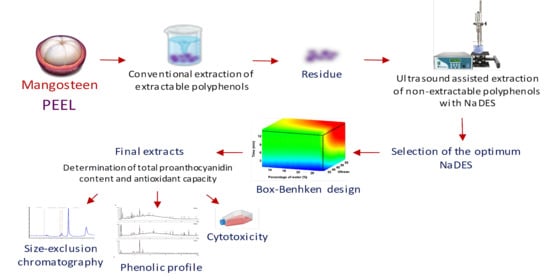
A Sustainable Approach for Extracting Non-Extractable Phenolic Compounds from Mangosteen Peel Using Ultrasound-Assisted Extraction and Natural Deep Eutectic Solvents
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Samples
2.2. Preparation of Natural Deep Eutectic Solvents (NaDES)
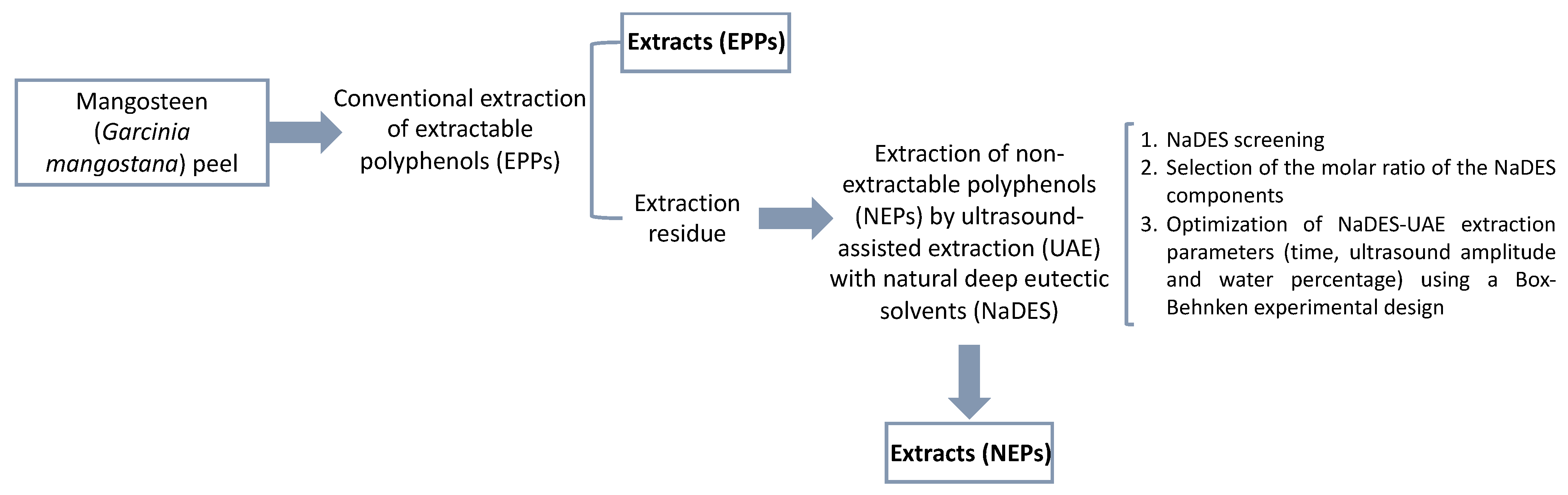
2.3. Extraction of Extractable Polyphenols (EPPs)
2.4. Extraction of Non-Extractable Polyphenols (NEPs)
2.5. Total Proanthocyanidin Content
2.5.1. DMAC Assay
2.5.2. Butanol/HCl Assay
2.6. Antioxidant Capacity Determination
2.6.1. ABTS Radical Assay
2.6.2. Capacity to Inhibit the Formation of a Hydroxyl Radical Assay
2.6.3. Ferric-Reducing Antioxidant Power (FRAP)
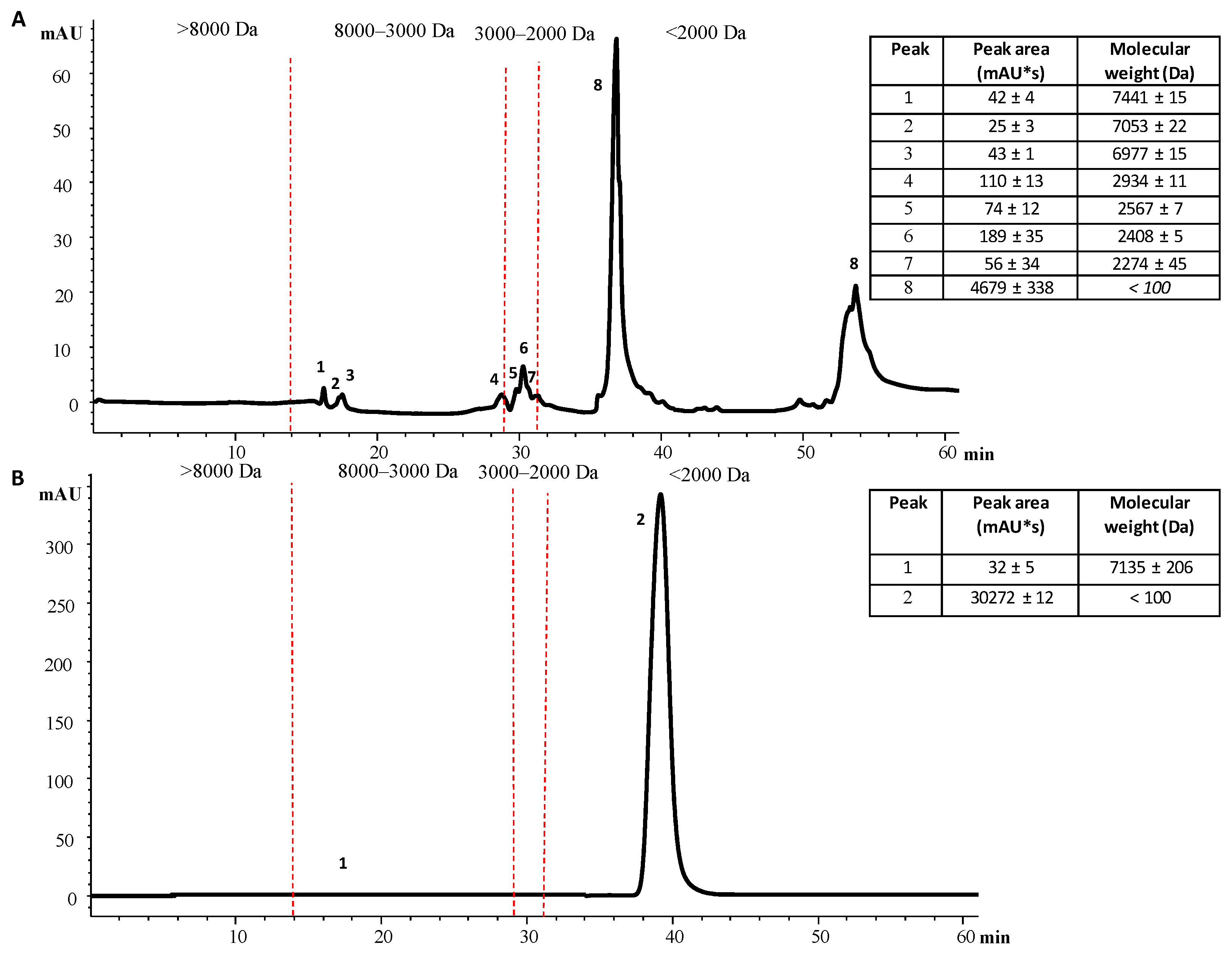
2.7. Determination of NEPs’ Molecular Weight from Mangosteen Peel Extracts by High-Performance Liquid Size-Exclusion Chromatography (HPLC-SEC)
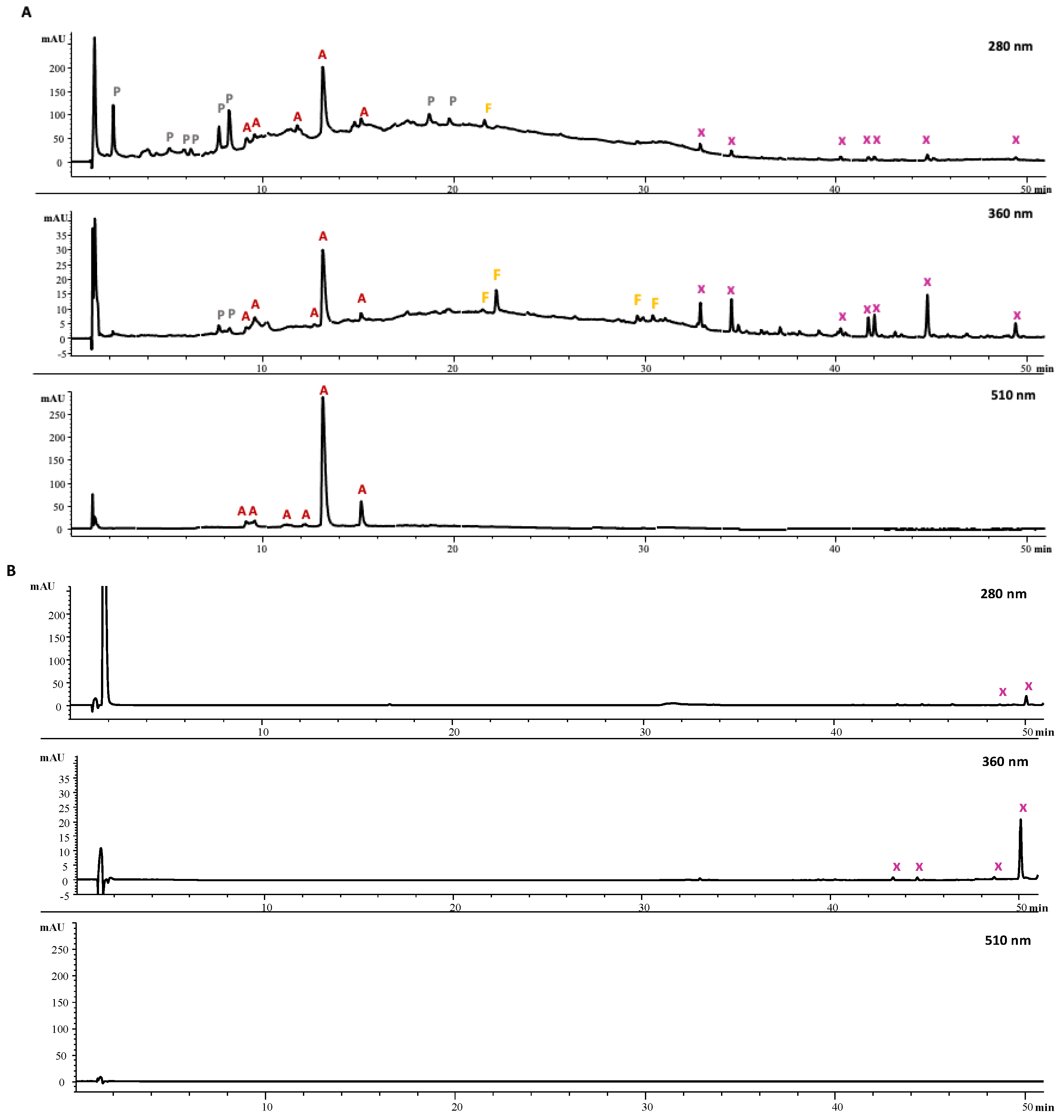
2.8. Analysis of Phenolic Compounds by HPLC-DAD
2.9. Cell Viability
2.10. Statistical Analysis
3. Results and Discussion
3.1. NaDES Screening for the Extraction of NEPs from Mangosteen Peel
3.2. Selection of the Molar Ratio of the NaDES Components
3.3. Optimization of the NaDES-UAE Extraction Parameters for NEPs from Mangosteen Peel Extraction Residue
3.4. Comparison of NEP Extraction by UAE-NaDES from the Extraction Residue of Mangosteen Peel with the Conventional Extraction of EPPs from Mangosteen Peel
3.5. Effect of NEP Extracts Obtained by UAE with ChCl:LA from the Extraction Residue of Mangosteen Peel on Viability in HeLa Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baiano, A. Recovery of biomolecules from food wastes: A review. Molecules 2014, 19, 14821–14842. [Google Scholar] [CrossRef] [PubMed]
- Rico, X.; Gullón, B.; Alonso, J.L.; Yáñez, R. Recovery of high value-added compounds from pineapple, melon, watermelon and pumpkin processing by-products: An overview. Food Res. Int. 2020, 132, 109086. [Google Scholar] [CrossRef]
- Esparza, I.; Jiménez-Moreno, N.; Bimbela, F.; Ancín-Azpilicueta, C.; Gandía, L.M. Fruit and vegetable waste management: Conventional and emerging approaches. J. Environ. Manag. 2020, 265, 110510. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Su, B.N.; Keller, W.J.; Mehta, R.G.; Kinghorn, D. Antioxidant Xanthones from pericarp of Garcinia mangostana (Mangosteen). J. Agric. Food. Chem. 2006, 54, 2077–2082. [Google Scholar] [CrossRef] [PubMed]
