Influences of the Pretreatments of Residual Biomass on Gasification Processes: Experimental Devolatilizations Study in a Fluidized Bed
Abstract
:1. Introduction
2. Materials and Methods
2.1. Investigated Biomasses
2.2. Bed Material and Conditions of Devolatilization Tests
2.3. Experimental Apparatus and Procedure for Devolatilization Tests
2.4. Processing of Devolatilization Data
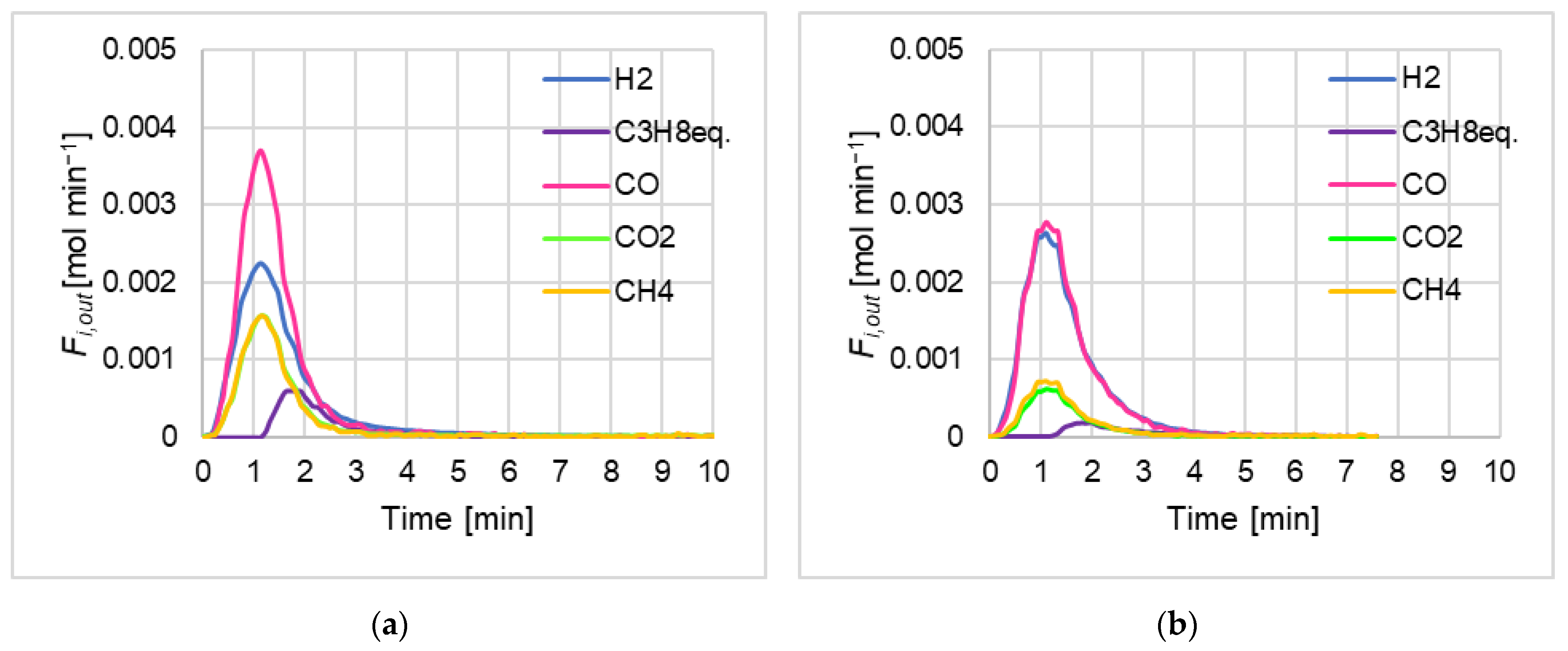
- for each set “biomass kind-temperature” and for each of the three repetitions, the highest released flow rate (i.e., peak top of Fi,out in Figure 1) was identified for each quantified species (i = H2, CO, CO2, CH4, and C3H8,eq.);
- a neighborhood of 7 Fi,out experimental points was selected, centered on the considered peak top;
- the arithmetic average (, Equation (4), where “p” superscript means “peak”) was calculated out of these 7 points.
