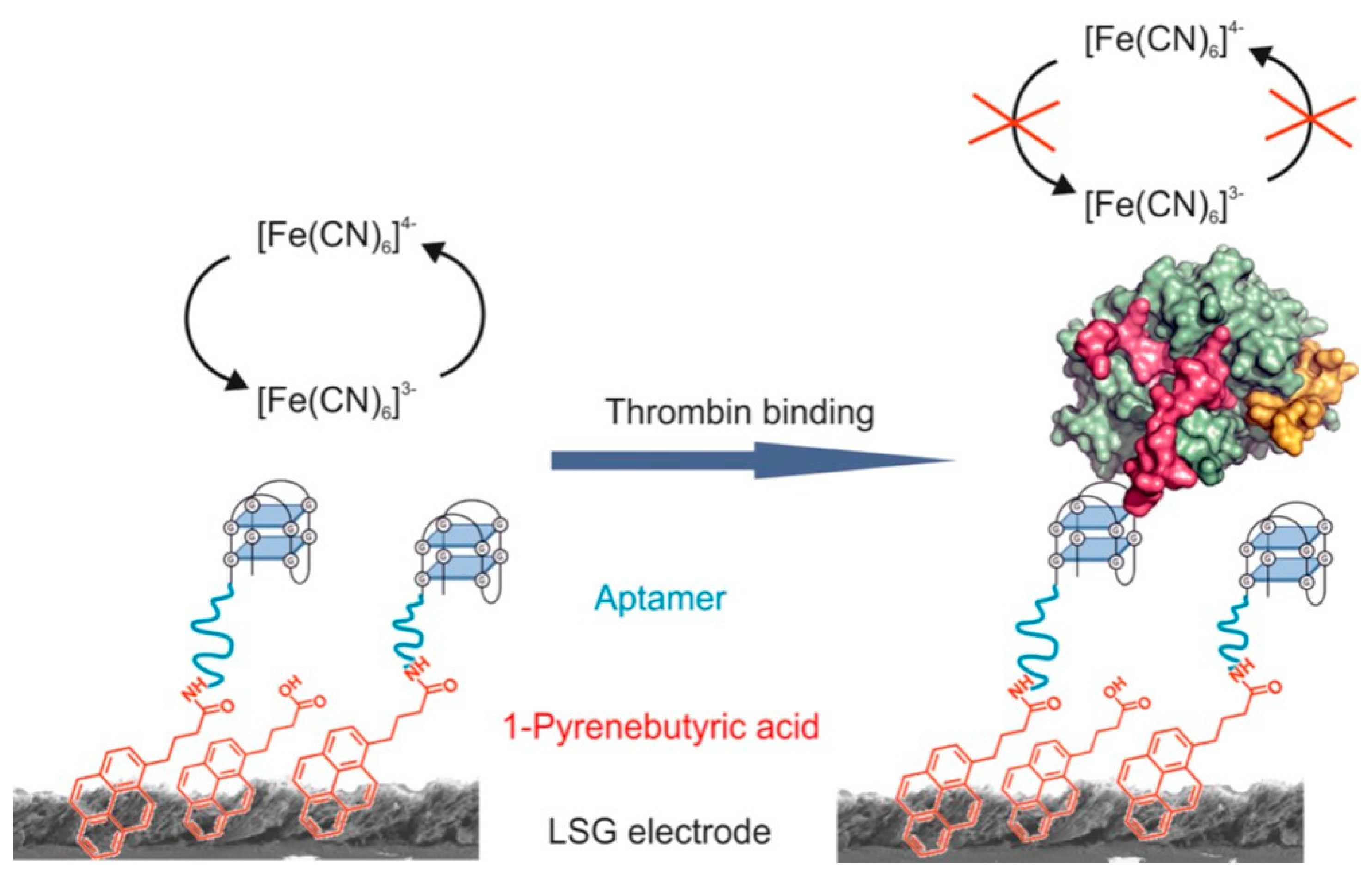
3.4. Biosensing
3.5. Pressure and Temperature Sensing
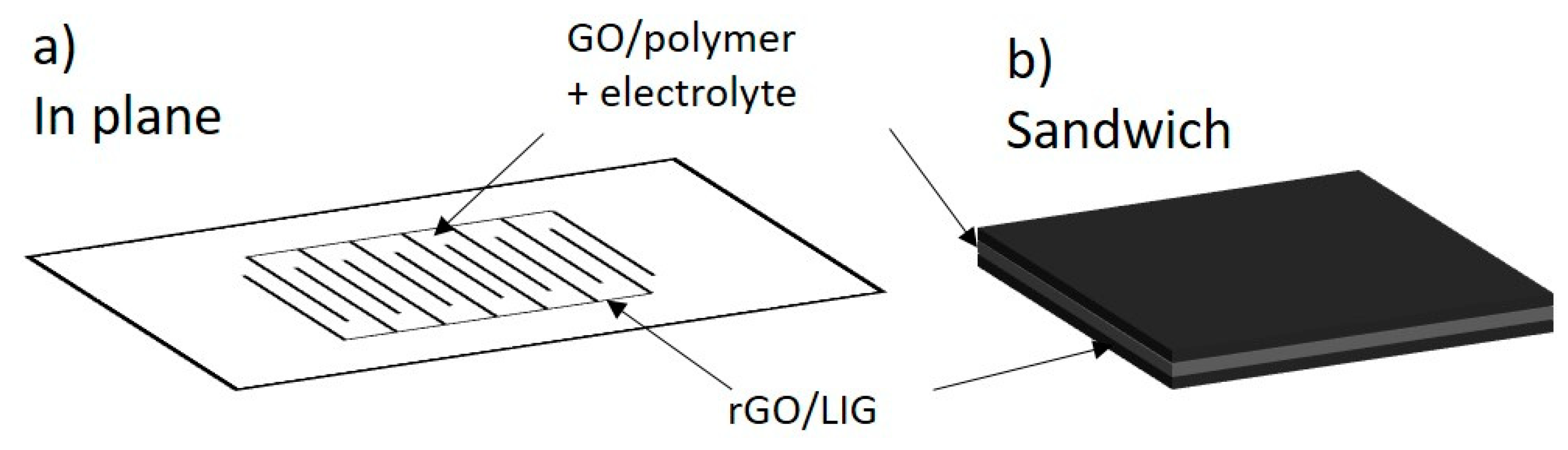
3.6. Energy Storage
3.7. Electrocatalysis
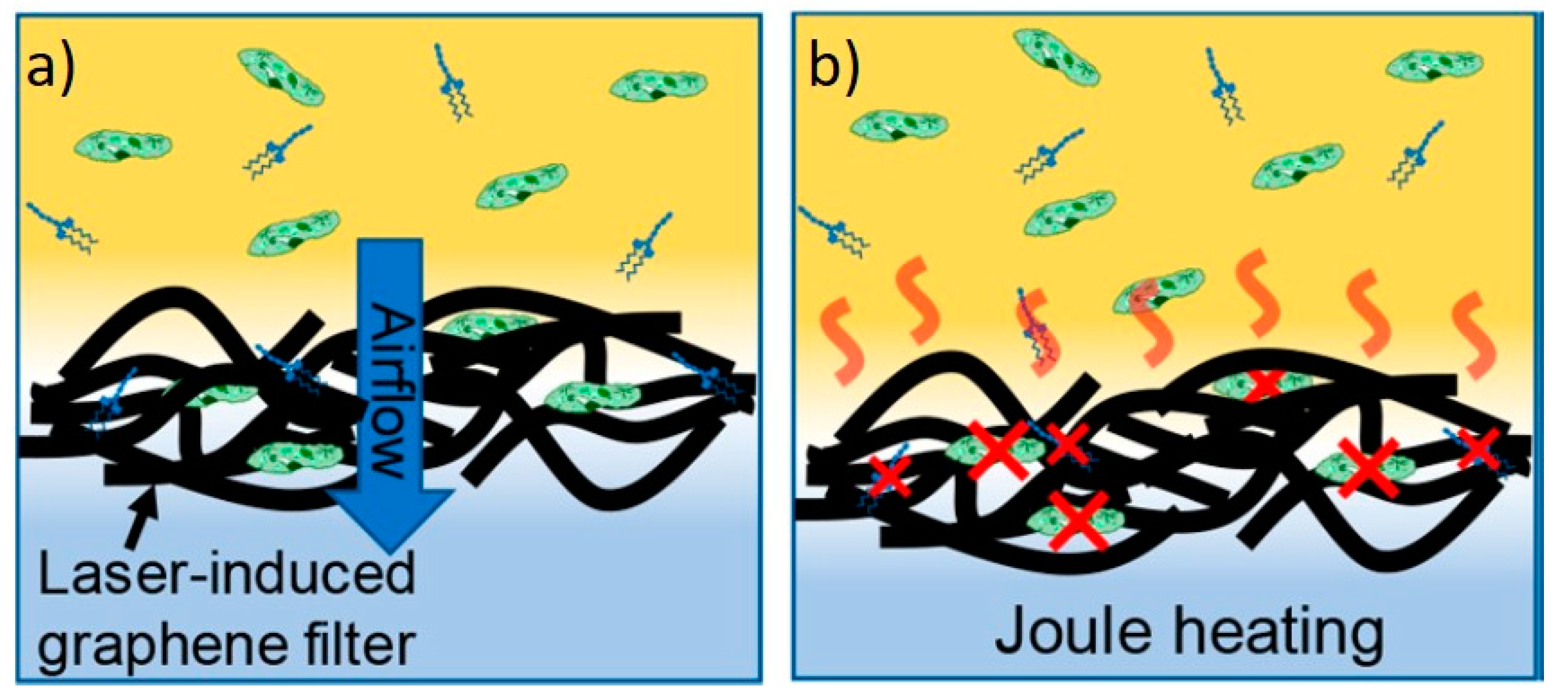
3.8. Environmental Applications
4. Conclusions and Perspectives
Funding
Data Availability Statement
Conflicts of Interest
References
- Novoselov, K.S.; Jiang, D.; Schedin, F.; Booth, T.J.; Khotkevich, V.V.; Morozov, S.V.; Geim, A.K. Two-dimensional atomic crystals. Proc. Natl. Acad. Sci. USA 2005, 102, 10451–10453. [Google Scholar] [CrossRef] [Green Version]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Katsnelson, M.I.; Grigorieva, I.V.; Dubonos, S.V.; Firsov, A.A. Two-dimensional gas of massless Dirac fermions in graphene. Nature 2005, 438, 197–200. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Reina, A.; Jia, X.; Ho, J.; Nezich, D.; Son, H.; Bulovic, V.; Dresselhaus, M.S.; Kong, J. Large Area, Few-Layer Graphene Films on Arbitrary Substrates by Chemical Vapor Deposition. Nano Lett. 2009, 9, 30–35. [Google Scholar] [CrossRef]
- Li, X.; Cai, W.; An, J.; Kim, S.; Nah, J.; Yang, D.; Piner, R.; Velamakanni, A.; Jung, I.; Tutuc, E.; et al. Large-Area Synthesis of High-Quality and Uniform Graphene Films on Copper Foils. Science 2009, 324, 1312. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.S.; Zhao, Y.; Jang, H.; Lee, S.Y.; Kim, J.M.; Kim, K.S.; Ahn, J.-H.; Kim, P.; Choi, J.-Y.; Hong, B.H. Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature 2009, 457, 706–710. [Google Scholar] [CrossRef]
- Bae, S.; Kim, H.; Lee, Y.; Xu, X.; Park, J.-S.; Zheng, Y.; Balakrishnan, J.; Lei, T.; Ri Kim, H.; Song, Y.I.; et al. Roll-to-roll production of 30-inch graphene films for transparent electrodes. Nat. Nanotechnol. 2010, 5, 574–578. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S.; McGovern, I.T.; Holland, B.; Byrne, M.; Gun’Ko, Y.K.; et al. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat. Nanotechnol. 2008, 3, 563–568. [Google Scholar] [CrossRef] [Green Version]
- Lotya, M.; Hernandez, Y.; King, P.J.; Smith, R.J.; Nicolosi, V.; Karlsson, L.S.; Blighe, F.M.; De, S.; Wang, Z.; McGovern, I.T.; et al. Liquid Phase Production of Graphene by Exfoliation of Graphite in Surfactant/Water Solutions. J. Am. Chem. Soc. 2009, 131, 3611–3620. [Google Scholar] [CrossRef] [Green Version]
- Lotya, M.; King, P.J.; Khan, U.; De, S.; Coleman, J.N. High-Concentration, Surfactant-Stabilized Graphene Dispersions. ACS Nano 2010, 4, 3155–3162. [Google Scholar] [CrossRef]
- Bourlinos, A.B.; Georgakilas, V.; Zboril, R.; Steriotis, T.A.; Stubos, A.K.; Trapalis, C. Aqueous-phase exfoliation of graphite in the presence of polyvinylpyrrolidone for the production of water-soluble graphenes. Solid State Commun. 2009, 149, 2172–2176. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef]