- Suttirak, W.; Manurakchinakorn, S. In vitro antioxidant properties of mangosteen peel extract. J. Food Sci. Technol. 2012, 51, 1–13. [Google Scholar] [CrossRef]
- Zadernowski, R.; Czaplicki, S.; Naczk, M. Phenolic acid profiles of mangosteen fruits (Garcinia mangostana). Food Chem. 2009, 112, 685–689. [Google Scholar] [CrossRef]
- Pothitirat, W.; Chomnawang, M.T.; Supabphol, R.; Gritsanapan, W. Comparison of bioactive compounds content, free radical scavenging and anti-acne inducing bacteria activities of extracts from the mangosteen fruit rind at two stages of maturity. Fitoterapia 2009, 80, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Pedraza-Chaverri, J.; Cárdenas-Rodríguez, N.; Orozco-Ibarra, M.; Pérez-Rojas, J.M. Medicinal properties of mangosteen (Garcinia mangostana). Food Chem. Toxicol. 2008, 46, 3227–3239. [Google Scholar] [CrossRef]
- Kusmayadi, A.; Adriani, L.; Abun, A.; Muchtaridi, M.; Tanuwiria, U.H. The effect of solvents and extraction time on total xanthone and antioxidant yields of mangosteen peel (Garcinia mangostana L.) extract. Drug Invent. Today 2018, 10, 2572–2576. [Google Scholar]
- Saputri, F.A.; Mayangsari, A.; Muchtaridi, M. The optimization of eluting condition of solid phase extraction method for ï¡-mangostin purification in mangosteen pericarp extract. Int. J. Appl. Pharm 2018, 10, 112–114. [Google Scholar]
- Bundeesomchok, K.; Filly, A.; Rakotomanomana, N.; Panichayupakaranant, P.; Chemat, F. Extraction of α-mangostin from Garcinia mangostana L. using alternative solvents: Computational predictive and experimental studies. LWT Food Sci. Technol. 2016, 65, 297–303. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Díaz-Rubio, M.E.; Saura-Calixto, F. Non-extractable polyphenols in plant foods: Nature, isolation, and analysis. In Polyphenols in Plants; Academic Press: Cambridge, MA, USA, 2014; pp. 203–218. [Google Scholar]
- Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bound phenolics in foods, a review. Food Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef]
- Han, Y.; Huang, M.; Li, L.; Cai, X.; Gao, Z.; Li, F.; Xiao, H. Non-extractable polyphenols from cranberries: Potential anti-inflammation and anti-colon-cancer agents. Food Funct. 2019, 10, 7714–7723. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Rodríguez, G.; Marina, M.L.; Plaza, M. Strategies for the extraction and analysis of non-extractable polyphenols from plants. J. Chromatogr. A 2017, 1514, 1–15. [Google Scholar] [CrossRef]