3. Results
3.1. Results from Devolatilization Tests: Integral-Average Quantities
3.2. Results from Devolatilization Peaks: Regression Analyses
4. Discussion
4.1. Integral-Average Quantities
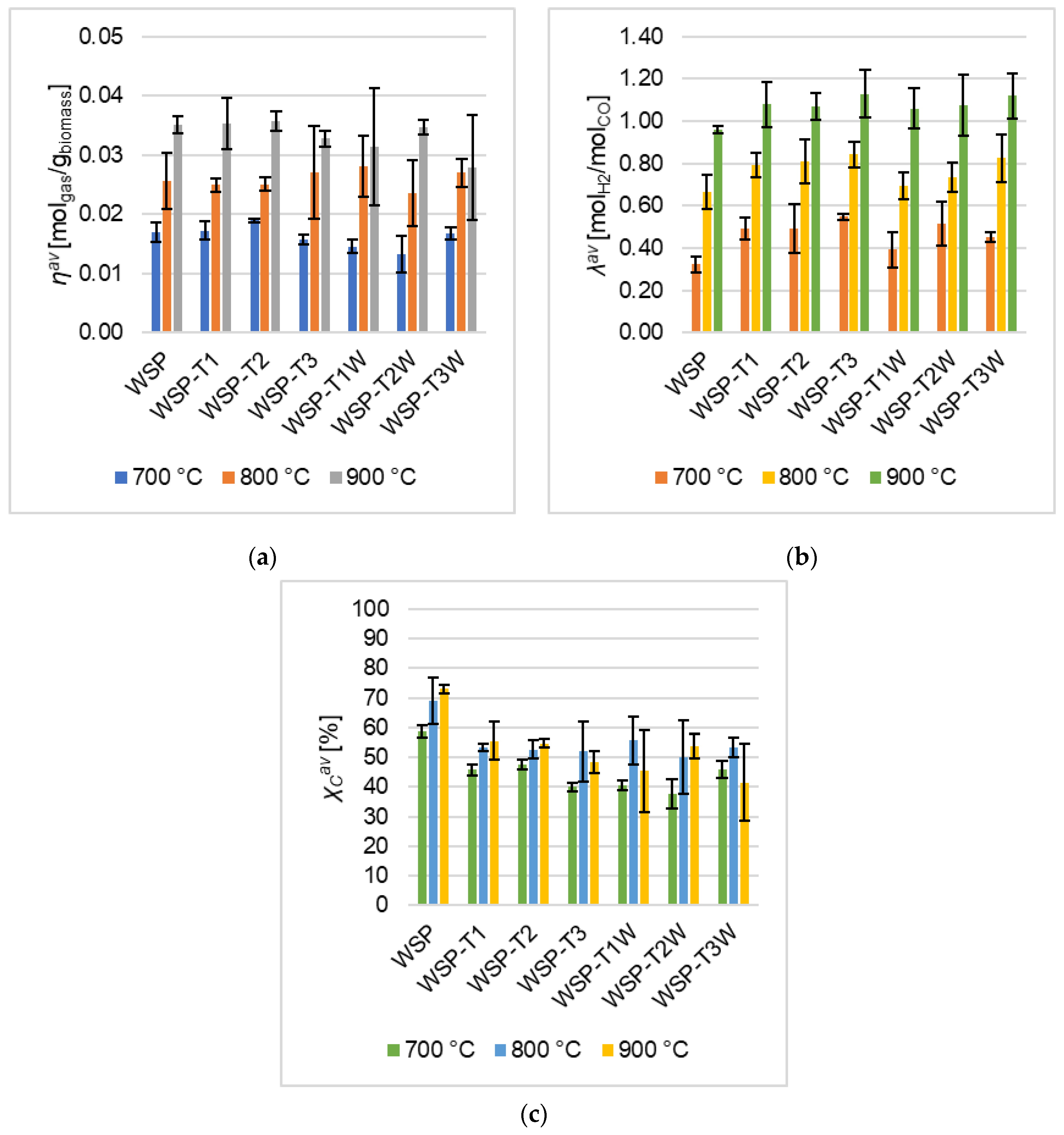
- the ηav of torrefied pellets was close to that of WSP (Figure 2a), with differences even less evident if standard deviations are taken into account;
- the λav of torrefied pellets is slightly higher than that of WSP (Figure 2b);
- with torrefied pellets, a substantial decrease of the emerged in comparison to the same quantity of WSP (Figure 2c); this is in agreement with the expected effects of the torrefaction pretreatment (defined elsewhere [17]). As highlighted by Fan et al. [33], torrefaction can lead to a reduction of carbon conversion in the thermochemical conversion of the biomass, because of devolatilization, polycondensation, and carbonization which occur during the pretreatment; as a matter of fact, Niu et al. [34] referred that torrefaction increased the elemental carbon content per unit of mass, because of the release of volatiles (such as water and CO2), which in turn made the biomass properties shift towards those of coal [35].
