Abstract
Aquaponics provides an alternative opportunity for the combined production of fish and plants. Most of the essential nutrients required for optimal plant growth can be supplied from the fish feed, except for K and Fe. These nutrients are usually inadequate in fish feed. In this study, red tilapia and rocket plants were co-cultivated in an aquaponics system along with the extra addition of K and Fe. Fish growth, morpho-anatomical characteristics, and K and Fe loading on fish gills were studied. Plant growth parameters were also determined. The addition of Fe and K slightly improved the produced fresh biomass of rocket per unit area and had no impact on tilapia growth and survival. No severe histological alterations in fish gills, liver, and midgut were detected. EDX analysis showed that the addition of K led to the enrichment of this specific ion in fish gills, but no effects of this accumulation were found on other aspects of fish growth and survival.
1. Introduction
Aquaponics is a multicultural technique that combines the simultaneous fish and plants culture in RAS systems, integrating aquaculture and hydroponics in a soilless system [1,2]. Aquaponic systems consist of the fish tank, the hydroponic cultivation tank, the mechanical filter, the biological filter, and the sump. The produced ammonia by the uneaten fish feed, fish feces, and fish urea is converted to usable by the plants’ nitrate through oxidization by nitrifying bacteria [3]. Nitrates are absorbed by the plants, and the purified water returns back to the fish tank.
Aquaponics is a sustainable solution with a small environmental footprint [4], as it uses less than 10% of the water used in conventional agriculture [2]. Independence from the soil is also an advantage of aquaponics over soil-based agriculture, as aquaponic systems may be located anywhere, with no need for suitable soil presence [5,6,7].
Fish, plants, and bacteria coexist in an aquaponic system. The type of the system, the filter size, fish species, fish biomass, number of plants, and plant species must be carefully chosen in order to have a successful production [8]. Tilapia species, carp, perch, and catfish, are the main species that are cultured in aquaponics systems [9,10,11], along with some crustacean species such as Cherax quadricarinatus and Procambarus spp. [12,13]. Among tilapia species, Oreochromis mossambicus is the most widely distributed. It is easy to keep and breed in captivity. The red tilapia (Oreochromis spp.) in aquaculture is a hybrid between O. mossambicus and either O. niloticus or O. hornorum [14]. In 2018 Oreochromis spp global aquaculture production was 1.03 million tons [15]. Tilapia is generally tolerant to pH, temperature, and dissolved oxygen changes.
Sometimes multispecies cultivation is more efficient in an aquaponic system. Different fish species used in identical aquaponic systems change oxygen levels and, along with the combined feed input, alter plant species growth [16]. Traditional aquaponics systems send nutrient-rich water from the fish tank to the plants and back (single loop). Decoupled aquaponics systems separate the aquaculture and aquaponics units. In these systems, the sludge from the fish tank is digested in the biological waste system, which provides the nutrients for the hydroponic system. The water sent from the RAS to the hydroponic unit is consequently replaced by clean water, which reduces nutrient concentrations and thus improves water quality [17]. Brackish water aquaponics systems are useful in areas where freshwater is limited. Brackish water aquaponics combines the culture of euryhaline fish species, such as sea bream or sea bass, in combination with a wide variety of plant organisms, such as algae or halophytes [18,19].
Until now, more than 150 species of vegetables, herbs, flowers, and small trees have been successfully used in aquaponics systems, with lettuce, tomato, basil, eggplant, pepper, or spinach to be the most widely used [20,21,22]. Since aquaponics is based on the usage of nutrients resulting from fish metabolism, significantly less fertilizers are required for the efficient cultivation of plants compared to classic hydroponics. Fish feed supplies most of the essential nutrients required for optimal plant growth except for calcium (Ca), potassium (K), and iron (Fe), which are usually inadequate and must be supplemented in aquaponic systems [23]. The relevant literature has documented the effects of K and Fe suboptimal availability to aquaponics-cultivated plants mainly in terms of yield reduction. Nevertheless, in a multicultural system, such as aquaponics, where the emphasis is given on the sustainability and biosafety of the products, crop and fish yield should not be the only criterion. Plant functional responses and fish well-being are also important. Here we report an experiment in which rocket plants were co-cultivated with tilapia fish under three different treatments: (i) no extra addition of nutrients, (ii) addition of Fe, and (iii) addition of Fe and K. Our aim was to focus on the effects that these input manipulations have on various aspects of fish well-being, in terms of morpho-anatomical characteristics and K and Fe loading on fish gills. Plant and fish growth parameters were also determined to evaluate the outcome of the external inputs.
2. Material and Methods
2.1. Aquaponic Systems Description
Nine autonomous aquaponics systems with a total volume of 630 L per system were used. Each system consisted of (i) a fish tank with a 400 L water volume, (ii) a 50 L hydroponic cultivation tank (112 × 73 × 20 cm) filled with clay pebble (8–16 mm) substrate, (iii) a biological sump filter of 180 L total volume, and (iv) a mechanical filter of 60 L. The sump filter was filled with 10 L of porous cylindrical substrate K1 (11 mm diameter each), which provided a large specific surface area (SSA) for nitrifying bacteria to colonize. A pump (HAILEA-hx-8830, 45 W, 2900 L/h, hmax 2.3 m) was placed in the last part of the filter to supply the aquaponic system with water through the filter (Q = 5.10 L/min). The mechanical filter consisted of three layers of fiberglass material, each layer 10 cm thick, in order to retain the solid residues from the fish tank (uneaten food and feces). Clay pebble substrate of the hydroponic tank also provided enough biofiltration, increasing the efficiency of the system. An air-lift pump (HAILEA, ACO-328, 70 L/min) was used to diffuse oxygen to the aquaponics system. Oxygen was equally distributed in the fish tank and the biological filter. A two-month period was required for the setup of the biological filter setup. According to Hirayama [24], 40–60 days are necessary for the establishment of bacteria and the efficient oxidation of ammonia to nitrate ions.
2.2. Experimental Design, Fish Rearing, and Plant Growth Conditions
In total, 270 red tilapias (Oreochromis spp.) were left to acclimatize in the experimental units for 15 days before the commencement of the experiment. Subsequently, the fish weight and length were measured, and fish were homogeneously distributed to the fish tanks in order to avoid statistically significant differences in initial weight and length among aquaponic systems. A total of 30 red tilapias were placed in each fish tank with an average weight of 8.74 ± 0.023 g and an average length of 8.30 ± 0.001 cm. The resulting average breeding density per fish tank was 655 g/m3. All experimental procedures were conducted according to the guidelines of the EU Directive 2010/63/EU regarding the protection of animals used for scientific purposes and were applied by FELASA accredited scientists (functions A–D). The experimental protocol was approved by the Ethics Committee and conducted at the registered experimental facility (EL-43BIO/exp-01) of the Laboratory of Aquaculture, Department of Ichthyology and Aquatic Environment, University of Thessaly (n. 329309/24–11-2020).
