Gelatin-Based Film Integrated with Copper Sulfide Nanoparticles for Active Packaging Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of Gelatin/CuSNP Nanocomposite Films
2.3. Film Characterization and Properties
2.3.1. Optical Properties
2.3.2. Surface Morphology and FTIR
2.3.3. Thickness, Mechanical Properties, and Thermal Stability
2.3.4. Water Vapor Permeability (WVP) and Water Contact Angle (WCA)
2.3.5. Antibacterial Activity
2.3.6. Statistical Analysis
3. Results and Discussion
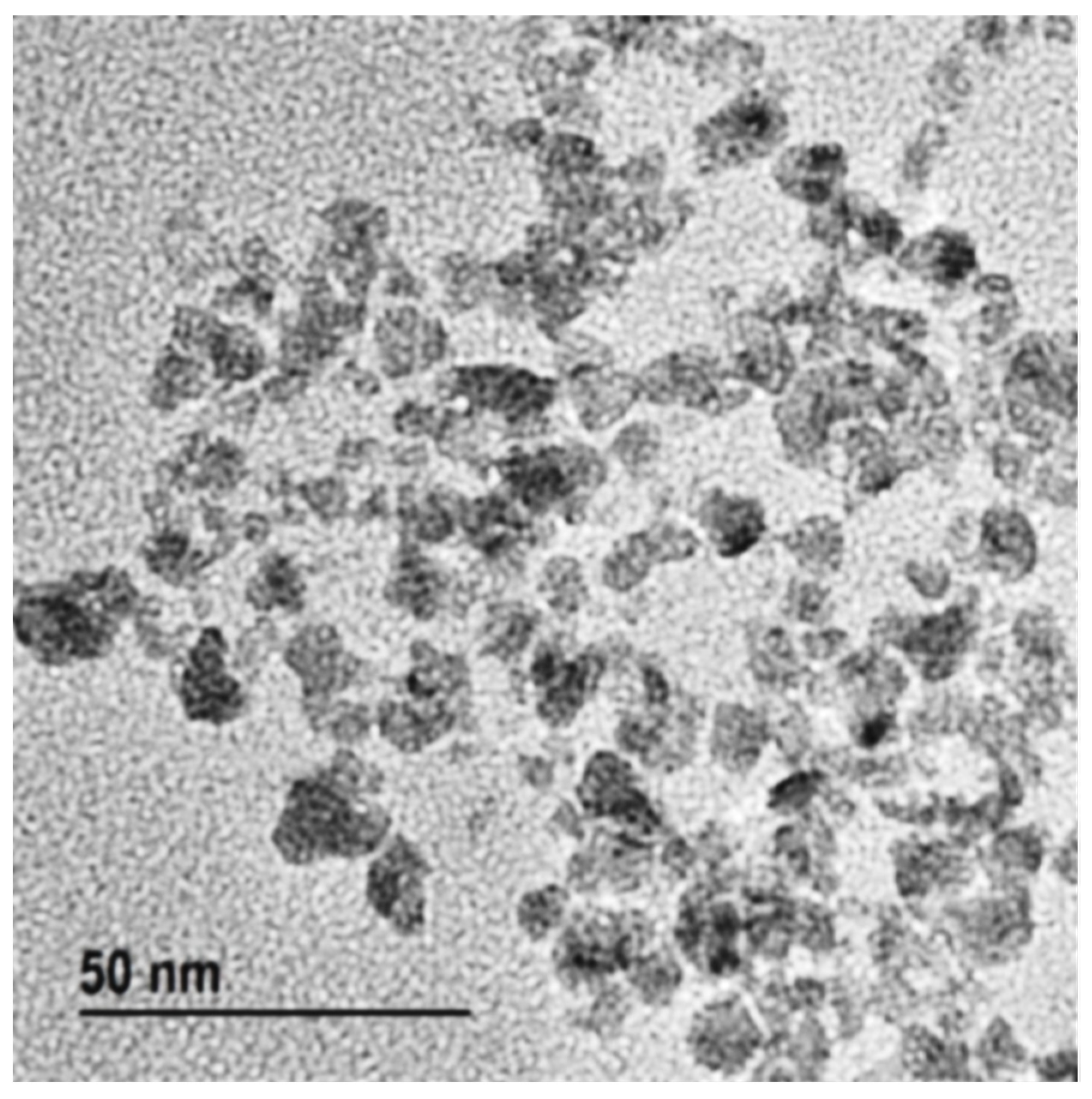
3.1. Morphology of CuSNP
3.2. Properties of the Nanocomposite Films
3.2.1. Optical Properties
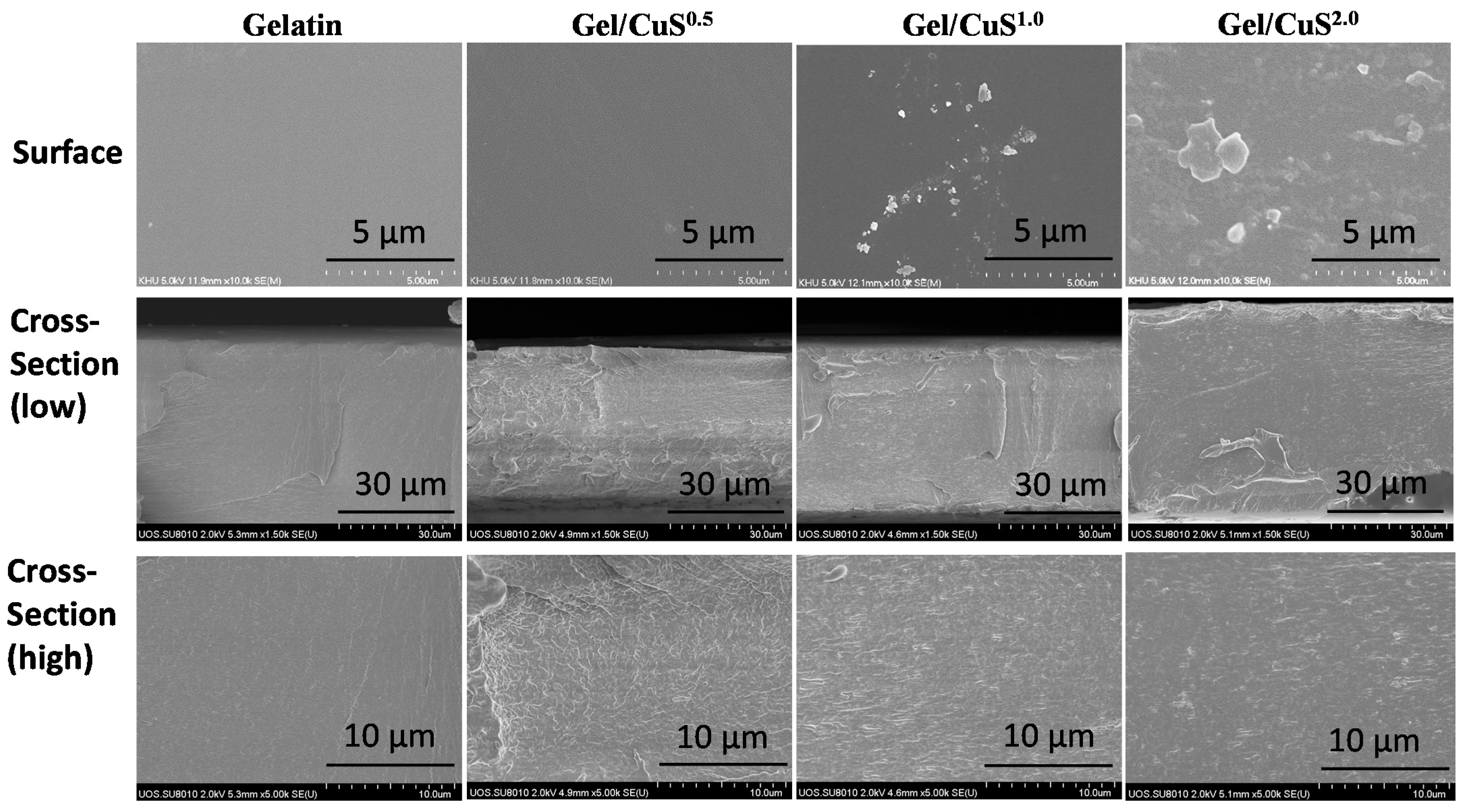
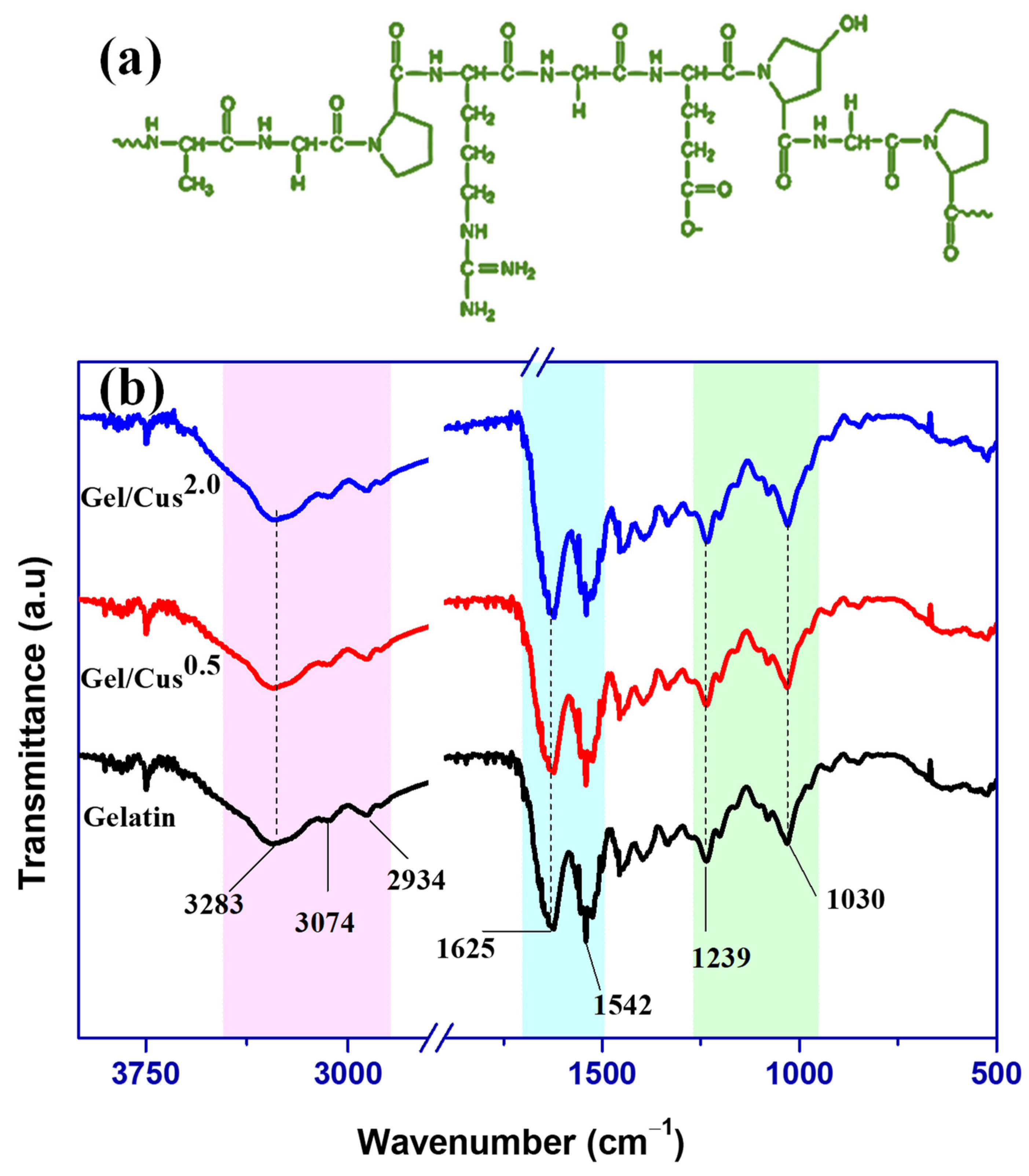
3.2.2. Morphology and FTIR
3.2.3. Mechanical Properties
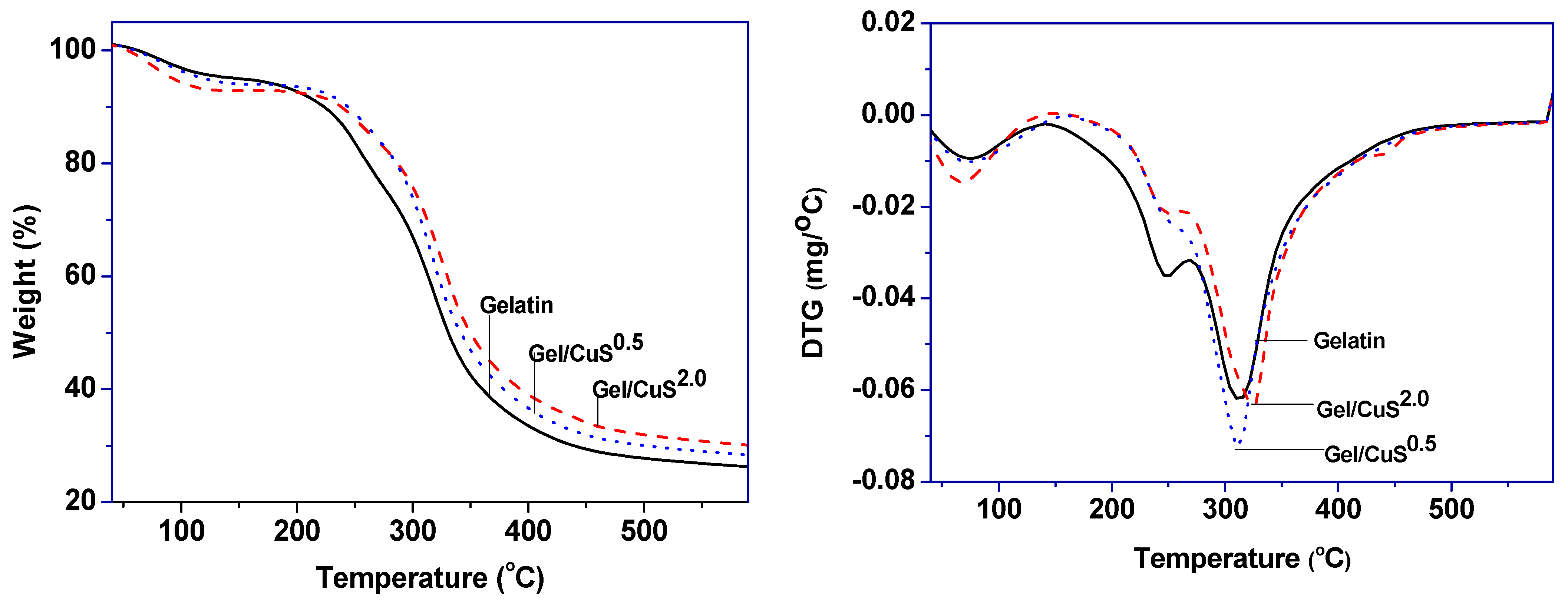
3.2.4. Thermostability
3.2.5. WVP and WCA
3.3. Antimicrobial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Licciardello, F. Packaging, Blessing in Disguise. Review on Its Diverse Contribution to Food Sustainability. Trends Food Sci. Technol. 2017, 65, 32–39. [Google Scholar] [CrossRef]
- Omerović, N.; Djisalov, M.; Živojević, K.; Mladenović, M.; Vunduk, J.; Milenković, I.; Knežević, N.Ž.; Gadjanski, I.; Vidić, J. Antimicrobial Nanoparticles and Biodegradable Polymer Composites for Active Food Packaging Applications. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2428–2454. [Google Scholar] [CrossRef]
- Nešić, A.; Cabrera-Barjas, G.; Dimitrijević-Branković, S.; Davidović, S.; Radovanović, N.; Delattre, C. Prospect of Polysaccharide-Based Materials as Advanced Food Packaging. Molecules 2019, 25, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, Use, and Fate of All Plastics Ever Made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [Green Version]
