Fabrication of Eco-Friendly Graphene Nanoplatelet Electrode for Electropolishing and Its Properties
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Melamine-GNP (M-GNP) and Film Fabrication
2.3. Characterization
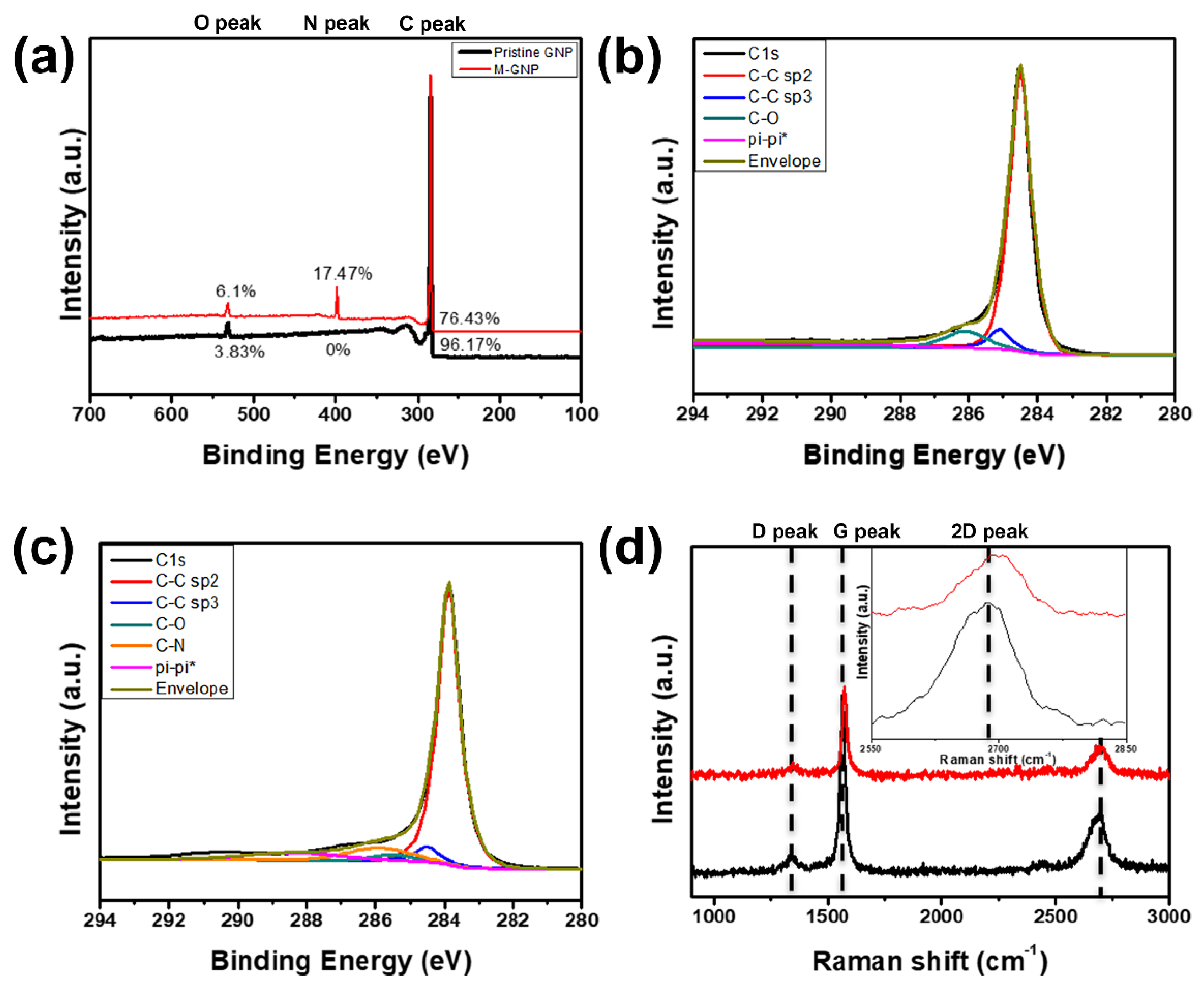
2.3.1. M-GNP Film Characterization
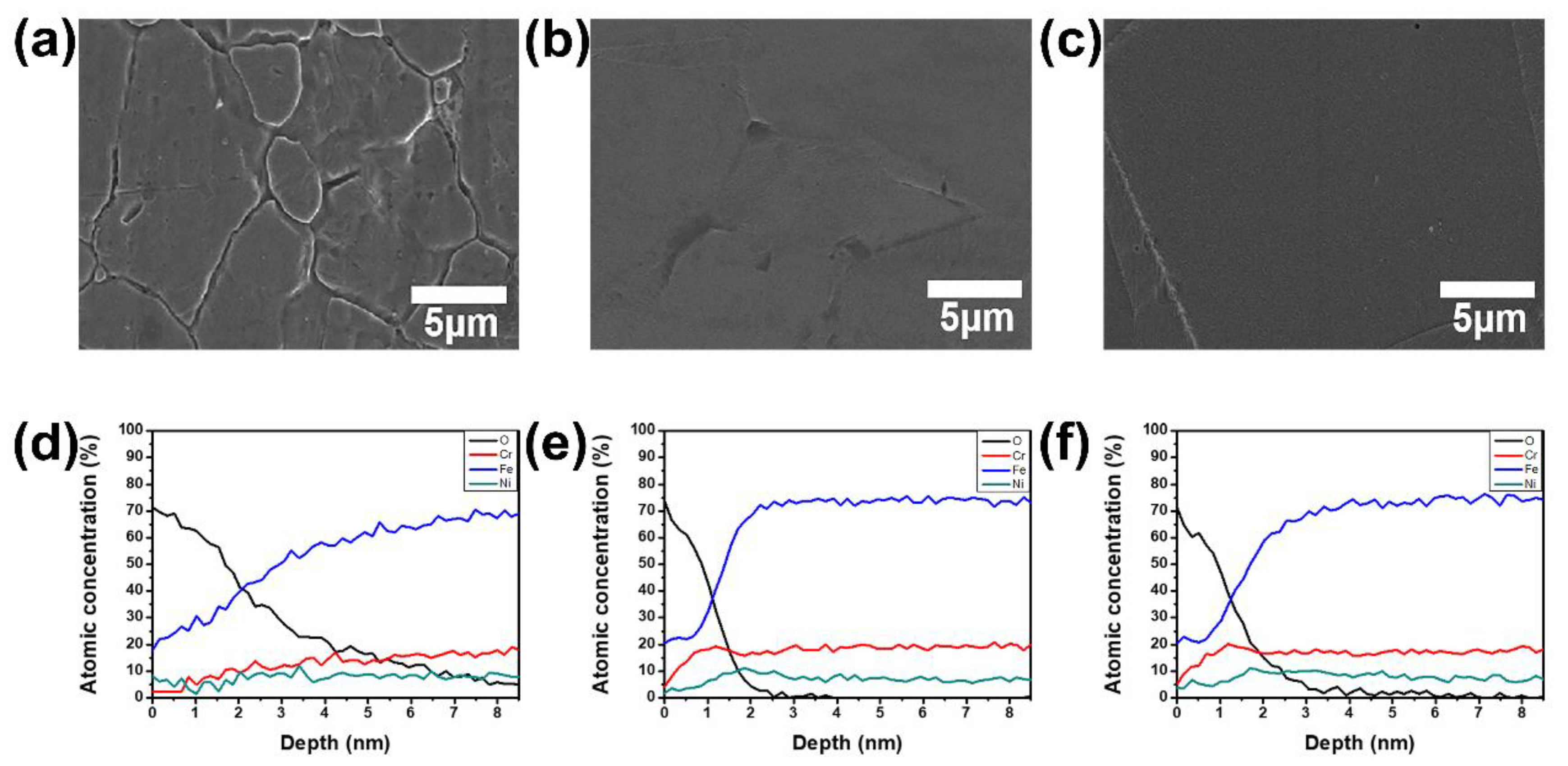
2.3.2. Electropolishing Characterization
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Leal, P.P.; Hurd, C.L.; Sander, S.G.; Armstrong, E.; Fernández, P.A.; Suhrhoff, T.J.; Roleda, M.Y. Copper pollution exacerbates the effects of ocean acidification and warming on kelp microscopic early life stages. Sci. Rep. 2018, 8, 14763. [Google Scholar] [CrossRef] [PubMed]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metal Toxicity and the Environment. Exp. Suppl. 2012. [Google Scholar] [CrossRef]
- Xu, L.; Zhou, Z.-F. Effects of Cu Pollution on the Expansion of an Amphibious Clonal Herb in Aquatic-Terrestrial Ecotones. PLoS ONE 2016, 11, e0164361. [Google Scholar] [CrossRef] [PubMed]
- Topsakal, M.; Şahin, H.; Ciraci, S. Graphene coatings: An efficient protection from oxidation. Phys. Rev. B 2012, 85, 155445. [Google Scholar] [CrossRef]
- Su, Y.; Kravets, V.G.; Wong, S.L.; Waters, J.; Geim, A.K.; Nair, R.R. Impermeable barrier films and protective coatings based on reduced graphene oxide. Nat. Commun. 2014, 5, 4843. [Google Scholar] [CrossRef]
- Dai, L. Functionalization of Graphene for Efficient Energy Conversion and Storage. Acc. Chem. Res. 2013, 46, 31–42. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef]
- Bhuyan, A.; Gregory, B.; Lei, H.; Yee, S.Y.; Gianchandani, Y.B. Pulse and DC Electropolishing of Stainless Steel for Stents and Other Devices. In Proceedings of the IEEE Sensors 2005, Irvine, CA, USA, 30 October–3 November 2005. [Google Scholar] [CrossRef]
