Vaccinium Species (Ericaceae): From Chemical Composition to Bio-Functional Activities
Abstract
1. Introduction
2. Traditional Uses of Vaccinium Species
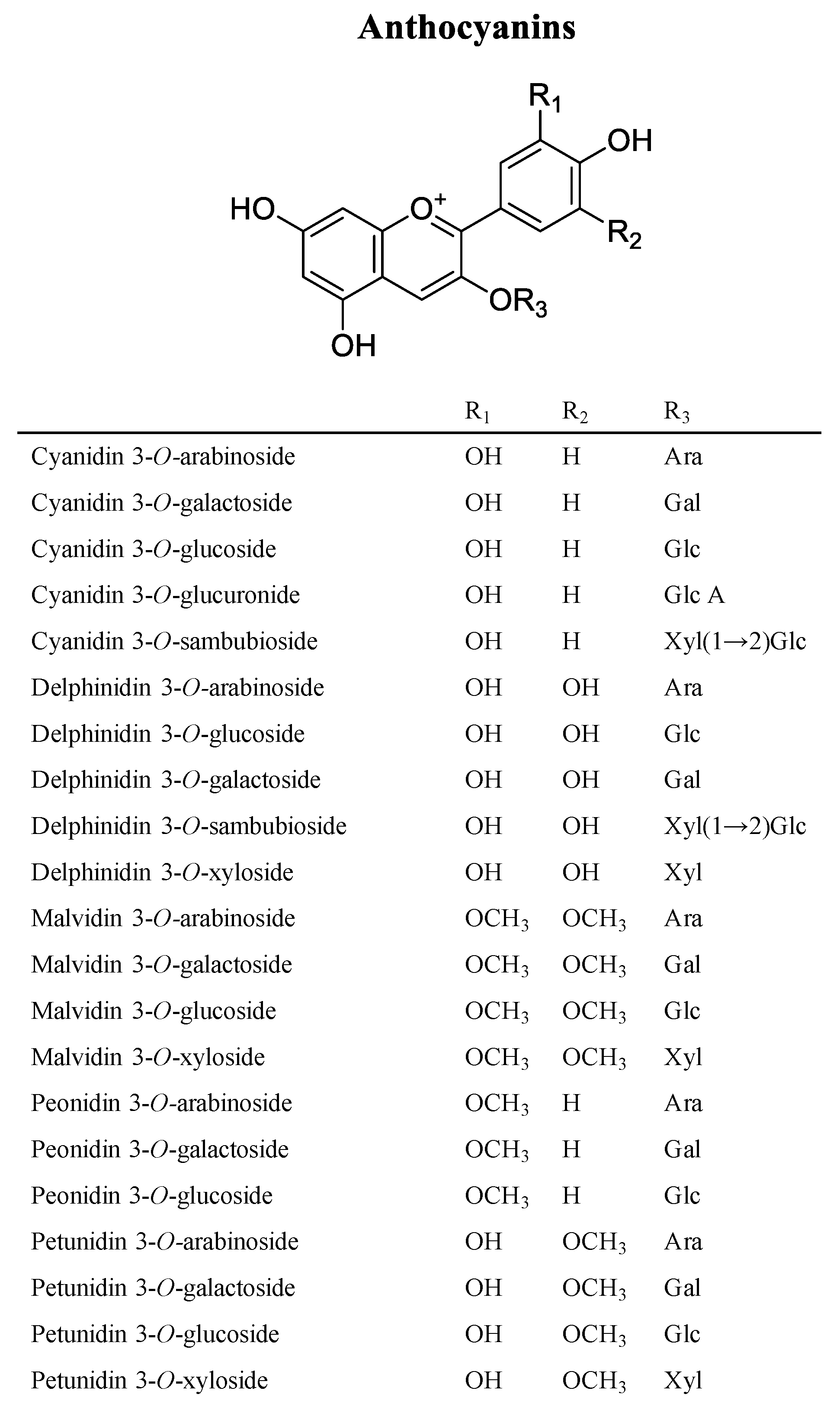
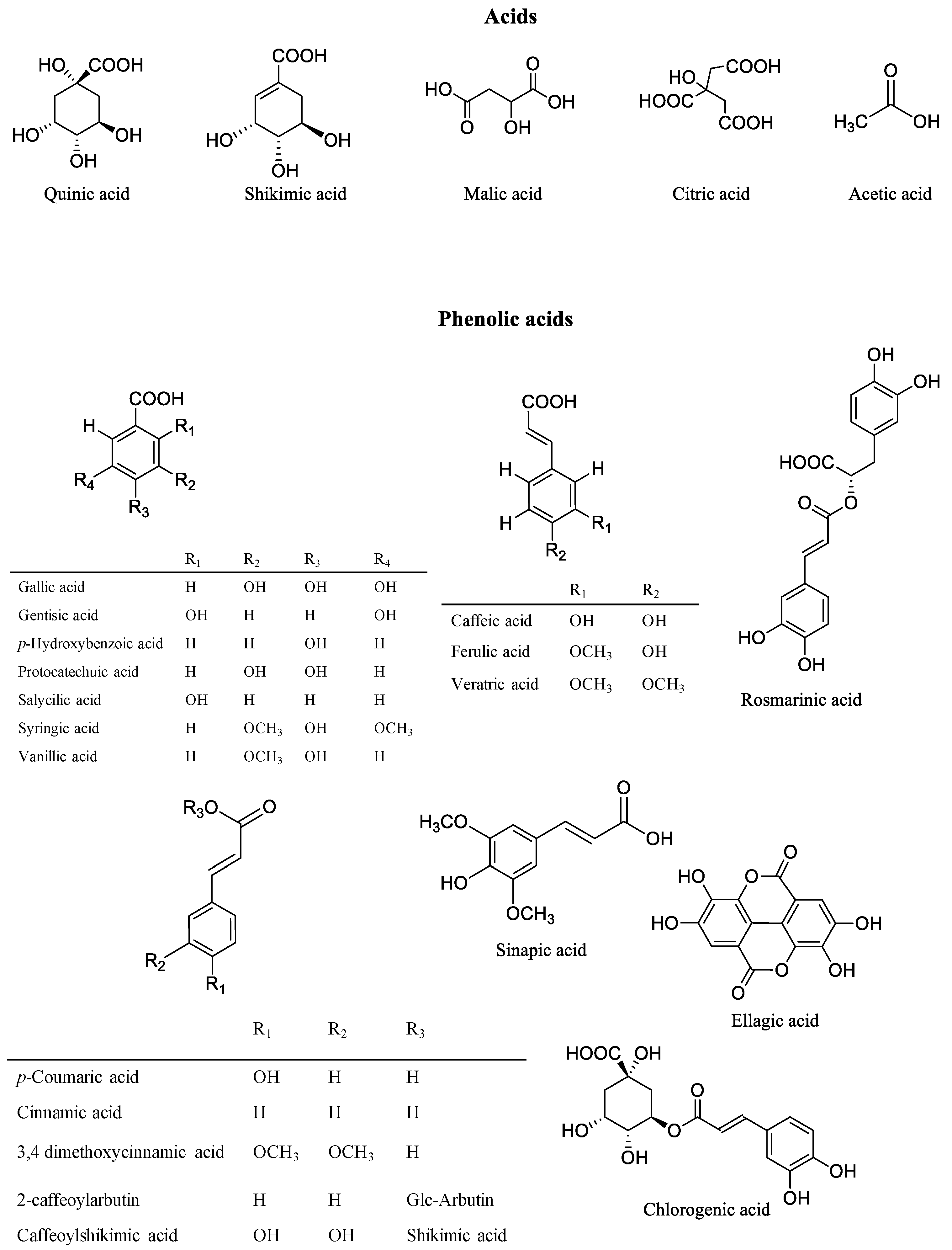
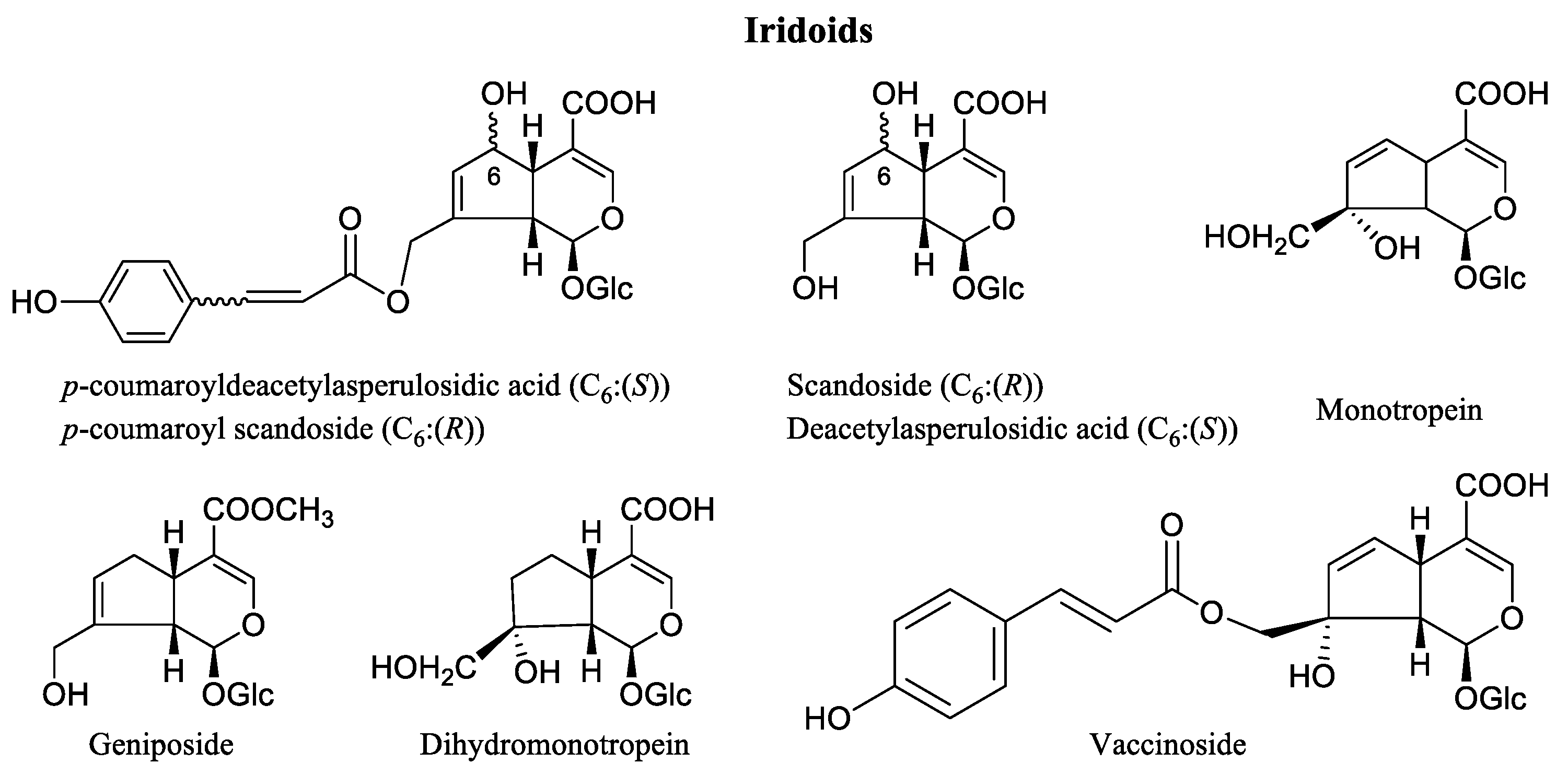
3. Phytochemicals of Vaccinium Fruits
4. The Chemical Profile of Vaccinium Leaves
5. Biological Properties of Vaccinium Species
5.1. Vaccinium and Diabetes
5.2. Vaccinium and Atherosclerosis
5.3. Vaccinium and Endothelial Dysfunction
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kloet, V.E. Manual of the flowering plants of Hawaii. Bishop Museum Spec. Publ. 1990, 83, 591–595. [Google Scholar]
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Valentine, D.H.; Walters, S.M.; Webb, D.A. Flora Europea; Cambridge University Press: Cambridge, UK, 1972; Volume 3, pp. 12–13. [Google Scholar]
- Colak, N.; Primetta, A.K.; Riihinen, K.R.; Jaakola, L.; Jiři Grúz, J.; Strnad, M.; Torun, H.; Ayaz, F.A. Phenolic compounds and antioxidant capacity in different-colored and non-pigmented berries of bilberry (Vaccinium myrtillus L.). Food Biosci. 2017, 20, 67–78. [Google Scholar] [CrossRef]
- Abreu, O.A.; Barreto, G.; Prieto, S. Vaccinium (Ericaceae): Ethnobotany and pharmacological potentials. Emir. J. Food A 2014, 26, 577–591. [Google Scholar] [CrossRef]
- Esposito, D.; Chen, A.; Grace, M.H.; Komarnytsky, S.; Lila, M.A. Inhibitory effects of wild blueberry anthocyanins and other flavonoids on biomarkers of acute and chronic inflammation in vitro. J. Agric. Food Chem. 2014, 62, 7022–7028. [Google Scholar] [CrossRef]
- Donnini, S.; Finetti, F.; Lusini, L.; Morbidelli, L.; Cheynier, V.; Barron, D.; Williamson, G.; Waltenberger, J.; Ziche, M. Divergent effects of quercetin conjugates on angiogenesis. Br. J. Nutr. 2006, 95, 1016–1023. [Google Scholar] [CrossRef]
- Tenuta, M.C.; Deguin, B.; Loizzo, M.R.; Dugay, A.; Acquaviva, R.; Malfa, G.A.; Bonesi, M.; Bouzidi, C.; Tundis, R. Contribution of flavonoids and iridoids to the hypoglycaemic, antioxidant, and nitric oxide (NO) inhibitory activities of Arbutus unedo L. Antioxidants 2020, 9, 184. [Google Scholar] [CrossRef] [PubMed]
- Brindisi, M.; Bouzidi, C.; Frattaruolo, L.; Loizzo, M.R.; Tundis, R.; Dugay, A.; Deguin, B.; Cappello, A.R.; Cappello, M.S. Chemical profile, antioxidant, anti-inflammatory, and anti-cancer effects of Italian Salvia rosmarinus spenn. methanol leaves extracts. Antioxidants 2020, 9, 826. [Google Scholar] [CrossRef]
- Brindisi, M.; Bouzidi, C.; Frattaruolo, L.; Loizzo, M.R.; Cappello, M.S.; Dugay, A.; Deguin, B.; Lauria, G.; Cappello, A.R.; Tundis, R. New Insights into the antioxidant and anti-inflammatory effects of Italian Salvia officinalis leaf and flower extracts in lipopolysaccharide and tumor-mediated inflammation models. Antioxidants 2021, 10, 311. [Google Scholar] [CrossRef]
- Wu, X.; Wang, T.T.Y.; Prior, R.L.; Pehrsson, P.R. Prevention of atherosclerosis by berries: The case of blueberries. J. Agric. Food Chem. 2018, 66, 9172–9188. [Google Scholar] [CrossRef]
- Kalt, W.; Cassidy, A.; Howard, L.R.; Krikorian, R.; Stull, A.J.; Tremblay, F.; Zamora-Ros, R. Recent research on the health benefits of blueberries and their anthocyanins. Adv. Nutr. 2020, 11, 224–236. [Google Scholar] [CrossRef]
- Kemper, K.J. Bilberry (Vaccinium myrtillus). Longwood Herb. Task Force 1999, 20115386, 55–71. [Google Scholar]
- Morazzoni, P.; Bombardelli, E. Vaccinium myrtillus L. Fitoterapia 1996, 68, 3–29. [Google Scholar]
- Frohne, D. Heidelbeerblätter; Teedrogen., M.W., Ed.; Wissenschaftliche Verlagsgesell: Stuttgart, Germany, 1990; pp. 217–219. [Google Scholar]
- Mustafa, B.; Hajdari, A.; Pieroni, A.; Pulaj, B.; Koro, X.; Quave, C.L. A cross-cultural comparison of folk plant uses among Albanians, Bosniaks, Gorani and Turks living in south Kosovo. J. Ethnobiol. Ethnomed. 2015, 12, 11–39. [Google Scholar] [CrossRef]
- Leduc, C.; Coonishish, J.; Haddad, P.; Cuerrier, A. Plants used by the Cree Nation of Eeyou Istchee (Quebec, Canada) for the treatment of diabetes: A novel approach in quantitative ethnobotany. J. Ethnopharmacol. 2006, 105, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Kari, P.R. Upper Tanana Ethnobotany; Alaska Historical Commission: Anchorage, Alaska, 1985. [Google Scholar]
- Standard for the Plant Drug of Heilongjiang Province; Heilongjiang Provincial Drug Administration: Harbin, China, 2001; p. 198.
- Mozaffarian, V. Identification of medicinal and aromatic plants of Iran. Farhang Moaser Tehran 2013, 391–392. [Google Scholar]