- Zhou, Y.; Bao, Q.; Varghese, B.; Tang, L.A.L.; Tan, C.K.; Sow, C.-H.; Loh, K.P. Microstructuring of Graphene Oxide Nanosheets Using Direct Laser Writing. Adv. Mater. 2010, 22, 67–71. [Google Scholar] [CrossRef]
- Ray, S.C. (Ed.) Chapter 2—Application and Uses of Graphene Oxide and Reduced Graphene Oxide. In Applications of Graphene and Graphene-Oxide Based Nanomaterials; William Andrew Publishing: Oxford, UK, 2015; pp. 39–55. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Stankovich, S.; Piner, R.D.; Chen, X.; Wu, N.; Nguyen, S.T.; Ruoff, R.S. Stable aqueous dispersions of graphitic nanoplatelets via the reduction of exfoliated graphite oxide in the presence of poly(sodium 4-styrenesulfonate). J. Mater. Chem. 2006, 16, 155–158. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, L.; Wei, S.; He, Y.; Xia, H.; Chen, Q.; Sun, H.-B.; Xiao, F.-S. Direct imprinting of microcircuits on graphene oxides film by femtosecond laser reduction. Nano Today 2010, 5, 15–20. [Google Scholar] [CrossRef]
- El-Kady, M.F.; Strong, V.; Dubin, S.; Kaner, R.B. Laser Scribing of High-Performance and Flexible Graphene-Based Electrochemical Capacitors. Science 2012, 335, 1326–1330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Zhang, J.B.; Peng, Z.W.; Li, Y.L.; Gao, C.T.; Ji, Y.S.; Ye, R.Q.; Kim, N.D.; Zhong, Q.F.; Yang, Y.; et al. High-Performance Pseudocapacitive Microsupercapacitors from Laser-Induced Graphene. Adv. Mater. 2016, 28, 838–845. [Google Scholar] [CrossRef]
- Lin, J.; Peng, Z.W.; Liu, Y.Y.; Ruiz-Zepeda, F.; Ye, R.Q.; Samuel, E.L.G.; Yacaman, M.J.; Yakobson, B.I.; Tour, J.M. Laser-induced porous graphene films from commercial polymers. Nat. Commun. 2014, 5, 5714. [Google Scholar] [CrossRef]
- Ye, R.; Chyan, Y.; Zhang, J.; Li, Y.; Han, X.; Kittrell, C.; Tour, J.M. Laser-Induced Graphene Formation on Wood. Adv. Mater. 2017, 29, 1702211. [Google Scholar] [CrossRef]
- Algozeeb, W.A.; Savas, P.E.; Luong, D.X.; Chen, W.; Kittrell, C.; Bhat, M.; Shahsavari, R.; Tour, J.M. Flash Graphene from Plastic Waste. ACS Nano 2020, 14, 15595–15604. [Google Scholar] [CrossRef]
- Kaidarova, A.; Kosel, J. Physical Sensors Based on Laser-Induced Graphene: A Review. IEEE Sens. J. 2021, 21, 12426–12443. [Google Scholar] [CrossRef]
- Li, G. Direct laser writing of graphene electrodes. J. Appl. Phys. 2020, 127, 010901. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Fei, Q.; Page, M.; Zhao, G.; Ling, Y.; Chen, D.; Yan, Z. Laser-induced graphene for bioelectronics and soft actuators. Nano Res. 2021. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Guo, W.; Cao, X.; Dou, Y.; Huang, L.; Song, Y.; Su, J.; Zeng, Z.; Ye, R. Laser-induced graphene for environmental applications: Progress and opportunities. Mater. Chem. Front. 2021. [Google Scholar] [CrossRef]
- Huang, L.; Su, J.; Song, Y.; Ye, R. Laser-Induced Graphene: En Route to Smart Sensing. Nano-Micro Lett. 2020, 12, 157. [Google Scholar] [CrossRef]
- Ye, R.; James, D.K.; Tour, J.M. Laser-Induced Graphene: From Discovery to Translation. Adv. Mater. 2019, 31, 1803621. [Google Scholar] [CrossRef]