- Ponto, L.; Datta, M.; Landolt, D. Electropolishing of iron-chromium alloys in phosphoric acid-sulphuric acid electrolytes. Surf. Coat. Technol. 1987, 30, 265–276. [Google Scholar] [CrossRef]
- Faust, C.L. Surface Preparation by Electropolishing. J. Electrochem. Soc. 1949, 95. [Google Scholar] [CrossRef]
- Kim, J.; Cha, J.; Jun, G.H.; Yoo, S.C.; Ryu, S.; Hong, S.H. Fabrication of Graphene Nanoplatelet/Epoxy Nanocomposites for Lightweight and High-Strength Structural Applications. Part. Part. Syst. Charact. 2018, 35, 1700412. [Google Scholar] [CrossRef]
- Teng, C.-C.; Ma, C.-C.M.; Lu, C.-H.; Yang, S.-Y.; Lee, S.-H.; Hsiao, M.-C.; Yen, M.-Y.; Chiou, K.-C.; Lee, T.-M. Thermal conductivity and structure of non-covalent functionalized graphene/epoxy composites. Carbon 2011, 49, 5107–5116. [Google Scholar] [CrossRef]
- Choi, E.-Y.; Han, T.H.; Hong, J.; Kim, J.E.; Lee, S.H.; Kim, H.W.; Kim, S.O. Noncovalent functionalization of graphene with end-functional polymers. J. Mater. Chem. 2010, 20, 1907–1912. [Google Scholar] [CrossRef]
- Cha, J.; Jun, G.H.; Park, J.K.; Kim, J.C.; Ryu, H.J.; Hong, S.H. Improvement of modulus, strength and fracture toughness of CNT/Epoxy nanocomposites through the functionalization of carbon nanotubes. Compos. Part B Eng. 2017, 129, 169–179. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.; Jin, S.; Shim, J.H.; Park, C.S.; Ryu, H.J.; Hong, S.H. Functionalization of carbon nanotubes for fabrication of CNT/epoxy nanocomposites. Mater. Des. 2016, 95, 1–8. [Google Scholar] [CrossRef]
- Ren, P.-G.; Yan, D.-X.; Chen, T.; Zeng, B.-Q.; Li, Z.-M. Improved properties of highly oriented graphene/polymer nanocomposites. J. Appl. Polym. Sci. 2011, 121, 3167–3174. [Google Scholar] [CrossRef]