- no evident influences emerged on gas yield (ηav, Equation (1), Figure 2);
4.2. Peak Quantities
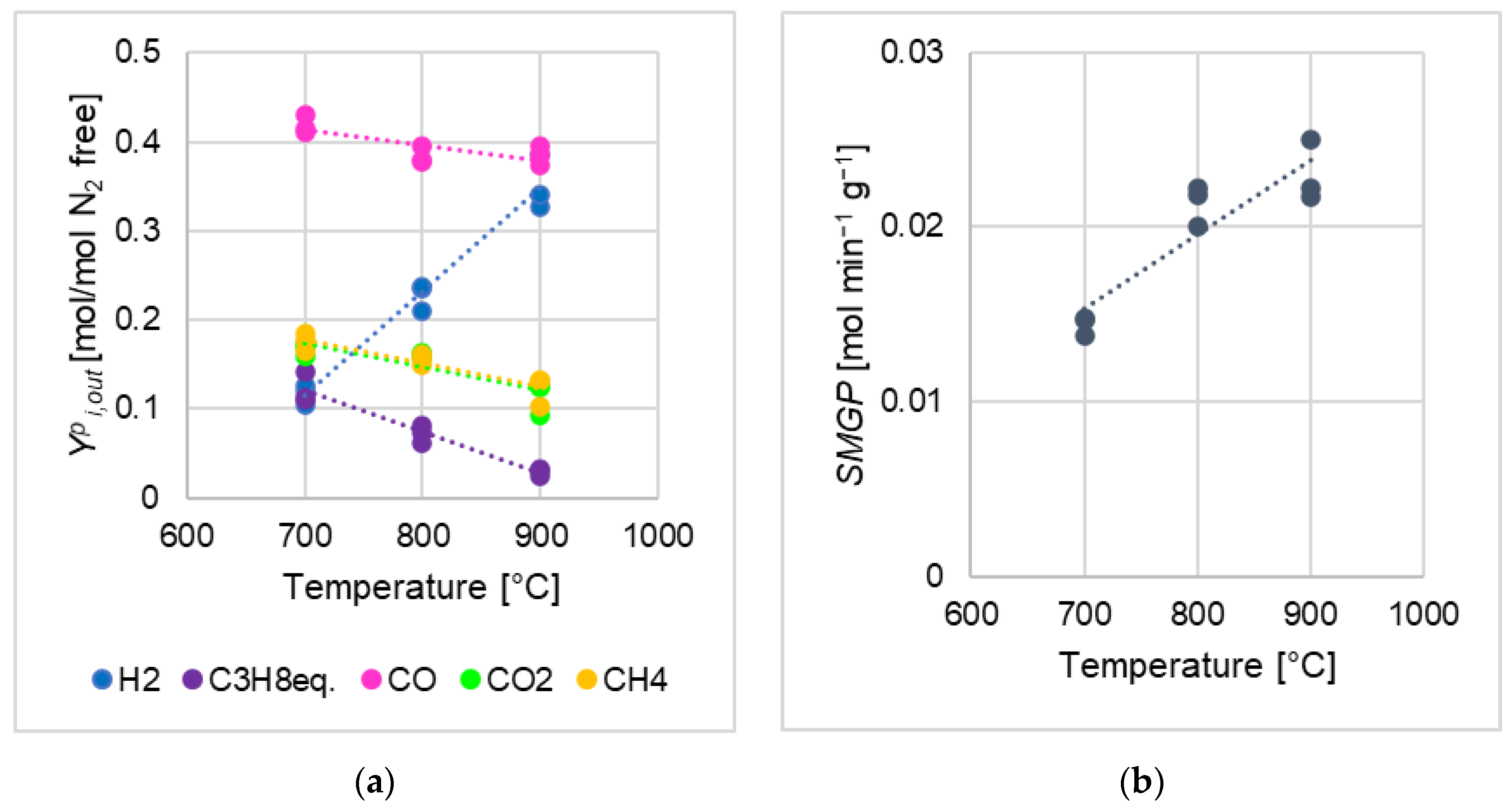
- , , (Equation (5)) decreased as the devolatilization temperature was increased, for all kinds of biomasses; qualitative identification analyses with the µGC AGILENT 490 found a high number of hydrocarbons species at 700 °C (i-C4H10, n-C4H10, C5H12, i-C5H12, n-C5H12, C6H6, C2H4/C2H2, C2H6, C3H8, C3H4), just some of them at 800 °C (n-C4H10, C6H6, C2H4/C2H2, C2H6, C3H8), while at 900 °C none of them was detected;
- (Equation (5)) increased as the temperature was increased for all kinds of biomasses;
- m is an index of the effects due to the variations of devolatilization temperature on the distribution in the syngas; for a generic gaseous species i, a positive m means that increases as the temperature was increased (and vice versa), and the higher of the absolute value of m, the more abrupt the variation due to temperature (somehow related to similar temperature effects represented by values of the Arrhenius activation energy);
- q may not have a proper physical-chemical interpretation for a given i species (anyway, it is likely interpretable analogously to the preexponential factor of Arrhenius function); in any case, being m equal, the higher the q the greater the tendency of a given biomass to release i;
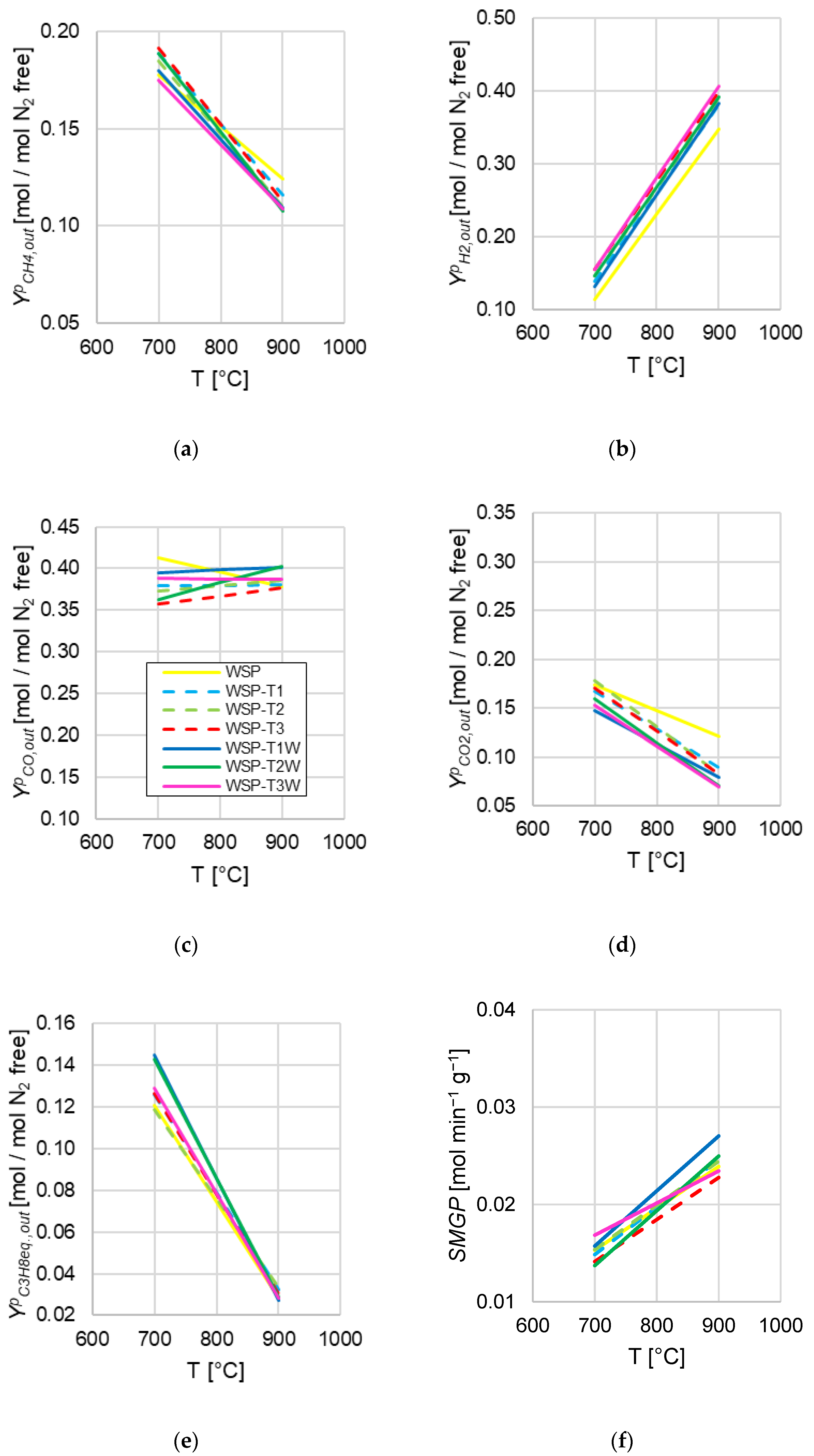
- WSP appeared as less likely to release H2 (Figure 6b) and more likely to release CO2 (Figure 6d) than pretreated wheat straw pellets; this could be related to the results of Qing et al. [38], who experimentally found that carbonaceous gases (CO2 and CO) are more easily released than H2 during the preliminary torrefaction pretreatment;
