Exercise and Manual Therapy for Diabetic Peripheral Neuropathy: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registry
2.2. Search and Information Sources
2.3. Eligibility Criteria
2.4. Selection of Studies
2.5. Data Extraction Process
2.6. Risk of Bias in Individual Studies
3. Results
3.1. Selection of Studies
3.2. Study Characteristics
3.2.1. Sample
3.2.2. Intervention
Exercise
Manual Therapy
Exercise and Manual Therapy
3.2.3. Dosage
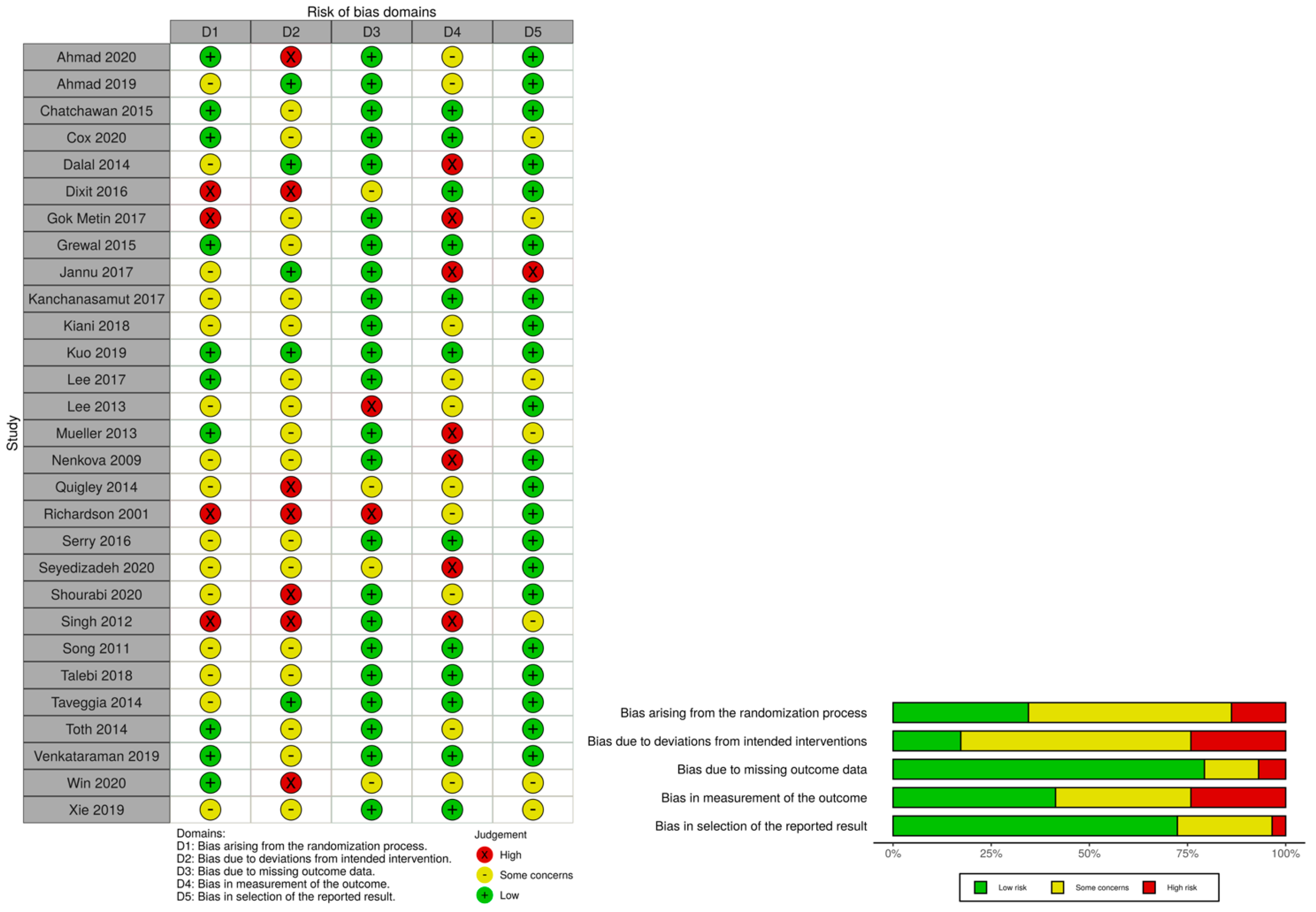
3.2.4. Evaluation of Methodological Quality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2005, 28, 7–42. [Google Scholar]
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care 2004, 27, 1047–1053. [Google Scholar] [CrossRef]
- Van Laake-Geelen, C.C.M.; Smeets, R.J.E.M.; Quadflieg, S.P.A.B.; Kleijnen, J.; Verbunt, J.A. The effect of exercise therapy combined with psychological therapy on physical activity and quality of life in patients with painful diabetic neuropathy: A systematic review. Scand. J. Pain 2019, 19, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Lineaweaver, W.C.; Chen, Z.; Hu, J.; Mullins, F.; Zhang, F. Surgical Decompression in the Treatment of Diabetic Peripheral Neuropathy: A Systematic Review and Meta-analysis. J. Reconstr. Microsurg. 2016, 33, 151–157. [Google Scholar] [CrossRef]
- Tadesse, D.B.; Gebrewahd, G.T.; Hailay, A.; Aberhe, W.; Mebrahtom, G.; Zereabruk, K.; Gebreayezgi, G.; Mariye, T.; Haile, T.G.; Gebremeskel, G.G.; et al. Diabetic Peripheral Neuropathy in Ethiopia: A Systematic Review and Meta-Analysis. J. Diabetes Res. 2021, 2021, 5304124. [Google Scholar]
- Castelli, G.; Desai, K.M.; Cantone, R.E. Peripheral Neuropathy: Evaluation and Differential Diagnosis. Am. Fam. Physician 2020, 102, 732–739. [Google Scholar]
- Vincent, A.M.; Brownlee, M.; Russell, J.W. Oxidative stress and programmed cell death in diabetic neuropathy. N. Y. Acad. Sci. 2002, 959, 368–383. [Google Scholar] [CrossRef] [PubMed]
- Samper Bernal, D.; Monerris Tabasco, M.M.; Homs Riera, M.; Soler Pedrola, M. Aetiology and management of diabetic peripheral neuropathy. Rev. Soc. Española Dolor. 2010, 17, 286–296. [Google Scholar] [CrossRef]
- Vázquez San Miguel, F.; Mauricio Puente, D.; Viadé Julià, J. Neuropatía diabética y pie diabético. Medicine 2016, 12, 971–981. [Google Scholar] [CrossRef]
- Wong, M.C.; Chung, J.W.Y.; Wong, T.K.S. Effects of treatments for symptoms of painful diabetic neuropathy: Systematic review. Br. Med. J. 2007, 335, 87–90. [Google Scholar] [CrossRef]