- Pervin, M.; Hasnat, M.A.; Lim, B.O. Antibacterial and antioxidant activities of Vaccinium corymbosum L. leaf extract. Asian Pac. J. Trop. Dis. 2013, 3, 444–453. [Google Scholar] [CrossRef]
- Pervin, M.; Hasnat, M.A.; Lim, J.H.; Lee, Y.M.; Kim, E.O.; Um, B.H.; Lim, B.O. Preventive and therapeutic effects of blueberry (Vaccinium corymbosum) extract against DSS-induced ulcerative colitis by regulation of antioxidant and inflammatory mediators. J. Nutr. Biochem. 2016, 28, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Branning, C.; Hakansson, A.; Ahrne, S.; Jeppsson, B.; Molin, G.; Nyman, M. Blueberry husks and multi-strain probiotics affect colonic fermentation in rats. Br. J. Nutr. 2009, 101, 859–870. [Google Scholar] [CrossRef]
- Karppinen, K.; Zoratti, L.; Nguyenquynh, N.; Häggman, H.; Jaakola, L. Molecular and metabolic mechanisms associated with fleshy fruit quality. Front. Plant Sci. 2016, 7, 657. [Google Scholar]
- Blumberg, J.B.; Camesano, T.A.; Cassidy, A.; Kris-Etherton, P.; Howell, A.; Manach, C.; Ostertag, L.M.; Sies, H.; Skulas-Ray, A.; Vita, J.A. Cranberries and their bioactive constituents in human health. Adv. Nutr. 2013, 4, 618–632. [Google Scholar] [CrossRef]
- Su, Z. Anthocyanins and flavonoids of Vaccinium, L. Pharm. Crops 2012, 3, 7–37. [Google Scholar] [CrossRef]
- Gao, L.; Mazza, G. Quantitation and distribution of simple and acylated anthocyanins and other phenolics in blueberries. J. Food Sci. 1994, 59, 1057–1059. [Google Scholar] [CrossRef]
- Beattie, J.; Crozier, A.; Duthie, G.G. Potential health benefits of berries. Curr. Nutr. Food Sci. 2005, 1, 71–86. [Google Scholar] [CrossRef]
- Borges, G.; Degeneve, A.; Mullen, W.; Crozier, A. Identification of flavonoid and phenolic antioxidants in black currants, blueberries, raspberries, red currants, and cranberries. J. Agric. Food Chem. 2010, 58, 3901–3909. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.J.; Howard, L.R.; Prior, R.L.; Clark, J.R. Flavonoid glycosides and antioxidant capacity of various blackberry, blueberry, and red grape genotypes determined by high-performance liquid chromatography/mass spectrometry. J. Sci. Food Agric. 2004, 84, 1771–1782. [Google Scholar] [CrossRef]
- Taruscio, T.G.; Barney, D.L.; Exon, J. Content and profile of flavanoid and phenolic acid compounds in conjunction with the antioxidant capacity for a variety of northwest Vaccinium berries. J. Agric. Food Chem. 2004, 52, 3169–3176. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Wang, S.Y. Oxygen radical absorbing capacity of phenolics in blueberries, cranberries, chokeberries, and lingonberries. J. Agric. Food Chem. 2003, 51, 502–509. [Google Scholar] [CrossRef]
- Suomalainen, H.; Keranen, A.J.A. The first anthocyanins appearing during the ripening of blueberries. Nature 1961, 191, 498–499. [Google Scholar] [CrossRef]
- Cabrita, L.; Froystein, N.A.; Andersen, O.M. Anthocyanin trisaccharides in blueberries of Vaccinium padifolium. Food Chem. 2000, 69, 33–36. [Google Scholar] [CrossRef]
- Du, Q.; Jerz, G.; Winterhalter, P. Isolation of two anthocyanin sambubiosides from bilberry (Vaccinium myrtillus) by high-speed counter-current chromatography. J. Chromatogr. A 2004, 1045, 59–63. [Google Scholar] [CrossRef]
- Spela, M.; Tomaz, P.; Lea, G.; Darinka, K.; Andreja, V.; Natasa, P.U.; Veronika, A. Phenolics in Slovenian bilberries (Vaccinium myrtillus L.) and blueberries (Vaccinium corymbosum L.). J. Agric. Food Chem. 2011, 59, 6998–7004. [Google Scholar]
- Scibisz, I.; Mitek, M. Influence of freezing process and frozen storage on anthocyanin contents of highbush blueberries. Food Sci. Technol. Qual. 2007, 5, 231–238. [Google Scholar]
- Wu, X.; Prior, R.L. Systematic identification and characterization of anthocyanins by HPLC-ESI-MS/MS in common foods in the United States: Fruits and berries. J. Agric. Food Chem. 2005, 53, 2589–2599. [Google Scholar] [CrossRef] [PubMed]
- Pappas, E.; Schaich, K.M. Phytochemicals of cranberries and cranberry products: Characterization, potential health effects, and processing stability. Crit. Rev. Food Sci. Nutr. 2009, 49, 741–781. [Google Scholar] [CrossRef]
- Andersen, O.M. Chromatographic separation of anthocyanins in cowberry (lingonberry) Vaccinium vites-idaea L. J. Food Sci. 1985, 50, 1230–1232. [Google Scholar] [CrossRef]
- Ek, S.; Kartimo, H.; Mattila, S.; Tolonen, A. Characterization of phenolic compounds from lingonberry (Vaccinium vitis-idaea L.). J. Agric. Food. Chem. 2006, 54, 9834–9842. [Google Scholar] [CrossRef] [PubMed]
