Abstract
Flowers have traditionally been used in the production of various dishes to improve the sensory aspects of food. In this study, the nutritional and functional composition of cactus flowers was evaluated for their possible use in the food industry. The objective of this research was also to identify the main secondary metabolites of flowers by HPLC/ESI/MS analyses. The findings indicated that the most abundant components of the flowers were carbohydrates, followed by ash and proteins. The highest antioxidant capacity, assessed by DPPH• and ABTS•+ assays, was observed in Echinocereus cinerascens, with values of 255.08 and 392.65 µM Trolox equivalents (TE)/g, respectively. These values were also correlated with a high content of phenolic compounds. Forty-four secondary metabolites were detected in total, of which the most predominant corresponded to compounds such as quercetin and isorhamnetin, as well as their derivatives, protocatechuic acid and apigenin. This study indicates that Cactaceae flowers could be a source of nutrients; in addition, the diversity of compounds and their good antioxidant properties suggest that they should be considered as new sources of natural antioxidant compounds in the elaboration of functional products.
1. Introduction
In recent years, there has been a great interest in incorporating edible flowers into human food [1]. Flowers have played an important role in food preparation, being frequently used as a fresh garnish in various dishes, or as a key ingredient in jams, cakes, yogurts, desserts, and ice creams, and even consumed directly as vegetables [2]. In these situations, the of using flowers is to improve the appearance, color, taste, and nutritional value of foods [3]. Throughout history, the use of flowers has been documented, mainly in food processing, in Roman, Chinese, Indian and Middle Eastern cultures [3]. The recent interest in including edible flowers in the human diet is mainly due to their nutritional contribution and the possible beneficial effects for health, as well as the likelihood of preventing some diseases associated with oxidative stress [4,5]. These benefits have been associated with the intake of different bioactive compounds such as flavonoids, anthocyanins, carotenes, terpenoids, alkaloids, vitamins, minerals, peptides, and essential oils [6,7]. Moreover, flowers have been shown to possess diverse biological properties, including antioxidant, antibacterial, antiproliferative, anti-obesity, hypoglycemic, neuroprotective, hepatoprotective, and anti-inflammatory activities [6,8].
Cactaceae are native plants of North America, with flowers which are also used in food. Cactaceae comprise about 1500 species, of which there are approximately 700 species in Mexico, grouped into 68 genera. They are considered an important part of the vegetation of arid and semiarid regions, playing an important role in culture and human populations; indigenous peoples use more than 150 species of Cactaceae, of which 50 are the most commonly cultivated for various purposes [9]. Among them, flowers for human consumption such as agave, garambullo and aloe flowers [10] are grown; these have high contents of bioactive compounds such as phenolic compounds, ascorbic acid, and carotenoids, to which good antioxidant capacities are attributed. In Mexico, in the state of Hidalgo, there is an arid surface area of 39% [11], where Cactaceae such as Cardón (CA) (Cylindropuntia rosea), Xoconostle Ulapa (XU) (Opuntia oligacantha), Xoconostle Cuaresmeño rosado (XC) (Opuntia matudae), and Pitaya (PI) (Echinocereus cinerascens) are very common. These Cactaceae are commercially important mainly for their fruits, although the flowers are also used as food mainly in rural communities in different types of traditional dishes, and are also consumed by animals as fodder [12]. However, in their contribution to the diet and as a health benefit to those who consume them, their nutraceutical properties are unknown. Similarly, the use of these flowers is limited by the difficulty of their application as a possible food ingredient, and the lack of knowledge about their nutritional and functional potentials. There are also no data on the identification of toxic compounds or allergenic and antinutritional factors that could compromise consumer health, or guarantee the quantities that are safe for consumption. In order to begin to obtain knowledge about the flowers of Cardón, Xoconostle and Pitaya as a possible source of bioactive compounds for use in the food industry, the objective of this work was the evaluation of their functional, nutritional composition, and the identification of their main metabolites by HPLC/ESI/MS.
2. Materials and Methods
2.1. Plant Material
Cardón (CA) (Cylindropuntia rosea), Xoconostle Ulapa (XU) (Opuntia oligacantha var Ulapa), Xoconostle Cuaresmeño (XC) (Opuntia matudae var Cuaresmeño rosado), and Pitaya (PI) (Echinocereus cinerascens) were collected at Tetepango, Hidalgo, Mexico (20°06′38″ N; 99°09′11″ W; 2100 m above sea level). The flowers were collected, stored in an ultra-freezer at −76 °C (model 703 Thermo-Scientific, Outside, MA, USA), and subsequently freeze-dried (model 79480 LABCONCO, Kansas City, MI, USA), and ground using a blade mill (Knife Mill Grindomix GM 200, Hahn, Germany). Samples were stored at 5 °C until further analysis.
2.2. Sample Preparation
Prior to antioxidant compound and activity determination, 0.1 g of lyophilized samples was mixed in 10 mL of an ethanol:water mixture (70:30, v/v), ultrasonicated for 15 min at a frequency of 40 kHz at 25 °C (model 32V118A Ultrasonicator bath LSS, China), and subsequently centrifuged at 16,500× g for 10 min at 5 °C (Centrifuge Thermo-Scientific, ST 16R, Osterode am Harz, Germany). The supernatant was then used for the corresponding analysis.
2.3. Nutritional Composition
The official AOAC [13] methods were used for analyses of moisture (925.09), fats (983.23), proteins (950.48), ashes (930.05) and crude fiber (985.29, 993.21). Total carbohydrates were calculated by difference. The energy was determined using the equation described by Chahdoura et al. [14]:
2.4. Determination of Total Carotenoids
The red (CR = capsanthin and capsorubin) and yellow (Cβ = ß-carotene, ß-cryptoxanthin, zeaxanthin) isochromatic fractions of the total carotenoids were evaluated according to Hornero-Mendez and Minguez-Mosquera [15]. Sample preparation was performed using 80% acetone. The absorbance of the samples was measured with a spectrophotometer at 472 nm and 508 nm, respectively. The red and yellow carotenoid measurement values are expressed as mg/g DW.