- Stein, C.; Eibel, B.; Sbruzzi, G.; Lago, P.D.; Plentz, R.D.M. Electrical stimulation and electromagnetic field use in patients with diabetic neuropathy: Systematic review and meta-analysis. Braz. J. Phys. Ther. 2013, 17, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Yammine, K.; Wehbe, R.; Assi, C. A systematic review on the efficacy of vitamin D supplementation on diabetic peripheral neuropathy. Clin. Nutr. 2020, 39, 2970–2974. [Google Scholar] [CrossRef]
- Colberg, S.R.; Sigal, R.J.; Fernhall, B.; Regensteiner, J.G.; Blissmer, B.J.; Rubin, R.R.; Chasan-Taber, L.; Albright, A.L.; Braun, B. Exercise and type 2 diabetes: The American College of Sports Medicine and the American Diabetes Association: Joint position statement. Diabetes Care 2010, 33, 147–167. [Google Scholar] [CrossRef] [PubMed]
- Association, A.D. Standards of Diabetes Care-2021. J. Clin. Appl. Res. Educ. 2021, 44, S21–S226. [Google Scholar]
- Matthews, B.G.; Hurn, S.E.; Harding, M.P.; Henry, R.A.; Ware, R.S. The effectiveness of non-surgical interventions for common plantar digital compressive neuropathy (Morton’s neuroma): A systematic review and meta-analysis. J. Foot Ankle Res. 2019, 12, 1–21. [Google Scholar] [CrossRef]
- Del Barrio, S.J.; Gracia, E.B.; García, C.H.; de Miguel, E.E.; Moreno, J.T.; Marco, S.R.; Laita, L.C. Tratamiento conservador en pacientes con síndrome del túnel carpiano con intensidad leve o moderada. Revisión sistemática. Neurología 2018, 33, 590–601. [Google Scholar] [CrossRef]
- Hernández-Secorún, M.; Montaña-Cortés, R.; Hidalgo-García, C.; Rodríguez-Sanz, J.; Corral-de-Toro, J.; Monti-Ballano, S.; Hamam-Alcober, S.; Tricás-Moreno, J.M.; Lucha-López, M.O. Effectiveness of conservative treatment according to severity and systemic disease in carpal tunnel syndrome: A systematic review. Int. J. Environ. Res. Public Health 2021, 18, 2365. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Moher, D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Herbert, R.; Moseley, A.; Sherrington, C.; Maher, C. Escala PEDro-Español. Physiotherapy 2000, 86, 55. [Google Scholar] [CrossRef]
- Sterne, J.A.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Risk of Bias Tools—Robvis (Visualization Tool). Cochrane Database of Systematic Reviews. Available online: https://mcguinlu.shinyapps.io/robvis/ (accessed on 10 March 2021).