- With regard to CH4 release (Figure 6a), a significant differentiation between WSP and pretreated wheat straw pellets emerged at 900 °C, with of WSP resulting as the highest value at that temperature;
- As far as hydrocarbons are concerned (C3H8eq, Figure 6e), at 700 °C WSP-T1W and WSP-T2W showed the highest , but at higher temperatures, the behavior of all biomasses became uniform;
- When considering torrefied (WSP-T1, WSP-T2, WSP-T3) and torrefied-washed pellets (WSP-T1W, WSP-T2W, WSP-T3W) as two groups, they showed a slight behavioral difference in terms of CO2 peak release (Figure 6d); for all other gas components, when individually considered, clear effects ascribable to the specific pre-treatment or the variation of torrefaction temperature could not be inferred;
- For each biomass, the H2/CO molar ratio related to peak analyses () was calculated as the ratio between the respective and straight lines in Figure 6b,c at a given temperature, obtaining the results summarized in Table 6; remarkably, a direct influence from torrefaction temperature emerged: the higher this parameter, the greater the H2/CO molar ratio related to peaks (with a negligible exception of the very close values of WSP-T2W and WSP-T3W at 700 °C), in fair agreement with evidences obtained by the analysis of integral-average quantities and the already cited literature evidence from Zhang et al. [36] (Section 4.1); in addition, relative percentage variation of H2/CO ratio related to peaks were calculated with WSP values as references (, Table 6), and WSP-T3 always exhibited the greatest variation at each temperature, with the absolutely most pronounced at 700 °C.