2.5. Determination of Chlorophyll
Chlorophyll a and b were determined according to the methodology described by Witham et al. [16], with slight modifications. Samples were prepared using 80% acetone. The absorbance was obtained at 645 and 663 nm (model 6715 UV–Vis Jenway spectrophotometer, Staffordshire, UK. The results are expressed as mg/g of DW.
2.6. Determination of Ascorbic Acid
The ascorbic acid content was evaluated as described by Dürüst et al. [17], with modifications. Samples (0.1 g) were prepared with 10 mL of 3% trichloroacetic acid (v/v). The samples were sonicated (model 3510 Branson, China) for 15 min at a frequency of 40 kHz. Subsequently, the samples were centrifuged at 10,000× g for 10 min; then, 2 mL of the supernatant was separated, to which 2 mL of the acetate buffer solution (pH = 4) was added. Briefly, 3 mL of dichloroindophenol and 15 mL of xylene were added. The absorbance was recorded in the spectrophotometer (model 6715 UV/Vis Jenway, Techne Inc., Staffordshire, UK) at a wavelength of 520 nm. The results were reported as milligrams of ascorbic acid per gram of dry weight (mg AA/g DW).
2.7. Determination of Total Phenol
The total phenolic content was determined by the Folin–Ciocalteu method described by Singleton and Rossi [18]. Ethanol at 80% was used for the preparation of the samples. Successively, 0.5 mL of the sample was mixed with 0.5 mL of the 50% Folin–Ciocalteu reagent in water. The mixture was allowed to stand for 7 min; then, 1.5 mL of 7.5% sodium carbonate was added, and the mixture was allowed to react in the dark for 60 min. The absorbance was determined at 725 nm using a UV–Vis spectrophotometer (Jenway, 6715, USA). The results are expressed as milligrams of gallic acid equivalents (mg GAE)/g DW.
2.8. Determination of Total Flavonoid
Flavonoids of flower samples were determined according to the method described by Rosales et al. [19], using the same solvent described for total phenol preparation. Later, 2 mL of the sample was mixed with 1.5 mL of sodium nitrite and 2 mL of water; this mixture was left to stand in the dark for 5 min. Then, 1.5 mL of aluminum trichloride and 1 mL of sodium hydroxide were added to the mixture, and it was allowed to stand for another 20 min. The absorbance was determined at 415 nm using a UV–Vis spectrophotometer (model 6715 UV/Vis Jenway, Techne Inc., Staffordshire, UK). The results are expressed as milligrams of quercetin equivalents (mg QE)/g DW.
2.9. Assessment of Antioxidant Activity
Antioxidant activity was determined using the 2,2-diphenyl-1-picrylhydraill (DPPH•) and 2,2′-azino-bis 3-ethylbenzothiazoline-6-sulfonic acid (ABTS•+) methods. Ethanol at 80% was used for sample preparation. The results are expressed as µM of Trolox equivalents (TE)/g dry weight. The DPPH• assay was performed in accordance with Brand-Williams et al. [20]. Briefly, 0.3 mL of the sample was mixed with 2.7 mL of DPPH• (6 × 10−5 M). The mixture was left in the dark for 1 h at 4 °C; then, the absorbance was determined at 517 nm. The ABTS•+ assay was performed following the method of Re et al. [21]. The ABTS•+ radical was produced by mixing 7 mM ABTS•+ solution with 10 mL of 2.45 mM potassium persulfate in complete darkness for 16 h with constant agitation. The radical ABTS•+ was diluted in 80% ethanol, reaching an absorbance value of 0.7 ± 0.02 at 734 nm. Later, 3.9 mL of diluted ABTS•+ radical was taken and mixed with 0.1 mL of the sample, and subsequently left for 6 min in the dark. The absorbance of the mixture at 734 nm was determined.
2.10. Extraction and Purification of Phenolic Compounds
Extracts were obtained according to the method described by Ascacio-Valdés et al. [22]. The extraction of phenolic compounds was carried out using a mixture of ethanol:water (70:30, v/v), with a 1:16 mass–volume ratio by means of ultrasound at 40 kHz (Branson, Mod. 2510, Marshall Scientific, New Hampshire, USA) for 20 min. The extracts were filtered and subsequently processed by column liquid chromatography (LC) with Amberlite XAD-16 to recover the polyphenolic fraction. Initially, elutions were carried out with distilled water, but then ethanol was used to recover the phenolic fraction of interest.
2.11. Identification of Phenolic Compounds by HPLC/ESI/MS
Following LC with Amberlite XAD-16, compounds were analyzed by high-performance liquid chromatography (HPLC) on a Varian HPLC system, including an automatic injector (VarianProStar 410, Palo Alto, CA, USA), a ternary pump (VarianProStar 230I, USA), and a photodiode array detector (PDA) (VarianProStar 330, Palo Alto, CA, USA). A liquid chromatography ion-trap mass spectrometer (Varian 500-MS IT Mass Spectrometer, CA, USA) equipped with an electrospray ion source was used. Samples (5 µL) were injected into a Denali C18 column (150 mm × 2.1 mm, 3 µm, Grace, USA). The oven temperature was kept at 30 °C. The eluents were formic acid (0.2%, v/v; solvent A) and acetonitrile (solvent B). The following gradient was applied: initial, 3% B; 0–5 min, 9% B linear; 5–15 min, 16% B linear; 15–45 min, 50% B linear. The column was washed and reconditioned. The flow rate was kept at 0.2 mL/min and the elution of phenolic compounds was controlled at 245, 280, 320 and 550 nm. All the effluent (0.2 mL/min) was injected into the source of the mass spectrometer, without division. All mass spectrometer experiments were performed in the [M-H]−1 negative mode. Nitrogen was used as the nebulizer gas and helium as the buffer gas. The ion source parameters were as follows: spraying voltage 5.0 kV; capillary voltage at 90 V; and temperature at 350 °C. Data were collected and processed using MS Workstation software (V 6.9).