- Jeya, J.; Chandrasekaran, M.; Venkatesan, S.P.; Sriram, V.; Britto, J.G.; Mageshwaran, G.; Durairaj, R.B. Scaling up Difficulties and Commercial Aspects of Edible Films for Food Packaging: A Review. Trends Food Sci. Technol. 2020, 100, 210–222. [Google Scholar] [CrossRef]
- Narasagoudr, S.S.; Hegde, V.G.; Chougale, R.B.; Masti, S.P.; Vootla, S.; Malabadi, R.B. Physico-Chemical and Functional Properties of Rutin Induced Chitosan/Poly (Vinyl Alcohol) Bioactive Films for Food Packaging Applications. Food Hydrocoll. 2020, 106096. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Preparation of Gelatin/Carrageenan-Based Color-Indicator Film Integrated with Shikonin and Propolis for Smart Food Packaging Applications. ACS Appl. Bio Mater. 2021, 4, 770–779. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.W. Carboxymethyl Cellulose-Based Antioxidant and Antimicrobial Active Packaging Film Incorporated with Curcumin and Zinc Oxide. Int. J. Biol. Macromol. 2020, 148, 666–676. [Google Scholar] [CrossRef]
- Mostafavi, F.S.; Zaeim, D. Agar-Based Edible Films for Food Packaging Applications—A Review. Int. J. Biol. Macromol. 2020, 159, 1165–1176. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Preparation of Antimicrobial and Antioxidant Gelatin/Curcumin Composite Films for Active Food Packaging Application. Colloids Surf. B Biointerfaces 2020, 188, 110761. [Google Scholar] [CrossRef]
- Mousazadeh, S.; Ehsani, A.; Moghaddas Kia, E.; Ghasempour, Z. Zinc Oxide Nanoparticles and Periodate Oxidation in Developing pH-Sensitive Packaging Film Based on Modified Gelatin. Food Packag. Shelf Life 2021, 28, 100654. [Google Scholar] [CrossRef]
- Huang, T.; Tu, Z.-C.; Shangguan, X.; Sha, X.; Wang, H.; Bansal, N. Fish gelatin modifications: A comprehensive review. Trends Food Sci. Technol. 2019, 86, 260–269. [Google Scholar] [CrossRef]