- Hokkanen, J.; Mattila, S.; Jaakola, L.; Pirttila, A.M.; Tolonen, A. Identification of phenolic compounds from lingonberry (Vaccinium vitis-idaea L.), bilberry (Vaccinium myrtillus L.) and hybrid bilberry (Vaccinium x intermedium Ruthe, L.) leaves. J. Agric. Food Chem. 2009, 57, 9437–9447. [Google Scholar] [CrossRef] [PubMed]
- Laetti, A.K.; Riihinen, K.R.; Jaakola, L. Phenolic compounds in berries and flowers of a natural hybrid between bilberry and lingonberry (Vaccinium intermedium Ruthe). Phytochemistry 2011, 72, 810–815. [Google Scholar] [CrossRef]
- Madhavi, D.L.; Bomser, J.; Smith, M.A.L.; Singleton, K. Isolation of bioactive constituents of Vaccinium myrtillus (bilberry) fruits and cell cultures. Plant Sci. 1998, 131, 95–103. [Google Scholar] [CrossRef]
- Pan, Y.F.; Qu, W.J.; Li, J.G.; Gu, Y.B. Qualitative and quantitative analysis of flavonoid aglycones from fruit residue of Vaccinium vitis-idaea L. by HPLC. Nat. Prod. Res. Develop. 2005, 17, 641–644. [Google Scholar]
- Latti, A.K.; Kainulainen, P.S.; Hayirlioglu-Ayaz, S.; Ayaz, F.A.; Riihinen, K.R. Characterization of anthocyanins in Caucasian blueberries (Vaccinium arctostaphylos L.) native to Turkey. J. Agric. Food Chem. 2009, 57, 5244–5249. [Google Scholar] [CrossRef] [PubMed]
- Nickavar, B.; Amin, G.; Salehi-Sormagi, M.H. Anthocyanins from Vaccinium arctostaphylos berries. Pharm. Biol. 2004, 42, 289–291. [Google Scholar] [CrossRef]
- Witzell, J.; Gref, R.; Näsholm, T. Plant-part specific and temporal variation in phenolic compounds of boreal bilberry (Vaccinium myrtillus) plants. Biochem. Syst. Ecol. 2003, 31, 115–127. [Google Scholar] [CrossRef]
- Laaksonen, O.; Sandell, M.; Kallio, H. Chemical factors contributing to orosensory profiles of bilberry (Vaccinium myrtillus) fractions. Eur. Food Res. Technol. 2010, 231, 271–285. [Google Scholar] [CrossRef]
- Cesoniene, L.; Daubaras, R.; Jasutiene, I.; Vencloviene, J.; Miliauskiene, I. Evaluation of the biochemical components and chromatic properties of the juice of Vaccinium macrocarpon Aiton and Vaccinium oxycoccos L. Plant Food Hum. Nutr. 2011, 66, 238–244. [Google Scholar] [CrossRef]
- Cui, Z.H.; Yuan, C.S. Flavones of Vaccinium uliginosum fruits. Fitoterapia 1992, 63, 283. [Google Scholar]
- Lehtonen, H.M.; Lehtinen, O.; Suomela, J.P.; Viitanen, M.; Kallio, H. Flavonol glycosides of sea buckthorn (Hippophae rhamnoides ssp. sinensis) and lingonberry (Vaccinium vitis-idaea) are bioavailable in humans and monoglucuronidated for excretion. J. Agric. Food Chem. 2010, 58, 620–627. [Google Scholar] [CrossRef]
- Latti, A.K.; Jaakola, L.; Riihinen, K.R.; Kainulainen, P.S. Anthocyanin and flavonol variation in bog bilberries (Vaccinium uliginosum L.) in Finlan. J. Agric. Food Chem. 2009, 58, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, P.; Guo, P.; Wang, Z.Y. Anthocyanin composition and content of the Vaccinium uliginosum berry. Food Chem. 2011, 125, 116–120. [Google Scholar] [CrossRef]
- Yang, G.X.; Fan, H.L.; Zheng, Y.N.; Li, Y.D. Separation and identification of the flavonoids in the fruit of Vaccinium uliginosum L. blueberry. J. Jilin. Agric. Univ. 2005, 27, 643–644. [Google Scholar]
- Sellappan, S.; Akoh, C.C.; Krewer, G. Phenolic compounds and antioxidant capacity of Georgia-grown blueberries and blackberries. J. Agric. Food Chem. 2002, 50, 2432–2438. [Google Scholar] [CrossRef] [PubMed]
- Zadernowski, R.; Naczk, M.; Nesterowicz, J. Phenolic acid profiles in some small berries. J. Agric. Food Chem. 2005, 53, 2118–2124. [Google Scholar] [CrossRef]
- Wang, C.; Zuo, Y. Ultrasound-assisted hydrolysis and gas chromatography-mass spectrometric determination of phenolic compounds in cranberry products. Food Chem. 2011, 128, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zuo, Y. GC-MS determination of flavonoids and phenolic and benzoic acids in human plasma after consumption of cranberry juice. J. Agric. Food Chem. 2004, 52, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Wang, C.; Zhan, J. Separation, characterization, and quantitation of benzoic and phenolic antioxidants in American cranberry fruit by GC-MS. J. Agric. Food Chem. 2002, 50, 3789–3794. [Google Scholar] [CrossRef]
- Ayaz, F.A.; Hayirlioglu-Ayaz, S.; Gruz, J.; Novak, O.; Strnad, M. Separation, characterization, and quantitation of phenolic acids in a little-known blueberry (Vaccinium arctostaphylos L.) fruit by HPLC-MS. J. Agric. Food Chem. 2005, 53, 8116–8122. [Google Scholar] [CrossRef] [PubMed]
- Dinda, B.; Debnath, S.; Harigaya, Y. Naturally occurring iridoids. A review, part 1. Chem. Pharm. Bull. 2007, 55, 159–222. [Google Scholar] [CrossRef] [PubMed]