- Turner, C. Sustainable analytical chemistry-more than just being green. Pure Appl. Chem. 2013, 85, 2217–2229. [Google Scholar] [CrossRef]
- Gullón, P.; Gullón, B.; Romaní, A.; Rocchetti, G.; Lorenzo, J.M. Smart advanced solvents for bioactive compounds recovery from agri-food by-products: A review. Trends Food Sci. Technol. 2020, 101, 182–197. [Google Scholar] [CrossRef]
- Fernández, M.A.; Espino, M.; Gómez, F.J.V.; Silva, M.F. Novel approaches mediated by tailor-made Green solvents for the extraction of phenolic compounds from agro-food industrial by-products. Food Chem. 2018, 239, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Koutsoukos, S.; Tsiaka, T.; Tzani, A.; Zoumpoulakis, P.; Detsi, A. Choline chloride and tartaric acid, a Natural Deep Eutectic Solvent for the efficient extraction of phenolic and carotenoid compounds. J. Clean. Prod. 2019, 241, 118384. [Google Scholar] [CrossRef]
- Dai, Y.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents providing enhanced stability of natural colorants from safflower (Carthamus tinctorius). Food Chem. 2014, 159, 116–121. [Google Scholar] [CrossRef]
- Bentley, J.; Olsen, E.K.; Moore, J.P.; Farrant, J.M. The phenolic profile extracted from the desiccation-tolerant medicinal shrub Myrothamnus flabellifolia using Natural Deep Eutectic Solvents varies according to the solvation conditions. Phytochemistry 2020, 173, 112323. [Google Scholar] [CrossRef] [PubMed]
- Chanioti, S.; Katsouli, M.; Tzia, C. Novel Processes for the Extraction of Phenolic Compounds from Olive Pomace and Their Protection by Encapsulation. Molecules 2021, 26, 1781. [Google Scholar] [CrossRef] [PubMed]
- Bosiljkov, T.; Dujmic, F.; Bubalo, M.C.; Hribar, J.; Vidrih, R.; Brncic, M.; Zlatic, E.; Redovnikovic, I.R.; Jokic, S. Natural deep eutectic solvents and ultrasound-assisted extraction: Green approaches for extraction of wine lees anthocyanins. Food Bioprod. Process. 2017, 102, 195–203. [Google Scholar] [CrossRef]
- Ameer, K.; Shahbaz, H.M.; Kwon, J.H. Green extraction methods for polyphenols from plant matrices and their byproducts: A review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 295–315. [Google Scholar] [CrossRef]