- Chatchawan, U.; Eungpinichpong, W.; Plandee, P.; Yamauchi, J. Effects of thai foot massage on balance performance in diabetic patients with peripheral neuropathy: A randomized parallel-controlled trial. Med. Sci. Monit. Basic Res. 2015, 21, 68–75. [Google Scholar]
- Cox, E.R.E.; Gajanand, T.; Burton, N.W.N.; Coombes, J.J.S.; Coombes, B.B.K. Effect of different exercise training intensities on musculoskeletal and neuropathic pain in inactive individuals with type 2 diabetes—Preliminary randomised controlled trial [with consumer summary]. Diabetes Res. Clin. Pract. 2020, 164, 108168. [Google Scholar] [CrossRef]
- Singh, P.P.; Bindra, S.; Singh, S.; Aggarwal, R.; Singh, J. Effect of Nerve Mobilization on Vibration Perception Threshold in Diabetic Peripheral Neuropathy. Indian J. Physiother. Occup. Ther. 2012, 6, 189–195. [Google Scholar]
- Taveggia, G.; Villafañe, J.H.; Vavassori, F.; Lecchi, C.; Borboni, A.; Negrini, S. Multimodal treatment of distal sensorimotor polyneuropathy in diabetic patients: A randomized clinical trial. J. Manip. Physiol. Ther. 2014, 37, 242–252. [Google Scholar] [CrossRef]
- Venkataraman, K.; Tai, B.C.; Khoo, E.Y.; Tavintharan, S.; Chandran, K.; Hwang, S.W.; Phua, M.S.; Wee, H.L.; Koh, G.C.; Tai, E.S. Short-term strength and balance training does not improve quality of life but improves functional status in individuals with diabetic peripheral neuropathy: A randomised controlled trial. Diabetologia 2019, 62, 2200–2210. [Google Scholar] [CrossRef]
- Win, M.M.T.M.M.; Fukai, K.; Nyunt, H.H.H.; Linn, K.Z.K. Hand and foot exercises for diabetic peripheral neuropathy: A randomized controlled trial. Nurs. Health Sci. 2020, 22, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Dixit, S.; Maiya, A.; Shastry, B.A.; Guddattu, V. Analysis of postural control during quiet standing in a population with diabetic peripheral neuropathy undergoing moderate intensity aerobic exercise training: A single blind, randomized controlled trial. Am. J. Phys. Med. Rehabil. 2016, 95, 516–524. [Google Scholar] [CrossRef]
- Grewal, G.S.; Schwenk, M.; Lee-Eng, J.; Parvaneh, S.; Bharara, M.; Menzies, R.A.; Talal, T.K.; Armstrong, D.G.; Najafi, B. Sensor-Based Interactive Balance Training with Visual Joint Movement Feedback for Improving Postural Stability in Diabetics with Peripheral Neuropathy: A Randomized Controlled Trial. Gerontology 2015, 61, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Jannu, C.; Prathap, S.; Puchchakayala, G.; Vahini, C.; Balaraju, K. Efficacy of Stability Trainer Exercises versus Wobble Board Exercises in Balance Re-Education in Patients with Diabetic Neuropathy. Indian J. Physiother. Occup. Ther. An. Int. J. 2017, 11, 12. [Google Scholar] [CrossRef]
- Kuo, L.C.; Yang, C.J.; Lin, C.F.; Jou, I.M.; Yang, Y.C.; Yeh, C.H.; Lin, C.C.; Hsu, H.Y. Effects of a task-based biofeedback training program on improving sensorimotor function in neuropathic hands in diabetic patients: A randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2019, 55, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Mueller, M.J.; Tuttle, L.J.; LeMaster, J.W.; Strube, M.J.; McGill, J.B.; Hastings, M.K.; Sinacore, D.R. Weight-bearing versus nonweight-bearing exercise for persons with diabetes and peripheral neuropathy: A randomized controlled trial. Arch. Phys. Med. Rehabil. 2013, 94, 829–838. [Google Scholar] [CrossRef]
- Nenkova, S.; Stewart, A.; Potterton, J.; Becker, P. The effects of isometric exercises and stretching on postural stability in Non–Insulin Dependent Diabetes Mellitus patients with diffuse symmetrical sensory motor neuropathy. S. Afr. J. Physiother. 2009, 65, 27–31. [Google Scholar] [CrossRef]
- Serry, Z.M.; Mossa, G.; Elhabashy, H.; Elsayed, S.; Elhadidy, R.; Azmy, R.M.; Mokhtar, A. Transcutaneous nerve stimulation versus aerobic exercise in diabetic neuropathy. Egypt J. Neurol. Psychiatry Neurosurg. 2016, 53, 124–129. [Google Scholar]
- Shourabi, P.; Bagheri, R.; Ashtary-Larky, D.; Wong, A.; Motevalli, M.S.; Hedayati, A.; Baker, J.S.; Rashidlamir, A. Effects of hydrotherapy with massage on serum nerve growth factor concentrations and balance in middle aged diabetic neuropathy patients. Complement. Ther. Clin. Pract. 2020, 39, 101141. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.K.; Sandman, D.; Vela, S. A focused exercise regimen improves clinical measures of balance in patients with peripheral neuropathy. Arch. Phys. Med. Rehabil. 2001, 82, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Lee, S.; Song, C. Whole-body vibration training improves balance, muscle strength and glycosylated hemoglobin in elderly patients with diabetic neuropathy. Tohoku J. Exp. Med. 2013, 231, 305–314. [Google Scholar] [CrossRef]