2.12. Statistical Analysis
Statistical analyses were performed using JMP 5.0.1 software (A Business Unit of SAS, Statistics Analysis System, v. 9.0). Data were analyzed using analysis of variance (ANOVA) and Tukey’s multiple comparison test; a significance level of p ≤ 0.05 was employed to establish significant differences between the samples. Values were expressed as the mean ± standard deviation, and all experiments were performed in triplicate.
3. Results and Discussion
3.1. Nutritional Composition
The nutritional composition of the flowers is presented in Table 1. The highest moisture content was found in flowers of Xoconoxtle Cuaresmeño (XC) (6.72 g/100 g of dry weight), and the lowest content (4.45 g/100 g of dry weight) was observed in flowers of Cardon (CA).

Table 1.
Nutritional composition of cactus flowers from Hidalgo, Mexico.
In general, flowers had the highest carbohydrate content, followed by ash and proteins. The carbohydrate contents ranged from 55.83 g/100 g DW (PI) to 67.44 g/ 100 g DW (CA). These values are higher than those reported by Ammar et al. [23] in flowers of nopal (Opuntia ficus-indica L.) and nopal tunero (Opuntia stricta), and by Navarro-González et al. [24] in flowers of paracress (Spilanthes oleracea L.), nasturtium (Tropaeolum majus L.) and Mexican marigold (Tagetes erecta L.). However, Fernandes et al. [3] reported similar values in edible flowers of erythrina (Erythrina caribaea) and rugosa rose (Rosa micrantha). Meanwhile, Pires et al. [25] reported higher values in marigold flowers (Calendula officinalis L.), dahlia (Dahlia mignon), Damascus rose (Rosa × damascena), and cornflower (Centaurea cyanus L.).
The ash content ranged from 11.21 g/100 g DW (CA) to 17.73 g/100 g DW (Xoconoxtle Ulapa (XU)). A relatively high ash content was found compared to values reported by González-Barrio et al. [26] in pansy flowers (Viola × wittrockiana) and dragoncillo (Antirrhinum majus L.), and by Pinedo-Espinosa et al. [10] in agave (Agave salmiana), aloe (Aloe vera), coral tree (Erythrina americana) and garambullo (Myrtillocactus geometrizans) flowers. On the other hand, Fernandes et al. [27] reported higher values in blue borage flowers (Borago officinalis L.) and in pansy flower varieties (Viola × wittrockiana).
The results of the protein and fat contents were consistent with those reported by Ammar et al. [23] in flowers of nopal (Opuntia ficus-indica L.) and nopal tunero (Opuntia stricta). On the other hand, Pires et al. [25] reported a lower protein content in cornflower flowers (Centaurea cyanus L.), dahlia (Dahlia mignon), calendula (Calendula officinalis L.), and Damascus rose (Rosa × damascena). However, the fat content was similar to values reported in Table 1. In other reports, higher protein and fat contents were found in nasturtium flowers (Tropaeolum majus L.), Begonia (Begonia × semperflorens), and yellow cosmos (Cosmos sulphureus) [28].
The results obtained for crude fiber, with values from 8.47 g/100 g DW (CA) to 11.52 g/100 g DW (XU, Pitaya (PI)), were lower compared than those reported by Ammar et al. [23] in flowers of nopal (Opuntia ficus-indica L.) and nopal tunero (Opuntia stricta) and from observations by González-Barrio et al. [26] in pansy flowers (Viola × wittrockiana) and dragoncillo (Antirrhinum majus L.). However, similar values were described in flowers from agave (Agave salmiana), aloe (Aloe vera), coral tree (Erythrina americana), and garambullo (Myrtillocactus geometrizans) reported by Pinedo-Espinosa et al. [10]. The flowers of CA, XU, XC and PI offered high energy values corresponding to 1300 kJ/100 g of fresh weight. This was due to the high content of carbohydrates present in the samples: higher than 55%. According to the obtained results, cactus flowers could be considered as a paired protein source in the diet, which is similar to other flowers and vegetables. In previous studies, it has been shown that edible flowers are a complementary nutritional source, with a composition similar to that of other plant foods, characterized by low lipid content, medium protein and fiber contents, and variable carbohydrate and mineral contents [25,26,28].
3.2. Content of Total Carotenoids
The contents of antioxidant compounds in the flowers of CA, XU, XC and PI are presented in Table 2. The red carotenoids (capsanthin and capsorubin) were the pigments with the highest presence in the flowers of XU, with values from 0.16 mg/g DW to 0.24 mg/g DW for PI, whereas the yellow carotenoids (β-carotene, β-cryptoxanthin and zeaxanthin), were only detected in XC with a minimum content of 0.02 mg/g DW. Thus far, there have been no reports of the carotenoid contents in these species of cacti; however, Chensom et al. [28] reported yellow carotenoids in yellow cosmos (Cosmos sulphureus) and spring (Primula × polyantha) flowers. Pinedo-Espinosa et al. [10] described higher contents of these secondary metabolites (red and yellow) in agave (Agave salmiana), aloe (Aloe vera), coral tree (Erythrina americana) and garambullo (Myrtillocactus geometrizans) flowers. The contents of total carotenoids shown in Table 2 are similar to those previously reported in pansy flowers (Viola × wittrockiana) and dragoncillo (Antirrhinum majus L.) [26]. However, for pitito flower (Tropaeolum pentaphyllum), cornflower (Centurea cyanus L.), elderberry (Sambucus nigra L.), chamomile (Matricaria L.) and Vulneraria (Anthyllis vulneraria L.), lower values were found, as reported by De Bona et al. [29] and Nowicka et al. [30]. Similar outcomes have been obtained in other studies, in which the carotenoid content varies according to the species of flower under study [31]. Carotenoids are found in all anatomical parts of the flower: sepals, pollen, anthers, stamens, and petals [32]. Consuming foods rich in carotenoids, including edible flowers, can alleviate diabetic retinopathy symptoms, improve glutathione peroxidation, and lower blood cholesterol levels, which ultimately contributes to efficient protection from nontransmissible chronic diseases [26,33].