- Alanon, M.E.; Ivanovic, M.; Gómez-Caravaca, A.M.; Arraéz-Román, D.; Segura-Carretero, A. Choline chloride derivative-based deep eutectic liquids as novel green alternative solvents for extraction of phenolic compounds from olive leaf. Arab. J. Chem. 2020, 13, 1685–1701. [Google Scholar] [CrossRef]
- Saratale, G.D.; Saratale, R.G.; Varjani, S.; Cho, S.K.; Chodake, G.S.; Kadam, A.; Mullar, S.I.; Bharagava, R.N.; Kim, D.S.; Shin, H.S. Development of ultrasound aided chemical pretreatment methods to enrich saccharification of wheat waste biomass for polyhydroxybutyrate production and its characterization. Ind. Crops Prod. 2020, 150, 112425. [Google Scholar] [CrossRef]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural Deep eutectic solvents—Solvents for the 21s century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Hernández-Corroto, E.; Plaza, M.; Marina, M.L.; García, M.C. Sustainable extraction of proteins and bioactive substances from pomegranate peel (Punica granatum L.) using pressurized liquids and deep eutectic solvents. Innov. Food Sci. Emerg. Technol. 2020, 60, 102314. [Google Scholar] [CrossRef]
- Barbieri, J.B.; Goltz, C.; Cavalheiro, F.B.; Toci, A.T.; Igarashi-Mafra, L.; Mafra, M.R. Deep eutectic solvents applied in the extraction and stabilization of rosemary (Rosmarinus officinalis L.) phenolic compounds. Ind. Crops Prod. 2020, 144, 112049. [Google Scholar] [CrossRef]
- Gullón, B.; Muñiz-Mouro, A.; Lú-Chau, T.A.; Moreira, M.T.; Lema, J.M.; Eibes, G. Green approaches for the extraction of antioxidants from eucalyptus leaves. Ind. Crops Prod. 2019, 138, 111473. [Google Scholar] [CrossRef]
- Pal, C.B.T.; Jadeja, G.C. Microwave-assisted deep eutectic solvent extraction of phenolic antioxidants from onion (Allium cepa L.) peel: A Box–Behnken design approach for optimization. J. Food Sci. Technol. 2019, 56, 4211–4223. [Google Scholar] [CrossRef]
- Duan, L.; Zhang, W.H.; Zhang, Z.H.; Liu, E.H.; Guo, L. Evaluation of natural deep eutectic solvents for the extraction of bioactive flavone C-glycosides from FlosTrollii. Microchem. J. 2019, 145, 180–186. [Google Scholar] [CrossRef]
- Tan, Y.T.; Ngoh, G.C.; Chua, A.S.M. Effect of functional groups in acid constituent of deep eutectic solvent for extraction of reactive lignin. Bioresour. Technol. 2019, 281, 359–366. [Google Scholar] [CrossRef]
- Zhou, H.C.; Lin, Y.M.; Wei, S.D.; Tam, N.F.Y. Structural diversity and antioxidant activity of condensed tannins fractionated from mangosteen pericarp. Food Chem. 2011, 129, 1710–1720. [Google Scholar] [CrossRef]