Fish were fed twice daily ad libitum with a commercial floating pellet diet (47.5% protein and 6.5% crude fat). Feeding was performed by hand. Fish tanks were cleaned daily by siphoning, and uneaten food was removed. Food consumption per fish tank was calculated by the difference between the amount fed and the amount of uneaten feed collected (corrected for leaching losses).
Rocket plants (Eruca vesicaria) were grown from seeds in an unheated greenhouse until the 6-true-leaf stage. A total of 108 rocket seedlings were chosen, showing no statistically significant differences in their morphometric characteristics (height, number of leaves). Twelve rocket plants were evenly placed in each hydroponic tank, 20 cm apart from each other. Plant positions were carefully selected to ensure the homogeneity of the light environment; each plant was exposed to 300–450 μmol m−2 s−1 of photosynthetically active radiation (PAR). The artificial light was supplied by a 600 W HPS lamp (SYLVANIA, 230 V) placed 65 cm above each growing area. The photoperiod (10 h light: 14 h dark) was controlled by a timer.
The Fe and K supplementation in the water of the aquaponics systems resulted in three treatments (three replicates per treatment):
(i) Control group, no nutrient addition.
(ii) Fe group, only Fe-DTPA was added (Fe target concentration 5 mg/L).
(iii) Fe + K group, Fe-DTPA and K2SO4 were added (target concentration 5 mg/L and 120 mg/L for Fe and K, respectively).
Fe and K target concentrations were selected according to Delaide et al. [25] and Nicoletto et al. [26] for leafy vegetables hydroponic cultivation. The photometric determination of Fe and K concentrations in the inlet point of the hydroponic cultivation tank was performed twice a week (Hach DR3900) and was followed by the necessary nutrient supplementation to ensure that the targeted Fe and K content was maintained in the recirculating water. The required amount of nutrients was dissolved in 4 L of chlorine-free water before added to the system. Ca foliar application (1 mL/m2) was performed twice a week in all groups to avoid Ca nutritional deficiencies.
2.3. Water Physicochemical Parameters and Quality Indicators
Water temperature (°C) and pH of fish tanks were recorded daily, while oxygen concentration (mg/L) and electrical conductivity (mS/cm) were recorded every three days. Temperature, pH, and oxygen concentration were measured with multimeter sensors (Hach, HQ40d), while electrical conductivity was measured with a conductivity meter (Crison, CM35).
Water quality measurements were performed at the inlet point of the hydroponic cultivation tank. Except for the above-mentioned Fe and K content, ammonium (NH4+), nitrate (NO3−), and nitrite (NO2−) ions were monitored once a week before the first fish feeding of the day. All measurements were performed using a Hach DR3900 photometer with special pre-weighted reagents.
2.4. Growth Indicators of Fish and Plants at the Final Harvest
The experiment lasted for 30 days. At the end of the experiment, fish anesthesia occurred, and final fish body weights and lengths were measured. Euthanasia of fish followed the EU Directive 2010/63/EU and FELASA guidelines and performed through an overdose of Tricaine methansulfonate (MS 222, 300+ mg/L).
The fish growth performance indicators were calculated as follows:
- Specific growth rate (SGR, %/day) = [(ln Wfin − ln Wi)/Δt] × 100
- Weight gain (WG, g) = Wfin − Wi
- Feed conversion ratio (FCR) = feed consumed/WG
where Win and Wfin are the initial and final weight of the fish, respectively, and Δt is the duration of the experiment in days.
Plant growth performance was determined by:
- Fresh leaves weight (g)
- Total fresh biomass yield (g/m2) = total fresh weight of aerial part/cultivated area
2.5. Fish Histopathology
Fish were placed immediately on ice after euthanasia. A total of 45 fish (5 per tank) were examined for histopathological alterations at the liver, midgut, and gills (second gill arch from the left side), as previously reported by Vlahos et al. [19]. Tissue samples first were fixed in Davidson’s fixative for 24 h at 4 °C followed by dehydration in graded series of ethanol, immersion in xylol, and embedding in paraffin wax. Tissue sections of 5–10 μm were stained with Hematoxylin-Eosin and examined under microscope (Axiostar plus Carl Zeiss Light Microscopy, Carl Zeiss Ltd., Gottingen, Germany). Total magnifications of 100× and 400× were used. A semi-quantitative grading system was used in order to quantify the histopathological alterations of the examined tissues [27]. Severity grading used the following system: Grade 0 (not remarkable), Grade 1 (minimal), Grade 2 (mild), Grade 3 (moderate), and Grade 4 (severe).
2.6. Gill EDX Analysis
In order to determine if iron (Fe) or potassium (K) were accumulated at the gills, the first gill arch from the right side from every sampled fish was removed, incinerated, and analyzed by energy dispersive spectroscopy (EDS). For each gill arch, three measurements were taken. For EDX analysis, a scanning electron microscope (Jeol JSM-6510 LV, Ltd., Tokyo, Japan) equipped with an X-ray analyzer (x-act Oxford, Abingdon, UK) was used.
2.7. Statistical Analysis
Values are presented as mean ± standard error (SEM). Normality test and homogeneity test were performed with Kolmogorov–Smirnov and Levene’s tests, respectively. To determine any significant differences between different treatments, one-way ANOVA was used, followed by Tukey’s post-hoc test. Statistical analyses were carried out using the software package IBM SPSS Statistics V22.
3. Results
3.1. Water Physicochemical Parameters and Quality Indicators
Water temperature, pH, and dissolved oxygen levels were kept constant during the experimental period and showed no significant differences (p > 0.05) between treatments (Table 1). The fluctuation of pH during the experimental period is shown in Figure 1. The mean values of NH4+ and NO3− at the inlet point of the hydroponic tank also showed no significant differences (p > 0.05) among the three treatments (Table 2, Figure 2). Mean Fe concentration was 3.61 ± 0.338 mg/L and 3.55 ± 0.354 mg/L for Fe and Fe + K group, respectively, while the mean K concentration for the Fe + K group was 112.00 ± 9.953 mg/L (Table 2, Figure 2).

Table 1.
Water physicochemical parameters in the fish tanks of the aquaponic systems during the study period (30 days) expressed as mean ± S.E.M. (n = 75 for T, pH and n = 39 for EC, O2). Means in a row followed by different superscript are significantly different (p > 0.05).