Table 2.
Antioxidant compounds in cactus flowers from Hidalgo, Mexico.
3.3. Content of Chlorophyll
Chlorophyll was detected in all the analyzed flowers, with values ranging from 16.43 mg/g DW (XU) to 29.03 mg/g DW (CA) (Table 2). The chlorophyll b values ranged from 21.52 mg/g DW (XU) to 36.99 mg/g DW (CA), while the content of total chlorophyll ranged from 37.95 mg/g DW (XU) to 66.02 mg/g DW (CA). Although there are no previous reports of the chlorophyll contents in these flowers, De Bona et al. [29] reported a chlorophyll a content of 0.51 mg/g DW and chlorophyll b content of 0.95 mg/g DW in pyrite flowers (Tropaeolum pentaphyllum).
3.4. Content of Ascorbic Acid
The highest content of ascorbic acid was detected in the PI flowers with 11.41 mg (AA)/g DW, whereas the rest of the flowers presented minimum concentrations (Table 2). The results in this study for CA, XU and XC flowers were lower compared to those reported in nasturtium flowers (Tropaeolum majus L.) [34], agave (Agave salmiana), aloe (Aloe vera), coral tree (Erythrina americana) and garambullo (Myrtillocactus geometrizans) [10]. Similarly, higher ascorbic acid contents were reported in other flowers including wild bergamot (Monarda didyma L.), dragonfly (Antirrhinum majus L.), Chinese carnation (Dianthus chinensis L.) and morning lily (Hemerocallis × hybrida) [35]. However, PI stands out for its high content of ascorbic acid compared to the data reported in the literature on edible flowers. In this regard, ascorbic acid is considered a natural organic compound, with powerful antioxidant properties [36].
3.5. Content of Total Phenols
Significant differences were observed in the phenol content in CA, XU and XC flowers (Table 2). The highest concentration of phenolic compounds was observed in PI, with a value up to eight times higher with respect to the flowers of CA, XU and XC.
These results are similar to those reported by Li et al. [37], who evaluated 51 edible flowers and reported phenol contents ranging between 0.13 and 11.48 mg of gallic acid equivalents (mg GAE)/g DW. On the other hand, Navarro-González et al. [24] described values between 6.64 and 26.63 mg GAE/g DW in other edible flowers; however, the values found in this study turned out to be lower than those reported by Kaisoon et al. [38], who stated values of 138.2 to 212.9 mg GAE/g DW in flowers from Thailand. Several studies have shown that edible flowers are an alternative source of phenolic compounds, and the high content of phenolic compounds has been directly correlated with antioxidant activity [26,39,40]. These compounds are widely distributed in vegetables, fruits, cereals, and edible flowers [41,42]; they are important antioxidant compounds due to their ability to eliminate free radicals [6,43].
3.6. Content of Total Flavonoids
PI presented the highest flavonoid content in comparison with the flowers of CA, XU and XC, with a difference in content up to fourfold higher (Table 2). High concentrations of flavonoids were found compared to those in studies reported by da Silva et al. [40] in 22 types of pansy flowers (Viola × wittrockiana), and by Pinedo-Espinosa et al. [10] in agave (Agave salmiana) and garambullo (Myrtillocactus geometrizans) flowers. The flavonoids present in edible flowers have biological importance, mainly as antioxidants with protective effects against cardiovascular diseases, antitumor activity, and anti-inflammation properties, among others [5,44]. Nevertheless, the antioxidant activity of flavonoids depends mainly on its chemical structure, where the positions and number of hydroxyl or methoxyl groups in the ring play an important role [45].
3.7. Antioxidant Activity
The antioxidant activity values by the DPPH• assay ranged from 11.23 to 255.08 µM Trolox equivalents (µM TE)/g DW; those of the ABTS•+ assay ranged from 20.47 to 392.65 µM TE/g DW (Table 3). The PI flowers exhibited a high antioxidant capacity in both methods, with values 10–22-fold higher than the rest of the flowers (CA, XU and XC). Conversely, the flowers of XU presented the minimum values in both methods. The results by means of the DPPH• test displayed high antioxidant activity compared to results reported by Zheng et al. [46] in flowers of cucumber (Cucumis sativus L.), globe amaranth (Gomphrena globosa L.), and day lily (Hermerocallis citrina). Nonetheless, they were lower compared to those reported by Chen et al. [47] in red plum (Prunus mume), globe amaranth (Gomphrena globosa L.) and carnation (Dianthus caryophyllus L.) flowers, as well as values reported by Barros et al. [48] in flowers of nasturtium (Tropaeolum majus L.), paracress (Spilanthes oleracea L.) and amaranth (Amaranthus hypochondriacus L.). Additionally, the antioxidant activity in petals of May rose (Rosa × centifolia), forget-me-not (Myosotis sylvatica) and rugosa rose (Rosa rugosa) was higher than that found in PI [46].

Table 3.
Antioxidant activity of cactus flowers from Hidalgo, Mexico.
The antioxidant activity values obtained using the ABTS•+ method, were found to be higher compared to the DPPH• assay, which can be explained by the differences in sensitivity between both methods [49]. Except for PI, the antioxidant activity of cactus flowers was higher compared to saffron flowers (Crocus sativus L.) and globe amaranth (Gomphrena globosa L.) reported by Zheng et al. [46], but lower than values reported by Chen et al. [47] in flowers of globe amaranth (Gomphrena globosa L.), calendula (Calendula officinalis L.), and red plum (Prunus mume).