- Condezo-Hoyos, L.; Mohanty, I.P.; Noratto, G.D. Assessing non-digestible compounds in applecultivars and their potential as modulators of obese faecal microbiota in vitro. Food Chem. 2014, 161, 208–215. [Google Scholar] [CrossRef]
- Zurita, J.; Díaz-Rubio, M.E.; Saura-Calixto, F. Improved procedure to determine non-extractable polymeric proanthocyanidins in plant foods. Int. J. Food Sci. Nutr. 2012, 63, 936–939. [Google Scholar] [CrossRef]
- Taha, F.S.; Wagdy, S.M.; Singer, F.A. Comparison between antioxidant activities of phenolic extracts from different parts of peanut. Life Sci. 2012, 99, 207–215. [Google Scholar]
- Montero, L.; Herrero, M.; Ibáñez, E.; Cifuentes, A. Profiling of phenolic compounds from different apple varieties using comprehensive two-dimensional liquid chromatography. J. Chromatogr. A 2013, 1313, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Jiménez, J.; Arranz, S.; Saura-Calixto, F. Proanthocyanidin content in foods is largely underestimated in the literature data: An approach to quantification of the missing proanthocyanidins. Food Res. Int. 2009, 42, 1381–1388. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Hernández -Corroto, E.; Marina, M.L.; García, M.C. Multiple protective effect of peptides released from Olea europaea and Prunus persica seeds against oxidative damage and cancer cell proliferation. Food Res. Int. 2018, 106, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Ajibola, C.F.; Fashakin, J.B.; Fagbemi, T.N.; Aluko, R.E. Effect of peptide size on antioxidant properties of African yam bean seed (Sphenostylis stenocarpa) protein hydrolysate fractions. Int. J. Mol. Sci. 2011, 12, 6685–6702. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Anee, T.I.; Fujita, M. Glutathione in plants: Biosynthesis and physiological role in environmental stress tolerance. Physiol. Mol. Biol. Plants 2017, 23, 249–268. [Google Scholar] [CrossRef]
- Domínguez-Rodríguez, G.; Marina, M.L.; Plaza, M. Enzyme-assisted extraction of bioactive non-extractable polyphenols from sweet cherry (Prunus avium L.) pomace. Food Chem. 2021, 339, 128086. [Google Scholar] [CrossRef]
- Domínguez-Rodríguez, G.; García, M.C.; Plaza, M.; Marina, M.L. Revalorization of Passiflora species peels as a sustainable source of antioxidant phenolic compounds. Sci. Total Environ. 2019, 696, 134030. [Google Scholar] [CrossRef]