5. Conclusions
- No evident influences on the integral-average gas yield emerged;
- All pretreated wheat straw pellets showed integral-average H2/CO molar ratios higher than those of untreated wheat straw: the highest value was recorded for wheat straw pellet torrefied at 270 °C (the highest explored devolatilization temperature);
- Integral-average carbon conversion of untreated wheat straw pellets was significantly higher that of pretreated pellets;
- The washing pretreatment after torrefaction did not produce significant improvements in term of integral-average gas yield, H2/CO molar ratio, and carbon conversion, when compared to only-torrefied ones.
- The higher the devolatilization temperature, the greater the H2 fraction in the syngas, at the expenses of CO2, CH4, and hydrocarbons;
- All pretreatments improved the H2/CO molar ratio related to peaks, in comparison to the same ratio obtained from untreated wheat straw;
- A direct influence from torrefaction temperature emerged on H2/CO molar ratio related to peaks, corroborating the less clear indication obtained by the integral average analyses.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EUR-Lex—32018L2001—EN—EUR-Lex. Available online: https://eur-lex.europa.eu/legal-content/en/TXT/?uri=CELEX:32018L2001 (accessed on 7 November 2020).
- Molino, A.; Larocca, V.; Chianese, S.; Musmarra, D. Biofuels production by biomass gasification: A review. Energies 2018, 11, 811. [Google Scholar] [CrossRef] [Green Version]
- Naik, S.N.; Goud, V.V.; Rout, P.K.; Dalai, A.K. Production of first and second generation biofuels: A comprehensive review. Renew. Sustain. Energy Rev. 2010, 14, 578–597. [Google Scholar] [CrossRef]
- Amaro, J.; Rosado, D.J.M.; Mendiburu, A.Z.; dos Santos, L.R.; de Carvalho, J.A. Modeling of syngas composition obtained from fixed bed gasifiers using Kuhn–Tucker multipliers. Fuel 2021, 287, 119068. [Google Scholar] [CrossRef]
- Dieringer, P.; Marx, F.; Alobaid, F.; Ströhle, J.; Epple, B. Process control strategies in chemical looping gasification-A novel process for the production of biofuels allowing for net negative CO2 emissions. Appl. Sci. 2020, 10, 4271. [Google Scholar] [CrossRef]
- Heidenreich, S.; Foscolo, P.U. New concepts in biomass gasification. Prog. Energy Combust. Sci. 2015, 46, 72–95. [Google Scholar] [CrossRef]
- Mattison, T.; Hildor, F.; Li, Y.; Linderholm, C. Negative emissions of carbon dioxide through chemical-looping combustion (CLC) and gasification (CLG) using oxygen carriers based on manganese and iron. Mitig. Adapt. Strateg. Glob. Chang. 2020, 25, 497–517. [Google Scholar] [CrossRef] [Green Version]
- Mendiara, T.; García-Labiano, F.; Abad, A.; Gayán, P.; de Diego, L.F.; Izquierdo, M.T.; Adánez, J. Negative CO2 emissions through the use of biofuels in chemical looping technology: A review. Appl. Energy 2018, 232, 657–684. [Google Scholar] [CrossRef]
- Mohamed, U.; Zhao, Y.; Yi, Q.; Shi, L.; Wei, G.; Nimmo, W. Evaluation of life cycle energy, economy and CO2 emissions for biomass chemical looping gasification to power generation. Renew. Energy 2021. [Google Scholar] [CrossRef]
- Di Giuliano, A.; Lucantonio, S.; Gallucci, K. Devolatilization of residual biomasses for chemical looping gasification in fluidized beds made up of oxygen-carriers. Energies 2021, 14, 311. [Google Scholar] [CrossRef]
- CLARA—Chemical Looping Gasification for Sustainable Production of Biofuels. Available online: https://clara-h2020.eu/ (accessed on 26 May 2021).