- Lee, K. Effects of whole-body vibration therapy on perception thresholds of type 2 diabetic patients with peripheral neuropathy: A randomized controlled trial. J. Phys. Ther. Sci. 2017, 29, 1684–1688. [Google Scholar] [CrossRef][Green Version]
- Kiani, N.; Marryam, M.; Malik, A.A.N.; Amjad, I. The effect of aerobic exercises on balance in diabetic neuropathy patients. J. Med. Sci. 2018, 26, 141–145. [Google Scholar]
- Ahmad, I.; Noohu, M.M.; Verma, S.; Singla, D.; Hussain, M.E. Effect of sensorimotor training on balance measures and proprioception among middle and older age adults with diabetic peripheral neuropathy. Gait Posture 2019, 74, 114–120. [Google Scholar] [CrossRef]
- Ahmad, I.; Verma, S.; Noohu, M.M.; Shareef, M.Y.; Ejaz Hussain, M. Sensorimotor and gait training improves proprioception, nerve function, and muscular activation in patients with diabetic peripheral neuropathy: A randomized control trial. J. Musculoskelet Neuronal Interact. 2020, 20, 234–248. [Google Scholar]
- Seyedizadeh, S.S.H.; Cheragh-Birjandi, S.; Hamedi Nia, M.R.M.; Cheragh-Birjandi, S.; Hamedi Nia, M.R.M. The Effects of Combined Exercise Training (Resistance-Aerobic) on Serum Kinesin and Physical Function in Type 2 Diabetes Patients with Diabetic Peripheral Neuropathy (Randomized Controlled Trials). J. Diabetes Res. 2020, 2020, 6978128. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Lu, L.; Zhou, X.; Zhong, C.; Ge, G.; Huang, H.; Zhang, X.; Zeng, Y. Effect of Gua Sha therapy on patients with diabetic peripheral neuropathy: A randomized controlled trial. Complement. Ther. Clin. Pract. 2019, 35, 348–352. [Google Scholar] [CrossRef]
- Talebi, G.A.; Saadat, P.; Javadian, Y.; Taghipour, M. Manual therapy in the treatment of carpal tunnel syndrome in diabetic patients: A randomized clinical trial. Casp. J. Intern. Med. 2018, 9, 283–289. [Google Scholar]
- Gok Metin, Z.; Arikan Donmez, A.; Izgu, N.; Ozdemir, L.; Arslan, I.E. Aromatherapy Massage for Neuropathic Pain and Quality of Life in Diabetic Patients. J. Nurs. Scholarsh. 2017, 49, 379–388. [Google Scholar] [CrossRef]
- Toth, C.; Brady, S.; Gagnon, F.; Wigglesworth, K. A randomized, single-blind, controlled, parallel assignment study of exercise versus education as adjuvant in the treatment of peripheral neuropathic pain. Clin. J. Pain 2014, 30, 111–118. [Google Scholar] [CrossRef]
- Quigley, P.A.; Bulat, T.; Schulz, B.; Friedman, Y.; Hart-Hughes, S.; Richardson, J.K.; Barnett, S. Exercise interventions, gait, and balance in older subjects with distal symmetric polyneuropathy: A three-group randomized clinical trial. Am. J. Phys. Med. Rehabil. 2014, 93, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Dalal, K.; Maran, V.B.; Pandey, R.M.; Tripathi, M. Determination of efficacy of reflexology in managing patients with diabetic neuropathy: A randomized controlled clinical trial. Evid. Based Complement. Altern. Med. 2014, 2014, 843036. [Google Scholar] [CrossRef]
- Song, C.H.; Petrofsky, J.S.; Lee, S.W.; Lee, K.J.; Yim, J.E. Effects of an exercise program on balance and trunk proprioception in older adults with diabetic neuropathies. Diabetes Technol. Ther. 2011, 13, 803–811. [Google Scholar] [CrossRef]
- Kanchanasamut, W.; Pensri, P. Effects of weight-bearing exercise on a mini-trampoline on foot mobility, plantar pressure and sensation of diabetic neuropathic feet; a preliminary study. Diabetes Foot Ankle 2017, 8, 1287239. [Google Scholar] [CrossRef] [PubMed]
- Boulton, A.J.; Vinik, A.I.; Arezzo, J.C.; Bril, V.; Feldman, E.L.; Freeman, R.; Malik, R.A.; Maser, R.E.; Sosenko, J.M.; Ziegler, D. Diabetic neuropathies: A statement of the American Diabetes Association. Diabetes Care 2005, 28, 956–962. [Google Scholar] [CrossRef]
- Amelia, R.; Wahyuni, A.S.; Yunanda, Y. Diabetic neuropathy among type 2 diabetes mellitus patients at amplas primary health care in Medan city. Open Access Maced. J. Med. Sci. 2019, 7, 3400–3403. [Google Scholar] [CrossRef] [PubMed]
- Forouhi, N.G.; Wareham, N.J. Epidemiology of diabetes. Medicine 2014, 42, 698–702. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, A.S.; Leet, J.; Egan, K.; Garza, R. Diabetic Neuropathy: A Critical, Narrative Review of Published Data from 2019. Curr. Pain Headache Rep. 2021, 25, 15. [Google Scholar] [CrossRef]