- Bhargava, N.; Sharanagat, V.S.; Mor, R.S.; Kumar, K. Active and Intelligent Biodegradable Packaging Films Using Food and Food Waste-Derived Bioactive Compounds: A Review. Trends Food Sci. Technol. 2020, 105, 385–401. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. New Insight into Melanin for Food Packaging and Biotechnology Applications. Crit. Rev. Food Sci. Nutr. 2021, in press. [Google Scholar] [CrossRef]
- Amjadi, S.; Emaminia, S.; Nazari, M.; Davudian, S.H.; Roufegarinejad, L.; Hamishehkar, H. Application of Reinforced ZnO Nanoparticle-Incorporated Gelatin Bionanocomposite Film with Chitosan Nanofiber for Packaging of Chicken Fillet and Cheese as Food Models. Food Bioprocess. Technol. 2019, 1–15. [Google Scholar] [CrossRef]
- Musso, Y.S.; Salgado, P.R.; Mauri, A.N. Smart Edible Films Based on Gelatin and Curcumin. Food Hydrocoll. 2017, 66, 8–15. [Google Scholar] [CrossRef] [Green Version]
- Kanmani, P.; Rhim, J.-W. Physical, Mechanical and Antimicrobial Properties of Gelatin Based Active Nanocomposite Films Containing AgNPs and Nanoclay. Food Hydrocoll. 2014, 35, 644–652. [Google Scholar] [CrossRef]
- Jamróz, E.; Kopel, P.; Juszczak, L.; Kawecka, A.; Bytesnikova, Z.; Milosavljevic, V.; Makarewicz, M. Development of Furcellaran-Gelatin Films with Se-AgNPs as an Active Packaging System for Extension of Mini Kiwi Shelf Life. Food Packag. Shelf Life 2019, 21, 100339. [Google Scholar] [CrossRef]
- Shankar, S.; Teng, X.; Li, G.; Rhim, J.-W. Preparation, Characterization, and Antimicrobial Activity of Gelatin/ZnO Nanocomposite Films. Food Hydrocoll. 2015, 45, 264–271. [Google Scholar] [CrossRef]
- Kanmani, P.; Rhim, J.-W. Physicochemical Properties of Gelatin/Silver Nanoparticle Antimicrobial Composite Films. Food Chem. 2014, 148, 162–169. [Google Scholar] [CrossRef]