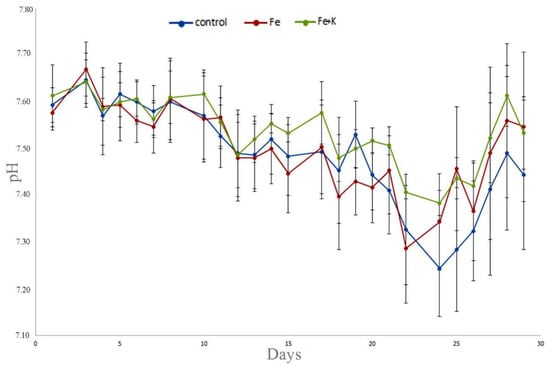
Figure 1.
pH fluctuation in the various treatments during the experimental period. Data are expressed as mean ± S.E.M (n = 3).

Table 2.
Water quality in the cultivation area of the aquaponic systems during the study period (30 days). Data are expressed as mean ± S.E.M. (n = 15). Means in a row followed by different superscript are significantly different (p > 0.05).
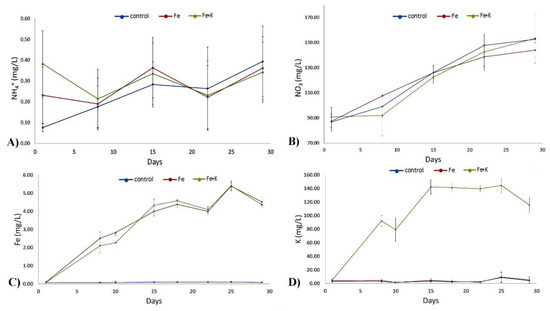
Figure 2.
The fluctuation of (A) NH4+, (B) NO3−, (C) Fe, and (D) K in the inlet point of the hydroponic tank of the various treatments during the experimental period. Data are expressed as mean ± S.E.M (n = 3).
3.2. Fish Growth Performance
The red tilapia growth performance is illustrated in Table 3. At the start of the experimental period, there were no significant differences in the means of red tilapia initial body weight (g) and length (cm) (p > 0.05) for all the groups (Table 3). Final weight, final length, weight gain, SGR, and FCR were similar (p > 0.05) for all three treatments (Table 3). The survival rate for the control, Fe, and Fe + K groups was 100%, 100%, and 99%, respectively.

Table 3.
Growth performance of red tilapia. Data are expressed as means ± S.E.M. Means in a row followed by different superscript are significantly different (p > 0.05).
3.3. Fish Histopathology
3.3.1. Liver
Liver histopathology of control and Fe treatments revealed minimal (grade 1) alterations (Table 4, Figure 3), such as granuloma, lipid accumulation to the liver cells, and pancreatic islet macrosteatosis. One fish from the Fe treatment showed focal inflammation signs (accumulation of leucocytes). Fe + K treatment showed mild (grade 2) alterations (Table 4, Figure 3), such as larger lipid accumulation to the liver cells, granuloma, regions with larger nuclei, necrotic regions, and regions with hemmoradge.

Table 4.
Severity score (0–4) for the observed histopathological alterations in fish liver, midgut, and gills.

Figure 3.
Histopathological examination of the liver. (A) Control group. Pancreatic islet macrosteatosis (arrow). (B) Fe group. Granuloma (arrow). (C) Fe group. Lipid droplets pressed some of the nuclei to the periphery of the hepatocytes (arrowhead). Some other nuclei appeared enlarged (arrow). (D) Fe group. One fish from this group showed focal inflammation signs (arrow). (E) Fe + K group. Necrotic region. (F) Fe + K group. Larger lipid accumulation.
3.3.2. Midgut
Midgut microscopic examination generally showed normal structure (grade 0) for all three treatments (Table 4, Figure 4). Noteworthy is that two fish from the Fe group exhibited histological abnormalities; an extremely large artery was observed in the first one and an abnormal stretching of the lamina propria in the other.
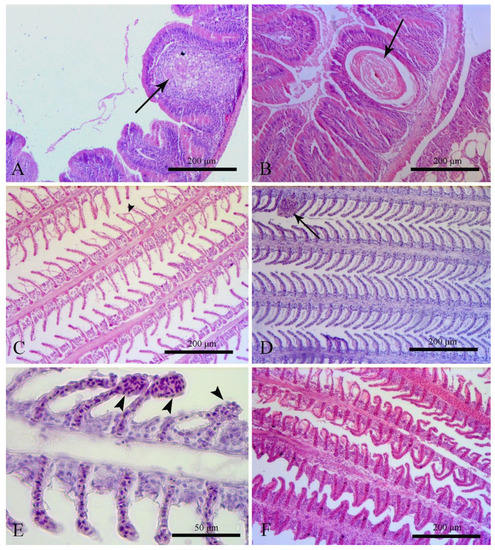
Figure 4.
(A) Fe-group midgut. In one fish, an extremely large artery was observed to a midgut villi (arrow). (B) Fe-group midgut. In one fish, a region with an abnormal stretching of the lamina propria was detected (arrow). (C) Control group-gills. Epithelium detachment at the secondary lamella (arrowhead) is considered an artifact. (D) Fe-group gills. Hyperemia of secondary lamella (arrow). (E) Fe + K-group gills. Telangiectasia of secondary lamellae (arrowhead). (F) Fe + K-group gills. Hyperplasia of primary lamellae.
3.3.3. Gills
3.4. Gills EDX Analysis
No Fe accumulation was detected (N.D.) at the fish gills of any treatment. K accumulation in the Fe + K treatment was almost seven times higher than the other two treatments (Table 5).

Table 5.
K and Fe accumulation in fish gills of all groups presented as At%. Data are expressed as means ± S.E.M (n = 15). Means in a row followed by different superscript are significantly different (p > 0.05).
3.5. Plant Growth Performance
The plant growth characteristics determined at the end of the 30-days experimental period are presented in Table 6. Rocket plants that received additional Fe showed the highest fresh weight of leaves, while control plants had statistically significant inferior growth compared to the other two groups. Fe treatment exhibited a trend for increased fresh biomass per m2, which is a measure of marketable yield. Although the differences were not statistically significant, they reached a 35% reduction in the produced fresh biomass in control plants and only a 13% reduction in Fe + K plants when compared to the Fe group. During the experimental period, signs of chlorosis were observed on the leaves of the control group plants.

Table 6.
Rocket growth performance as assessed by the fresh weight of leaves per plant and the marketable produced biomass per unit area. Data are expressed as means ± S.E.M (n = 36 for leaves fresh weight and n = 3 for total produced biomass). Means in a row followed by different superscript are significantly different (p > 0.05).
4. Discussion
4.1. Abiotic Factors
In the present study, the co-cultivation of red tilapia and rockets was performed in an aquaponic system for 30 days. The supplementation of Fe alone and its combination with K was examined in relation to no external inputs treatment (control). The effects of the various treatments on water quality indicators, fish growth performance, fish histopathology alteration markers, Fe and K loading in fish gills, and plant growth parameters were assessed.