PI, CA, XU and XC flowers could be considered as rich sources of antioxidant compounds with possible beneficial effects on health. Edible flowers have been shown to be a rich source of bioactive compounds such as anthocyanins, vitamins, carotenoids, polyphenols, and flavonoids [6].
3.8. Phenolic Compound Identification by HPLC/ESI/MS
The extraction yields of bioactive compounds obtained for the hydroethanol extract were, 11.53% for Cardón, 16.92% for Xoconoste ‘Ulapa’, 12.18% for Xoconostle ‘Cuaresmeño’ rosado and 27.23% for Pitaya.
It was possible to detect 28 total compounds, of which 19 compounds were unique. The main compounds identified were flavonols, flavones, anthocyanins, catechins, hydroxybenzoic acids, hydroxycinnamic acids, methoxyflavones, methoxycinnamic acids, methoxyflavonols, lignans, and proanthocyanidin trimers. Table 4 shows the tentative identification of phenolic compounds, where the peaks separated by HPLC/ESI/MS were identified according to their molecular weight using the VARIAN Work Station database (version 2.0). In Figure 1, the chromatographic profiles of phenolic compounds separated by HPLC are presented.

Table 4.
Identification of polyphenolic compounds by HPLC/ESI/MS of cactus flowers from Hidalgo, Mexico.
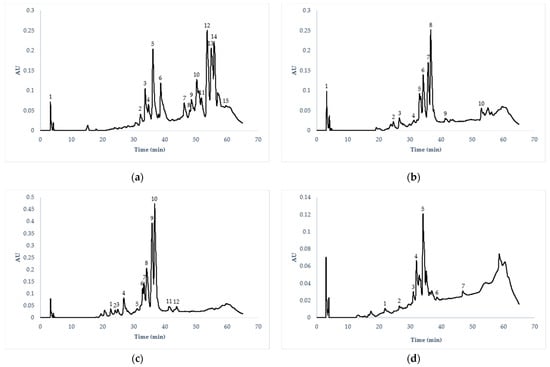
Figure 1.
Chromatographic profiles of the main phenolic compounds of cactus flowers from Hidalgo, Mexico. (a) Cylindropuntia rosea (CA): (1, 10, 15) hydroxycinnamic acids, (5) anthocyanins, (2, 3, 4, 6, 12) flavonols, (7, 11, 14) hydroxybenzoic acids, (13) methoxyflavones, (9) proanthocyanidin trimers. (b) Opuntia oligacantha (XU): (1, 2, 3) methoxycinnamic acids, (4, 10) methoxyflavones, (6) flavonols, (7, 8) methoxyflavonols. (c) Opuntia matudae (XC): (1, 2) methoxycinnamic acids, (4) lignans, (5) methoxyflavones, (8) flavonols, (9, 10) methoxyflavonols, (12) anthocyanins. (d) Echinocereus cinerascens (PI): (1, 2) catechins, (3, 4, 5) flavanols, (7) methoxyflavonols.
The presence of 15 compounds was detected in CA, while 10, 12 and 17 compounds were discovered in XU, XC, and PI, respectively. All elutions were recorded at 280 nm. According to the chromatogram, the main compounds detected in the CA flowers were identified as flavanols (peak 12, with Rt 54.47 min and m/z 301, identified as quercetin), followed by hydroxybenzoic acids (peak 14, with Rt 56.59 min and m/z 315, identified as protocatechuic acid 4-O-glucoside), and methoxyflavones (peak 13, with Rt 55.73 and m/z 283, identified as Geraldone).
In the flowers of XU and XC, the main compounds detected were identified as isorhamnetin 3-O-glucoside methoxyflavonols (peak 8, with Rt 37.63 and m/z 476.9 in XU, and peak 10, with Rt 37.67 min and m/z 476.9 in XC), followed by isorhamnetin 3-O-glucoside 7-O-rhamnoside (peak 7, with Rt 36.67 and m/z 622.9 in XU, and peak 9, with Rt 37.73 and m/z 622.9 in XC), and flavanols such as quercetin 3-O-glucoside (peak 6, with Rt 35.24 and m/z 462.8 in XU and peak 8, with Rt 35.13 min and m/z 462.9 in XC). In PI flowers, the main compound detected was identified as flavone apigenin 6, 8-di-C-glucoside (peak 5, with Rt 35.10 min and m/z 592.1), followed by flavonols such as quercetin 3-O-xylosil-glucoronide (peak 4, with Rt 32.87 and m/z 609.1), and methoxyflavonols such as 3,7-dimethylquercetin (peak 7, with Rt 48.14 and m/z 329.2).
Quercetin and its derivatives were the main secondary metabolites found in the flowers analyzed in this study; it is a natural flavonoid widely distributed in plants, fruits and vegetables in a glycosylated form. The glycosides and rutinosides improve the biological activity of quercetin aglycone, with potential pharmacological effects such as the prevention of chronic diseases and some types of cancer due to its antioxidant action against free radical damage [50].
Isorhamnetin and its derivatives were also detected; according to the literature, it has been shown that they have preventive effects against neurodegenerative diseases, in addition to exhibiting pharmacological effects against cardiovascular diseases [51,52]. It has been reported that isorhamnetin and its derivatives have potential anti-inflammatory activity through the suppression of cellular infiltration, inhibiting the production of nitric oxide, the activity of COX-2, and the secretion of cytosines [53].
Other important compounds such as derivatives of protocatechuic acid and apigenin were also detected. These compounds also have pharmacological effects against some diseases. Protocatechuic acid and its derivatives act as antioxidant, anti-inflammatory, antimicrobial, anticancer, and antidiabetic agents [54,55]. Studies in cell cultures and animal models have also revealed that apigenin and its derivatives exhibit anticancer effects by preventing cell proliferation in various malignant tumors (liver, pancreas, blood, prostate, breast, thyroid, skin), in addition to its antioxidant, antiviral, antidepressant, antimicrobial, cardioprotective, antimutagenic, and anti-inflammatory activities [56,57].