- Wan, Z.; Streed, E.W.; Lobino, M.; Wang, S.; Sang, R.T.; Cole, I.S.; Thiel, D.V.; Li, Q. Laser-Reduced Graphene: Synthesis, Properties, and Applications. Adv. Mater. Technol. 2018, 3, 1700315. [Google Scholar] [CrossRef]
- Stanford, M.G.; Zhang, C.; Fowlkes, J.D.; Hoffman, A.; Ivanov, I.N.; Rack, P.D.; Tour, J.M. High-Resolution Laser-Induced Graphene. Flexible Electronics beyond the Visible Limit. ACS Appl. Mater. Interfaces 2020, 12, 10902–10907. [Google Scholar] [CrossRef]
- Cote, L.J.; Cruz-Silva, R.; Huang, J. Flash Reduction and Patterning of Graphite Oxide and Its Polymer Composite. J. Am. Chem. Soc. 2009, 131, 11027–11032. [Google Scholar] [CrossRef]
- Choi, S.-J.; Kim, S.-J.; Kim, I.-D. Ultrafast optical reduction of graphene oxide sheets on colorless polyimide film for wearable chemical sensors. NPG Asia Mater. 2016, 8, e315. [Google Scholar] [CrossRef]
- Trusovas, R.; Ratautas, K.; Račiukaitis, G.; Barkauskas, J.; Stankevičienė, I.; Niaura, G.; Mažeikienė, R. Reduction of graphite oxide to graphene with laser irradiation. Carbon 2013, 52, 574–582. [Google Scholar] [CrossRef]
- Strong, V.; Dubin, S.; El-Kady, M.F.; Lech, A.; Wang, Y.; Weiller, B.H.; Kaner, R.B. Patterning and Electronic Tuning of Laser Scribed Graphene for Flexible All-Carbon Devices. ACS Nano 2012, 6, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- El-Kady, M.F.; Kaner, R.B. Scalable fabrication of high-power graphene micro-supercapacitors for flexible and on-chip energy storage. Nat. Commun. 2013, 4, 1475. [Google Scholar] [CrossRef] [PubMed]
- Arul, R.; Oosterbeek, R.N.; Robertson, J.; Xu, G.; Jin, J.; Simpson, M.C. The mechanism of direct laser writing of graphene features into graphene oxide films involves photoreduction and thermally assisted structural rearrangement. Carbon 2016, 99, 423–431. [Google Scholar] [CrossRef]
- Guo, L.; Jiang, H.-B.; Shao, R.-Q.; Zhang, Y.-L.; Xie, S.-Y.; Wang, J.-N.; Li, X.-B.; Jiang, F.; Chen, Q.-D.; Zhang, T.; et al. Two-beam-laser interference mediated reduction, patterning and nanostructuring of graphene oxide for the production of a flexible humidity sensing device. Carbon 2012, 50, 1667–1673. [Google Scholar] [CrossRef]
- Huang, L.; Liu, Y.; Ji, L.-C.; Xie, Y.-Q.; Wang, T.; Shi, W.-Z. Pulsed laser assisted reduction of graphene oxide. Carbon 2011, 49, 2431–2436. [Google Scholar] [CrossRef]
- Buccheri, M.A.; D’Angelo, D.; Scalese, S.; Spanò, S.F.; Filice, S.; Fazio, E.; Compagnini, G.; Zimbone, M.; Brundo, M.V.; Pecoraro, R.; et al. Modification of graphene oxide by laser irradiation: A new route to enhance antibacterial activity. Nanotechnology 2016, 27, 245704. [Google Scholar] [CrossRef] [PubMed]
- Yazdi, A.Z.; Navas, I.O.; Abouelmagd, A.; Sundararaj, U. Direct Creation of Highly Conductive Laser-Induced Graphene Nanocomposites from Polymer Blends. Macromol. Rapid Commun. 2017, 38, 1700176. [Google Scholar] [CrossRef]