- Choi, Y.H.; Verpoorte, R. Green solvents for the extraction of bioactive compounds from natural products using ionic liquids and deep eutectic solvents. Curr. Opin. Food Sci. 2019, 26, 87–93. [Google Scholar] [CrossRef]
- Žlabur, J.Š.; Voća, S.; Brnč, M.; Rimac-Brnč, S. New Trends in Food Technology for Green Recovery of Bioactive Compounds From Plant Materials. In Role of Materials Science in Food Bioengineering; Academic Press: Cambridge, MA, USA, 2018; pp. 1–36. [Google Scholar]
- Alanon, M.E.; Ivanovic, M.; Pimentel-Mora, S.; Borrás-Linares, I.; Arraez-Roman, D.; Segura-Carretero, A. A novel sustainable approach for the extraction of value-added compounds from Hibiscus sabdariffa L. calyces by natural deep eutectic solvents. Food Res. Int. 2020, 137, 109646. [Google Scholar] [CrossRef] [PubMed]
- Ivanocic, M.; Alanon, M.E.; Arráerz-Román, D.; Segura-Carretero, A. Enhanced and green extraction of bioactive compounds from Lippia citriadora by tailor-made natural deep eutectic solvents. Food Res. Int. 2018, 111, 67–76. [Google Scholar] [CrossRef]
- Loarce, L.; Oliver-Simancas, R.; Marchante, L.; Díaz-Maroto, M.C.; Alanon, M.E. Implementation of subcritical water extraction with natural deep eutectic solvents for sustainable extraction from winemaking by-products. Food Res. Int. 2020, 137, 109728. [Google Scholar] [CrossRef]
- Wang, Y.; Singh, A.P.; Hurst, W.J.; Glinski, J.A.; Koo, H.; Vorsa, N. Influence of degree-of-polymerization and linkage on the quantification of proanthocyanidins using 4-dimethylaminocinnamaldehyde (DMAC) assay. J. Agric. Food Chem. 2016, 64, 2190–2199. [Google Scholar] [CrossRef]
- Shay, P.E.; Trofymow, J.A.; Constabel, C.P. An improved butanol-HCl assay for quantification of water-soluble, acetone: Methanol-soluble, and insoluble proanthocyanidins (condensed tannins). Plant Methods 2017, 13, 63. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.Y.; Wain, A.; Groombridge, L.; Grimes, E. Forensic identification of urine using the DMAC test: A method validation study. Sci. Justice 2012, 52, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Radošević, K.; Ćurko, N.; Gaurina Srček, V.; Bubalo, M.; Tomašević, M.; Kovačević Ganić, K.; Radojčić Redovniković, I. Natural deep eutectic solvents as beneficial extractants for enhancement of plant extracts bioactivity. LWT Food Sci. Technol. 2016, 73, 45–51. [Google Scholar] [CrossRef]
- Kalpna, R.; Mital, K. Vegetable and fruit peels as a novel source of antioxidants. J. Med. Plants Res. 2011, 5, 63–71. [Google Scholar]