Compounds of biological importance were discovered in this investigation, and this is relevant due to the scarcity of previous studies present in the literature regarding the identification of compounds in these species of cactus flowers.
4. Conclusions
The flowers of Cardón (Cylindropuntia rosea), Xoconostle ‘Ulapa’ (Opuntia oligacantha), Xoconostle ‘Cuaresmeño’ rosado (Opuntia matudae) and Pitaya (Echinocereus cinerascens) presented acceptable contents of proteins, ashes and carbohydrates, with nutritional compositions similar to calendula, nasturtium and pansy flowers. The flowers studied are a promising source of antioxidant compounds, highlighting the Pitaya flower due to a higher content of phenols and flavonoids, which favored greater antioxidant effects. In the profile of the polyphenolic compounds, the main compounds found were quercetin, quercetin 3-O-acetyl-rhamnoside, protocatechuic acid 4-O-glucoside, isorhamnetin 3-O-glucoside, isorhamnetin 3-O-glucoside 7-O-rhamnoside, apigenin 6,8-di-C-glucoside and quercetin 3-O-xylosyl-glucuronide. According to the results reported in this study, these flowers can be an alternative source of nutrients and antioxidant compounds, and could therefore be included in the development of functional foods; however, it is recommended for complementary studies to be carried out, such as identifying the median lethal dose (LD50), as well as any possible antinutritional components.
Author Contributions
Conceptualization, C.A.P.-N., J.A.-M. and A.D.H.-F.; methodology, C.A.P.-N., A.D.H.-F. and J.A.A.-V.; investigation, C.A.P.-N., A.D.H.-F.; resources, A.D.H.-F. and R.G.C.-M.; writing—original draft preparation, C.A.P.-N.; writing—review and editing, A.D.H.-F., J.A.-M. and E.R.-M.; visualization, A.D.H.-F.; supervision, A.D.H.-F. and J.A.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The author C.A.P.N. thanks Consejo Nacional de Ciencia y Tecnología (CONACyT/Scholarship No. 733096) for the grant provided for this research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Matyjaszczyk, E.; Śmiechowska, M. Edible flowers. Benefits and Risks Pertaining to Their Consumption. Trends Food Sci. Technol. 2019, 91, 670–674. [Google Scholar] [CrossRef]
- Takahashi, J.A.; Rezende, F.A.G.G.; Moura, M.A.F.; Dominguete, L.C.B.; Sande, D. Edible flowers: Bioactive profile and its potential to be used in food development. Food Res. Int. 2020, 129, 108868. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, L.; Casal, S.; Pereira, J.A.; Saraiva, J.A.; Ramalhosa, E. Edible Flowers: A Review of the Nutritional, Antioxidant, Antimicrobial Properties and Effects on Human Health. J. Food Compost. Anal. 2017, 60, 38–50. [Google Scholar] [CrossRef]
- Grzeszczuk, M.; Stefaniak, A.; Pachlowska, A. Biological value of various edible flower species. Acta Sci. Pol. Hortorum Cultus 2016, 15, 109–119. [Google Scholar]
- Nanda, B.L. Antioxidant and Anticancer Activity of Edible Flowers. JDDT 2019, 9, 290–295. [Google Scholar] [CrossRef]
- Kumari, P.; Ujala; Bhargava, B. Phytochemicals from edible flowers: Opening a new arena for healthy lifestyle. J. Funct. Foods 2021, 78, 104375. [Google Scholar] [CrossRef]
- Zheng, J.; Lu, B.; Xu, B. An update on the health benefits promoted by edible flowers and involved mechanisms. Food Chem. 2021, 340, 127940. [Google Scholar] [CrossRef]
- Pires, T.C.S.P.; Dias, M.I.; Barros, L.; Calhelha, R.C.; Alves, M.J.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Edible flowers as sources of phenolic compounds with bioactive potential. Food Res. Int. 2018, 105, 580–588. [Google Scholar] [CrossRef] [Green Version]
- Salazar, J.R.; Loza-Mejía, M.A.; Soto-Cabrera, D. Chemistry, biological activities and in silico bioprospection of sterols and triterpenes from Mexican columnar cactaceae. Molecules 2020, 25, 1649. [Google Scholar] [CrossRef] [Green Version]
- Pinedo-Espinoza, J.M.; Gutiérrez-Tlahque, J.; Santiago-Saenz, Y.O.; Aguirre-Mancilla, C.L.; Reyes-Fuentes, M.; López-Palestina, C.U. Nutritional Composition, Bioactive Compounds and Antioxidant Activity of Wild Edible Flowers Consumed in Semiarid Regions of Mexico. Plant Foods Hum. Nutr. 2020, 75, 413–419. [Google Scholar] [CrossRef]
- Olson, D.M.; Dinerstein, E.; Wikramanayake, E.D.; Burgess, N.D.; Powell, G.V.; Underwood, E.C.; Kassem, K.R. Ecorregiones terrestres del mundo: Un nuevo mapa de la vida en la Tierra Un nuevo mapa global de ecorregiones terrestres proporciona una herramienta innovadora para conservar la biodiversidad. BioScience 2001, 51, 933–938. [Google Scholar] [CrossRef]
- Pérez-Escandón, B.E.; Villacencio-Nieto, M.A.; Ramírez-Aguirre, A. Lista de las Plantas Útiles del Estado de Hidalgo, Primera edn. Universidad Autónoma del Estado de Hidalgo, Hidalgo 2003. Available online: https://books.google.com.mx/books/about/Lista_de_las_plantas_%C3%BAtiles_del_Estado.html?id=m5L3tqHwGn8C&redir_esc=y (accessed on 10 June 2021).