- Singh, S.P.; Li, Y.; Zhang, J.; Tour, J.M.; Arnusch, C.J. Sulfur-Doped Laser-Induced Porous Graphene Derived from Polysulfone-Class Polymers and Membranes. ACS Nano 2018, 12, 289–297. [Google Scholar] [CrossRef]
- Lamberti, A.; Serrapede, M.; Ferraro, G.; Fontana, M.; Perrucci, F.; Bianco, S.; Chiolerio, A.; Bocchini, S. All-SPEEK flexible supercapacitor exploiting laser-induced graphenization. 2d Mater. 2017, 4, 035012. [Google Scholar] [CrossRef]
- Yuan, M.; Luo, F.; Wang, Z.; Li, H.; Rao, Y.; Yu, J.; Wang, Y.; Xie, D.; Chen, X.; Wong, C.-P. Facile and Scalable Fabrication of High-Performance Microsupercapacitors Based on Laser-Scribed In Situ Heteroatom-Doped Porous Graphene. ACS Appl. Mater. Interfaces 2021, 13, 22426–22437. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Ye, R.; Chyan, Y.; Wang, T.; Zhang, C.; Shi, L.; Zhang, T.; Zhao, Y.; Tour, J.M. Laser-Induced Graphene from Wood Impregnated with Metal Salts and Use in Electrocatalysis. ACS Appl. Nano Mater. 2018, 1, 5053–5061. [Google Scholar] [CrossRef]
- Chyan, Y.; Ye, R.; Li, Y.; Singh, S.P.; Arnusch, C.J.; Tour, J.M. Laser-Induced Graphene by Multiple Lasing: Toward Electronics on Cloth, Paper, and Food. ACS Nano 2018, 12, 2176–2183. [Google Scholar] [CrossRef]
- Beckham, J.L.; Li, J.T.; Stanford, M.G.; Chen, W.; McHugh, E.A.; Advincula, P.A.; Wyss, K.M.; Chyan, Y.; Boldman, W.L.; Rack, P.D.; et al. High-Resolution Laser-Induced Graphene from Photoresist. ACS Nano 2021. [Google Scholar] [CrossRef]
- Li, Z.; Lu, L.; Xie, Y.; Wang, W.; Lin, Z.; Tang, B.; Lin, N. Preparation of Laser-Induced Graphene Fabric from Silk and Its Application Examples for Flexible Sensor. Adv. Eng. Mater. 2021, 2100195. [Google Scholar] [CrossRef]
- Mahmood, F.; Zhang, H.; Lin, J.; Wan, C. Laser-Induced Graphene Derived from Kraft Lignin for Flexible Supercapacitors. ACS Omega 2020, 5, 14611–14618. [Google Scholar] [CrossRef]
- Dong, Y.; Rismiller, S.C.; Lin, J. Molecular dynamic simulation of layered graphene clusters formation from polyimides under extreme conditions. Carbon 2016, 104, 47–55. [Google Scholar] [CrossRef] [Green Version]
- Sokolov, D.A.; Shepperd, K.R.; Orlando, T.M. Formation of Graphene Features from Direct Laser-Induced Reduction of Graphite Oxide. J. Phys. Chem. Lett. 2010, 1, 2633–2636. [Google Scholar] [CrossRef]
- Scardaci, V.; Fichera, L.; Fragalà, M.E.; Tuccitto, N.; Marletta, G.; Compagnini, G. Reduction of Graphene Oxide by Laser Scribing in Different Atmospheres and Application in Humidity Sensing. J. Nanomater. 2020, 2020, 4946954. [Google Scholar] [CrossRef]
- Scardaci, V.; Compagnini, G. Raman Spectroscopy Investigation of Graphene Oxide Reduction by Laser Scribing. C 2021, 7, 48. [Google Scholar] [CrossRef]