In an aquaponic system, the water temperature setting is dependent on the fish and plant species. Tilapia can live in waters with a wide range of temperature, 17–35 °C, with the optimum temperature range for normal development, reproduction, and growth to be about 25–32 °C, depending on the fish species, size, and genetic variation [28,29,30,31,32,33]. In the present study, the water temperature was kept at 22.8–22.9 °C, meeting the requirements of both tilapia and rocket plants. pH management is also important in an aquaponics system. In hydroponics, the pH is generally set between 4.5 and 6. In RAS systems, the pH is between 7.0 and 8.0 in order to meet the requirements for both fish and nitrifying bacteria. The pH of aquaponics systems is a compromise between the above-mentioned diverse requirements of its living components; the optimal pH range appears to be 6.0–7.5 [34]. pH higher than 7.5 causes reduced micronutrient and phosphorus solubility; thus, plant uptake of certain nutrients is restricted [34,35]. Optimum availability for K occurs at pH range 6–8, while the optimum availability for Fe is at pH range 4–6 [34]. The pH in our study was kept almost constant over time (Figure 1) for all the treatments. The mean values were 7.49–7.54. Temperature and pH values in aquaponic systems are important parameters for plant needs/nutrition and for fish welfare.
DO is very important for the fish, plant roots, and bacteria. Plant roots require lower DO values than fish. Most of the fish need DO 5 mg/L or more, while tilapia can tolerate very low DO concentrations, i.e., 1.0–1.5 mg/L [36]. A general rule is 6 mg/L for cold-water fish and 4 mg/L for warm water fish [37]. Plant roots and nitrifying bacteria require at least 3 mg/L DO [34]. So, in an aquaponic system, if the DO is set to meet the fish requirements, the requirements for plants and bacteria are also met. In the present study, the oxygen levels were 8.41 ± 0.042, 8.36 ± 0.032 and 8.32 ± 0.036 for the control, Fe, and Fe + K treatments, respectively.
EC appeared higher at the Fe + K treatment, where fertilization occurred. EC indicates the number of charged ions circulating in the water column, so the more ions (nutrients) circulating, the higher the value of conductivity [38,39].
In an aquaponic system, the fish feed is the main nutrient source [40]. Fish metabolize the feed, and their feces along with the uneaten food, become the nutrient source for plants through oxidation of the produced ammonia to nitrate by the nitrifying bacteria. It has been suggested that 80% of the required nutrients for plants can be provided by the fish feed [41]. The rest 20% concern nutrients that are absent or in low levels in fish feed, such as Ca, K and Fe, and should be supplemented either directly to the water or as foliar fertilization. Nitrogen can be absorbed by plants in two forms, nitrate or ammonium, depending on the concentration and the plant physiology [42]. In the present study, the low ammonium mean values and the gradual rise of nitrate levels (Figure 2) proved the efficiency of the filter in oxidizing the produced ammonia. During the 30-days study period, the mean nitrate concentration (mg/L) was 122.56 ± 7.367, 120.82 ± 5.911, 120.32 ± 8.584 for the control, Fe, and Fe + K treatment, respectively. The daily supply of 13.47–14.03 g of fish feed provided efficient nitrogen for plant nutrition. According to Santamaria et al. [43], leaf number and yield of the rocket are low with NH4 nutrition, whereas they reach the highest values with the 50:50 NH4:NO3 ratio. In their study, water and N-use efficiencies increased in the rocket with the increase in NO3-N percentage in the nutrient solution. In our work, the mean Fe concentration (mg/L) for the 30-days study period was 3.61 ± 0.338 for Fe treatment and 3.55 ± 0.354 for Fe + K treatment, while the K concentration (mg/L) for the Fe + K treatment was 112.00 ± 9.953. These values are close to the optimum target values of 5 mg/L for Fe and 120 mg/L for K.
4.2. Fish Growth Performance
During the 30-day study period, red tilapias were fed twice daily ad libitum. For fish, FCR is used for the conventional measure of livestock production efficiency, while SGR is connected to fish growth. Lower FCR values and higher SGR values indicate a suitable growth performance. Red tilapia in the aquaponic systems of the present study showed high growth performance as judged by FCR and SGR values, which were similar along the three treatments. Additionally, the supplementation of Fe and K did not affect fish survival. All the above-mentioned results indicate that the addition of Fe or K did not affect fish growth. There are few articles examining the impact of fertilizers on tilapia growth. Rafiee et al. [44], working in aquaponics with a combination of lettuce and red tilapia, reported different fish survival rates between the treatment of no inputs and the treatment of Fe, K, Ca, Mg, Mn, P, and Zn addition (58% and 73%, respectively). Additionally, the latter treatment resulted in higher FCR and lower SGR compared to the treatment of no external nutrients. Ru et al. [33] reported 31.5% increased feed consumption and 14.3% higher FCR of Nile tilapia reared in an aquaponic system where nutrient supplementation occurred. The growth performance indices of the present study (survival, FCR, and SGR) indicate better fish growth compared to the two above-mentioned works [44,45]. Moreover, the absence of differences between treatments in all other measured fish growth parameters, such as weight gain, final weight, and length, highlights the neutral effect of Fe and K supplementation on fish development.
4.3. Fish Histopathology
Histological studies of farmed fish in aquaponics systems are limited. Vlahos et al. [16] performed the histopathological examination in sea breams in a brackish water aquaponic system, while Nozzi et al. [45] examined the histopathology of sea bass in a freshwater aquaponic system. Nevertheless, the experimental design of these studies did not include nutrient supplementation.
Fat deposition in the fish liver is affected by the dietary lipid content. Lower dietary lipid content results in lower liver lipid content and smaller and lesser lipid droplets [46]. In the present study, red tilapia were fed ad libidum twice daily with a fish feed containing 6.5% crude fat. Minimal lipid accumulation was detected at the liver of control and Fe treatment and mild lipid accumulation to the Fe + K treatment. No steatosis sign was detected in any of the treatments. Liver histopathology of the Fe + K treatment also showed the presence of granulomas, regions with larger nuclei, necrotic regions, and regions with haemmoradge. These alterations, by the frequency of their appearance in the examined fish, were characterized as mild. Since the liver is an important store of energy reserves, where dissection is possible, the hepatosomatic index (HIS) is often used as an estimate of the energy status of the fish [47,48]. According to Singh and Srivastava [49] (2017), a decrease in HIS has a relation to toxicity on fish. Values between 1.3% and 1.97% have been reported for red tilapia (Oreochromis spp.) [50,51,52,53]. Our values of HIS are in agreement with these studies, indicating no toxicity. Midgut had a normal structure in all three treatments. Individual lesions were detected in the midgut in 2 of the 15 examined fish and were characterized as random and non-specific.