- Tundis, R.; Loizzo, M.R.; Menichini, F.; Statti, G.A.; Menichini, F. Biological and pharmacological activities of iridoids: Recent developments. Mini Rev. Med. Chem. 2008, 8, 399–420. [Google Scholar] [CrossRef]
- Wang, C.; Gong, X.; Bo, A.; Zhang, L.; Zhang, M.; Zang, E.; Zhang, C.; Li, M. Iridoids: Research advances in their phytochemistry, biological activities, and pharmacokinetics. Molecules 2020, 25, 287. [Google Scholar] [CrossRef]
- Heffels, P.; Müller, L.; Schieber, A.; Weber, F. Profiling of iridoid glycosides in Vaccinium species by UHPLC-MS. Food Res. Int. 2017, 100, 462–468. [Google Scholar] [CrossRef]
- Tenuta, M.C.; Malfa, G.A.; Marco, B.; Rosaria, A.; Loizzo, M.R.; Dugay, A.; Bouzidi, C.; Tomasello, B.; Tundis, A.; Deguin, B. LC-ESI-QTOF-MS profiling, protective effects on oxidative damage, and inhibitory activity of enzymes linked to type 2 diabetes and nitric oxide production of Vaccinium corymbosum L. (Ericaceae) extracts. J. Berry Res. 2020, 10, 603–622. [Google Scholar] [CrossRef]
- Turner, A.; Chen, S.N.; Nikolic, D.; van Breemen, R.; Farnsworth, N.R.; Pauli, G.F. Coumaroyl iridoids and a depside from cranberry (Vaccinium macrocarpon). J. Nat. Prod. 2007, 70, 253–258. [Google Scholar] [CrossRef]
- Leisner, C.P.; Kamileen, M.O.; Conway, M.E.; O.‘Connor, S.E.; Buell, C.R. Differential iridoid production as revealed by a diversity panel of 84 cultivated and wild blueberry species. PLoS ONE 2017, 12, e0179417. [Google Scholar]
- Ma, C.; Dastmalchi, K.; Flores, G.; Wu, S.B.; Pedraza-Peñalosa, P.; Long, C.; Kennelly, E.J. Antioxidant and metabolite profiling of North American and neotropical blueberries using LC-TOF-MS and multivariate analyses. J. Agric. Food Chem. 2013, 61, 3548–3559. [Google Scholar] [CrossRef]
- Kondo, M.; MacKinnon, S.L.; Craft, C.C.; Matchett, M.D.; Hurta, R.A.; Neto, C.C. Ursolic acid and its esters: Occurrence in cranberries and other Vaccinium fruit and effects on matrix metalloproteinase activity in DU145 prostate tumor cells. J. Sci. Food Agric. 2011, 91, 789–796. [Google Scholar] [CrossRef]
- Chu, W.; Gao, H.; Cao, S.; Fang, X.; Chen, H.; Xiao, S. Composition and morphology of cuticular wax in blueberry (Vaccinium spp.) fruits. Food Chem. 2017, 219, 436–442. [Google Scholar] [CrossRef]
- Ayaz, F.A.; Kadioglu, A.; Bertoft, E.; Acar, C.; Turna, I. Effect of fruit maturation on sugar and organic acid composition in two blueberries (Vaccinium arctostaphylos and V. myrtillus) native to Turkey. New Zealand. J. Crop Hort. Sci. 2001, 29, 137–141. [Google Scholar] [CrossRef]
- Huopalahti, R.; Järvenpää, E.P.; Katina, K. A novel solid-phase extraction-hplc method for the analysis of anthocyanin and organic acid composition of finish cranberry. J. Liquid Chrom. Related Technol. 2000, 23, 2695–2701. [Google Scholar] [CrossRef]
- Kalt, W.; McDonald, J.E. Chemical composition of lowbush blueberry cultivars. J. Am. Soc. Hort. Sci. 1996, 121, 142–146. [Google Scholar] [CrossRef]
- Riihinen, K.; Jaakola, L.; Karenlampi, S.; Hohtola, A. Organ-specific distribution of phenolic compounds in bilberry (Vaccinium myrtillus) and “northblue” blueberry (Vaccinium corymbosum × V. angustifolium). Food Chem. 2008, 110, 156–160. [Google Scholar] [CrossRef]
- Teleszko, M.; Wojdyło, A. Comparison of phenolic compounds and antioxidant potential between selected edible fruits and their leaves. J. Funct. Foods 2015, 14, 736–746. [Google Scholar] [CrossRef]
- Ferlemi, A.V.; Mermigki, P.G.; Makri, O.E.; Anagnostopoulos, D.; Koulakiotis, N.S.; Margarity, M.; Tsarbopoulos, A.; Georgakopoulos, C.D.; Lamari, F.N. Cerebral area differential redox response of neonatal rats to selenite-induced oxidative stress and to concurrent administration of highbush blueberry leaf polyphenols. Neurochem. Res. 2015, 40, 2280–2292. [Google Scholar] [CrossRef]
- Ferlemi, A.V.; Lamari, F.N. Berry leaves: An alternative source of bioactive natural products of nutritional and medicinal value. Antioxidants 2016, 5, 17. [Google Scholar] [CrossRef]
- Wang, L.J.; Wu, J.; Wang, H.X.; Li, S.S.; Zheng, X.C.; Du, H.; Xu, Y.J.; Wang, L.S. Composition of phenolic compounds and antioxidant activity in the leaves of blueberry cultivars. J. Funct. Foods 2015, 16, 295–304. [Google Scholar] [CrossRef]
- Ieri, F.; Martini, S.; Innocenti, M.; Mulinacci, N. Phenolic distribution in liquid preparations of Vaccinium myrtillus L. and Vaccinium vitis idaea L. Phytochem. Anal. 2013, 24, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Sidorova, Y.; Shipelin, V.; Mazo, V.; Zorin, S.; Petrov, N.; Kochetkova, A. Hypoglycemic and hypolipidemic effect of Vaccinium myrtillus L. leaf and Phaseolus vulgaris L. seed coat extracts in diabetic rats. Nutrition 2017, 41, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Mzhavanadze, V.V. Kaempferol glycosides from the leaves of the Cancasian bilberry, Vaccinium arctostaphylos. Soobshch Akad Nauk Gruz SSR 1971, 62, 445–447. [Google Scholar]