- Li, Y.L.; Luong, D.X.; Zhang, J.B.; Tarkunde, Y.R.; Kittrell, C.; Sargunaraj, F.; Ji, Y.S.; Arnusch, C.J.; Tour, J.M. Laser-Induced Graphene in Controlled Atmospheres: From Superhydrophilic to Superhydrophobic Surfaces. Adv. Mater. 2017, 29, 1700496. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Lv, C.; Aoyagi, E.; Ogawa, S.; Watanabe, A. Laser Direct Writing of a High-Performance All-Graphene Humidity Sensor Working in a Novel Sensing Mode for Portable Electronics. ACS Appl. Mater. Interfaces 2018, 10, 23987–23996. [Google Scholar] [CrossRef]
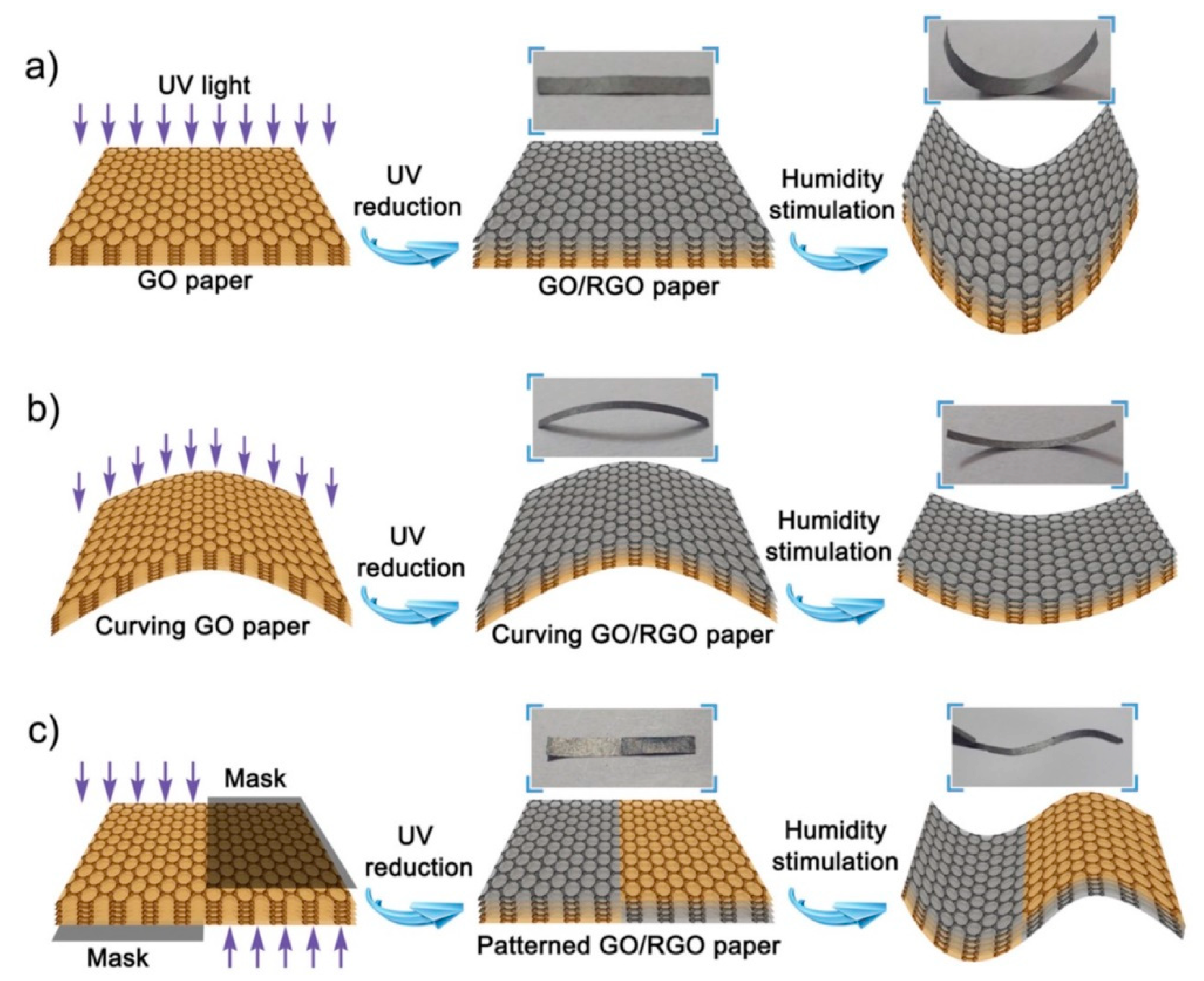
- Han, D.-D.; Zhang, Y.-L.; Liu, Y.; Liu, Y.-Q.; Jiang, H.-B.; Han, B.; Fu, X.-Y.; Ding, H.; Xu, H.-L.; Sun, H.-B. Bioinspired Graphene Actuators Prepared by Unilateral UV Irradiation of Graphene Oxide Papers. Adv. Funct. Mater. 2015, 25, 4548–4557. [Google Scholar] [CrossRef]
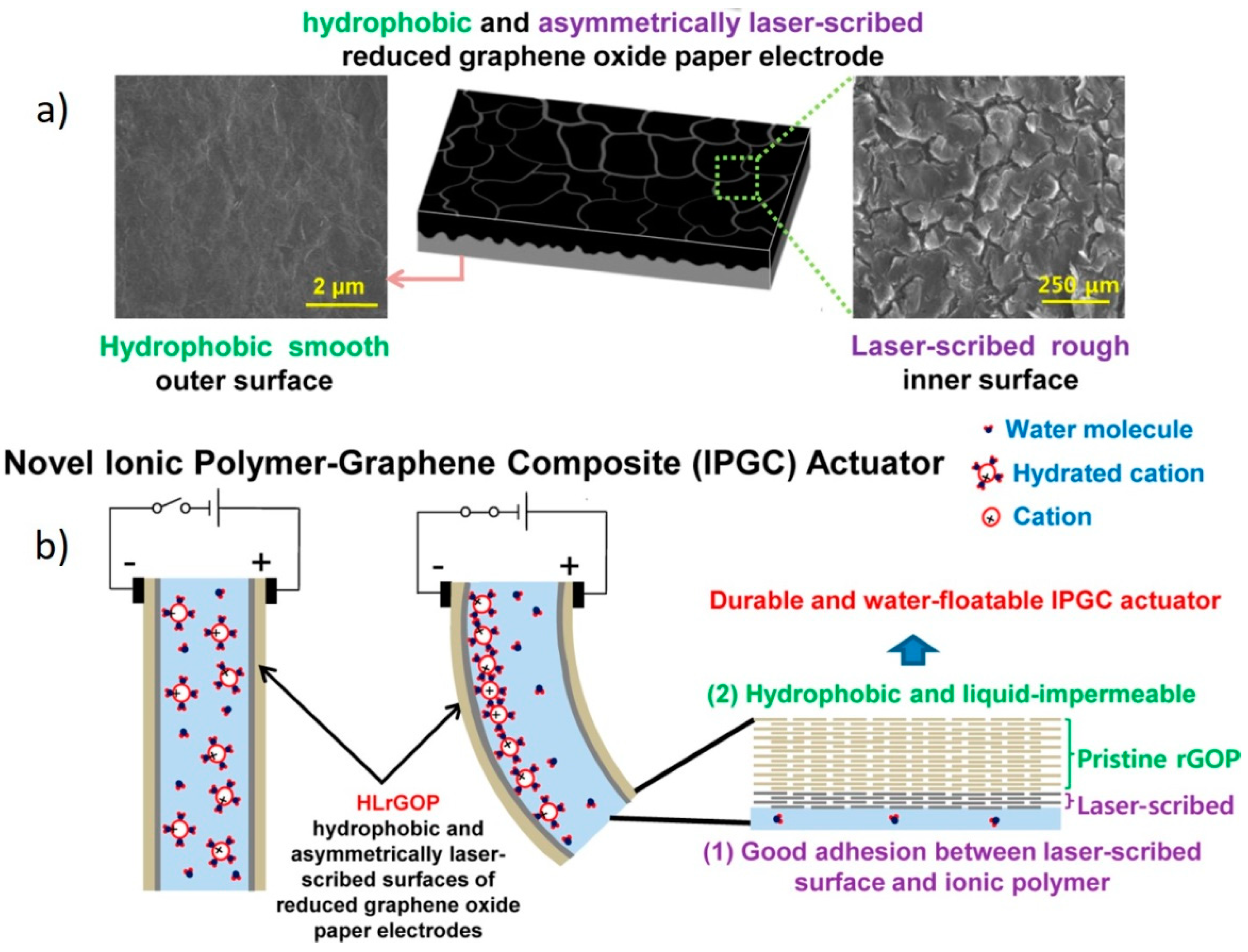
- Kim, J.; Jeon, J.-H.; Kim, H.-J.; Lim, H.; Oh, I.-K. Durable and Water-Floatable Ionic Polymer Actuator with Hydrophobic and Asymmetrically Laser-Scribed Reduced Graphene Oxide Paper Electrodes. ACS Nano 2014, 8, 2986–2997. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Niu, Z.; Chen, J.; Zhai, Y.; Xu, Y.; Luo, S. Freestanding laser induced graphene paper based liquid sensors. Carbon 2019, 153, 472–480. [Google Scholar] [CrossRef]
- Tian, H.; Shu, Y.; Cui, Y.-L.; Mi, W.-T.; Yang, Y.; Xie, D.; Ren, T.-L. Scalable fabrication of high-performance and flexible graphene strain sensors. Nanoscale 2014, 6, 699–705. [Google Scholar] [CrossRef]
- Rahimi, R.; Ochoa, M.; Yu, W.; Ziaie, B. Highly Stretchable and Sensitive Unidirectional Strain Sensor via Laser Carbonization. ACS Appl. Mater. Interfaces 2015, 7, 4463–4470. [Google Scholar] [CrossRef]
- Jeong, S.-Y.; Lee, J.-U.; Hong, S.-M.; Lee, C.-W.; Hwang, S.-H.; Cho, S.-C.; Shin, B.-S. Highly Skin-Conformal Laser-Induced Graphene-Based Human Motion Monitoring Sensor. Nanomaterials 2021, 11, 951. [Google Scholar] [CrossRef]
- Groo, L.; Nasser, J.; Inman, D.J.; Sodano, H.A. Transfer printed laser induced graphene strain gauges for embedded sensing in fiberglass composites. Compos. Part B: Eng. 2021, 219, 108932. [Google Scholar] [CrossRef]
- Carvalho, A.F.; Fernandes, A.J.S.; Leitão, C.; Deuermeier, J.; Marques, A.C.; Martins, R.; Fortunato, E.; Costa, F.M. Laser-Induced Graphene Strain Sensors Produced by Ultraviolet Irradiation of Polyimide. Adv. Funct. Mater. 2018, 28, 1805271. [Google Scholar] [CrossRef]
- Sun, B.; McCay, R.N.; Goswami, S.; Xu, Y.; Zhang, C.; Ling, Y.; Lin, J.; Yan, Z. Gas-Permeable, Multifunctional On-Skin Electronics Based on Laser-Induced Porous Graphene and Sugar-Templated Elastomer Sponges. Adv. Mater. 2018, 30, 1804327. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.-Q.; Tian, H.; Liu, Y.; Ju, Z.-Y.; Pang, Y.; Chen, Y.-Q.; Wang, D.-Y.; Tian, X.-G.; Yan, J.-C.; Deng, N.-Q.; et al. An intelligent artificial throat with sound-sensing ability based on laser induced graphene. Nat. Commun. 2017, 8, 14579. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.; Wang, S.; Wu, J.; Yu, Y.; Li, R.; Eda, S.; Chen, J.; Feng, G.; Lawrie, B.; Hu, A. Bisphenol A Sensors on Polyimide Fabricated by Laser Direct Writing for Onsite River Water Monitoring at Attomolar Concentration. ACS Appl. Mater. Interfaces 2016, 8, 17784–17792. [Google Scholar] [CrossRef] [PubMed]