- Faba, L.; Díaz, E.; Ordóñez, S. Recent developments on the catalytic technologies for the transformation of biomass into biofuels: A patent survey. Renew. Sustain. Energy Rev. 2015, 51, 273–287. [Google Scholar] [CrossRef]
- The Concept: From Biomass to Biofuel—CLARA. Available online: https://clara-h2020.eu/the-concept/ (accessed on 17 October 2020).
- Marx, F.; Dieringer, P.; Ströhle, J.; Epple, B. Design of a 1 MWth pilot plant for chemical looping gasification of biogenic residues. Energies 2021, 14, 2581. [Google Scholar] [CrossRef]
- Di Giuliano, A.; Funcia, I.; Pérez-Vega, R.; Gil, J.; Gallucci, K. Novel application of pretreatment and diagnostic method using dynamic pressure fluctuations to resolve and detect issues related to biogenic residue ash in chemical looping gasification. Processes 2020, 8, 1137. [Google Scholar] [CrossRef]
- Biomass Pre-Treatment—CLARA. Available online: https://clara-h2020.eu/biomass-pre-treatment/ (accessed on 9 November 2020).
- Dieringer, P.; Funcia, I.; Soleimani, A.; Liese, T. Public Report II. Available online: https://clara-h2020.eu/wp-content/uploads/2020/11/CLARA_PublicReport2.pdf (accessed on 26 May 2021).
- Tumuluru, J.S.; Sokhansanj, S.; Hess, J.R.; Wright, C.T.; Boardman, R.D. A review on biomass torrefaction process and product properties for energy applications. Ind. Biotechnol. 2011, 7, 384–401. [Google Scholar] [CrossRef] [Green Version]
- Ru, B.; Wang, S.; Dai, G.; Zhang, L. Effect of torrefaction on biomass physicochemical characteristics and the resulting pyrolysis behavior. Energy Fuels 2015, 29, 5865–5874. [Google Scholar] [CrossRef]
- Stelte, W.; Nielsen, N.P.K.; Hansen, H.O.; Dahl, J.; Shang, L.; Sanadi, A.R. Reprint of: Pelletizing properties of torrefied wheat straw. Biomass Bioenergy 2013, 53, 105–112. [Google Scholar] [CrossRef]
- Cen, K.; Zhang, J.; Ma, Z.; Chen, D.; Zhou, J.; Ma, H. Investigation of the relevance between biomass pyrolysis polygeneration and washing pretreatment under different severities: Water, dilute acid solution and aqueous phase bio-oil. Bioresour. Technol. 2019, 278, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Jand, N.; Foscolo, P.U. Decomposition of wood particles in fluidized beds. Ind. Eng. Chem. Res. 2005, 44, 5079–5089. [Google Scholar] [CrossRef]
- Malsegna, B.; Di Giuliano, A.; Gallucci, K. Experimental study of absorbent hygiene product devolatilization in a bubbling fluidized bed. Energies 2021, 14, 2399. [Google Scholar] [CrossRef]
- Shadle, L. F luidized B ed C hemical L ooping. Available online: https://clara-h2020.eu/deliverables/ (accessed on 26 May 2021).
- Unidad de Pretratamiento—Cener BIO2C. Available online: https://www.bio2c.es/es/unidad-de-pretratamiento/ (accessed on 11 May 2021).