- Association of Official Analytical Chemists International (AOAC). Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Chahdoura, H.; Morales, P.; Barreira, J.C.; Barros, L.; Fernández-Ruiz, V.; Ferreira, I.C.; Achour, L. Dietary fiber, mineral elements profile and macronutrients composition in different edible parts of Opuntia microdasys (Lehm.) Pfeiff and Opuntia macrorhiza (Engelm.). LWT Food Sci. Technol. 2015, 64, 446–451. [Google Scholar] [CrossRef] [Green Version]
- Hornero-Méndez, D.; Mínguez-Mosquera, M.I. Rapid spectrophotometric determination of red and yellow isochromic carotenoid fractions in paprika and red pepper oleoresins. J. Agric. Food Chem. 2001, 49, 3584–3588. [Google Scholar] [CrossRef] [PubMed]
- Witham, F.F.; Blaydes, D.F.; Devlin, R.M. Experiments in Plant Physiology; Van Nostrand Rteinhold Company: New York, NY, USA, 1971; pp. 241–242. [Google Scholar]
- Dürüst, N.; Sümengen, D.; Dürüst, Y. Ascorbic acid and element contents of foods of Trabzon (Turkey). J. Agric. Food Chem. 1997, 45, 2085–2087. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Rosales, M.A.; Cervilla, L.M.; Sánchez-Rodríguez, E.; Rubio-Wilhelmi, M.D.M.; Blasco, B.; Ríos, J.J.; Ruiz, J.M. The effect of environmental conditions on nutritional quality of cherry tomato fruits: Evaluation of two experimental Mediterranean greenhouses. J. Sci. Food Agr. 2011, 91, 152–162. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C.L.W.T. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Bio. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Ascacio-Valdés, J.; Aguilera-Carbó, A.; Martínez-Hernández, J.; Rodríguez-Herrera, R.; Aguilar, C. Euphorbia antisyphilitica residues as a new source of ellagic acid. Chem. Pap. 2010, 64, 528–532. [Google Scholar] [CrossRef]
- Ammar, I.; Ennouri, M.; Bali, O.; Attia, H. Characterization of two prickly pear species flowers growing in Tunisia at four flowering stages. LWT Food Sci. Technol. 2014, 59, 448–454. [Google Scholar] [CrossRef]
- Navarro-González, I.; González-Barrio, R.; García-Valverde, V.; Bautista-Ortín, A.B.; Periago, M.J. Nutritional composition and antioxidant capacity in edible flowers: Characterisation of phenolic compounds by HPLC-DAD-ESI/MSn. Int. J. Mol. Sci. 2014, 16, 805–822. [Google Scholar] [CrossRef] [Green Version]
- Pires, T.C.; Dias, M.I.; Barros, L.; Ferreira, I.C. Nutritional and chemical characterization of edible petals and corresponding infusions: Valorization as new food ingredients. Food Chem. 2017, 220, 337–343. [Google Scholar] [CrossRef] [Green Version]
- González-Barrio, R.; Periago, M.J.; Luna-Recio, C.; Garcia-Alonso, F.J.; Navarro-González, I. Chemical composition of the edible flowers, pansy (Viola wittrockiana) and snapdragon (Antirrhinum majus) as new sources of bioactive compounds. Food Chem. 2018, 252, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, L.; Ramalhosa, E.; Pereira, J.A.; Saraiva, J.A.; Casal, S. Borage, camellia, centaurea and pansies: Nutritional, fatty acids, free sugars, vitamin E, carotenoids and organic acids characterization. Food Res. Int. 2020, 132, 109070. [Google Scholar] [CrossRef]
- Chensom, S.; Okumura, H.; Mishima, T. Primary Screening of Antioxidant Activity, Total Polyphenol Content, Carotenoid Content, and Nutritional Composition of 13 Edible Flowers from Japan. Prev. Nutr. Food Sci. 2019, 24, 171–178. [Google Scholar] [CrossRef] [PubMed]
- De Bona, G.S.; Boschetti, W.; Bortolin, R.C.; Vale, M.G.; Moreira, J.C.; de Rios, A.O.; Flôres, S.H. Characterization of dietary constituents and antioxidant capacity of Tropaeolum pentaphyllum Lam. J. Food Sci. Technol. 2017, 54, 3587–3597. [Google Scholar] [CrossRef] [PubMed]
- Nowicka, P.; Wojdyło, A. Anti-Hyperglycemic and Anticholinergic Effects of Natural Antioxidant Contents in Edible Flowers. Antioxidants 2019, 8, 308. [Google Scholar] [CrossRef] [Green Version]
- Kamalambigeswari, R.; Rebecca, L.J. Extraction of major carotenoids from flower petals. Int. J. Pharm. Sci. Res. 2016, 39, 37–39. [Google Scholar]
- Britton, G. Functions of Intact Carotenoids. In Carotenoids; Britton, G., Liaaen-Jensen, S., Pfander, H., Eds.; Birkhäuser: Basel, Switzerland, 2008; Volume 4, pp. 189–212. [Google Scholar] [CrossRef]
- Matějková, J.; Petříková, K. Variation in content of carotenoids and vitamin C in carrots. Not. Sci. Biol. 2010, 2, 88–91. [Google Scholar] [CrossRef] [Green Version]
- Garzón, G.A.; Wrolstad, R.E. Major anthocyanins and antioxidant activity of Nasturtium flowers (Tropaeolum majus). Food Chem. 2009, 114, 44–49. [Google Scholar] [CrossRef]
- Stefaniak, A.; Grzeszczuk, M.E. Nutritional and biological value of five edible flower species. Not. Bot. Horti Agrobo. 2019, 47, 128–134. [Google Scholar] [CrossRef] [Green Version]