- Nayak, P.; Kurra, N.; Xia, C.; Alshareef, H.N. Highly Efficient Laser Scribed Graphene Electrodes for On-Chip Electrochemical Sensing Applications. Adv. Electron. Mater. 2016, 2, 1600185. [Google Scholar] [CrossRef]
- Fenzl, C.; Nayak, P.; Hirsch, T.; Wolfbeis, O.S.; Alshareef, H.N.; Baeumner, A.J. Laser-Scribed Graphene Electrodes for Aptamer-Based Biosensing. ACS Sens. 2017, 2, 616–620. [Google Scholar] [CrossRef]
- Santos, N.F.; Pereira, S.O.; Moreira, A.; Girão, A.V.; Carvalho, A.F.; Fernandes, A.J.S.; Costa, F.M. IR and UV Laser-Induced Graphene: Application as Dopamine Electrochemical Sensors. Adv. Mater. Technol. 2021, 6, 2100007. [Google Scholar] [CrossRef]
- Vanegas, D.C.; Patiño, L.; Mendez, C.; Oliveira, D.A.; Torres, A.M.; Gomes, C.L.; McLamore, E.S. Laser Scribed Graphene Biosensor for Detection of Biogenic Amines in Food Samples Using Locally Sourced Materials. Biosensors 2018, 8. [Google Scholar] [CrossRef] [Green Version]
- Soares, R.R.A.; Hjort, R.G.; Pola, C.C.; Parate, K.; Reis, E.L.; Soares, N.F.F.; McLamore, E.S.; Claussen, J.C.; Gomes, C.L. Laser-Induced Graphene Electrochemical Immunosensors for Rapid and Label-Free Monitoring of Salmonella enterica in Chicken Broth. ACS Sens. 2020, 5, 1900–1911. [Google Scholar] [CrossRef]
- Marques, A.C.; Cardoso, A.R.; Martins, R.; Sales, M.G.F.; Fortunato, E. Laser-Induced Graphene-Based Platforms for Dual Biorecognition of Molecules. ACS Appl. Nano Mater. 2020, 3, 2795–2803. [Google Scholar] [CrossRef]
- Kazemzadeh, R.; Andersen, K.; Motha, L.; Kim, W.S. Highly Sensitive Pressure Sensor Array With Photothermally Reduced Graphene Oxide. IEEE Electron Device Lett. 2015, 36, 180–182. [Google Scholar] [CrossRef]
- Kazemzadeh, R.; Woo Soo, K. Flexible Temperature Sensor with Laser Scribed Graphene Oxide. In Proceedings of the 14th IEEE International Conference on Nanotechnology, Bangkok, Thailand, 18–21 August 2014; pp. 420–423. [Google Scholar]
- Gholami Laelabadi, K.; Moradian, R.; Manouchehri, I. One-Step Fabrication of Flexible, Cost/Time Effective, and High Energy Storage Reduced Graphene Oxide@PANI Supercapacitor. ACS Appl. Energy Mater. 2020, 3, 5301–5312. [Google Scholar] [CrossRef]
- Yu, X.; Li, N.; Zhang, S.; Liu, C.; Chen, L.; Han, S.; Song, Y.; Han, M.; Wang, Z. Ultra-thick 3D graphene frameworks with hierarchical pores for high-performance flexible micro-supercapacitors. J. Power Sources 2020, 478, 229075. [Google Scholar] [CrossRef]
- Cai, F.; Tao, C.-A.; Li, Y.; Yin, W.; Wang, X.; Wang, J. Effects of amount of graphene oxide and the times of LightScribe on the performance of all-solid-state flexible graphene-based micro-supercapacitors. Mater. Res. Express 2017, 4, 036304. [Google Scholar] [CrossRef]
