Production of Porous Ceramic Materials from Spent Fluorescent Lamps
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Procedure
2.3. Analytical Methods
- IMS-103 and IMS-120: Ultra-scientific multi-standards; multi-element calibration standard-3, 100 mL (IMS-103): 10 mg/l of Sb, Au, Pt, Rh, Hf, Ru, Ir, Te, Pd, and Sn; matrix 10% HCl/1% HNO3; COD: 8500-6948.
- CCS-5: Inorganic-Ventures; multi-standard, 100 mL: 100.00 ± 0.70 µg/mL of B, Ge, Hf, Mo, Nb, P, Re, S, Sb, Si, Sn, Ta, Ti, W, and Zr; matrix HNO3 7.14% + 1% HF v/v.
- Ag1: Agilent; multi-element calibration standard-1, 100 mL: 10 mg/l of Ce, Dy, Er, Eu, Gd, Ho, La, Lu, Nd, Pr, Sc, Sm, Tb, Th, Tm, Y, and Yb; matrix 5% HNO3; N° 8500-6944.
- IV-ICPMS-71A: Inorganic-Ventures; multi-standard, 100 mL: 10 mg/L of Ag, Al, As, B, Ba, Be, Ca, Cd, Ce, Co, Cr, Cs, Cu, Dy, Er, Eu, Fe, Ga, Gd, Ho, K, La, Lu, Mg, Mn, Na, Nd, Ni, P, Pb, Pr, Rb, S, Se, Sm, Sr, Th, Tl, Tm, U, V, Yb, and Zn.
- Tb: ICP TraceCERT®-44881; Terbium standard for ICP: 1000 mg/L of Tb in 2% nitric acid, prepared with high purity Tb4O7, HNO3, and water.