Gills are the main respiratory organ of fish. Changes in dissolved oxygen and water temperature can lead to the rapid and reversible morphological change of the gills. Several species such as Oncorhynchus mykiss, Rutilus rutilus, Perca fluviatilis, Anguilla anguilla, Ambloplites rupestris, Micropterus salmoides, and Carassius carassius have been reported to be able to reduce their respiratory area and their oxygen demands as well [54,55,56,57,58,59]. Red tilapia is a warm water fish and can tolerate low dissolved oxygen values of about 4 mg/L [37]. The primary lamellae hyperplasia observed at the present study in all treatments may be a physiological result as the dissolved oxygen was between 8.32 and 8.41 mg/L, higher than the tilapia’s lower demands. Yavuzcan Yildiz et al. [8] reported that in an aquaponic system, the high level of suspended solids could provoke damage to the gill structure, such as epithelial detachment, hyperplasia, lamella fusion, and reduction in epithelial volume. In the present study, the histopathological examination of the gills revealed similar results as Yavuzcan Yildiz et al. [8] described. Uneaten food and feces were daily siphoned; however, a breakdown in small particles still occurred. These particles are very difficult to collect and are potentially dangerous.
In general, the very limited cases of mild histopathological alterations in liver, midgut, and gills and, moreover, their complete absence in the majority of fish indicate that tilapias of our study were well adapted to the aquaponics system.
4.4. Gills EDX Analysis
Freshwater fish can absorb waterborne metals through their gill epithelia due to the binding capacity of the mucous layer that covers the gill arches and their close contact with the surrounding environment [60,61]. Gills absorb active ions; thus, their oxygen absorption and osmoregulation capability can be affected, leading to numerous histopathological changes in the gill [62]. Wepener et al. [63] reported that when Tilapia sparrmanii was exposed to 319 mg/L iron concentration, the iron in gill tissue increased significantly after only 2 h exposure, remained elevated during the 72 h exposure period, and returned to control values after 96 h of exposure. In our research, red tilapias were exposed to significantly lower iron concentrations (3.61 mg/L and 3.55 mg/L for the Fe and Fe + K treatment, respectively), and no iron accumulation was detected at the gills after 30 days.
High potassium concentrations disrupt various physiological functions of fish, such as osmoregulation, acid-base balance, muscle contraction, and nerve function [64,65,66]. According to Mount et al. [67], a 305.6 mg/L potassium concentration could be lethal for fathead minnow. Davidson et al. [68] noticed gill irritation at concentrations of 110–130 mg/L K. In our research, the mean K concentration in the water was 112.00 mg/L for the Fe + K treatment, while for the control and Fe treatments were 3.81 mg/L and 4.44 mg/L, respectively. This fact was well reflected in the K accumulation in gills of the Fe + K treatment fish, where the K detected by EDX was seven times higher than the other two treatments. This high accumulation did not seem to affect the gill histomorphology, as mild histopathological alterations were detected in all three treatments. No mortalities were observed, indicating that the K concentration was not lethal.
4.5. Plant Growth Performance
Leaves fresh weight of the rocket plants that received additional Fe during their cultivation outweigh the plants of the other two treatments; however, the differences between this group and Fe + K group were not statistically significant. Control plants showed inferior growth in terms of fresh weight of the aerial parts, but also in the produced biomass per unit area, which is the measure of the system productivity. Nutrient amendments have been reported to enhance the productivity of the aquaponic systems, which are inherently deficient in Fe and K [33]. Nevertheless, it is important to examine and target the minimal possible perturbations of the system, hence the minimal inputs that sustain the productivity without compromising the sustainability of aquaponics. It was proved in our experimental setup that the addition of only Fe is adequate to succeed in optimum growth. K addition seems to be not indispensable for rocket growth. Considering the fact that K seems to accumulate to the gills, and maybe this accumulation can have long-term effects on fish health, we would suggest that in aquaponics systems with rocket cultivation, no extra K addition is needed.
There are a few articles working with a rocket in aquaponics, but their experimental questions are far from the target of the present study. Indeed, the educational use of aquaponics system [69] and life cycle assessment modeling [70] necessitated the use of rocket plants in mixture with many other crops; thus, the results could not be compared with ours. Barbosa et al. [71] examined rocket growth under two different system water volumes, and their results indicated poor growth performance reaching 8–15 times lower leaves fresh weight compared to ours, mainly due to extremely high sowing density of seedlings. Finally, Lennard and Ward [72] concluded that rocket does not adapt to aquaponic culture as well as other herbs, because they found increased plant growth in the hydroponics system. Our results do not corroborate these findings since the growth of rocket across all treatments in the present study showed 2–3 times higher values compared with both hydroponics and aquaponics as reported in the experiment of Lennard and Ward [72].
5. Conclusions
The results of the present study indicate that the addition of Fe and K slightly improved the produced fresh biomass of rocket per unit area, with the supplementation of only Fe being the minimal input that accelerated growth. Additionally, nutrients input had no impact on tilapia growth, survival and caused no remarkable histological alterations. Thus, although the addition of K led to the enrichment of this specific ion in fish gills, no effects of this accumulation were found on other aspects of fish growth and survival. All the above-mentioned results indicate that Fe fertilization can improve the production of a rocket, while K addition seems to be not indispensable for rocket growth. Both K and Fe do not impact tilapia growth and health parameters examined here and thus would not endanger the food safety of aquaponics products.
Author Contributions
P.B. and E.L. designed the study. P.S., E.T. and T.V. performed the experiments. E.L., E.T. and T.V. performed the plant growth parameters analysis and P.B and P.S. the histology analysis. P.S., P.B., E.L. and E.T. performed the data analysis. S.Z. performed the EDX analysis. P.B., E.L. and S.Z drafted the manuscript. All authors checked and approved the final version of the manuscript.