- Kader, F.; Rovel, B.; Girardin, M.; Metche, M. Fractionation and identification of the phenolic compounds of highbush blueberries (Vaccinium corymbosum L.). Food Chem. 1996, 55, 35–40. [Google Scholar] [CrossRef]
- Scibisz, I.; Mitek, M. Antioxidant activity and phenolic compound content in dried highbush blueberries (Vaccinium corymbosum L.). Zywnosc 2006, 13, 68–76. [Google Scholar]
- Martz, F.; Jaakola, L.; Julkunen-Tiitto, R.; Stark, S. Phenolic composition and antioxidant capacity of bilberry (Vaccinium myrtillus) leaves in Northern Europe following foliar development and along environmental gradients. J. Chem. Ecol. 2010, 36, 1017–1028. [Google Scholar] [CrossRef]
- Neto, C.C.; Salvas, M.R.; Autio, W.R.; van den Heuvel, J.E. Variation in concentration of phenolic acid derivatives and quercetin glycosides in foliage of cranberry that may play a role in pest deterrence. J. Am. Soc. Hortic. Sci. 2010, 135, 494–500. [Google Scholar] [CrossRef]
- Mzhavanadze, V.V.; Targamadze, I.L.; Dranik, L.I. Phenolic compounds of the leaves of Vaccinium arctostaphylos. Chem. Nat. Comp. 2004, 8, 125–126. [Google Scholar] [CrossRef]
- Szakiel, A.; Paczkowski, C.; Huttunen, S. Triterpenoid content of berries and leaves of bilberry Vaccinium myrtillus from Finland and Poland. J. Agric. Food Chem. 2012, 60, 11839–11849. [Google Scholar] [CrossRef]
- Ramassamy, C. Emerging role of polyphenolic compounds in the treatment of neurodegenerative diseases: A review of their intracellular targets. Eur. J. Pharmacol. 2006, 545, 51–64. [Google Scholar] [CrossRef]
- Miller, K.; Feucht, W.; Schmid, M. Bioactive compounds of strawberry and blueberry and their potential health effects based on human intervention studies: A brief overview. Nutrients 2019, 11, 1510. [Google Scholar] [CrossRef] [PubMed]
- Mantzorou, M.; Zarros, A.; Vasios, G.; Theocharis, S.; Pavlidou, E.; Giaginis, C. Cranberry: A promising natural source of potential nutraceuticals with anticancer activity. Anticancer Agents Med. Chem. 2019, 19, 1672–1686. [Google Scholar] [CrossRef] [PubMed]
- Del Bó, C.; Riso, P.; Campolo, J.; Møller, P.; Loft, S.; Klimis-Zacas, D.; Brambilla, A.; Rizzolo, A.; Porrini, M. A single portion of blueberry (Vaccinium corymbosum L.) improves protection against DNA damage but not vascular function in healthy male volunteers. Nutr. Res. 2013, 33, 220–227. [Google Scholar] [CrossRef]
- Vinson, J.A.; Bose, P.; Proch, J.; Al Kharrat, H.; Samman, N. Cranberries and cranberry products: Powerful in vitro, ex vivo, and in vivo sources of antioxidants. J. Agric. Food Chem. 2008, 56, 5884–5891. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Vieira, A. Protective activities of Vaccinium antioxidants with potential relevance to mitochondrial dysfunction and neurotoxicity. Neurotoxicology 2007, 28, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Torri, E.; Lemos, M.; Caliari, V.A.L.; Kassuya, C.; Bastos, J.K.; Andrade, S.F. Anti-inflammatory and antinociceptive properties of blueberry extract (Vaccinium corymbosum). J. Pharm. Pharmacol. 2007, 59, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.R.; Pereira, R.; Figueiredo, I.; Freitas, V.; Dinis, T.C.; Almeida, L.M. Comparison of anti-inflammatory activities of an anthocyanin-rich fraction from Portuguese blueberries (Vaccinium corymbosum L.) and 5-aminosalicylic acid in a TNBS-induced colitis rat model. PLoS ONE 2017, 12, e0174116. [Google Scholar] [CrossRef] [PubMed]
- Marziano, C.; Genet, G.; Hirschi, K.K. Vascular endothelial cell specification in health and disease. Angiogenesis 2021. [Google Scholar] [CrossRef]
- Matsuzawa, Y.; Lerman, A. Endothelial dysfunction and coronary artery disease: Assessment, prognosis, and treatment. Coron. Artery Dis. 2014, 25, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Daiber, A.; Steven, S.; Weber, A.; Shuvaev, V.V.; Muzykantov, V.R.; Laher, I.; Li, H.; Lamas, S.; Münzel, T. Targeting vascular (endothelial) dysfunction. Br. J. Pharmacol. 2017, 174, 1591–1619. [Google Scholar] [CrossRef]
- Alfaras, I.; Di Germanio, C.; Bernier, M.; Csiszar, A.; Ungvari, Z.; Lakatta, E.G.; de Cabo, R. Pharmacological strategies to retard cardiovascular aging. Circ. Res. 2016, 118, 1626–1642. [Google Scholar] [CrossRef]