- Schmid, A.B.; Bland, J.D.P.; Bhat, M.A.; Bennett, D.L.H. The relationship of nerve fibre pathology to sensory function in entrapment neuropathy. Brain 2014, 137, 3186–3199. [Google Scholar] [CrossRef]
- Gu, Y.; Dennis, S.M.; Kiernan, M.C.; Harmer, A.R. Aerobic exercise training may improve nerve function in type 2 diabetes and pre-diabetes: A systematic review. Diabetes Metab. Res. Rev. 2019, 35, e3099. [Google Scholar] [CrossRef] [PubMed]
- Ites, K.I.; Anderson, E.J.; Cahill, M.L.; Kearney, J.A.; Post, E.C.; Gilchrist, L.S. Balance interventions for Diabetic Peripheral Neuropathy: A systematic review. J. Geriatr. Phys. Ther. 2011, 34, 109–116. [Google Scholar] [CrossRef]
- Zhu, G.C.; Tsai, K.L.; Chen, Y.W.; Hung, C.H. Neural mobilization attenuates mechanical allodynia and decreases proinflammatory cytokine concentrations in rats with painful diabetic neuropathy. Phys. Ther. 2018, 98, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Larkin, M.E.; Barnie, A.; Braffett, B.H.; Cleary, P.A.; Diminick, L.; Harth, J.; Gatcomb, P.; Golden, E.; Lipps, J.; Lorenzi, G.; et al. Musculoskeletal complications in type 1 diabetes. Diabetes Care 2014, 37, 1863–1869. [Google Scholar] [CrossRef]
- Bialosky, J.E.; Bishop, M.D.; Price, D.D.; Robinson, M.E.; Vincent, K.R.; George, S.Z. A randomized sham-controlled trial of a neurodynamic technique in the treatment of carpal tunnel syndrome. J. Orthop. Sports Phys. Ther. 2009, 39, 709–723. [Google Scholar] [CrossRef] [PubMed]
- Lima, C.R.; Martins, D.F.; Reed, W.R. Physiological responses induced by manual therapy in animal models: A scoping review. Front. Neurosci. 2020, 14, 430. [Google Scholar] [CrossRef]

| Database | Search Strategy | Filters |
|---|---|---|
| PubMed | “Diabetes Mellitus” [Mesh] AND (“Diabetes Complications” [Mesh] OR “Peripheral Nervous System Diseases” [Mesh] OR “Diabetic Neuropathies” [Mesh]) AND (“Musculoskeletal Manipulations” [Mesh] OR “Therapy, Soft Tissue” [Mesh] OR “Manual therapy” OR “Physical Therapy” OR “Resistance Training” [Mesh] OR “Exercise Therapy” [Mesh]) |
|
| Web of Science | “Diabetes Mellitus” AND (“Diabetes Complications” OR “Peripheral Nervous System Diseases” OR “Diabetic Neuropathies”) AND (“Musculoskeletal Manipulations” OR “Therapy, Soft Tissue” OR “Manual therapy” OR “Physical Therapy” OR “Resistance Training” OR “Exercise Therapy”) |
|
| SCOPUS | “Diabetes Mellitus” AND (“Diabetes Complications” OR “Peripheral Nervous System Diseases” OR “Diabetic Neuropathies”) AND (“Musculoskeletal Manipulations” OR “Therapy, Soft Tissue” OR “Manual therapy” OR “Physical Therapy” OR “Resistance Training” OR “Exercise Therapy”) AND “Clinical Trial” |
|
| Cochrane Library | “Diabetes Mellitus” AND (“Diabetes Complications” OR “Peripheral Nervous System Diseases” OR “Diabetic Neuropathies”) AND (“Musculoskeletal Manipulations” OR “Therapy, Soft Tissue” OR “Manual therapy” OR “Physical Therapy” OR “Resistance Training” OR “Exercise Therapy”) |
|
| PEDro | “diabetic neuropathy” |
|
| Study | N (H/M) | Inclusion Criteria | Exclusion Criteria |
|---|---|---|---|
| a. Exercise Intervention | |||
| Ahmad (2020) | 38 (25/13) |
|
|
| Ahmad (2020) | 37 (24/13) |
|
|
| Cox (2020) | 32 (19/13) |
|
|
| Dixit (2016) | 82 (-) |
|
|
| Grewal (2015) | 35 (16/19) |
|
|
| Jannu (2017) | 50 (28/22) |
|
|
| Kanchanasamut (2017) | 21 (-) |
|
|
| Kiani (2018) | 38 (14/24) |
|
|
| Kuo (2019) | 38 (21/17) |
|
|
| Lee (2017) | 60 (37/22) |
|
|
| Lee (2013) | 55 (24/31) |
|
|
| Mueller (2013) | 29 (17/12) |
|
|
| Nenkova (2009) | 40 (14/26) |
|
|
| Quigley (2014) | 99 (15/84) |
|
|
| Richardson (2001) | 16 (12/4) |
|
|
| Serry (2016) | 60 (28/32) |
|
|
| Seyedizadeh (2020) | 24 (0/24) |
|
|
| Song (2011) | 38 (15/23) |
|
|
| Taveggia (2014) | 27 (10/17) |
|
|
| Toth (2014) | 54 (22/32) |
|
|
| Venkataraman (2019) | 143 (-) |
|
|
| Win (2020) | 75 (18/57) |
|
|
| b. Manual Therapy Intervention | |||
| Chatchawan (2015) | 60 (20/40) |
|
|
| Dalal (2014) | 58 (31/27) |
|
|
| Gok Metin (2017) | 46 (11/35) |
|
|
| Singh (2012) | 30 (30/0) |
|
|
| Talebi (2018) | 30 (-) |
|
|
| Xie (2019) | 119 (63/56) |
|
|
| c. Combinate intervention | |||
| Shourabi (2020) | 42 (-) |
|
|
| Study | Groups | Dose | Description | Outcomes | Results | |
|---|---|---|---|---|---|---|
| a. Exercise intervention | ||||||
| Ahmad (2020) | G1: Intervention—Exercise. G2: Control. |
|
|
| G1: +NCV peroneal/tibial; -Distal tibial latency. EO: TA, MG, MF t·gp (p < 0.05). ↓MF/↑TA treadmill. Co-contraction (p < 0.05). G2: +NCV tibial, +DL tibial. ↑TA-MF treadmill (p < 0.05). G1 vs. G2: Significant difference in all proprioception angles for G1. | Balance: Improvement in both groups, better for G1. Function: Not measured. Pain: Not measured. |