Funding
This research is co-financed by Greece and the European Union (European Social Fund-ESF) through the Operational Programme “Human Resources Development, Education and Lifelong Learning 2014–2020” in the context of the project “Aquaponic: When fish meet plants” (MIS 5048927).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki. The experimental protocol was approved by the Ethics Committee and conducted at the registered experimental facility (EL-43BIO/exp-01) of the Laboratory of Aquaculture, Department of Ichthyology and Aquatic Environment, University of Thessaly (n. 329309/24–11-2020).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rakocy, J.E.; Bailey, D.S.; Shultz, R.C.; Thoman, E.S. Update on tilapia and vegetable production in the UVI aquaponic system. In Proceedings of the Sixth International Symposium on Tilapia in Aquaculture, Manila, Philippines, 11 July 2004. [Google Scholar]
- Somerville, C.; Cohen, M.; Pantanella, E.; Stankus, A.; Lovatelli, A. Small-Scale Aquaponic Food Production: Integrated Fish and Plant Farming; FAO Fisheries and Aquaculture Technical Paper; FAO: Rome, Italy, 2014; Volume I, p. 589. [Google Scholar]
- Cebron, A.; Garnier, J. Nitrobacter and Nitrospira genera as representatives of nitrite-oxidizing bacteria: Detection, quantification and growth along the lower Seine River (France). Water Res. 2005, 39, 4979–4992. [Google Scholar] [CrossRef]
- Tyson, R.V.; Treadwell, D.D.; Simonne, E.H. Opportunities and challenges to sustainability in aquaponic systems. Hortechnology 2011, 21, 6–13. [Google Scholar] [CrossRef]
- Blidariu, F.; Grozea, A. Increasing the economic efficiency and sustainability of indoor fish farming by means of aquaponics—review. Anim. Sci. Biotechnol. 2011, 44, 1–8. [Google Scholar]
- Love, D.C.; Fry, J.P.; Li, X.; Hill, E.S.; Genello, L.; Semmens, K.; Thompson, R.E. Commercial aquaponics production and profitability: Findings from an international survey. Aquaculture 2015, 435, 67–74. [Google Scholar] [CrossRef]
- Love, D.C.; Uhl, M.S.; Genello, L. Energy and water use of a small-scale raft aquaponics system in Baltimore, Maryland, United States. Aquac. Eng. 2015, 68, 19–27. [Google Scholar] [CrossRef]
- Yavuzcan Yildiz, H.; Robaina, L.; Pirhonen, J.; Mente, E.; Domínguez, D.; Parisi, G. Fish Welfare in Aquaponic Systems: Its Relation to Water Quality with an Emphasis on Feed and Faeces—A Review. Water 2017, 9, 13. [Google Scholar] [CrossRef]
- Bittsanszky, A.; Uzinger, N.; Gyulai, G.; Mathis, A.; Junge, R.; Villarroel, M.; Kotzen, B.; Kőmíves, T. Nutrient supply of plants in aquaponic systems. Ecocycles 2016, 2, 17–20. [Google Scholar] [CrossRef]
- Nuwansi, K.K.T.; Verma, A.K.; Prakash, C.; Tiwari, V.K.; Chandrakant, M.H.; Shete, A.P.; Prabhath, G.P.W.A. Effect of water flow rate on polyculture of koi carp (Cyprinus carpio var. koi) and goldfish (Carassius auratus) with water spinach (Ipomoea aquatica) in recirculating aquaponic system. Aquac. Int. 2016, 24, 385–393. [Google Scholar] [CrossRef]
- Andriani, Y.; Dhahiyat, Y.; Zahidah, Z.; Zidni, I. The effect of stocking density ratio of fish on water plant productivity in aquaponics culture system. Nusant. Biosci. 2017, 9, 31–35. [Google Scholar] [CrossRef]
- Diver, S.; Rinehart, L. Aquaponics-Integration of Hydroponics with Aquaculture; Attra: Butte, MT, USA, 2000; pp. 1–16. [Google Scholar]
- Saha, S.; Monroe, A.; Day, M.R. Growth, yield, plant quality and nutrition of basil (Ocimum basilicum L.) under soilless agricultural systems. Ann. Agricult. Sci. 2016, 61, 181–186. [Google Scholar] [CrossRef]
- Global Invasive Species Database. Species Profile: Oreochromis Mossambicus. 2021. Available online: http://www.iucngisd.org/gisd/species.php?sc=131 (accessed on 10 May 2021).
- FAO. The State of World Fisheries and Aquaculture 2020; Sustainability in Action; FAO: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Knaus, U.; Palm, H.W. Effects of the fish species choice on vegetables in aquaponics under spring-summer conditions in northern Germany (Mecklenburg Western Pomerania). Aquaculture 2017, 473, 62–73. [Google Scholar] [CrossRef]
- Goddek, S.; Joyce, A.; Wuertz, S.; Körner, O.; Bläser, I.; Reuter, M.; Keesman, K.J. Decoupled aquaponics systems. In Aquaponics Food Production Systems; Goddek, S., Joyce, A., Kotzen, B., Burnell, G.M., Eds.; Springer: Cham, Switzerland, 2019; pp. 201–229. [Google Scholar]
- Boxman, S. Resource Recovery through Halophyte Production in Marine Aquaponics: An Evaluation of the Nutrient Cycling and the Environmental Sustainability of Aquaponics. Ph.D. Thesis, University of South Florida, Tampa, FL, USA, 2015. [Google Scholar]
- Vlahos, N.; Levizou, E.; Stathopoulou, P.; Berillis, P.; Antonopoulou, E.; Bekiari, V.; Krigas, N.; Kormas, K.; Mente, E. An Experimental Brackish Aquaponic System Using Juvenile Gilthead Sea Bream (Sparus aurata) and Rock Samphire (Crithmum maritimum). Sustainability 2019, 11, 4820. [Google Scholar] [CrossRef]
- Endut, A.; Jusoh, A.; Ali, N.; Wan Nik, W.B.; Hassan, A. A study on the optimal hydraulic loading rate and plant ratios in recirculation aquaponic system. Bioresour. Technol. 2010, 101, 1511–1517. [Google Scholar] [CrossRef]
- Palm, W.H.; Bissa, K.; Knaus, U. Significant factors a ecting the economic sustainability of closed aquaponics systems. Part II: Fish and plant growth. AACL Bioflux 2014, 7, 162–175. [Google Scholar]
- Khater, E.G.; Bahnasawy, A.H.; Shams, A.E.S.; Hassaan, M.S.; Hassan, Y.A. Utilization of effuent fishfarms in tomato cultivation. Ecol. Eng. 2015, 83, 199–207. [Google Scholar] [CrossRef]
- Rakocy, J.E. Ten Guidelines for Aquaponic Systems. Aquaponics J. 2007, 1, 14–17. [Google Scholar]
- Hirayama, K. Water control by filtration in closed culture systems. Aquaculture 1974, 4, 369–385. [Google Scholar] [CrossRef]