- Hg: 1000 mg/L (HNO3 12% w/w) Fluka.
- Re (internal standard): Standard for ICP 1 72.00 72.00. 1000 mg/L Re in 2% nitric acid, prepared with high purity Re metal, HNO3 and water. COD: 39957.
- Ge (internal standard): Standard for ICP TraceCERT®, 1000 mg/L Ge in 2% nitric acid (contains HF traces), prepared with high purity Ge metal, HNO3, HF and water. COD: 5419.
- The ICP–MS internal standard mixture consisted of 6Li, 45Sc, 72Ge, 103Rh, 115In, 159Tb, 175Lu, and 209Bi at 10 μg/mL in 5% HNO3 (Agilent Technologies, UK).
3. Results and Discussion
3.1. Mineralogical Analysis of Starting Powders
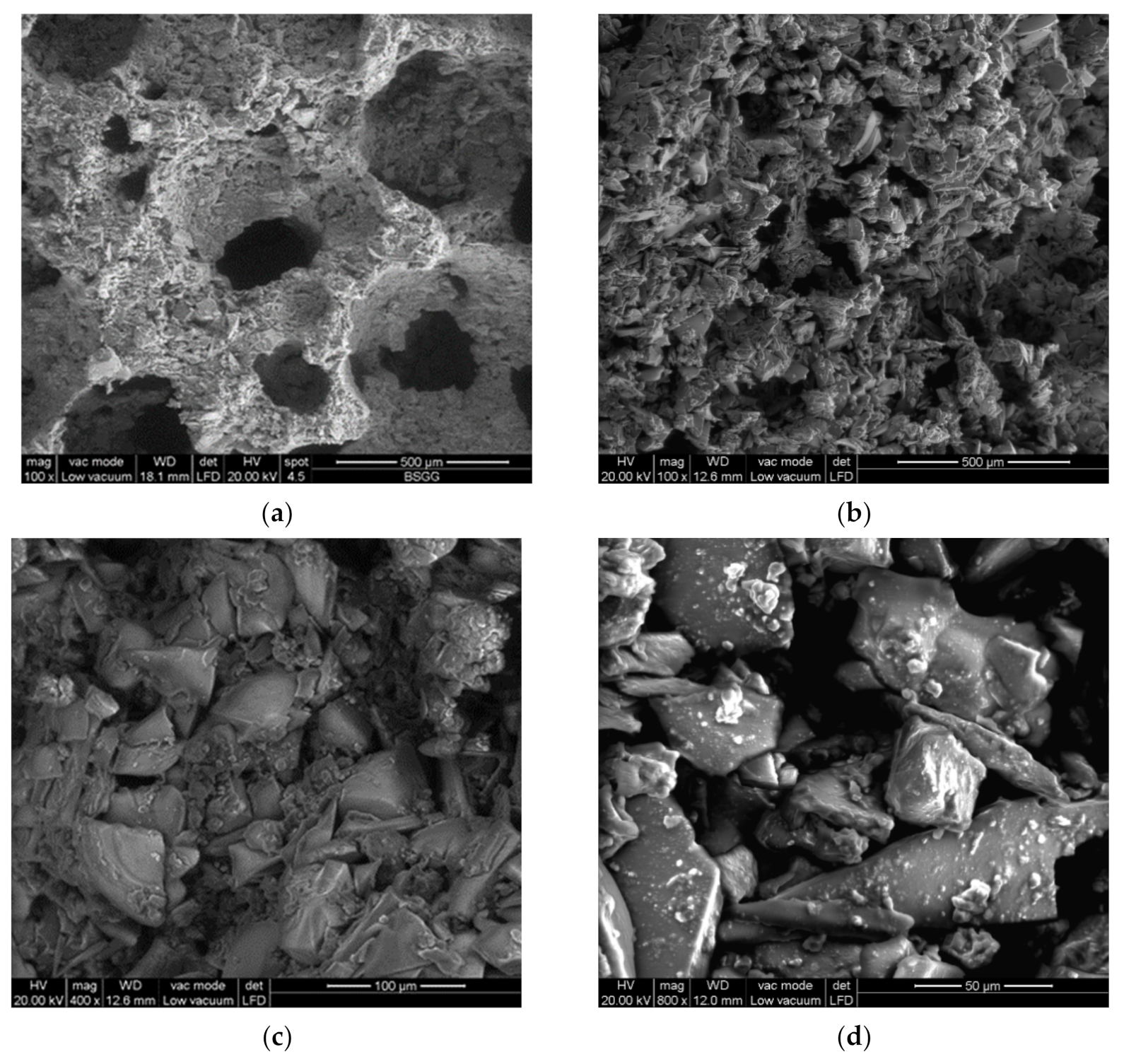
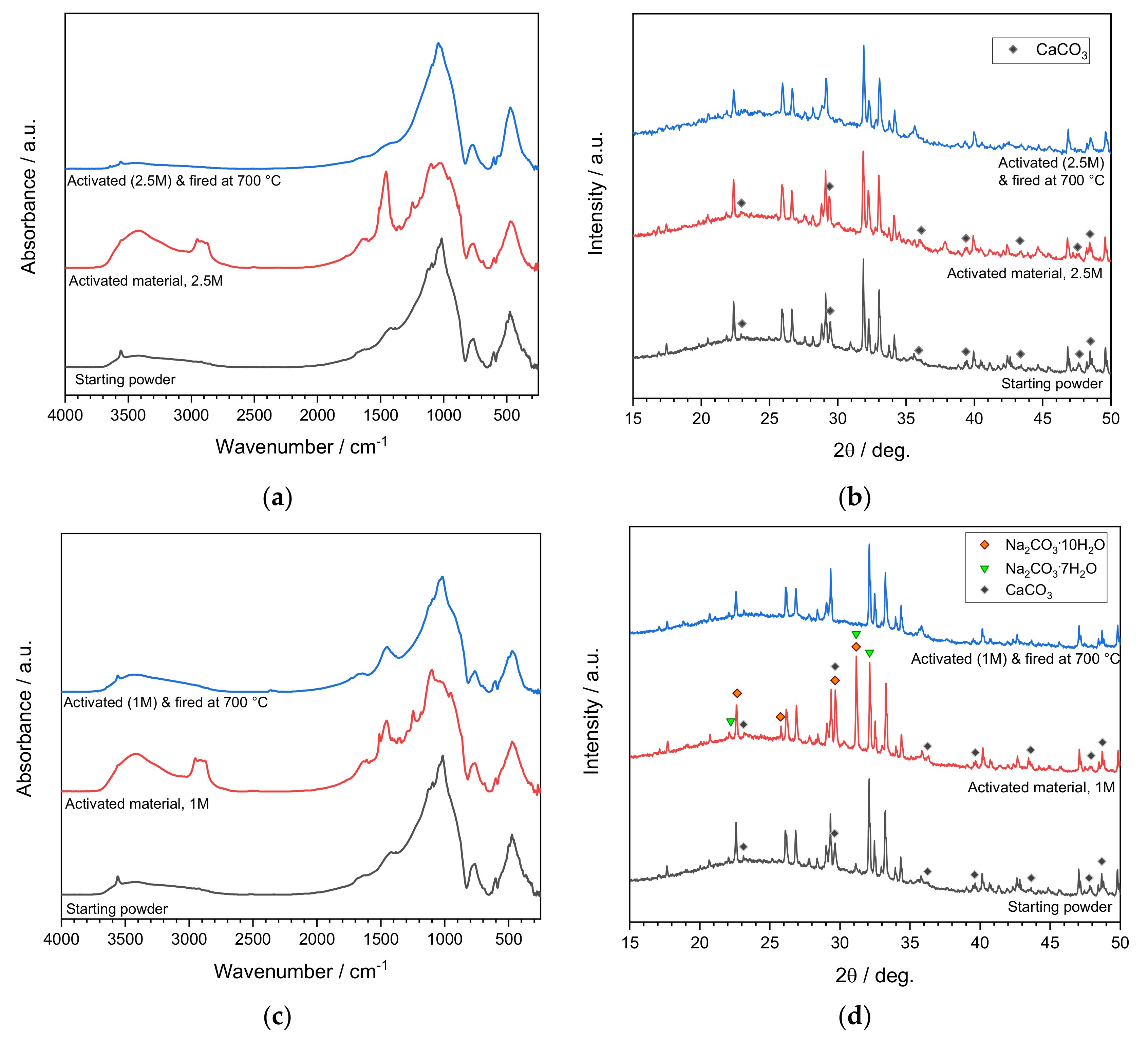
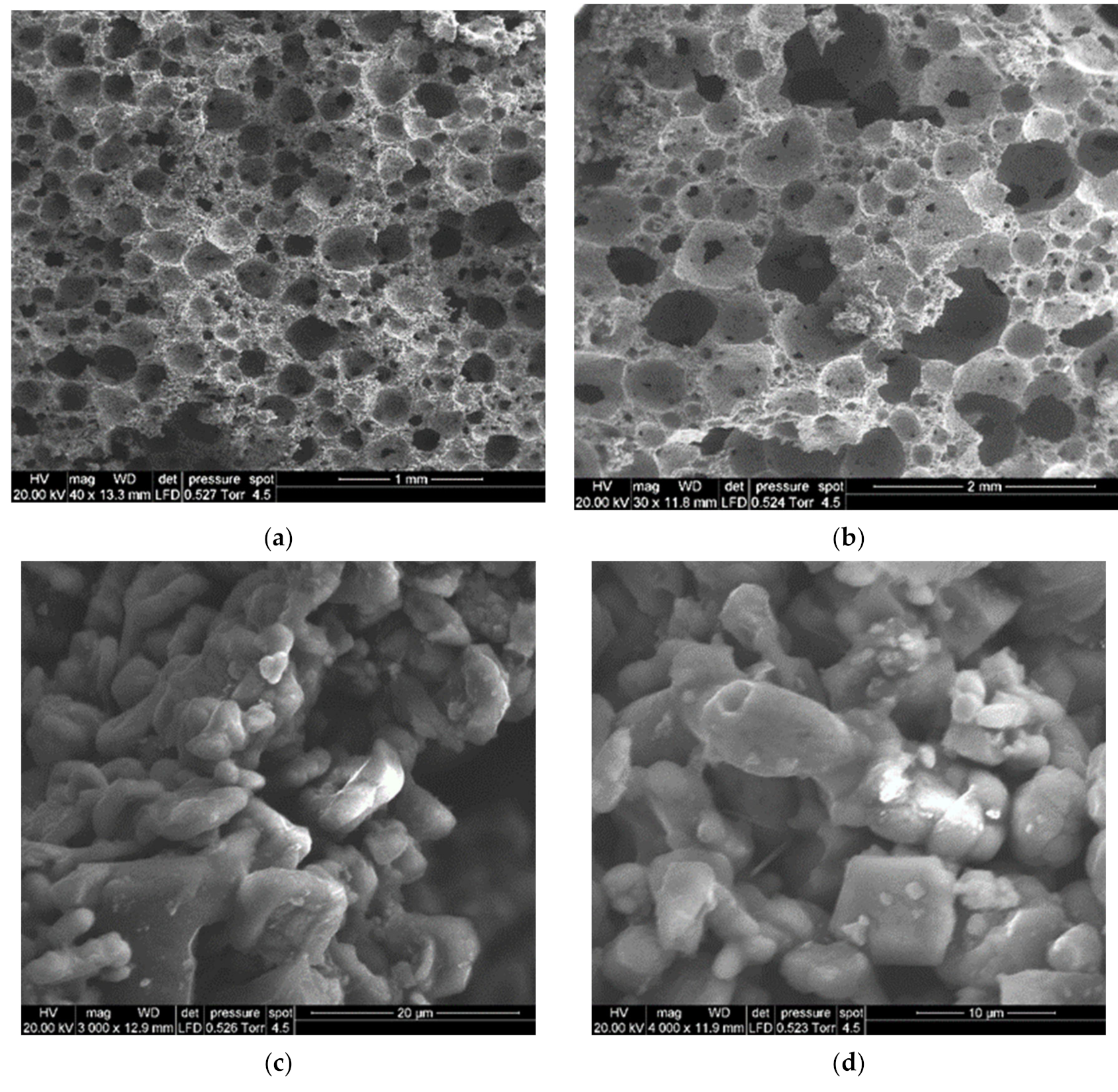
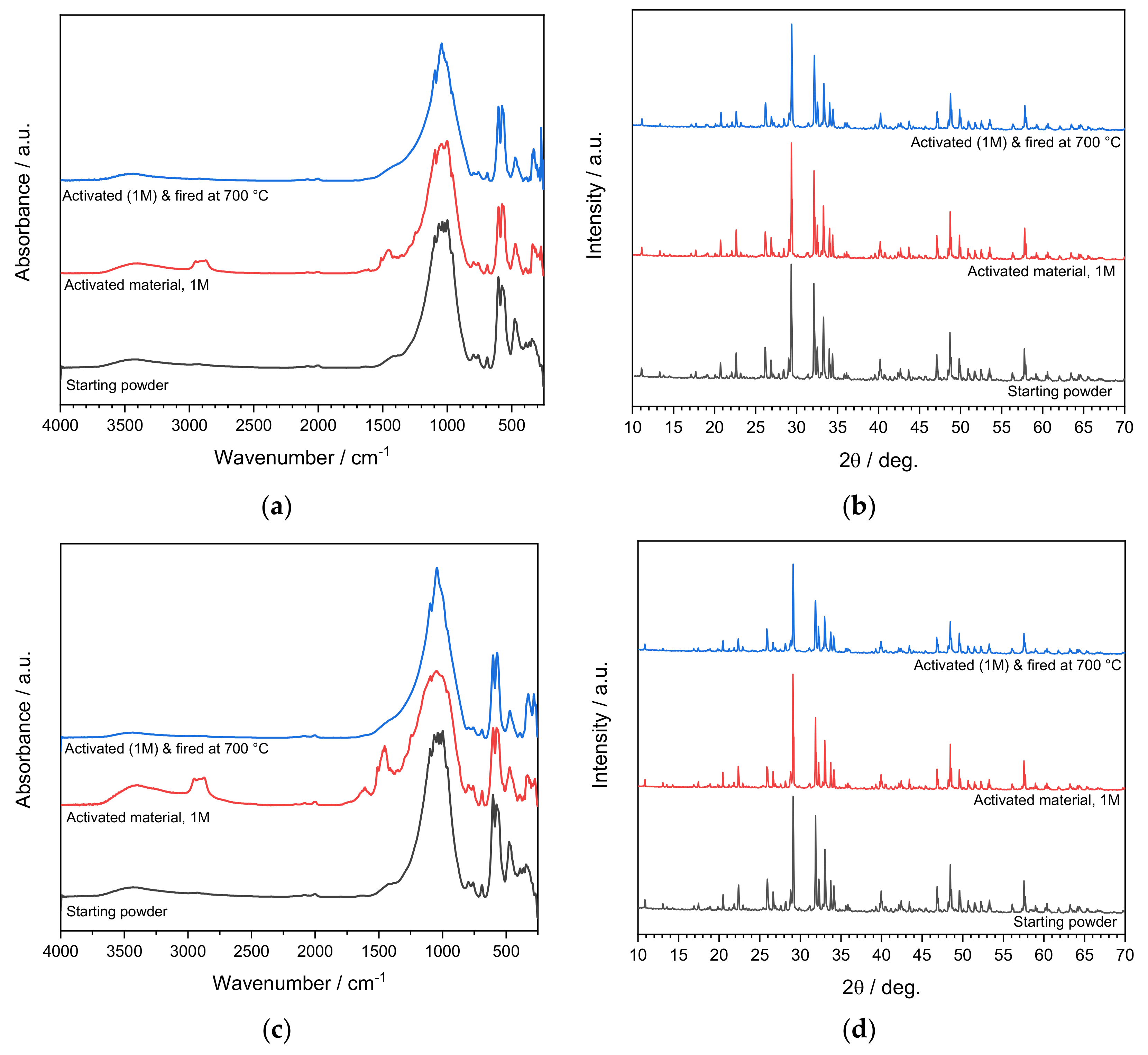
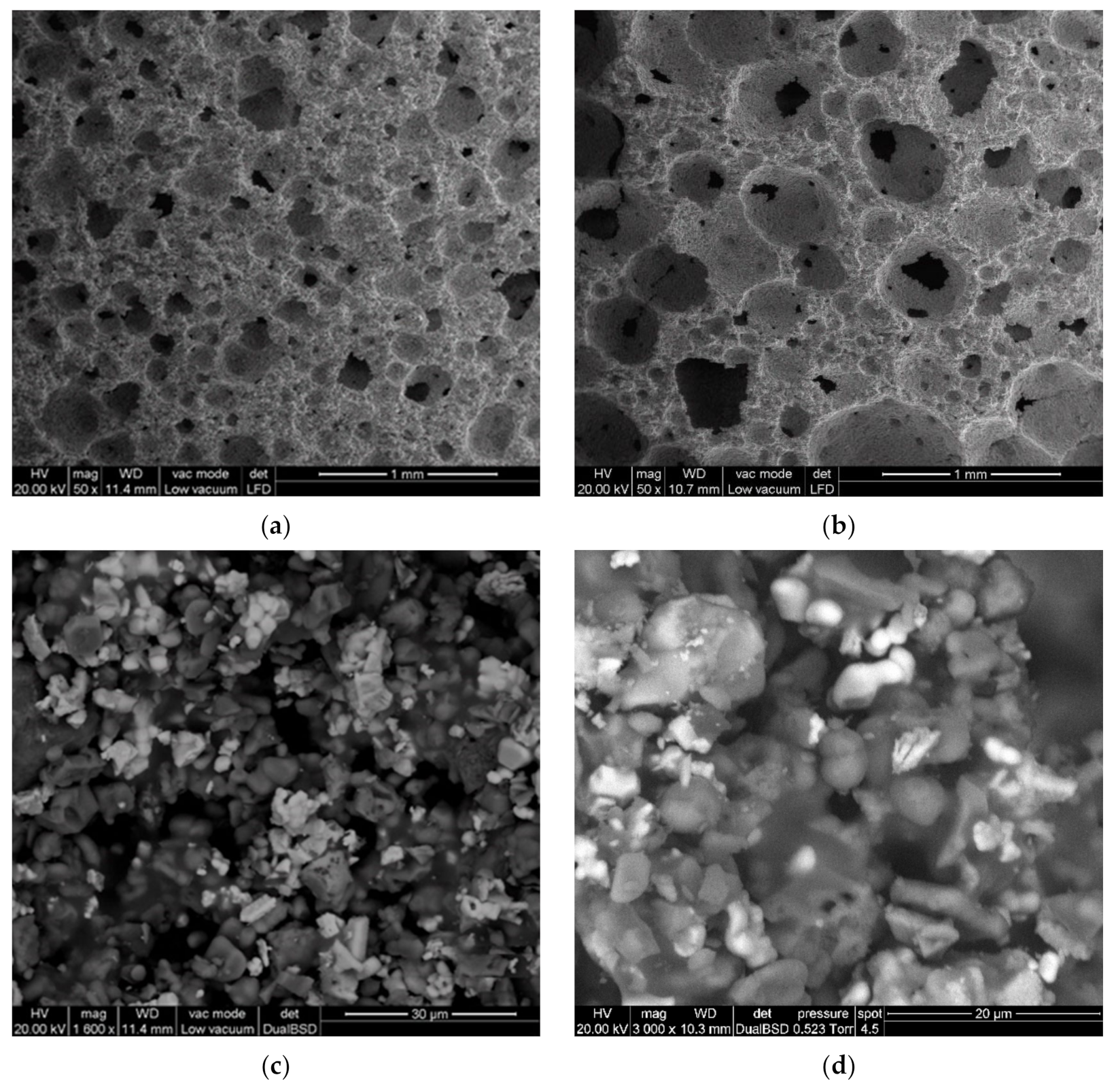
3.2. Activation of Coarse Powders (SFL > 20 μm)
3.3. Activation of Fine Powders (SFL < 20 μm)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Ashby, M.F. Materials and the Environment: Eco-Informed Material Choice, 2nd ed.; Buttersworth-Heinemann: Oxford, UK, 2013. [Google Scholar] [CrossRef]