- Du, J.; Cullen, J.J.; Buettner, G.R. Ascorbic acid: Chemistry, biology and the treatment of cancer. Biochim. Biophys. Acta Rev. Cancer 2012, 1826, 443–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, A.N.; Li, S.; Li, H.B.; Xu, D.P.; Xu, X.R.; Chen, F. Total Phenolic Contents and Antioxidant Capacities of 51 Edible and Wild Flowers. J. Funct. Foods 2014, 6, 319–330. [Google Scholar] [CrossRef]
- Kaisoon, O.; Konczak, I.; Siriamornpun, S. Potential Health Enhancing Properties of Edible Flowers from Thailand. Food Res. Int. 2012, 46, 563–571. [Google Scholar] [CrossRef]
- Kaisoon, O.; Siriamornpun, S.; Weerapreeyakul, N.; Meeso, N. Phenolic compounds and antioxidant activities of edible flowers from Thailand. J. Funct. Foods 2011, 3, 88–99. [Google Scholar] [CrossRef]
- da Silva, L.A.; Fischer, S.Z.; Zambiazi, R.C. Proximal Composition, Bioactive Compounds Content and Color Preference of Viola × wittrockiana Flowers. Int. J. Gastron. Food Sci. 2020, 22, 100236. [Google Scholar] [CrossRef]
- Tiwari, B.K.; Brunton, N.P.; Brennan, C.S. Sources of Phytochemicals. In Handbook of Plant Food Phytochemicals Source, Stability and Extraction, 1st ed.; Tiwari, B.K., Brunton, N.P., Brennan, C.S., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2013; pp. 105–137. [Google Scholar]
- Chen, G.L.; Chen, S.G.; Xiao, Y.; Fu, N.L. Antioxidant Capacities and Total Phenolic Contents of 30 Flowers. Ind. Crops Prod. 2018, 111, 430–445. [Google Scholar] [CrossRef]
- Vuolo, M.M.; Lima, V.S.; Maróstica, M.R., Jr. Phenolic Compounds. Bioact. Compd. 2019, 33–50. [Google Scholar] [CrossRef]
- Kwon, J.H.; Oh, H.J.; Lee, D.S.; In, S.J.; Seo, K.H.; Jung, J.W.; Cha, B.J.; Lee, D.Y.; Baek, N.I. Pharmacological Activity and Quantitative Analysis of Flavonoids Isolated from the Flowers of Begonia Semperflorens Link et Otto. Appl. Biol. Chem. 2019, 62. [Google Scholar] [CrossRef]
- Fan, J.; Zhu, W.; Kang, H.; Ma, H.; Tao, G. Flavonoid constituents and antioxidant capacity in flowers of different Zhongyuan tree penoy cultivars. J. Funct. Foods 2012, 4, 147–157. [Google Scholar] [CrossRef]
- Zheng, J.; Yu, X.; Maninder, M.; Xu, B. Total Phenolics and Antioxidants Profiles of Commonly Consumed Edible Flowers in China. Int. J. Food Prop. 2018, 21, 1524–1540. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.L.; Chen, S.G.; Xie, Y.Q.; Chen, F.; Zhao, Y.Y.; Luo, C.X.; Gao, Y.Q. Total phenolic, flavonoid and antioxidant activity of 23 edible flowers subjected to in vitro digestion. J. Funct. Foods 2015, 17, 243–259. [Google Scholar] [CrossRef]
- Barros, R.G.C.; Andrade, J.K.S.; Pereira, U.C.; de Oliveira, C.S.; Rezende, Y.R.R.S.; Silva, T.O.M.; Nogueira, J.P.; Gualberto, N.C.; Araujo, H.C.S.; Narain, N. Phytochemicals screening, antioxidant capacity and chemometric characterization of four edible flowers from Brazil. Food Res. Int. 2020, 130, 108899. [Google Scholar] [CrossRef]
- Gouveia, S.C.; Castilho, P.C. Phenolic composition and antioxidant capacity of cultivated artichoke, Madeira cardoon and artichoke-based dietary supplements. Food Res. Int. 2012, 48, 712–724. [Google Scholar] [CrossRef]
- Lesjak, M.; Beara, I.; Simin, N.; Pintać, D.; Majkić, T.; Bekvalac, K.; Mimica-Dukić, N. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Funct. Foods 2018, 40, 68–75. [Google Scholar] [CrossRef]
- Ishola, I.O.; Osele, M.O.; Chijioke, M.C.; Adeyemi, O.O. Isorhamnetin enhanced cortico-hippocampal learning and memory capability in mice with scopolamine-induced amnesia: Role of antioxidant defense, cholinergic and BDNF signaling. Brain Res. 2019, 1712, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Gong, G.; Guan, Y.Y.; Zhang, Z.L.; Rahman, K.; Wang, S.J.; Zhou, S.; Zhang, H. Isorhamnetin: A review of pharmacological effects. Biomed. Pharmacother. 2020, 128, 110301. [Google Scholar] [CrossRef] [PubMed]
- Antunes-Ricardo, M.; Gutiérrez-Uribe, J.A.; López-Pacheco, F.; Alvarez, M.M.; Serna-Saldívar, S.O. In vivo anti-inflammatory effects of isorhamnetin glycosides isolated from Opuntia ficus-indica (L.) Mill cladodes. Ind. Crop. Prod. 2015, 76, 803–808. [Google Scholar] [CrossRef]
- Semaming, Y.; Pannengpetch, P.; Chattipakorn, S.C.; Chattipakorn, N. Pharmacological properties of protocatechuic acid and its potential roles as complementary medicine. Evid. Based Complementary Alt. 2015, 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krzysztoforska, K.; Mirowska-Guzel, D.; Widy-Tyszkiewicz, E. Pharmacological effects of protocatechuic acid and its therapeutic potential in neurodegenerative diseases: Review on the basis of in vitro and in vivo studies in rodents and humans. Nutr. Neurosci. 2019, 22, 72–82. [Google Scholar] [CrossRef]
- Zheng, X.; Yu, L.; Yang, J.; Yao, X.; Yan, W.; Bo, S.; Wang, G. Synthesis and anti-cancer activities of apigenin derivatives. Med. Chem. 2014, 10, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Madunić, J.; Madunić, I.V.; Gajski, G.; Popić, J.; Garaj-Vrhovac, V. Apigenin: A dietary flavonoid with diverse anticancer properties. Cancer Lett. 2018, 413, 11–22. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).