- Delaide, B.; Goddek, S.; Gott, J.; Soyeurt, H.; Jijakli, M.H. Lettuce (Lactuca sativa L. var. Sucrine) growth performance in complemented aquaponic solution outperforms hydroponics. Water 2016, 8, 467. [Google Scholar] [CrossRef]
- Nicoletto, C.; Maucieri, C.; Mathis, A.; Schmautz, Z.; Komives, T.; Sambo, P.; Junge, R. Extension of aquaponic water use for NFT baby-leaf production: Mizuna and rocket salad. Agronomy 2018, 8, 75. [Google Scholar] [CrossRef]
- Johnson, R.; Wolf, J.; Braunbeck, T. Guidance Document on the Diagnosis of Endocrine-Related Histopathology in Fish Gonads; Organisation for Economic Co-operation and Development: Paris, France, 2009; p. 96. [Google Scholar]
- Philippart, J.C.; Ruwet, J.C. Ecology and distribution of tilapias. In The Biology and Culture of Tilapias; ICLARM Conference Proceedings 7; ICLARM-International Center for Living Aquatic Resources Management: Abbassa, Egypt, 1982; pp. 15–59. [Google Scholar]
- El-Sayed, A.F.M. Effects of stocking density and feeding levels on growth and feed efficiency of Nile tilapia (Oreochromis niloticus L.) fry. Aquaculture research 2002, 33, 621–626. [Google Scholar] [CrossRef]
- Al-Hafedh, Y.S.; Alam, A.; Beltagi, M.S. Food production and water conservation in a recirculating aquaponic system in Saudi Arabia at different ratios of fish feed to plants. J. World Aquac. Soc. 2008, 39, 510–520. [Google Scholar] [CrossRef]
- García-Trejo, J.F.; Peña-Herrejon, G.A.; Soto-Zarazúa, G.M.; Mercado-Luna, A.; Alatorre-Jácome, O.; Rico-García, E. Effect of stocking density on growth performance and oxygen consumption of Nile tilapia (Oreochromis niloticus) under greenhouse conditions. Lat. Am. J. Aquat. Res. 2016, 44, 177–183. [Google Scholar] [CrossRef]
- da Silva Cerozi, B.; Fitzsimmons, K. The effect of pH on phosphorus availability and speciation in an aquaponics nutrient solution. Bioresour. Technol. 2016, 219, 778–781. [Google Scholar] [CrossRef] [PubMed]
- Ru, D.; Liu, J.; Hu, Z.; Zou, Y.; Jiang, L.; Cheng, X.; Lv, Z. Improvement of aquaponic performance through micro-and macro-nutrient addition. Environ. Sci. Pollut. Res. 2017, 24, 16328–16335. [Google Scholar] [CrossRef] [PubMed]
- Lennard, W.; Goddek, S. Aquaponics: The basics. In Aquaponics Food Production Systems; Goddek, S., Joyce, A., Kotzen, B., Burnell, G.M., Eds.; Springer: Cham, Switzerland, 2019; pp. 113–143. [Google Scholar]
- Tyson, R.; Simonne, E.; White, J.; Lamb, E. Reconciling water quality parameters impacting nitrification in aquaponics: The pH levels. Proc. Fla. State Horticult. Soc. 2004, 117, 79–83. [Google Scholar]
- Abdel-Tawwab, M.; Hagras, A.E.; Elbaghdady, H.A.M.; Monier, M.N. Effects of dissolved oxygen and fish size on Nile tilapia, Oreochromis niloticus (L.): Growth performance, whole-body composition, and innate immunity. Aquac. Int. 2015, 23, 1261–1274. [Google Scholar] [CrossRef]
- Wedemeyer, G.A. Physiology of Fish in Intensive Culture Systems; Springer: Boston, MA, USA, 1996. [Google Scholar]
- Lennard, W. Aquaponic System Design Parameters: Basic System Water Chemistry; Aquaponic Fact Sheet Series—System Water Chemistry; Aquaponic Solutions: Melbourne, Australia, 2012. [Google Scholar]
- Dunwoody, R.K. Aquaponics and Hydroponics: The Effects of Nutrient Source and Hydroponic Subsystem Design on Sweet Basil Production. Master’s Thesis, Department of Biology and Agriculture, University of Central Missouri, Warrensburg, MO, USA, 2013. [Google Scholar]
- Rakocy, J.E.; Masser, M.P.; Losordo, T.M. Recirculating Aquaculture Tank Production Systems: Aquaponics—Integrating Fish and Plant Culture; SRAC Publication No. 454; Southern Regional Aquaculture Center: Stoneville, MS, USA, 2006. [Google Scholar]
- Lennard, W. Commercial Aquaponic Systems: Integrating Recirculating Fish Culture with Hydroponic Plant Production; Wilson Lennard Ed: Blackrock, VIC, Australia, 2017. [Google Scholar]
- Eck, M.; Körner, O.; Jijakli, M.H. Nutrient cycling in aquaponics systems. In Aquaponics Food Production Systems; Goddek, S., Joyce, A., Kotzen, B., Burnell, G.M., Eds.; Springer: Cham, Switzerland, 2019; pp. 231–246. [Google Scholar]
- Santamaria, P.; Elia, A.; Papa, G.; Serio, F. Nitrate and ammonium nutrition in chicory and rocket salad plants. J. Plant Nutr. 1998, 21, 1779–1789. [Google Scholar] [CrossRef]
- Rafiee, G.R.; Ros Saad, C.; Kamarudin, M.S.; Ismail, M.R.; Sijam, K. Effects of supplementary nutrient in an aquaponic system for production of ornamental red tilapia (Oreochromis Sp.) and lettuce (Lactuca sativa var longifolia). Surv. Fish. Sci. 2019, 5, 65–75. [Google Scholar] [CrossRef]
- Nozzi, V.; Strofaldi, S.; Piquer, I.F.; Di Crescenzo, D.; Olivotto, I.; Carnevali, O. Amyloodinum ocellatum in Dicentrarchus labrax: Study of infection in salt water and freshwater aquaponics. Fish Shellfish. Immunol. 2016, 57, 179–185. [Google Scholar] [CrossRef]
- Valaroutsou, E.; Voudanta, E.; Mente, E.; Berillis, P. A microscope and image analysis study of the liver and exocrine pancreas of sea bream Sparus aurata fed different diets. Int. J. Zool. Res. 2013, 3, 54. [Google Scholar]
- Wootton, R.J.; Evans, G.W.; Mills, L.A. Annual cycle in female three-spined sticklebacks (Gasterosteus aculeatus L.) from an upland and lowland population. J. Fish Biol. 1978, 12, 331–343. [Google Scholar] [CrossRef]
- Campbell, S.; Love, R.M. Energy reserves of male and female haddock (Melanogrunzmus aeglefinus L.) from the Moray Firth. J. Du Cons. Int. Pour L’explorution De Lu Mer 1978, 38, 120–121. [Google Scholar] [CrossRef]
- Singh, S.; Srivastava, A. Variations in hepatosomatic index (HSI) and gonadosomatic index (GSI) in fish heteropneustes fossilis exposed to higher sub-lethal concentration to arsenic and copper. J. Ecophysiol. Occup. Health 2017, 15, 89–93. [Google Scholar]