- Payandehpeyman, J.; Mazaheri, M.; Khamehchi, M. Prediction of electrical conductivity of polymer-graphene nanocomposites by developing an analytical model considering interphase, tunneling and geometry effects. Compos. Commun. 2020, 21, 100364. [Google Scholar] [CrossRef]
- Chen, H.; Müller, M.B.; Gilmore, K.J.; Wallace, G.G.; Li, D. Mechanically Strong, Electrically Conductive, and Biocompatible Graphene Paper. Adv. Mater. 2008, 20, 3557–3561. [Google Scholar] [CrossRef]
- Lee, E.-S. Machining Characteristics of the Electropolishing of Stainless Steel (STS316L). Int. J. Adv. Manuf. Technol. 2000, 16, 591–599. [Google Scholar] [CrossRef]
- Rohly, K.; Istephanous, N.; Belu, A.; Untereker, D.; Coscio, M.; Heffelfinger, J.; Thomas, R.; Allen, J.; Francis, R.; Robinson, A.; et al. Effect of Time, Temperature, and Solution Composition on the Passivation of 316L Stainless Steel for Biomedical Applications. Mater. Sci. Forum 2003, 426–432, 3017–3022. [Google Scholar] [CrossRef]
- Nazneen, F.; Galvin, P.; Arrigan, D.W.M.; Thompson, M.; Benvenuto, P.; Herzog, G. Electropolishing of medical-grade stainless steel in preparation for surface nano-texturing. J. Solid State Electrochem. 2012, 16, 1389–1397. [Google Scholar] [CrossRef]
- Lin, C.-C.; Hu, C.-C. Electropolishing of 304 stainless steel: Surface roughness control using experimental design strategies and a summarized electropolishing model. Electrochim. Acta. 2008, 53, 3356–3363. [Google Scholar] [CrossRef]
- Awad, A.M.; Ghany, N.A.A.; Dahy, T.M. Removal of tarnishing and roughness of copper surface by electropolishing treatment. Appl. Surf. Sci. 2010, 256, 4370–4375. [Google Scholar] [CrossRef]
- Haïdopoulos, M.; Turgeon, S.; Sarra-Bournet, C.; Laroche, G.; Mantovani, D. Development of an optimized electrochemical process for subsequent coating of 316 stainless steel for stent applications. J. Mater. Sci. Mater. Med. 2006, 17, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Stefanov, P.; Stoychev, D.; Stoycheva, M.; Gonzalez-Elipe, A.R.; Marinova, T. XPS, SEM and TEM characterization of stainless-steel 316L surfaces after electrochemical etching and oxidizing. Surf. Interface Anal. 1999, 28, 106–110. [Google Scholar] [CrossRef]