- Gao, W.; Singh, N.; Song, L.; Liu, Z.; Reddy, A.L.M.; Ci, L.; Vajtai, R.; Zhang, Q.; Wei, B.; Ajayan, P.M. Direct laser writing of micro-supercapacitors on hydrated graphite oxide films. Nat. Nanotechnol. 2011, 6, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Moon, K.-S.; Li, J.; Xie, Y.; Liu, J.; Sun, Z.; Lu, L.; Tang, Y.; Wong, C.-P. Laser-oxidized Fe3O4 nanoparticles anchored on 3D macroporous graphene flexible electrodes for ultrahigh-energy in-plane hybrid micro-supercapacitors. Nano Energy 2020, 77, 105058. [Google Scholar] [CrossRef]
- Peng, Z.; Lin, J.; Ye, R.; Samuel, E.L.G.; Tour, J.M. Flexible and Stackable Laser-Induced Graphene Supercapacitors. ACS Appl. Mater. Interfaces 2015, 7, 3414–3419. [Google Scholar] [CrossRef]
- Peng, Z.; Ye, R.; Mann, J.A.; Zakhidov, D.; Li, Y.; Smalley, P.R.; Lin, J.; Tour, J.M. Flexible Boron-Doped Laser-Induced Graphene Microsupercapacitors. ACS Nano 2015, 9, 5868–5875. [Google Scholar] [CrossRef]
- Song, W.X.; Zhu, J.X.; Gan, B.H.; Zhao, S.Y.; Wang, H.; Li, C.J.; Wang, J. Flexible, Stretchable, and Transparent Planar Microsupercapacitors Based on 3D Porous Laser-Induced Graphene. Small 2018, 14, 1702249. [Google Scholar] [CrossRef]
- Steele, B.C.H.; Heinzel, A. Materials for fuel-cell technologies. Nature 2001, 414, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Tee, S.Y.; Win, K.Y.; Teo, W.S.; Koh, L.-D.; Liu, S.; Teng, C.P.; Han, M.-Y. Recent Progress in Energy-Driven Water Splitting. Adv. Sci. 2017, 4, 1600337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Sun, Y.; Qin, Y.; Zhang, W.; Wang, L.; Luo, M.; Yang, H.; Guo, S. Recent Advances on Water-Splitting Electrocatalysis Mediated by Noble-Metal-Based Nanostructured Materials. Adv. Energy Mater. 2020, 10, 1903120. [Google Scholar] [CrossRef]
- Zhang, J.; Ren, M.; Wang, L.; Li, Y.; Yakobson, B.I.; Tour, J.M. Oxidized Laser-Induced Graphene for Efficient Oxygen Electrocatalysis. Adv. Mater. 2018, 30, 1707319. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Peng, C.; Luo, W.; Lv, M.; Li, X.; Li, D.; Huang, Q.; Fan, C. Graphene-Based Antibacterial Paper. ACS Nano 2010, 4, 4317–4323. [Google Scholar] [CrossRef]
- Singh, S.P.; Li, Y.; Be’er, A.; Oren, Y.; Tour, J.M.; Arnusch, C.J. Laser-Induced Graphene Layers and Electrodes Prevents Microbial Fouling and Exerts Antimicrobial Action. ACS Appl. Mater. Interfaces 2017, 9, 18238–18247. [Google Scholar] [CrossRef]
- Stanford, M.G.; Li, J.T.; Chen, Y.; McHugh, E.A.; Liopo, A.; Xiao, H.; Tour, J.M. Self-Sterilizing Laser-Induced Graphene Bacterial Air Filter. ACS Nano 2019, 13, 11912–11920. [Google Scholar] [CrossRef]
- Rathinam, K.; Singh, S.P.; Li, Y.; Kasher, R.; Tour, J.M.; Arnusch, C.J. Polyimide derived laser-induced graphene as adsorbent for cationic and anionic dyes. Carbon 2017, 124, 515–524. [Google Scholar] [CrossRef]