| Ahmad (2020) | G1: Intervention—Exercise. G2: Control. |
|
|
| G1 vs. G2: COP range + Proprioception front for G1 (p < 0.05). Significantly different t effect for all outcomes except COP sway VF F–B, OLS EO right. Effect—age for OLS EO-EC left, EC right, COP sway WVF F–B. Group—affects all outcomes except COP sway. T effect—age for OLS EO-EC left and EC right. (p < 0.05). | Balance: Improvement in both groups, better for G1. Function: No improvement. Pain: Not measured. |
| Cox (2020) | G1: Intervention—Exercise C-MICT. G2: Intervention—Exercise C-HIIT. G3: Control. |
|
|
| G1: 96.5% adherence. G2: 97.9% adherence. G1 vs. G3: Significant difference in pain intensity for G1 (p < 0.05). G2 vs. G3: Significant difference in pain intensity for G2 (p < 0.05). Adverse effect: C-HIIT ↑risk of adverse events and msk for 100 h train. | Balance: Not measured. Function: No improvement. Pain: Better results for intervention groups. |
| Dixit (2016) | G1: Intervention—Exercise. G2: Control. |
|
|
| G1 vs. G2: Significant difference in oscillatory velocity ECF x-axis and EOF ML (p < 0.05) for G1. | Balance: Greater improvement in G1. Function: Not measured. Pain: Not measured. |
| Grewal (2015) | G1: Intervention—Exercise. G2: Control. |
|
|
| G1: >26.09% improvement in balance outcomes. Between −0.04/27.68% of change for SF-12 and ADL. G2: Between −34.29/23.03% of change for outcomes. G1 vs. G2: Significant difference in EO (not CoM AP sway), EC ankle sway, SF-12 mental component for G1. (p < 0.05). | Balance: Improvement in both groups. Better for G1. Function: Improvement in both groups. Better for G1. Pain: Not measured. |
| Jannu (2017) | G1: Intervention—Exercise WooB. G2: Intervention—Exercise ST. |
|
|
| G1: No significant differences (p > 0.05). G2: Significant differences (p < 0.05) for BBS/TUG. G1 vs. G2: Significant difference for G2 (p < 0.05). | Balance: Better for G2. Function: Better for G2. Pain: Not measured. |
| Kanchanasamut (2017) | G1: Intervention—Exercise. G2: Control. |
|
|
| G1: Significant improvement (p < 0.05) flex-Ext 1st MTP (0–8 wk/0–20 wk); peak plantar pressure lateral left, medial right forefoot (0–20 wk/8–20 wk); flex 1st MTP left (8–20 wk). G2: No significant improvement (p > 0.05). G1 vs. G2: Significant difference in flex 1st MTP (20 wk), ext 1st MTP left (20 wk), pressure perception left (8 wk) and vibration left (8–20 wk) and right (20 wk) for G1 (p < 0.05). | Balance: Better for G1 at 8 and 20 wk. Function: Better for G1 at 20 wk. Pain: Not measured. |
| Kiani (2018) | G1: Intervention—Aerobic. G2: Intervention—Balance. |
|
|
| G1 vs. G2: Significant difference in BBT (6 wk), FRT and BRT (3 wk/6 wk) for G1. | Balance: Better for G1 at 3 and 6 wk. Function: Better for G1 at 3 and 6 wk. Pain: Not measured. |
| Kuo (2019) | G1: Intervention—Biofeedback. G2: Intervention—Multimodal. |
|
|
| G1 vs. G2: Significant difference in S2PD, D2PD, % and maximum pinch strength, Purdue Pegboard Test, Diabetes-39 (control, sexual function, energy and mobility) for G1. | Balance: Not measured. Function: Better for G1. Pain: Not measured. |
| Lee (2017) | G1: Intervention—Vibratory exercise. G2: Intervention—Strength exercise. |
|
|
| G1: Significant improvement (p < 0.05) in VPT G2: No significant improvement (p > 0.05). G1 vs. G2: Significant differences (p < 0.05) in VPT for G1. | Balance: Not measured. Function: Improvement in G1. Better for G1. Pain: Not measured. |
| Lee (2013) | G1: Intervention—Exercise WBV. G2: Intervention—Exercise BE2. G3: Control. |
|
|
| G1: Significant improvement (p < 0.05) in HbA1c, postural sway, OLS, FRT, BBS, TUG, FTSTS. G2: Significant improvement (p < 0.05) in postural sway, OLS, FRT, BBS, TUG, FTSTS. G3: No significant improvement (p > 0.05) G1 vs. G2: Significant differences (p < 0.05) in HbA1c, postural sway, OLS, BBS, TUG, FTSTS for G1. G1 vs. G3: Significant differences (p < 0.05) in OLS for G1. G2 vs. G3: Significant differences (p < 0.05) in OLS for G2. | Balance: Improvement in G1 and G2. Better for G1 vs. G2 and G3; of G2 vs. G3. Function: Improvement in G1 and G2. Better for G1 vs. G2. Pain: Not measured. |
| Mueller (2013) | G1: Intervention—Exercise WB. G2: Intervention—Exercise NWB. |
|
|
| G1 vs. G2: Significant differences (p < 0.05) in 6MWD for G1, and in HbA1c for G2 (p < 0.05). | Balance: Not measured. Function: Better for G1 in motor function. Better for G2 in physiological function. Pain: Not measured. |