- Shankar, S.; Wang, L.-F.; Rhim, J.-W. Effect of Melanin Nanoparticles on the Mechanical, Water Vapor Barrier, and Antioxidant Properties of Gelatin-Based Films for Food Packaging Application. Food Packag. Shelf Life 2019, 21, 100363. [Google Scholar] [CrossRef]
- Kumari, S.; Singh, B.N.; Srivastava, P. Effect of Copper Nanoparticles on Physico-Chemical Properties of Chitosan and Gelatin-Based Scaffold Developed for Skin Tissue Engineering Application. 3 Biotech 2019, 9, 102. [Google Scholar] [CrossRef]
- Zheng, J.P.; Li, P.; Ma, Y.L.; Yao, K. De Gelatin/Montmorillonite Hybrid Nanocomposite. I. Preparation and Properties. J. Appl. Polym. Sci. 2002, 86, 1189–1194. [Google Scholar] [CrossRef]
- He, Q.; Zhang, Y.; Cai, X.; Wang, S. Fabrication of Gelatin–TiO2 Nanocomposite Film and Its Structural, Antibacterial and Physical Properties. Int. J. Biol. Macromol. 2016, 84, 153–160. [Google Scholar] [CrossRef]
- Sahraee, S.; Milani, J.M.; Ghanbarzadeh, B.; Hamishehkar, H. Effect of Corn Oil on Physical, Thermal, and Antifungal Properties of Gelatin-Based Nanocomposite Films Containing Nano Chitin. LWT Food Sci. Technol. 2017, 76, 33–39. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Z.; Liu, Y.; Li, N.; Wang, W.; Gao, J. Preparation of Reduced Graphene Oxide/Gelatin Composite Films with Reinforced Mechanical Strength. Mater. Res. Bull. 2012, 47, 2245–2251. [Google Scholar] [CrossRef]
- Roy, S.; Van Hai, L.; Kim, H.C.; Zhai, L.; Kim, J. Preparation and Characterization of Synthetic Melanin-like Nanoparticles Reinforced Chitosan Nanocomposite Films. Carbohydr. Polym. 2020, 231, 115729. [Google Scholar] [CrossRef]
- Jamróz, E.; Khachatryan, G.; Kopel, P.; Juszczak, L.; Kawecka, A.; Krzyściak, P.; Kucharek, M.; Bębenek, Z.; Zimowska, M. Furcellaran Nanocomposite Films: The Effect of Nanofillers on the Structural, Thermal, Mechanical and Antimicrobial Properties of Biopolymer Films. Carbohydr. Polym. 2020, 116244. [Google Scholar] [CrossRef]