- Mensah, G.A.; Wei, G.S.; Sorlie, P.D.; Fine, L.J.; Rosenberg, Y.; Kaufmann, P.G.; Mussolino, M.E.; Hsu, L.L.; Addou, E.; Engelgau, M.M.; et al. Decline in cardiovascular mortality: Possible causes and implications. Circ. Res. 2017, 120, 366–380. [Google Scholar] [CrossRef]
- Cravotto, G.; Boffa, L.; Genzini, L.; Garella, D. Phytotherapeutics: An evaluation of the potential of 1000 plants. J. Clin. Pharm. Ther. 2010, 35, 11–48. [Google Scholar] [CrossRef] [PubMed]
- Martineau, L.C.; Couture, A.; Spoor, D.; Benhaddou-Andaloussi, A.; Harris, C.; Meddah, B.; Leduc, C.; Burt, A.; Vuong, T.; Mai Le, P.; et al. Anti-diabetic properties of the Canadian lowbush blueberry Vaccinium angustifolium Ait. Phytomedicine 2006, 13, 612–623. [Google Scholar] [CrossRef]
- Chan, S.W.; Chu, T.T.W.; Choi, S.W.; Benzie, I.F.F.; Tomlinson, B. Impact of short-term bilberry supplementation on glycemic control, cardiovascular disease risk factors, and antioxidant status in Chinese patients with type 2 diabetes. Phytother. Res. 2021. [Google Scholar] [CrossRef]
- Shahcheraghi, S.H.; Aljabali, A.A.A.; Al Zoubi, M.S.; Mishra, V.; Charbe, N.B.; Haggag, Y.A.; Shrivastava, G.; Almutary, A.G.; Alnuqaydan, A.M.; Barh, D.; et al. Overview of key molecular and pharmacological targets for diabetes and associated diseases. Life Sci. 2021, 278, 119632. [Google Scholar] [CrossRef]
- McDougall, G.J.; Shpiro, F.; Dobson, P.; Smith, P.; Blake, A.; Stewart, D. Different polyphenolic components of soft fruits inhibit alpha-amylase and alpha-glucosidase. J. Agric. Food Chem. 2005, 53, 2760–2766. [Google Scholar] [CrossRef]
- Bljajić, K.; Petlevski, R.; Vujić, L.; Čačić, A.; Šoštarić, N.; Jablan, J.; Saraiva de Carvalho, S.; Zovko Končić, M. Chemical composition, antioxidant and α-glucosidase-inhibiting activities of the aqueous and hydroethanolic extracts of Vaccinium myrtillus leaves. Molecules 2017, 22, 703. [Google Scholar] [CrossRef]
- Karcheva-Bahchevanska, D.P.; Lukova, P.K.; Nikolova, M.M.; Mladenov, R.D.; Iliev, I.N. Effect of extracts of bilberries (Vaccinium myrtillus L.) on amyloglucosidase and α-glucosidase activity. Folia Med. 2017, 59, 197–202. [Google Scholar] [CrossRef]
- Li, H.; Park, H.M.; Ji, H.S.; Han, J.; Kim, S.K.; Park, H.Y.; Jeong, T.S. Phenolic-enriched blueberry-leaf extract attenuates glucose homeostasis, pancreatic beta-cell function, and insulin sensitivity in high-fat diet-induced diabetic mice. Nutr. Res. 2020, 73, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Cásedas, G.; Les, F.; Gómez-Serranillos, M.P.; Smith, C.; López, V. Anthocyanin profile, antioxidant activity and enzyme inhibiting properties of blueberry and cranberry juices: A comparative study. Food Funct. 2017, 8, 4187–4193. [Google Scholar] [CrossRef]
- Cutler, B.R.; Gholami, S.; Chua, J.S.; Kuberan, B.; Anandh Babu, P.V. Blueberry metabolites restore cell surface glycosaminoglycans and attenuate endothelial inflammation in diabetic human aortic endothelial cells. Int. J. Cardiol. 2018, 261, 155–158. [Google Scholar] [CrossRef]
- Huang, W.; Yao, L.; He, X.; Wang, L.; Li, M.; Yang, Y.; Wan, C. Hypoglycemic activity and constituents analysis of blueberry (Vaccinium corymbosum) fruit extracts. Diabetes Metab. Syndr. Obes. 2018, 11, 357–366. [Google Scholar] [CrossRef]
- Huang, W.; Yan, Z.; Li, D.; Ma, Y.; Zhou, J.; Sui, Z. Antioxidant and anti-inflammatory effects of blueberry anthocyanins on high glucose-induced human retinal capillary endothelial cells. Oxidative Med. Cell Longev. 2018, 2018, 1862462. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Hutabarat, R.P.; Chai, Z.; Zheng, T.; Zhang, W.; Li, D. Antioxidant blueberry anthocyanins induce vasodilation via PI3K/Akt signaling pathway in high-glucose-induced human umbilical vein endothelial cells. Int. J. Mol. Sci. 2020, 21, 1575. [Google Scholar] [CrossRef]
- Song, Y.; Huang, L.; Yu, J. Effects of blueberry anthocyanins on retinal oxidative stress and inflammation in diabetes through Nrf2/HO-1 signaling. J. Neuroimmunol. 2016, 301, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Jafarizade, M.; Kahe, F.; Sharfaei, S.; Momenzadeh, K.; Pitliya, A.; Tajrishi, F.Z.; Singh, P.; Chi, G. The role of interleukin-27 in atherosclerosis: A contemporary review. Cardiology 2021, 19, 1–13. [Google Scholar] [CrossRef]
- Cignarella, A.; Nastasi, M.; Cavalli, E.; Puglisi, L. Novel lipid-lowering properties of Vaccinium myrtillus L. leaves, a traditional antidiabetic treatment, in several models of rat dyslipidaemia: A comparison with ciprofibrate. Thromb. Res. 1996, 84, 311–322. [Google Scholar] [CrossRef]