- Ng, W.K.; Lim, H.A.; Lim, S.L.; Ibrahim, C.O. Nutritive value of palm kernel meal pretreated with enzyme or fermented with Trichoderma koningii (Oudemans) as a dietary ingredient for red hybrid tilapia (Oreochromis sp.). Aquac. Res. 2002, 33, 1199–1207. [Google Scholar] [CrossRef]
- Ng, W.K.; Koh, C.B.; Sudesh, K.; Siti-Zahrah, A. Effects of dietary organic acids on growth, nutrient digestibility and gut microflora of red hybrid tilapia, Oreochromis sp., and subsequent survival during a challenge test with Streptococcus agalactiae. Aquac. Res. 2009, 40, 1490–1500. [Google Scholar] [CrossRef]
- Low, K.H.; Zain, S.M.; Abas, M.R. Evaluation of metal concentrations in red tilapia (Oreochromis spp) from three sampling sites in Jelebu, Malaysia using principal component analysis. Food Anal. Methods 2011, 4, 276–285. [Google Scholar] [CrossRef]
- Wan-Mohtar, W.A.A.Q.I.; Taufek, N.M.; Yerima, G.; Rahman, J.; Thiran, J.P.; Subramaniam, K.; Sabaratnam, V. Effect of bioreactor-grown biomass from Ganoderma lucidum mycelium on growth performance and physiological response of red hybrid tilapia (Oreochromis sp.) for sustainable aquaculture. Org. Agric. 2021, 11, 327–335. [Google Scholar] [CrossRef]
- McCormick, J.H.; Jensen, K.M.; Leino, R.L. Survival, blood osmolality and gill morphology of juvenile yellow perch, rock bass, black crappie, and largemouth bass exposed to acidified soft water. Trans. Am. Fish. Soc. 1989, 118, 386–399. [Google Scholar] [CrossRef]
- Leino, R.L.; McCormick, J.H. Responses of juvenile largemouth bass to different pH and aluminum levels at overwintering temperatures—effects on gill morphology, electrolyte balance, scale calcium, liver glycogen, and depot fat. Can. J. Zool. 1993, 71, 531–543. [Google Scholar] [CrossRef]
- Lappivaara, J.; Nikinmaa, M.; Tuurala, H. Arterial oxygentension and the structure of the secondary lamellae of the gills in rainbow-trout (Oncorhynchus mykiss) after acute exposure to zinc and during recovery. Aquat. Toxicol. 1995, 32, 321–331. [Google Scholar] [CrossRef]
- Haaparanta, A.; Valtonen, E.T.; Hoffmann, R.W. Gill anomalies of perch and roach from four lakes differing in water quality. J. Fish Biol. 1997, 50, 575–591. [Google Scholar] [CrossRef]
- Sollid, J.; De Angelis, P.; Gundersen, K.; Nilsson, G.E. Hypoxia induces adaptive and reversible gross-morphological changes in crucian carp gills. J. Exp. Biol. 2003, 206, 3667–3673. [Google Scholar] [CrossRef] [PubMed]
- Berillis, P.; Mente, E.; Nikouli, E.; Makridis, P.; Grundvig, H.; Bergheim, A.; Gausen, M. Improving aeration for efficient oxygenation in sea bass sea cages. Blood, brain and gill histology. Open Life Sci. 2016, 11, 270–279. [Google Scholar] [CrossRef]
- Palaniappan, P.R.; Sabhanayakam, S.; Krishnakumar, N.; Vadivelu, M. Morphological changes due to lead exposure and the influence of DMSA on the gill tissues of the freshwater fish, Catla catla. Food Chem. Toxicol. 2008, 46, 2440–2444. [Google Scholar] [CrossRef] [PubMed]
- Aldoghachi, M.A.; Azirun, M.S.; Yusoff, I.; Ashraf, M.A. Ultrastructural effects on gill tissues induced in red tilapia Oreochromis sp. by a waterborne lead exposure. Saudi J. Biol. Sci. 2016, 23, 634–641. [Google Scholar] [CrossRef]
- Lehtinen, K.; Klingstedt, G. X-ray microanalysis in the scanning electron microscope on fish gills affected by acidic, heavy metal containing industrial effluents. Aquat. Toxicol. 1983, 3, 93–102. [Google Scholar] [CrossRef]
- Wepener, V.; Van Vuren, J.H.J.; Du Preez, H.H. Uptake and distribution of a copper, iron and zinc mixture in gill, liver and plasma of a freshwater teleost, Tilapia sparrmanii. Water SA 2001, 27, 99–108. [Google Scholar] [CrossRef]
- Evans, D.H. Teleost fish osmoregulation: What have we learned since August Krogh, Homer Smith, and Ancel Keys. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2008, 295, 704–713. [Google Scholar] [CrossRef]
- Opoku-Okrah, C.; Acquah, B.K.S.; Dogbe, E.E. Changes in potassium and sodium concentrations in stored blood. Pan Afr. Med. J. 2015, 20. [Google Scholar] [CrossRef]
- Borvinskaya, E.V.; Sukhovskaya, I.V.; Vasil’eva, O.B.; Nazarova, M.A.; Smirnov, L.P.; Svetov, S.A.; Krutskikh, N.V. Whitefish (Coregonus lavaretus) Response to Varying Potassium and Sodium Concentrations: A Model of Mining Water Toxic Response. Mine Water Environ. 2016, 36, 393–400. [Google Scholar] [CrossRef]
- Mount, D.R.; Gulley, D.D.; Hockett, J.R.; Garrison, T.D.; Evans, J.M. Statistical models to predict the toxicity of major ions to Ceriodaphnia dubia, Daphnia magna and Pimephales promelas (fathead minnows). Environ. Toxicol. Chem. 1997, 16, 2009–2019. [Google Scholar] [CrossRef]
- Davidson, J.; Good, C.; Welsh, C.; Summerfelt, S.T. Abnormal swimming behavior and increased deformities in rainbow trout Oncorhynchus mykiss cultured in low exchange water recirculating aquaculture systems. Aquac. Eng. 2011, 45, 109–117. [Google Scholar] [CrossRef]
- Maucieri, C.; Forchino, A.A.; Nicoletto, C.; Junge, R.; Pastres, R.; Sambo, P.; Borin, M. Life cycle assessment of a micro aquaponic system for educational purposes built using recovered material. J. Clean. Prod. 2018, 172, 3119–3127. [Google Scholar] [CrossRef]
- Chen, P.; Zhu, G.; Kim, H.J.; Brown, P.B.; Huang, J.Y. Comparative life cycle assessment of aquaponics and hydroponics in the Midwestern United States. J. Clean. Prod. 2020, 275, 122888. [Google Scholar] [CrossRef]
- Barbosa, P.T.L.; Povh, J.A.; Silva, A.; do Nascimento, L.; Ventura, A.S.; Stringhetta, G.R.; Laice, L.M.; de Oliveira, A.F.; de Carvalho, T. Performance of Nile Tilapia and vegetables Grown in Different Aquaponic Volumes. J. Agric. Stud. 2020, 8, 497–506. [Google Scholar] [CrossRef]
- Lennard, W.; Ward, J. A comparison of plant growth rates between an NFT hydroponic system and an NFT aquaponic System. Horticulturae 2019, 5, 27. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).