- Wang, J.; Tarapata, C.J.; Fitz, M.J. Electro-polishing fixture and electrolyte solution for polishing stents and method. U.S. Patent No. 6,375,826, 23 April 2002. [Google Scholar]
- Lee, S.-J.; Lai, J.-J. The effects of electropolishing (EP) process parameters on corrosion resistance of 316L stainless steel. J. Mater. Process. Technol. 2003, 140, 206–210. [Google Scholar] [CrossRef]
- Thierry, B.; Tabrizian, M.; Trepanier, C.; Savadogo, O.; Yahia, L. Effect of surface treatment and sterilization processes on the corrosion behavior of NiTi shape memory alloy. J. Biomed. Mater. Res. 2000, 51, 685–693. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, J.; Yoon, W.; Chung, B.; Jeon, G.; Ryu, S. Fabrication of Eco-Friendly Graphene Nanoplatelet Electrode for Electropolishing and Its Properties. Appl. Sci. 2021, 11, 3224. https://doi.org/10.3390/app11073224
Jeong J, Yoon W, Chung B, Jeon G, Ryu S. Fabrication of Eco-Friendly Graphene Nanoplatelet Electrode for Electropolishing and Its Properties. Applied Sciences. 2021; 11(7):3224. https://doi.org/10.3390/app11073224
Chicago/Turabian StyleJeong, Junyoung, Wanjun Yoon, Bongjin Chung, Giyoung Jeon, and Seongwoo Ryu. 2021. "Fabrication of Eco-Friendly Graphene Nanoplatelet Electrode for Electropolishing and Its Properties" Applied Sciences 11, no. 7: 3224. https://doi.org/10.3390/app11073224
APA StyleJeong, J., Yoon, W., Chung, B., Jeon, G., & Ryu, S. (2021). Fabrication of Eco-Friendly Graphene Nanoplatelet Electrode for Electropolishing and Its Properties. Applied Sciences, 11(7), 3224. https://doi.org/10.3390/app11073224