- Basu, A.; Lyons, T.J. Strawberries, blueberries, and cranberries in the metabolic syndrome: Clinical perspectives. J. Agric. Food Chem. 2012, 60, 5687–5692. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Gu, L.; Hager, T.; Hager, A.; Wilkes, S.; Howard, L. Purified berry anthocyanins but not whole berries normalize lipid parameters in mice fed an obesogenic high fat diet. Mol. Nutr. Food Res. 2009, 53, 1406–1418. [Google Scholar] [CrossRef] [PubMed]
- Kalt, W.; Foote, K.; Fillmore, S.A.; Lyon, M.; Van Lunen, T.A.; McRae, K.B. Effect of blueberry feeding on plasma lipids in pigs. Br. J. Nutr. 2008, 100, 70–78. [Google Scholar] [CrossRef]
- Zagayko, A.L.; Kolisnyk, T.Y.; Chumak, O.I.; Ruban, O.A.; Koshovyi, O.M. Evaluation of anti-obesity and lipid-lowering properties of Vaccinium myrtillus leaves powder extract in a hamster model. J. Basic Clin. Physiol. Pharmacol. 2018, 29, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, T.C.; Moura, E.G.; de Oliveira, E.; Soares, P.N.; Guarda, D.S.; Bernardino, D.N.; Ai, X.X.; Rodrigues, V.D.S.T.; de Souza, G.R.; da Silva, A.J.R.; et al. Cranberry (Vaccinium macrocarpon) extract treatment improves triglyceridemia, liver cholesterol, liver steatosis, oxidative damage and corticosteronemia in rats rendered obese by high fat diet. Eur. J. Nutr. 2018, 57, 1829–1844. [Google Scholar] [CrossRef]
- Wu, X.; Kang, J.; Xie, C.; Burris, R.; Ferguson, M.E.; Badger, T.M.; Nagarajan, S. Dietary blueberries attenuate atherosclerosis in apolipoprotein E-deficient mice by upregulating antioxidant enzyme expression. J. Nutr. 2010, 140, 1628–1632. [Google Scholar] [CrossRef]
- Matziouridou, C.; Marungruang, N.; Nguyen, T.D.; Nyman, M.; Fåk, F. Lingonberries reduce atherosclerosis in Apoe-/- mice in association with altered gut microbiota composition and improved lipid profile. Mol. Nutr. Food Res. 2016, 60, 1150–1160. [Google Scholar] [CrossRef]
- Xie, C.; Kang, J.; Chen, J.R.; Lazarenko, O.P.; Ferguson, M.E.; Badger, T.M.; Nagarajan, S.; Wu, X. Lowbush blueberries inhibit scavenger receptors CD36 and SR-A expression and attenuate foam cell formation in ApoE-deficient mice. Food Funct. 2011, 2, 588–594. [Google Scholar] [CrossRef]
- Xie, C.; Kang, J.; Chen, J.R.; Nagarajan, S.; Badger, T.M.; Wu, X. Phenolic acids are in vivo atheroprotective compounds appearing in the serum of rats after blueberry consumption. J. Agric. Food Chem. 2011, 59, 10381–10387. [Google Scholar] [CrossRef]
- Heitzer, T.; Schlinzig, T.; Krohn, K.; Meinertz, T.; Münzel, T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation 2001, 104, 2673–2678. [Google Scholar] [CrossRef]
- Curtis, P.J.; van der Velpen, V.; Berends, L.; Jennings, A.; Feelisch, M.; Umpleby, A.M.; Evans, M.; Fernandez, B.O.; Meiss, M.S.; Minnion, M.; et al. Blueberries improve biomarkers of cardiometabolic function in participants with metabolic syndrome-results from a 6-month, double-blind, randomized controlled trial. Am. J. Clin. Nutr. 2019, 109, 1535–1545. [Google Scholar] [CrossRef] [PubMed]
- Del Bo’, C.; Porrini, M.; Fracassetti, D.; Campolo, J.; Klimis-Zacas, D.; Riso, P. A single serving of blueberry (V. corymbosum) modulates peripheral arterial dysfunction induced by acute cigarette smoking in young volunteers: A randomized-controlled trial. Food Funct. 2014, 5, 3107–3116. [Google Scholar] [CrossRef]
- Del Bo’, C.; Deon, V.; Campolo, J.; Lanti, C.; Parolini, M.; Porrini, M.; Klimis-Zacas, D.; Riso, P. A serving of blueberry (V. corymbosum) acutely improves peripheral arterial dysfunction in young smokers and non-smokers: Two randomized, controlled, crossover pilot studies. Food Funct. 2017, 8, 4108–4117. [Google Scholar] [CrossRef]
- Stull, A.J.; Cash, K.C.; Champagne, C.M.; Gupta, A.K.; Boston, R.; Beyl, R.A.; Johnson, W.D.; Cefalu, W.T. Blueberries improve endothelial function, but not blood pressure, in adults with metabolic syndrome: A randomized, double- blind, placebo-controlled clinical trial. Nutrients 2015, 7, 4107–4123. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Xia, M.; Yang, Y.; Liu, F.; Li, Z.; Hao, Y.; Mi, M.; Jin, T.; Ling, W. Purified anthocyanin supplementation improves endothelial function via NO-cGMP activation in hypercholesterolemic individuals. Clin. Chem. 2011, 57, 1524–1533. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Pang, W.; He, C.; Li, Y.; Jiang, Y.; Guo, C. Blueberry anthocyanin-enriched extracts attenuate fine particulate matter (PM 2.5)-induced cardiovascular dysfunction. J. Agric. Food Chem. 2017, 65, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Vendrame, S.; Tsakiroglou, P.; Kristo, A.S.; Schuschke, D.A.; Klimis-Zacas, D. Wild blueberry consumption attenuates local inflammation in the perivascular adipose tissue of obese Zucker rats. Appl. Physiol. Nutr. Metab. 