- Basumatary, I.B.; Mukherjee, A.; Katiyar, V.; Kumar, S. Biopolymer-Based Nanocomposite Films and Coatings: Recent Advances in Shelf-Life Improvement of Fruits and Vegetables. Crit. Rev. Food Sci. Nutr. 2020, in press. [Google Scholar] [CrossRef]
- Jafarzadeh, S.; Jafari, S.M. Impact of Metal Nanoparticles on the Mechanical, Barrier, Optical and Thermal Properties of Biodegradable Food Packaging Materials. Crit. Rev. Food Sci. Nutr. 2020, in press. [Google Scholar] [CrossRef]
- Chawla, R.; Sivakumar, S.; Kaur, H. Antimicrobial Edible Films in Food Packaging: Current Scenario and Recent Nanotechnological Advancements—A Review. Carbohydr. Polym. Technol. Appl. 2021, 2, 100024. [Google Scholar] [CrossRef]
- Etxabide, A.; Uranga, J.; Guerrero, P.; De la Caba, K. Development of active gelatin films by means of valorisation of food processing waste: A review. Food Hydrocoll. 2017, 68, 92–198. [Google Scholar] [CrossRef]
- Ku, G.; Zhou, M.; Song, S.; Huang, Q.; Hazle, J.; Li, C. Copper Sulfide Nanoparticles as a New Class of Photoacoustic Contrast Agent for Deep Tissue Imaging at 1064 Nm. ACS Nano 2012, 6, 7489–7496. [Google Scholar] [CrossRef] [Green Version]
- Dharsana, U.S.; Varsha, M.K.N.S.; Behlol, A.A.K.; Veerappan, A.; Thiagarajan, R.; Sai Varsha, M.K.N.; Khan Behlol, A.A.; Veerappan, A.; Thiagarajan, R. Sulfidation Modulates the Toxicity of Biogenic Copper Nanoparticles. RSC Adv. 2015, 5, 30248–30259. [Google Scholar] [CrossRef]
- Goel, S.; Chen, F.; Cai, W. Synthesis and Biomedical Applications of Copper Sulfide Nanoparticles: From Sensors to Theranostics. Small 2014, 10, 631–645. [Google Scholar] [CrossRef]
- Saldanha, P.L.; Brescia, R.; Prato, M.; Li, H.; Povia, M.; Manna, L.; Lesnyak, V. Generalized One-Pot Synthesis of Copper Sulfide, Selenide-Sulfide, and Telluride-Sulfide Nanoparticles. Chem. Mater. 2014, 26, 1442–1449. [Google Scholar] [CrossRef]