| Nenkova (2009) | G1: Intervention—Exercise. G2: Control. |
|
|
| G1 vs. G2: Significant differences (p < 0.05) in all outcomes for G1. | Balance: Not measured. Function: Better for G1. Pain: Not measured. |
| Quigley (2014) | G1: Intervention—Exercise FBT. G2: Intervention—Exercise TC. G3: Control. |
|
|
| G1: Significant improvement (p < 0.05) in peak ankle PF power, peak ground reaction force-anterior and posterior, step width and variability. G2: Significant improvement (p < 0.05) in TUG, step width and step time. G3: No significant improvement (p > 0.05) | Balance: Improvement in G1 and G2. Function: Improvement in G1 and G2. Pain: Not measured. |
| Richardson (2001) | G1: Intervention—Lower quadrant. G2: Intervention—Upper quadrant. |
|
|
| G1: Significant improvement (p < 0.05) in all outcomes except ABC scale. G2: No significant improvement (p > 0.05). | Balance: Improvement in G1. Function: Improvement in G1. Pain: Not measured. |
| Serry (2016) | G1: Intervention—Exercise. G2: Intervention—TENS. G3: Control. |
|
|
| G1: Significant improvement (p < 0.05) in VAS. G2: Significant improvement (p < 0.05) in VAS. G3: No significant improvement (p > 0.05). | Balance: Not measured. Function: No improvement. Pain: Improvement in G1 and G2. |
| Seyedizadeh (2020) | G1: Intervention—Exercise. G2: Control. |
|
|
| G1: Significant improvement (p < 0.05) in lower limb strength. G2: Significant improvement (p < 0.05) in lower limb strength. G1 vs. G2: Significant differences (p < 0.05) in aerobic resistance and lower limb strength for G1 (p < 0.05). | Balance: No improvement. Function: Improvement in G1 and G2. Better for G1. Pain: Not measured. |
| Song (2011) | G1: Intervention—Exercise + Education. G2: Control. |
|
|
| G1: Significant improvement (p < 0.05) for all outcomes. G2: No significant improvement (p > 0.05). | Balance: Improvement in G1. Function: Improvement in G1. Pain: Not measured. |
| Taveggia (2014) | G1: Intervention—Exercise. G2: Control. |
|
|
| G1: Significant improvement (p < 0.05) in 6MWT, FIM, SpO2. G2: Significant improvement (p < 0.05) in 6MWT, FIM, SpO2. G1 vs. G2: Significant differences in Tinneti scale walk and FEO2 for G1 (p < 0.05). | Balance: Improvement in G1 and G2. Better for G1. Function: Improvement in G1 and G2. Better for G1. Pain: Not measured. |
| Toth (2014) | G1: Intervention—Exercise. G2: Control |
|
|
| G1: Improvement in all outcomes, not PGIC/CGI. G2: Any improvement. | Balance: Not measured. Function: Improvement in G1. Pain: Improvement in G1. |
| Venkataraman (2019) | G1: Intervention—Exercise. G2: Control. |
|
|
| G1 vs. G2: Significant differences in HRQoL pain, TUG, FTSTS, ABC scale, muscular strength (ankle and knee) for G1 (p < 0.05). | Balance: Better for G1. Function: Better for G1. Pain: Better for G1. |
| Win (2020) | G1: Intervention—Exercise. G2: Control. |
|
|
| G1 vs. G2: Significant differences in PNQ (motor area) for G1 (p < 0.05). | Balance: No significant difference. Function: Better for G1. Pain: No significant difference. |
| b. Manual Therapy Intervention | ||||||
| Chatchawan (2015) | G1: Intervention—Thai Massage. G2: Control. |
|
|
| G1: Significant improvement (p < 0.05) in all outcomes at 1–2 wk, except OLS and SWMT on 1 wk. G2: Significant improvement (p < 0.05) in ROM after 1 wk and all outcomes after 2 wk. | Balance: Improvement in G1 at 1 wk. Function: Improvement in G1 and G2. Pain: Not measured. |
| Dalal (2014) | G1: Intervention—Reflexology. G2: Control. |
|
|
| G1: Significant improvement (p < 0.05) in all outcomes. G2: Significant improvement (p < 0.05) in all outcomes. G1 vs. G2: Significant differences in all outcomes for G1 (p < 0.05). | Balance: Not measured. Function: Improvement in both groups. Better for G1. Pain: Improvement in both groups. Better for G1. |
| Gok Metin (2017) | G1: Intervention—Aromatherapy. G2: Control |
|
|
| G1: Significant improvement (p < 0.05) for VAS, QoL. G2: No significant improvement (p < 0.05). G1 vs. G2: Significant differences for VAS and QoL for G1 (p < 0.05). | Balance: Not measured. Function: Improvement in G1. Better for G1. Pain: Improvement in G1. Better for G1. |
| Singh (2012) | G1: Intervention—Neurodynamic. G2: Control. |
|
|
| G1 vs. G2: Significant differences for VPT 1st MTP right head for G1 (p < 0.05). | Balance: Not measured. Function: Better for G1. Pain: Not measured. |
| Talebi (2018) | G1: Intervention—Manual therapy. G2: Intervention—TENS. |