- Gangwar, M.; Gautam, M.K.; Sharma, A.K.; Tripathi, Y.B.; Goel, R.K.; Nath, G. Antioxidant capacity and radical scavenging effect of polyphenol rich Mallotus philippinensis fruit extract on human erythrocytes: An in vitro study. Sci. World J. 2014, 2014, 279451. [Google Scholar] [CrossRef] [PubMed]
- El Kantar, S.; Rajha, H.N.; Boussetta, N.; Vorobiev, E.; Maroun, R.G.; Louka, N. Green extraction of polyphenols from grapefruit peels using high voltage electrical discharges, deep eutectic solvents, and aqueous glycerol. Food Chem. 2019, 295, 165–171. [Google Scholar] [CrossRef]
- Garcia, A.; Rodriguez-Juan, E.; Rodriguez-Gutierrez, G.; Rios, J.J.; Fernandez-Bolanos, J. Extraction of phenolic compounds from virgin olive oil by deep eutectic solvents (DESs). Food Chem. 2016, 197, 554–561. [Google Scholar] [CrossRef]
- Lorrain, B.; Pechamat, L.; Teissedre, P.L. Evolution of analysis of polyphenols from grapes, wines, and extracts. Molecules 2013, 18, 1076–1100. [Google Scholar] [CrossRef]
- Hayyan, M.; Hashim, M.A.; Hayyan, A.; Al-Saadi, M.A.; AlNashef, I.M.; Mirghani, M.E.E.; Saheed, O.K. Are deep eutectic solvents benign or toxic? Chemosphere 2013, 90, 2193–2195. [Google Scholar] [CrossRef]


| Component 1 (HBA) | Component 2 (HBD) | Abbreviation | Molar Ratio | Reference |
|---|---|---|---|---|
| Choline chloride | Glycerol | ChCl-gly | 1:2 | [29] |
| Choline chloride | Ethylene glycol | ChCl-EG | 1:2 | [30] |
| Choline chloride | Urea | ChCl-urea | 1:2 | [31] |
| Choline chloride | Sorbitol | ChCl-sorb | 1:1 | [32] |
| Choline chloride | Lactic acid | ChCl-LA | 1:2 | [33] |
| Choline chloride | Citric acid | ChCl-CA | 2:1 | [34] |
| Choline chloride | Formic acid | ChCl-FA | 1:2 | [33] |
| NaDES | DMAC (mg Epicatechin/100 g Sample) | Butanol/HCl (mg Epicatechin/100 g Sample) | Hydroxyl Radical (% of Inhibition) | % Scavenging of ABTS Radicals |
|---|---|---|---|---|
| ChCl:FA | 18 ± 1 b | 1767 ± 197 b,c | 71 ± 17 a | 41 ± 2 d |
| ChCl:LA | 25.4 ± 0.6 a | 2447 ± 237 a | 24 ± 5 d | 34 ± 2 e |
| ChCl:sorb | 5 ± 1 f | 759 ± 52 d | 58 ± 10 b | 49 ± 5 c |
| ChCl:EG | 9.2 ± 0.9 e | 623 ± 110 e | 39 ± 8 c | 65 ± 4 a |
| ChCl:CA | 15 ± 2 d | 1493 ± 72 c | 25 ± 2 g | |
| ChCl:urea | 415 ± 57 e | 9 ± 4 e | 61 ± 2 b | |
| ChCl:gly | 16.5 ± 0.5 c | 1954 ± 184 b | 10 ± 4 e | 30 ± 6 f |