| Author and Ref. | Analyte | Functionalization Type | Limit of Detection |
|---|---|---|---|
| Cheng [66] | Bisphenol-A | Aptamer | Atto-/Femtomolar |
| Fenzl [68] | Thrombin | Aptamer | Picomolar |
| Marques [72] | AA, AMOX | Polymers | Nanomolar |
| Nayak [67] | AA, DA, UA | Pt nanoparticles | Micromolar |
| Santos [69] | DA | - | Sub-micromolar |
| Soares [71] | Salmonella | Antibodies | 13 colony-forming units |
| Vanegas [70] | Biogenic amines | Cu nanocubes | Micromolar |
| Author and Ref. | Configuration/Material | Electrolyte | Specific Capacitance | Power Density (Max) | Discharge Time (Max) | Stability (Cycles) |
|---|---|---|---|---|---|---|
| Cai [77] | In-plane/GO | H2SO4/PVA | 3 mFcm−2 | - | 60 s (0.05 mAcm−2) | 1000 |
| El-Kady [19] | Sandwich/GO | H3PO4/PVA | 5 mFcm−2 | 3 Wcm−3 | 10 s | 10,000 |
| El-Kady [36] | In-plane/GO | H2SO4/PVA | 2.35 mFcm−2 | 200 Wcm−3 | 30 s | 10,000 |
| Gao [78] | In-plane + Sandwich/GO | Na2SO4 or TEABF4 | 3 mFcm−2 | 2.4 Wcm−3 | - | 10,000 |
| Laelabadi [75] | In-plane/GO + PANI | H2SO4/PVA | 72 mFcm−2 | 81.4 mWcm−3 | 3000 s (35 μAcm−2) | 1000 |
| Lamberti [43] | In-plane/LIG | Na2SO4 | 0.34 mFcm−2 | 0.01 mWcm−2 | 200 s (0.625 μAcm−2) | - |
| Li [20] | In-plane/LIG | LiCl/PVA or H2SO4/PVA | 361 mFcm−2 | 11.8 mWcm−2/2.89 Wcm−3 | 150 s (0.25 mAcm−2) | 2000 |
| Li [54] | In-plane/LIG | H2SO4/PVA | 37 mFcm−2 | 2 mWcm−2 | 15 s (0.5 mAcm−2) | - |
| Lin [21] | In-plane/LIG | H2SO4/ionic liquid | 4 mFcm−2 | 9 mWcm−2/3 Wcm−3 | 25 s (0.2 mAcm−2) | 9000 |
| Liu [79] | In-plane/LIG + Fe3O4 | H2SO4/PVA | 719 mFcm−2 | 0.3 mWcm−2 | - | 900 |
| Mahmood [49] | In-plane/LIG | H2SO4/PVA | 0.88 mFcm−2 | 25μWcm−2 | 60 s (0.01 mAcm−2) | 10,000 |
| Peng [80] | Sandwich/LIG | H2SO4/PVA | 9 mFcm−2 | 9 mWcm−2/4 Wcm−3 | 30 s (0.5 mAcm−2) | 8000 |
| Peng [81] | In-plane/LIG | H2SO4/PVA | 16.5 mFcm−2 | 4 Wcm−3 | 9 s (1 mAcm−2) | 12,000 |
| Song [82] | In-plane/LIG | potassium polyacrylate/KOH gel electrolyte | 0.79 mFcm−2 | - | 7 s (75 μAcm−2) | 10,000 |
| Ye [22] | In-plane/LIG | KOH | 320 mFcm−2 | - | 600 s (1 mAcm−2) | 400 |
| Yu [76] | In-plane/LIG + PPy | H2SO4/PVA | 2412 mFcm−2 | 0.325 mWcm−2 | 4000 s (0.5 mAcm−2) | 10,000 |
| Yuan [44] | In-plane/LIG | H2SO4/PVA | 60.6 mFcm−2 | - | 200 s (0.5 mAcm−2) | 20,000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scardaci, V. Laser Synthesized Graphene and Its Applications. Appl. Sci. 2021, 11, 6304. https://doi.org/10.3390/app11146304
Scardaci V. Laser Synthesized Graphene and Its Applications. Applied Sciences. 2021; 11(14):6304. https://doi.org/10.3390/app11146304
Chicago/Turabian StyleScardaci, Vittorio. 2021. "Laser Synthesized Graphene and Its Applications" Applied Sciences 11, no. 14: 6304. https://doi.org/10.3390/app11146304
APA StyleScardaci, V. (2021). Laser Synthesized Graphene and Its Applications. Applied Sciences, 11(14), 6304. https://doi.org/10.3390/app11146304