- Rincón Romero, A.; Giacomello, G.; Pasetto, M.; Bernardo, E. Novel ‘inorganic gel casting’ process for the manufacturing of glass foams. J. Eur. Ceram. Soc. 2017, 37, 2227–2234. [Google Scholar] [CrossRef]
- Bonifazi, G.; Serranti, S. Imaging spectroscopy based strategies for ceramic glass contaminants removal in glass recycling. Waste Manag. 2006, 26, 627–639. [Google Scholar] [CrossRef] [PubMed]
- Farcomeni, A.; Serranti, S.; Bonifazi, G. Non-parametric analysis of infrared spectra for recognition of glass and glass ceramic fragments in recycling plants. Waste Manag. 2008, 28, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Rincón Romero, A.; Marangoni, M.; Cetin, S.; Bernardo, E. Recycling of inorganic waste in monolithic and cellular glass-based materials for structural and functional applications. J. Chem. Technol. Biotechnol. 2016, 91, 1946–1961. [Google Scholar] [CrossRef] [Green Version]
- Petersen, R.R.; König, J.; Yue, Y. The viscosity window of the silicate glass foam production. J. Non-Cryst. Solids 2017, 456, 49–54. [Google Scholar] [CrossRef]
- Chinnam, R.K.; Francis, A.A.; Will, J.; Bernardo, E.; Boccaccini, A.R. Review. Functional glasses and glass-ceramics derived from iron rich waste and combination of industrial residues. J. Non-Cryst. Solids 2013, 365, 63–74. [Google Scholar] [CrossRef]
- Rey-Raap, N.; Gallardo, A. Removal of mercury bonded in residual glass from spent fluorescent lamps. J. Environ. Manag. 2013, 115, 175–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belardi, G.; Ippolito, N.; Piga, L.; Serracino, M. Investigation on the status of rare earth elements contained in the powder of spent fluorescent lamps. Thermochim. Acta 2014, 591, 22–30. [Google Scholar] [CrossRef]
- Krishnamurthy, N.; Gupta, C.K. Extractive Metallurgy of Rare Earths, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar] [CrossRef]
- Song, X.; Chang, M.H.; Pecht, M. Rare-Earth elements in lighting and optical applications and their recycling. JOM 2013, 65, 1276–1282. [Google Scholar] [CrossRef]
- Deshmane, V.G.; Islam, S.Z.; Bhave, R.R. Selective recovery of rare earth elements from a wide range of e-waste and process scalability of membrane solvent extraction. Environ. Sci. Technol. 2020, 54, 550–558. [Google Scholar] [CrossRef]