- Gibilaro, L.G. Fluidization-Dynamics; Butterworth-Heinemann: Oxford, UK; Woburn, MA, USA, 2001. [Google Scholar]
- Yang, W.-C. Handbook of Fluidization and Fluid-Particle Systems; Marcel Dekker: New York, NY, USA, 2003; ISBN 082470259X. [Google Scholar]
- Grace, J.R. Fluidized bed hydrodynamics. In Handbook of Multiphase Systems; Hemisphere Publishing Corp.: London, UK, 1982; pp. 6–8. [Google Scholar]
- Werther, J.; Ogada, T.; Borodulya, V.A.; Dikalenko, V.I. Devolatilisation and combustion characteristics of sewage sludge in a bubbling fluidized bed furnace. In The Institute of Energy’s Second International Conference on Combustion & Emissions Control; Elsevier: Amsterdam, The Netherlands, 1995; pp. 149–158. [Google Scholar]
- Zeng, X.; Wang, Y.; Yu, J.; Wu, S.; Zhong, M.; Xu, S.; Xu, G. Coal pyrolysis in a fluidized bed for adapting to a two-stage gasification process. Energy Fuels 2011, 25, 1092–1098. [Google Scholar] [CrossRef]
- Condori, O.; García-Labiano, F.; de Diego, L.F.; Izquierdo, M.T.; Abad, A.; Adánez, J. Biomass chemical looping gasification for syngas production using ilmenite as oxygen carrier in a 1.5 kWth unit. Chem. Eng. J. 2021, 405, 126679. [Google Scholar] [CrossRef]
- Wang, S.; Song, T.; Yin, S.; Hartge, E.U.; Dymala, T.; Shen, L.; Heinrich, S.; Werther, J. Syngas, tar and char behavior in chemical looping gasification of sawdust pellet in fluidized bed. Fuel 2020, 270, 117464. [Google Scholar] [CrossRef]
- Fan, Y.; Tippayawong, N.; Wei, G.; Huang, Z.; Zhao, K.; Jiang, L.; Zheng, A.; Zhao, Z.; Li, H. Minimizing tar formation whilst enhancing syngas production by integrating biomass torrefaction pretreatment with chemical looping gasification. Appl. Energy 2020, 260, 114315. [Google Scholar] [CrossRef]
- Niu, Y.; Lv, Y.; Lei, Y.; Liu, S.; Liang, Y.; Wang, D.; Hui, S. Biomass torrefaction: Properties, applications, challenges, and economy. Renew. Sustain. Energy Rev. 2019, 115, 109395. [Google Scholar] [CrossRef]
- Medic, D.; Darr, M.; Shah, A.; Potter, B.; Zimmerman, J. Effects of torrefaction process parameters on biomass feedstock upgrading. Fuel 2012, 91, 147–154. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, J.; Guo, W.; Wu, Z.; Wang, Z.; Yang, B. Effect of torrefaction pretreatment on biomass chemical looping gasification (BCLG) characteristics: Gaseous products distribution and kinetic analysis. Energy Convers. Manag. 2021. [Google Scholar] [CrossRef]
- Chen, D.; Mei, J.; Li, H.; Li, Y.; Lu, M.; Ma, T.; Ma, Z. Combined pretreatment with torrefaction and washing using torrefaction liquid products to yield upgraded biomass and pyrolysis products. Bioresour. Technol. 2017, 228, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhou, J.; Liu, B.; Mei, Q.; Luo, Z. Influence of torrefaction pretreatment on biomass gasification technology. Chin. Sci. Bull. 2011, 56, 1449–1456. [Google Scholar] [CrossRef] [Green Version]


| Name of Biomass | Characteristic of the Pellet |
|---|---|
| WSP | Wheat Straw Pellet |
| WSP-T1 | Wheat Straw Pellet—Torrefied at T1 = 250 °C |
| WSP-T2 | Wheat Straw Pellet—Torrefied at T2 = 260 °C |
| WSP-T3 | Wheat Straw Pellet—Torrefied at T3 = 270 °C |
| WSP-T1W | Wheat Straw Pellet—Torrefied at T1 and Washed |
| WSP-T2W | Wheat Straw Pellet—Torrefied at T2 and Washed |
| WSP-T3W | Wheat Straw Pellet—Torrefied at T3 and Washed |
| Material | Sand | |
|---|---|---|
| dp (μm) | 212–250 | |
| ρp (kg m−3) | 2.6 × 103 | |
| T (°C) | umf (cm s−1) | Generalized Geldart Group [27] |
| 700 | 2.4 | B |
| 800 | 4.4 | B |
| 900 | 2.9 | B |
| Species of Gas | m [Mol Mol N2 Free−1 °C−1] | q [Mol Mol N2 Free−1] |