- Li, Y.; Lu, W.; Huang, Q.; Li, C.; Chen, W. Copper Sulfide Nanoparticles for Photothermal Ablation of Tumor Cells. Nanomedicine 2010, 5, 1161–1171. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Hu, N.; Wang, X.; Zhang, Y.S.; Sun, R. Copper Sulfide Nanoparticle/Cellulose Composite Paper: Room-Temperature Green Fabrication for NIR Laser-Inducible Ablation of Pathogenic Microorganisms. ACS Sustain. Chem. Eng. 2017, 5, 2648–2655. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Fabrication of Copper Sulfide Nanoparticles and Limonene Incorporated Pullulan/Carrageenan-Based Film with Improved Mechanical and Antibacterial Properties. Polymers 2020, 12, 2665. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W.; Jaiswal, L. Bioactive Agar-Based Functional Composite Film Incorporated with Copper Sulfide Nanoparticles. Food Hydrocoll. 2019, 93, 156–166. [Google Scholar] [CrossRef]
- Li, F.; Liu, Y.; Cao, Y.; Zhang, Y.; Zhe, T.; Guo, Z.; Sun, X.; Wang, Q.; Wang, L. Copper Sulfide Nanoparticle-Carrageenan Films for Packaging Application. Food Hydrocoll. 2020, 109, 106094. [Google Scholar] [CrossRef]
- Li, M.; Fu, S.; Basta, A.H. Light-induced shape-memory polyurethane composite film containing copper sulfide nanoparticles and modified cellulose nanocrystals. Carbohydr. Polym. 2020, 230, 115676. [Google Scholar] [CrossRef]
- Roy, S.; Shankar, S.; Rhim, J.-W. Melanin-Mediated Synthesis of Silver Nanoparticle and Its Use for the Preparation of Carrageenan-Based Antibacterial Films. Food Hydrocoll. 2019, 88, 237–246. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Effect of CuS Reinforcement on the Mechanical, Water Vapor Barrier, UV-Light Barrier, and Antibacterial Properties of Alginate-Based Composite Films. Int. J. Biol. Macromol. 2020, 164, 37–44. [Google Scholar] [CrossRef]