- Klinger, J.M. Rare earth elements: Development, sustainability and policy issues. Extr. Ind. Soc. 2018, 5, 1–7. [Google Scholar] [CrossRef]
- Lukowiak, A.; Zur, L.; Tomala, R.; LamTran, T.N.; Bouajaj, A.; Strek, W.; Righini, G.C.; Wickleder, M.; Ferrari, M. Rare earth elements and urban mines: Critical strategies for sustainable development. Ceram. Int. 2020, 16, 26247–26250. [Google Scholar] [CrossRef]
- Innocenzi, V. Treatment of spent fluorescent lamps, cathode-ray tubes, and spent catalysts by hydrometallurgical procedures. In Waste Electrical and Electronic Equipment Recycling: Aqueous Recovery Methods; Vegliò, F., Birloaga, I., Eds.; Woodhead Publishing: Sawston, UK, 2018; pp. 139–160. [Google Scholar] [CrossRef]
- Yurramendi, L.; Gijsemans, L.; Forte, F.; Aldana, J.L.; del Río, C.; Binnemans, K. Enhancing rare-earth recovery from lamp phosphor waste. Hydrometallurgy 2019, 187, 38–44. [Google Scholar] [CrossRef]
- Ramteke, D.D.; Hujova, M.; Kraxner, J.; Galusek, D.; Rincón Romero, A.; Falcone, R.; Bernardo, E. Up-cycling of ‘unrecyclable’ glasses in glass-based foams by weak alkali-activation, gel casting and low-temperature sintering. J. Clean. Prod. 2021, 278, 123985. [Google Scholar] [CrossRef]
- Rincón Romero, A.; Salvo, M.; Bernardo, E. Up-cycling of vitrified bottom ash from MSWI into glass-ceramic foams by means of ‘inorganic gel casting’ and sinter-crystallization. Constr. Build. Mater. 2018, 192, 133–140. [Google Scholar] [CrossRef]
- Vaitkus, A.; Merkys, A.; Gražulis, S. Validation of the Crystallography Open Database using the Crystallographic Information Framework. J. Appl. Crystallogr. 2021, 54, 661–672. [Google Scholar] [CrossRef]
- Standard UNI EN 12457-2:2002. Characterisation of Waste—Leaching—Compliance Test for Leaching of Granular Waste Materials and Sludges—Part 2: One Stage Batch Test at a Liquid to Solid Ratio of 10 L/kg for Materials with Particle Size Below 4 mm (Without or With Size Reduction). Available online: https://standards.iteh.ai/catalog/standards/cen/db6fbdf3-1de7-457c-a506-46c4898e3f09/en-12457-2-2002 (accessed on 29 June 2021).