| H2 | 1.170 × 10−3 | −7.050 × 10−1 |
| C3H8eq. | −4.622× 10−4 | 4.440 × 10−1 |
| CO | −1.730 × 10−4 | 5.340 × 10−1 |
| CO2 | −2.686 × 10−4 | 3.622 × 10−1 |
| CH4 | −2.672 × 10−4 | 3.648 × 10−1 |
| SMGP | m [mol min−1 g−1 °C−1] | q [mol min−1 g−1] |
| 4.293 × 10−1 | −1.475 × 10−2 |
| Type of Biomass | Species of Gas | m (Mol Mol N2 Free−1 °C−1) | q (Mol Mol N2 Free−1) |
|---|---|---|---|
| WSP-T1 | H2 | 1.213 × 10−3 | −7.106 × 10−1 |
| C3H8eq | −4.654 × 10−4 | 4.514 × 10−1 | |
| CO | 8.298 × 10−6 | 3.733 × 10−1 | |
| CO2 | −3.932 × 10−4 | 4.429 × 10−1 | |
| CH4 | −3.631 × 10−4 | 4.429 × 10−1 | |
| WSP-T2 | H2 | 1.217 × 10−3 | −7.064 × 10−1 |
| C3H8eq | −4.272 × 10−4 | 4.177 × 10−1 | |
| CO | 6.025 × 10−5 | 3.311 × 10−1 | |
| CO2 | −4.774 × 10−4 | 5.125 × 10−1 | |
| CH4 | −3.721 × 10−4 | 4.450 × 10−1 | |
| WSP-T3 | H2 | 1.221 × 10−3 | −6.999 × 10−1 |
| C3H8eq | −4.802 × 10−4 | 4.624 × 10−1 | |
| CO | 9.178 × 10−5 | 2.936 × 10−1 | |
| CO2 | −4.400 × 10−4 | 4.780 × 10−1 | |
| CH4 | −3.924 × 10−4 | 4.659 × 10−1 | |
| WSP-T1W | H2 | 1.255 × 10−3 | −7.466 × 10−1 |
| C3H8eq | −5.867 × 10−4 | 5.553 × 10−1 | |
| CO | 2.963 × 10−5 | 3.747 × 10−1 | |
| CO2 | −3.447 × 10−4 | 3.892 × 10−1 | |
| CH4 | −3.535 × 10−4 | 4.274 × 10−1 | |
| WSP-T2W | H2 | 1.224 × 10−3 | −7.107 × 10−1 |
| C3H8eq | −5.705 × 10−4 | 5.420 × 10−1 | |
| CO | 1.980 × 10−4 | 2.247 × 10−1 | |
| CO2 | −4.458 × 10−4 | 4.715 × 10−1 | |
| CH4 | −4.053 × 10−4 | 4.725 × 10−1 | |
| WSP-T3W | H2 | 1.256 × 10−3 | −7.236 × 10−1 |
| C3H8eq | −5.044 × 10−4 | 4.821 × 10−1 | |
| CO | −3.523× 10−6 | 3.903 × 10−1 | |
| CO2 | −4.187 × 10−4 | 4.457 × 10−1 | |
| CH4 | −3.295 × 10−4 | 4.054 × 10−1 |
| Biomass | m [Mol Min−1 g−1 °C−1] | q [Mol Min−1 g−1] |
| WSP-T1 | 4.882 × 10−5 | −1.933 × 10−2 |
| WSP-T2 | 4.534 × 10−5 | −1.636 × 10−2 |
| WSP-T3 | 4.312 × 10−5 | −1.605 × 10−2 |
| WSP-T1W | 5.648 × 10−5 | −2.380 × 10−2 |
| WSP-T2W | 5.618 × 10−5 | −2.560 × 10−2 |
| WSP-T3W | 3.297 × 10−5 | −6.241 × 10−3 |
| Biomass | 700 °C | 800 °C | 900 °C | |||
|---|---|---|---|---|---|---|
| (molH2 molCO−1) | (%) | (molH2 molCO−1) | (%) | (molH2 molCO−1) | (%) | |
| WSP | 0.28 | 0 | 0.58 | 0 | 0.92 | 0 |
| WSP-T1 | 0.37 | 32 | 0.68 | 17 | 1.00 | 9 |
| WSP-T2 | 0.39 | 41 | 0.70 | 21 | 1.01 | 10 |
| WSP-T3 | 0.43 | 57 | 0.75 | 29 | 1.06 | 15 |
| WSP-T1W | 0.33 | 21 | 0.65 | 11 | 0.95 | 4 |
| WSP-T2W | 0.40 | 46 | 0.70 | 20 | 0.97 | 5 |
| WSP-T3W | 0.40 | 45 | 0.73 | 24 | 1.05 | 14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lucantonio, S.; Di Giuliano, A.; Gallucci, K. Influences of the Pretreatments of Residual Biomass on Gasification Processes: Experimental Devolatilizations Study in a Fluidized Bed. Appl. Sci. 2021, 11, 5722. https://doi.org/10.3390/app11125722
Lucantonio S, Di Giuliano A, Gallucci K. Influences of the Pretreatments of Residual Biomass on Gasification Processes: Experimental Devolatilizations Study in a Fluidized Bed. Applied Sciences. 2021; 11(12):5722. https://doi.org/10.3390/app11125722
Chicago/Turabian StyleLucantonio, Stefania, Andrea Di Giuliano, and Katia Gallucci. 2021. "Influences of the Pretreatments of Residual Biomass on Gasification Processes: Experimental Devolatilizations Study in a Fluidized Bed" Applied Sciences 11, no. 12: 5722. https://doi.org/10.3390/app11125722
APA StyleLucantonio, S., Di Giuliano, A., & Gallucci, K. (2021). Influences of the Pretreatments of Residual Biomass on Gasification Processes: Experimental Devolatilizations Study in a Fluidized Bed. Applied Sciences, 11(12), 5722. https://doi.org/10.3390/app11125722