| NaDES | DMAC (mg Epicatechin/100 g Sample) | Butanol/HCl (mg Epicatechin/100 g Sample) | Hydroxyl Radical (% of Inhibition) | % Scavenging of ABTS Radicals |
|---|---|---|---|---|
| ChCl:LA | ||||
| 1:1 | 13.7 ± 0.5 d | 1230 ± 182 b | 33 ± 5 a,b | 13.9 ± 0.8 d |
| 1:2 | 25.4 ± 0.6 a | 2447 ± 237 a | 24 ± 5 b | 34 ± 2 c |
| 1:3 | 18.7 ± 0.6 b | 2277 ± 386 a | 24 ± 4 b | 15 ± 1 d |
| ChCl:EG | ||||
| 1:2 | 9.2 ± 0.9 e | 623 ± 110 c | 39 ± 8 a | 65 ± 4 a |
| 1:3 | 16.9 ± 0.5 c | 2187 ± 436 a | 31 ± 3 a | 39 ± 1 b |
| Water Percentage (%) | Ultrasound Amplitude (%) | Extraction Time (min) | |||
| UAE optimal extraction conditions | 18.8 | 60.0 | 15.0 | ||
| Response Variables | UAE Theoretical Values | UAE Experimental Values | Conventional Extraction | ||
| DMAC (mg epicatechin/100 g sample) | 219.95 | 238 ± 21 | 2.4 ± 0.2 | ||
| Butanol/HCl (mg epicatechin/100 g sample) | 102,200 | 37,638 ± 6389 | 1017 ± 57 | ||
| Scavenging capacity of ABTS radicals (%) | 105.57 | 113 ± 10 | 14 ± 1 | ||
| Hydroxyl radicals (% of hydroxyl radical inhibition) | 61.14 | 66 ± 9 | 5.0 ± 0.4 | ||
| FRAP (mg GSH/g sample) | 153.28 | 283 ± 17 | 1.58 ± 0.08 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plaza, M.; Domínguez-Rodríguez, G.; Sahelices, C.; Marina, M.L. A Sustainable Approach for Extracting Non-Extractable Phenolic Compounds from Mangosteen Peel Using Ultrasound-Assisted Extraction and Natural Deep Eutectic Solvents. Appl. Sci. 2021, 11, 5625. https://doi.org/10.3390/app11125625
Plaza M, Domínguez-Rodríguez G, Sahelices C, Marina ML. A Sustainable Approach for Extracting Non-Extractable Phenolic Compounds from Mangosteen Peel Using Ultrasound-Assisted Extraction and Natural Deep Eutectic Solvents. Applied Sciences. 2021; 11(12):5625. https://doi.org/10.3390/app11125625
Chicago/Turabian StylePlaza, Merichel, Gloria Domínguez-Rodríguez, Cristina Sahelices, and María Luisa Marina. 2021. "A Sustainable Approach for Extracting Non-Extractable Phenolic Compounds from Mangosteen Peel Using Ultrasound-Assisted Extraction and Natural Deep Eutectic Solvents" Applied Sciences 11, no. 12: 5625. https://doi.org/10.3390/app11125625
APA StylePlaza, M., Domínguez-Rodríguez, G., Sahelices, C., & Marina, M. L. (2021). A Sustainable Approach for Extracting Non-Extractable Phenolic Compounds from Mangosteen Peel Using Ultrasound-Assisted Extraction and Natural Deep Eutectic Solvents. Applied Sciences, 11(12), 5625. https://doi.org/10.3390/app11125625