- Badocco, D.; Lavagnini, I.; Mondin, A.; Tapparo, A.; Pastore, P. Limit of detection in the presence of instrumental and non-instrumental errors: Study of the possible sources of error and application to the analysis of 41 elements at trace levels by induced coupled plasma-mass spectrometry technique. Spectroc. Acta Part B Atom. Spectr. 2015, 107, 178–184. [Google Scholar] [CrossRef]
- Ronda, C. Rare-Earth Phosphors: Fundamentals and Applications. Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Jayaramaiah, J.R.; Lakshminarasappa, B.N.; Nagabhushana, B.M. Luminescence studies of europium doped yttrium oxide nano phosphor. Sens. Actuator B Chem. 2012, 173, 234–238. [Google Scholar] [CrossRef]
- Alajlani, Y.; Can, N. Novel Dy and Sm activated BaSi2O5 phosphors: Insights into the structure, intrinsic and extrinsic luminescence, and influence of doping concentration. J. Lumines. 2021, 230, 117718. [Google Scholar] [CrossRef]
- Owens, C.L.; Nash, G.R.; Hadler, K.; Fitzpatrick, R.S.; Anderson, C.G.; Wall, F. Apatite enrichment by rare earth elements: A review of the effects of surface properties. Adv. Colloid Interface Sci. 2019, 265, 14–28. [Google Scholar] [CrossRef]
- Raposo, C.; Carvalhinho Windmöller, C.; Alves Durão Júnior, W. Mercury speciation in fluorescent lamps by thermal release analysis. Waste Manag. 2003, 23, 879–886. [Google Scholar] [CrossRef]
- Rabelo Monich, P.; Rincón Romero, A.; Höllen, D.; Bernardo, E. Porous glass-ceramics from alkali activation and sinter-crystallization of mixtures of waste glass and residues from plasma processing of municipal solid waste. J. Clean Prod. 2018, 188, 871–878. [Google Scholar] [CrossRef]
- García Lodeiro, I.; Macphee, D.E.; Palomo, A.; Fernández-Jiménez, A. Effect of alkalis on fresh C–S–H gels. FTIR analysis. Cem. Concr. Res. 2009, 39, 147–153. [Google Scholar] [CrossRef]
- Black, L.; Breen, C.; Yarwood, J.; Garbev, K.; Stemmermann, P.; Gasharova, B. Structural features of C–S–H(I) and its carbonation in air—a Raman spectroscopic study. Part II: Carbonated phases. J. Am. Ceram. Soc. 2007, 90, 908–917. [Google Scholar] [CrossRef]
- Elsayed, H.; Rincón Romero, A.; Ferroni, L.; Gardin, C.; Zavan, B.; Bernardo, E. Bioactive Glass-Ceramic Scaffolds from Novel ‘Inorganic Gel Casting’ and Sinter-Crystallization. Materials 2017, 10, 171. [Google Scholar] [CrossRef]
- Datta, S.; Vero, S.E.; Hettiarachchi, G.M.; Johannesson, K. Tungsten contamination of soils and sediments: Current state of science. Curr. Pollut. Rep. 2017, 3, 55–64. [Google Scholar] [CrossRef]
- Ventrella, A.; Smeacetto, F.; Salvo, M.; Ferraris, M. A New glass coating for foam glass. Int. J. Appl. Ceram. Technol. 2011, 8, 187–193. [Google Scholar] [CrossRef]
- Ansys Granta EduPack. 2021. Available online: https://www.grantadesign.com/ (accessed on 29 June 2021).


| Oxide | SFL > 20 μm | SFL < 20 μm | SLG [2] |
|---|---|---|---|
| Na2O | 15.2 | − | 13 |
| MgO | 2.6 | − | 2.1 |
| Al2O3 | 4.0 | 8.9 | 2.4 |
| SiO2 | 57.3 | 11.1 | 70.8 |
| P2O5 | 3.5 | 20.0 | − |
| K2O | 1.1 | 0.2 | 1.1 |
| CaO | 9.7 | 34.7 | 9.4 |
| BaO | 2.1 | 6.4 | 0.2 |
| Eu2O3 | 0.3 | 1.1 | − |
| Fe2O3 | 1.8 | 1.0 | 0.3 |
| Y2O3 | 2.4 | 10.8 | − |
| Cl2O | − | 0.1 | − |
| La2O3 | − | 1.8 | − |
| Ce2O3 | − | 2.0 | − |
| MnO | − | 0.8 | − |
| Tb2O3 | − | 0.9 | − |
| ZnO | − | 0.1 | 0.1 |
| B2O3 | − | − | 0.1 |