2016, 41, 1045–1051. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, C.S.; Lee, Y.M.; Sohn, E.; Jo, K.; Kim, J.S. Vaccinium myrtillus extract prevents or delays the onset of diabetes-induced blood-retinal barrier breakdown. Int. J. Food Sci. Nutr. 2015, 66, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Mastantuono, T.; Starita, N.; Sapio, D.; D’Avanzo, S.A.; Di Maro, M.; Muscariello, E.; Paterni, M.; Colantuoni, A.; Lapi, D. The Effects of Vaccinium myrtillus extract on hamster pial microcirculation during hypoperfusion-reperfusion injury. PLoS ONE 2016, 11, e0150659. [Google Scholar] [CrossRef] [PubMed]
- Bharat, D.; Cavalcanti, R.R.M.; Petersen, C.; Begaye, N.; Cutler, B.R.; Assis Costa, M.M.; Gomes Ramos, R.; Ferreira, M.R.; Li, Y.; Bharath, L.P.; et al. Blueberry metabolites attenuate lipotoxicity-induced endothelial dysfunction. Mol. Nutr. Food Res. 2018, 62, 1–17. [Google Scholar] [CrossRef]
- Park, S.H.; Jeong, S.; Chung, H.T.; Pae Pterostilbene, H.E. An active constituent of blueberries, stimulates Nitric Oxide production via activation of endothelial nitric oxide synthase in human umbilical vein endothelial cells. Plant Foods Hum. Nutr. 2015, 70, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhu, Y.; Li, C.; Sui, Z.; Min, W. Effect of blueberry anthocyanins malvidin and glycosides on the antioxidant properties in endothelial cells. Oxidative Med. Cell. Longev. 2016, 2016, 1591803. [Google Scholar] [CrossRef]
- Morgan, K.M.; Loloi, J.; Songdej, N. Cranberry supplementation as a cause of major intraoperative bleeding during vascular surgery due to aspirin-like platelet inhibition. Blood Coagul. Fibrinolysis 2020, 31, 402–404. [Google Scholar]
- Izzo, A.A.; Hoon-Kim, S.; Radhakrishnan, R.; Williamson, E.M. A critical approach to evaluating clinical efficacy, adverse events and drug interactions of herbal remedies. Phytother. Res. 2016, 30, 691–700. [Google Scholar] [CrossRef] [PubMed]
| Vaccinium | Traditional Uses | Part Used | References |
|---|---|---|---|
| V. myrtillus | Fevers and coughs | Fruits | [12] |
| Antidiabetic and anti-inflammatory diabetic | Leaves | [13,14] | |
| Respiratory inflammations | Leaves and fruits | [15] | |
| Stomatitis | Fruits | [12] | |
| Eye inflammation | Fruits | [15] | |
| Intestinal and liver disorders | Fruits | [12] | |
| Hepatitis | Fruits | [15] | |
| Digestive and urinary tract disorders | Fruits | [15] | |
| Renal stones | Leaves and fruits | [12,15] | |
| Antiseptic, astringent, tonic | Fruits | [13] | |
| Anti-anemic | Leaves and fruits | [15] | |
| V. vitis idaea | Antipyretic | Leaves and fruits | [15] |
| Sore eyes, abscesses, toothache, thrush and snow blindness | Fruits | [16] | |
| Colds, coughs and sore throats | Fruits | [17] | |
| Anti-inflammatory properties in urinary tract | Leaves | [15] | |
| Respiratory system infections | Stems and leaves | [18] | |
| Frequent urination | Fruits | [16] | |
| Urinary tract infection properties | Fruits | [15] | |
| Kidney stones | Fruits | [15] | |
| Anti-inflammatory | Stems and leaves | [18] | |
| Wound healing, anti-rheumatic, anti-convulsant, diuretic and anti-diabetic | Leaves and fruits | [15] | |
| V. arctostaphylos | Anti-hypertensive and anti-diabetic | Leaves and fruits | [19] |
| V. corymbosum | Anti-diabetic, antioxidant, and anti-inflammatory | Fruits | [20,21] |
| Gastrointestinal disorders | Fruits | [22] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tundis, R.; Tenuta, M.C.; Loizzo, M.R.; Bonesi, M.; Finetti, F.; Trabalzini, L.; Deguin, B. Vaccinium Species (Ericaceae): From Chemical Composition to Bio-Functional Activities. Appl. Sci. 2021, 11, 5655. https://doi.org/10.3390/app11125655
Tundis R, Tenuta MC, Loizzo MR, Bonesi M, Finetti F, Trabalzini L, Deguin B. Vaccinium Species (Ericaceae): From Chemical Composition to Bio-Functional Activities. Applied Sciences. 2021; 11(12):5655. https://doi.org/10.3390/app11125655
Chicago/Turabian StyleTundis, Rosa, Maria C. Tenuta, Monica R. Loizzo, Marco Bonesi, Federica Finetti, Lorenza Trabalzini, and Brigitte Deguin. 2021. "Vaccinium Species (Ericaceae): From Chemical Composition to Bio-Functional Activities" Applied Sciences 11, no. 12: 5655. https://doi.org/10.3390/app11125655
APA StyleTundis, R., Tenuta, M. C., Loizzo, M. R., Bonesi, M., Finetti, F., Trabalzini, L., & Deguin, B. (2021). Vaccinium Species (Ericaceae): From Chemical Composition to Bio-Functional Activities. Applied Sciences, 11(12), 5655. https://doi.org/10.3390/app11125655