- Pereda, M.; Ponce, A.G.; Marcovich, N.E.; Ruseckaite, R.A.; Martucci, J.F. Chitosan-Gelatin Composites and Bi-Layer Films with Potential Antimicrobial Activity. Food Hydrocoll. 2011, 25, 1372–1381. [Google Scholar] [CrossRef]
- Yin, Y.; Li, Z.; Sun, Y.; Yao, K. A Preliminary Study on Chitosan/Gelatin Polyelectrolyte Complex Formation. J. Mater. Sci. 2005, 40, 4649–4652. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, P.; Wang, P.; Wang, Z.; Luo, X. Using copper sulfide nanoparticles as cross-linkers of tumor microenvironment responsive polymer micelles for cancer synergistic photo-chemotherapy. Nanoscale 2021, 13, 3723–3736. [Google Scholar] [CrossRef]
- Vogler, E.A. Structure and reactivity of water at biomaterial surfaces. Adv. Colloid Interface Sci. 1998, 74, 69–117. [Google Scholar] [CrossRef]
- Reinsch, B.C.; Levard, C.; Li, Z.; Ma, R.; Wise, A.; Gregory, K.B.; Brown, G.E.; Lowry, G.V. Sulfidation of Silver Nanoparticles Decreases Escherichia Coli Growth Inhibition. Environ. Sci. Technol. 2012, 46, 6992–7000. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Melanin-Mediated Synthesis of Copper Oxide Nanoparticles and Preparation of Functional Agar/CuO NP Nanocomposite Films. J. Nanomater. 2019, 2019, 2840517. [Google Scholar] [CrossRef]
- Ahmed, K.B.A.; Anbazhagan, V. Synthesis of Copper Sulfide Nanoparticles and Evaluation of in Vitro Antibacterial Activity and in Vivo Therapeutic Effect in Bacteria-Infected Zebrafish. RSC Adv. 2017, 7, 36644–36652. [Google Scholar] [CrossRef] [Green Version]


| Films | Gelatin (g) | Glycerol (g) | CuSNP (mg) |
|---|---|---|---|
| Gelatin | 4 | 1.2 | 0 |
| Gel/CuS0.5 | 4 | 1.2 | 20 |
| Gel/CuS1.0 | 4 | 1.2 | 40 |
| Gel/CuS2.0 | 4 | 1.2 | 80 |
| Films | L | a | b | ΔE | T280 (%) | T660 (%) |
|---|---|---|---|---|---|---|