| Sample | Molarity NaOH (M) | S/L (%) | SLG (wt.%) | Density (g/cm3) | Porosity (%) | Compressive Strength (MPa) |
|---|---|---|---|---|---|---|
| GF-1 | 2.5 | 68 | − | ρgeom: 0.57 ± 0.02 ρapp: 2.57 ± 0.07 ρt: 2.63 ± 0.01 | Total: 78.4 Open: 77.9 Closed: 0.5 | 5.8 ± 0.6 |
| GF-2 | 1.0 | 68 | − | ρgeom: 0.53 ± 0.01 ρapp: 2.58 ± 0.05 ρt: 2.61 ± 0.01 | Total: 79.9 Open: 79.7 Closed: 0.2 | 4.2 ± 0.5 |
| GF-3 | 1.0 | 68 | 10 | ρgeom: 0.85 ± 0.01 ρapp: 3.26 ± 0.01 ρt: 3.27 ± 0.01 | Total: 74.0 Open: 73.9 Closed: 0.1 | 2.2 ± 0.2 |
| GF-4 | 1.0 | 66 | 10 | ρgeom: 0.60 ± 0.01 ρapp: 3.12 ± 0.01 ρt: 3.22 ± 0.01 | Total: 81.5 Open: 80.9 Closed: 0.6 | 1.0 ± 0.1 |
| Element | GF-1 | GF-2 | GF-3 | GF-4 | Limit Values (mg/kg) | ||
|---|---|---|---|---|---|---|---|
| Recycled | Inert | Non-Hazardous | |||||
| Ba | 8.5 | 5.5 | 2.8 | 1.3 | <20 | <20 | 20–100 |
| Cr | 0.11 | 0.27 | 0.05 | 0.08 | <0.3 | <0.5 | 0.5–10 |
| Mo | 0.06 | 0.06 | 0.05 | 0.08 | <0.5 | <0.5 | 0.5–10 |
| V | 0.002 | 0.01 | 0.02 | 0.04 | <1 | <0.5 | 0.5–10 |
| Cu | 0.00199 | <0.00131 | <0.00131 | 0.00141 | <1 | <2 | 2–50 |
| Ni | <6.6 × 10−4 | <6.6 × 10−4 | <6.6 × 10−4 | <6.6 × 10−4 | <0.4 | <0.4 | 0.4–10 |
| Co | <0.3 × 10−4 | <0.3 × 10−4 | <0.3 × 10−4 | <0.3 × 10−4 | <1 | ||
| W | 3.41 | 8.75 | 0.94 | 1.38 | <1.5 | ||
| Hg | 0.004 | 0.010 | 0.001 | 0.002 | <0.04 | <0.01 | 0.01–0.2 |
| Cd | <0.3 × 10−4 | <0.3 × 10−4 | <0.3 × 10−4 | <0.4 × 10−4 | <0.04 | <0.04 | 0.04–0.1 |
| Y | <1.1 × 10−4 | 2.7 × 10−4 | 3.3 × 10−4 | 3.9 × 10−4 | <0.01 | ||
| Eu | 9.4 × 10−4 | 6.0 × 10−4 | 3.2 × 10−4 | 1.8 × 10−4 | <0.01 | ||
| La | 1.0 × 10−4 | 0.6 × 10−4 | 1.5 × 10−4 | 1.2 × 10−4 | <0.01 | ||
| Nb | 0.5 × 10−4 | 1.2 × 10−4 | 0.1 × 10−4 | 0.2 × 10−4 | <0.01 | ||
| Gd | 0.4 × 10−4 | 0.3 × 10−4 | 0.8 × 10−4 | 1.1 × 10−4 | <0.01 | ||
| Sm | 0.3 × 10−4 | 0.2 × 10−4 | 0.1 × 10−4 | 0.1 × 10−4 | <0.01 | ||
| Ce | <0.1 × 10−4 | <0.1 × 10−4 | 0.7 × 10−4 | 1.2 × 10−4 | <0.01 | ||
| U | <0.1 × 10−4 | <0.1 × 10−4 | 0.1 × 10−4 | 0.1 × 10−4 | <0.01 | ||
| Tb | <0.2 × 10−4 | <0.2 × 10−4 | 0.2 × 10−4 | 0.4 × 10−4 | <0.01 | ||
| Cl- | 28.6 | 10.4 | 20.2 | 18.0 | 800 | 800 | 15,000 |
| F- | 1.42 | 2.2 | 3.7 | 0.58 | 10 | 150 | |
| SO42- | 9.6 | 5.7 | 12.1 | 8.4 | 2500 | 1500 | 20,000 |
| pH | 12.68 | 12.18 | 11.81 | 8.55 | |||
| Conduct. (μS/cm) | 876 | 482 | 274 | 395 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosson, E.; Rincón Romero, A.; Badocco, D.; Zorzi, F.; Sgarbossa, P.; Bertani, R.; Pastore, P.; Bernardo, E. Production of Porous Ceramic Materials from Spent Fluorescent Lamps. Appl. Sci. 2021, 11, 6056. https://doi.org/10.3390/app11136056
Rosson E, Rincón Romero A, Badocco D, Zorzi F, Sgarbossa P, Bertani R, Pastore P, Bernardo E. Production of Porous Ceramic Materials from Spent Fluorescent Lamps. Applied Sciences. 2021; 11(13):6056. https://doi.org/10.3390/app11136056
Chicago/Turabian StyleRosson, Egle, Acacio Rincón Romero, Denis Badocco, Federico Zorzi, Paolo Sgarbossa, Roberta Bertani, Paolo Pastore, and Enrico Bernardo. 2021. "Production of Porous Ceramic Materials from Spent Fluorescent Lamps" Applied Sciences 11, no. 13: 6056. https://doi.org/10.3390/app11136056
APA StyleRosson, E., Rincón Romero, A., Badocco, D., Zorzi, F., Sgarbossa, P., Bertani, R., Pastore, P., & Bernardo, E. (2021). Production of Porous Ceramic Materials from Spent Fluorescent Lamps. Applied Sciences, 11(13), 6056. https://doi.org/10.3390/app11136056