|
|
| G1: Significant improvement (p < 0.05) in all outcomes. G2: Significant improvement (p < 0.05) in VAS and BCQT-SSS. G1 vs. G2: Significant differences in SSS, FSS and MNT for G1 (p < 0.05). | Balance: Not measured. Function: Improvement in both groups. Better for G1. Pain: Improvement in both groups. |
| Xie (2019) | G1: Intervention—Gua Sha therapy. G2: Control. |
|
|
| G1: Significant improvement (p < 0.05) in all outcomes and follow-ups. G1 vs. G2: Significant differences in VPT and ABI (4 wk); all outcomes (8/12 wk) for G1 (p < 0.05). | Balance: Not measured. Function: Improvement in G1. Better for G1. Pain: Improvement in G1. |
| c. Combinate intervention | ||||||
| Shourabi (2020) | G1: Intervention—Aquatic exercise. G2: Intervention—AE + M. G3: Intervention—Massage. G4: Control. |
|
|
| G1: Significant improvement (p < 0.05) in nerve growth factor and BBS, and between G4 in favour of G1. G2: Significant improvement (p < 0.05) in all outcomes and between G4 in favour of G2. Signficant difference in BBS and nerve growth factor between G1 and G3, in favour of G2. G3: Significant improvement (p < 0.05) in nerve growth factor and BBS. | Balance: Improvement in G1, G2 and G3. Better for G1 and G2 vs. G4, and G2 vs. G1 and G3. Function: Improvement in G1, G2 and G3. Better for G1 and G2 vs. G4, and G2 vs. G1 and G3. Pain: Not measured. |
| Study | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ahmad (2020) | X | X | X | X | X | X | X | X | X | 9 | ||
| Ahmad (2019) | X | X | X | X | X | 5 | ||||||
| Chatchawan (2015) | X | X | X | X | X | X | X | X | 8 | |||
| Cox (2020) | X | X | X | X | X | X | X | 7 | ||||
| Dalal (2014) | X | X | X | X | X | X | 6 | |||||
| Dixit (2016) | X | X | X | X | X | X | 6 | |||||
| Gok Metin (2017) | X | X | X | X | X | X | X | X | 8 | |||
| Grewal (2015) | X | X | X | X | X | X | X | X | 8 | |||
| Jannu (2017) | X | X | X | X | 5 | |||||||
| Kanchanasamut (2017) | X | X | X | X | 4 | |||||||
| Kiani (2018) | X | X | X | X | X | 5 | ||||||
| Kuo (2019) | X | X | X | X | X | X | X | X | X | 9 | ||
| Lee (2017) | X | X | X | X | X | X | X | 7 | ||||
| Lee (2013) | X | X | X | X | X | X | X | 7 | ||||
| Mueller (2013) | X | X | X | X | X | X | X | X | X | 9 | ||
| Nenkova (2009) | X | X | X | X | 4 | |||||||
| Quigley (2014) | X | X | X | X | X | X | X | 7 | ||||
| Richardson (2001) | X | X | X | X | 4 | |||||||
| Serry (2016) | X | X | X | X | X | 5 | ||||||
| Seyedizadeh (2020) | X | X | X | X | X | X | X | X | 8 | |||
| Shourabi (2020) | X | X | X | X | X | 5 | ||||||
| Singh (2012) | X | X | X | X | 4 | |||||||
| Song (2011) | X | X | X | X | X | X | 6 | |||||
| Talebi (2018) | X | X | X | X | X | X | X | 7 | ||||
| Taveggia (2014) | X | X | X | X | X | X | X | 8 | ||||
| Toth (2014) | X | X | X | X | X | X | X | X | X | 9 | ||
| Venkataraman (2019) | X | X | X | X | X | 7 | ||||||
| Win (2020) | X | X | X | X | X | 5 | ||||||
| Xie (2019) | X | X | X | X | X | X | X | X | 8 | |||
| Mean | 6.6 | |||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Secorún, M.; Vidal-Peracho, C.; Márquez-Gonzalvo, S.; Corral-de-Toro, J.; Müller-Thyssen-Uriarte, J.; Rodríguez-Sanz, J.; Lucha-López, M.O.; Tricás-Moreno, J.M.; Hidalgo-García, C. Exercise and Manual Therapy for Diabetic Peripheral Neuropathy: A Systematic Review. Appl. Sci. 2021, 11, 5665. https://doi.org/10.3390/app11125665
Hernández-Secorún M, Vidal-Peracho C, Márquez-Gonzalvo S, Corral-de-Toro J, Müller-Thyssen-Uriarte J, Rodríguez-Sanz J, Lucha-López MO, Tricás-Moreno JM, Hidalgo-García C. Exercise and Manual Therapy for Diabetic Peripheral Neuropathy: A Systematic Review. Applied Sciences. 2021; 11(12):5665. https://doi.org/10.3390/app11125665
Chicago/Turabian StyleHernández-Secorún, Mar, Concepción Vidal-Peracho, Sergio Márquez-Gonzalvo, Jaime Corral-de-Toro, Julián Müller-Thyssen-Uriarte, Jacobo Rodríguez-Sanz, María Orosia Lucha-López, José Miguel Tricás-Moreno, and César Hidalgo-García. 2021. "Exercise and Manual Therapy for Diabetic Peripheral Neuropathy: A Systematic Review" Applied Sciences 11, no. 12: 5665. https://doi.org/10.3390/app11125665
APA StyleHernández-Secorún, M., Vidal-Peracho, C., Márquez-Gonzalvo, S., Corral-de-Toro, J., Müller-Thyssen-Uriarte, J., Rodríguez-Sanz, J., Lucha-López, M. O., Tricás-Moreno, J. M., & Hidalgo-García, C. (2021). Exercise and Manual Therapy for Diabetic Peripheral Neuropathy: A Systematic Review. Applied Sciences, 11(12), 5665. https://doi.org/10.3390/app11125665