| Gelatin | 91.1 ± 0.1 d | −0.7 ± 0.1 a | 6.6 ± 0.2 a | 2.3 ± 0.3 a | 18.2 ± 0.1 d | 89.5 ± 0.3 d |
| Gel/CuS0.5 | 83.7 ± 1.0 c | −1.3 ± 0.2 b | 9.5 ± 0.6 b | 10.0 ± 1.1 b | 11.0 ± 0.9 c | 78.1 ± 0.5 c |
| Gel/CuS1.0 | 66.5 ± 3.0 b | −2.1 ± 0.3 c | 18.2 ± 1.6 c | 29.2 ± 3.4 c | 3.9 ± 0.8 b | 61.3 ± 1.8 b |
| Gel/CuS2.0 | 55.7 ± 4.0 a | −1.35 ± 0.3 b | 19.1 ± 0.7 c | 39.3 ± 4.0 d | 1.0 ± 0.1 a | 39.5 ± 2.1 a |
| Films | Thickness (μm) | TS (MPa) | EB (%) | EM (GPa) | WCA (Deg.) | WVP (× 10−9 g·m/m2·Pa·s) |
|---|---|---|---|---|---|---|
| Gelatin | 51.8 ± 0.9 ab | 45.0 ± 2.7 a | 8.6 ± 1.7 b | 1.5 ± 0.04 a | 63.4 ± 0.8 a | 2.26 ± 0.37 b |
| Gel/CuS0.5 | 49.8 ± 0.3 a | 58.1 ± 2.4 b | 6.0 ± 0.1 a | 2.3 ± 0.04 b | 63.2 ± 0.5 a | 1.98 ± 0.03 ab |
| Gel/CuS1.0 | 55.6 ± 4.3 b | 65.1 ± 2.4 c | 7.2 ± 0.4 ab | 2.3 ± 0.12 b | 61.1 ± 1.4 a | 1.84 ± 0.09 ab |
| Gel/CuS2.0 | 56.5 ± 3.4 b | 63.4 ± 3.8 c | 6.7 ± 0.2 a | 2.2 ± 0.05 b | 61.4 ± 1.8 a | 1.64 ± 0.14 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roy, S.; Rhim, J.-W. Gelatin-Based Film Integrated with Copper Sulfide Nanoparticles for Active Packaging Applications. Appl. Sci. 2021, 11, 6307. https://doi.org/10.3390/app11146307
Roy S, Rhim J-W. Gelatin-Based Film Integrated with Copper Sulfide Nanoparticles for Active Packaging Applications. Applied Sciences. 2021; 11(14):6307. https://doi.org/10.3390/app11146307
Chicago/Turabian StyleRoy, Swarup, and Jong-Whan Rhim. 2021. "Gelatin-Based Film Integrated with Copper Sulfide Nanoparticles for Active Packaging Applications" Applied Sciences 11, no. 14: 6307. https://doi.org/10.3390/app11146307
APA StyleRoy, S., & Rhim, J.-W. (2021). Gelatin-Based Film Integrated with Copper Sulfide Nanoparticles for Active Packaging Applications. Applied Sciences, 11(14), 6307. https://doi.org/10.3390/app11146307







