Deep Eutectic Solvents for the Extraction of Bioactive Compounds from Natural Sources and Agricultural By-Products
Abstract
:Featured Application
Abstract
1. Introduction
2. Toxicity, Bioactivity and Biodegradability
3. DESs for the Extraction of Bioactive Compounds from Plants, Fruit and Vegetables
4. Valorization of Agricultural By-Products Using DESs
5. Using DES to Extract Bioactive Compounds from Other Natural Sources
6. Conclusions and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. Save Food for a Better Climate; FAO: Quebec City, QC, Canada, 2017. [Google Scholar]
- Benvenutti, L.; Zielinski, A.A.F.; Ferreira, S.R.S. Which Is the Best Food Emerging Solvent: IL, DES or NADES? Trends Food Sci. Technol. 2019, 90, 133–146. [Google Scholar] [CrossRef]
- Kunz, W.; Häckl, K. The Hype with Ionic Liquids as Solvents. Chem. Phys. Lett. 2016, 661, 6–12. [Google Scholar] [CrossRef]
- Jablonský, M.; Škulcová, A.; Malvis, A.; Šima, J. Extraction of Value-Added Components from Food Industry Based and Agro-Forest Biowastes by Deep Eutectic Solvents. J. Biotechnol. 2018, 282, 46–66. [Google Scholar] [CrossRef]
- Choi, Y.H.; van Spronsen, J.; Dai, Y.; Verberne, M.; Hollmann, F.; Arends, I.W.C.E.; Witkamp, G.J.; Verpoorte, R. Are Natural Deep Eutectic Solvents the Missing Link in Understanding Cellular Metabolism and Physiology? Plant Physiol. 2011, 156, 1701–1705. [Google Scholar] [CrossRef] [Green Version]
- Wei, Z.; Qi, X.; Li, T.; Luo, M.; Wang, W.; Zu, Y.; Fu, Y. Application of Natural Deep Eutectic Solvents for Extraction and Determination of Phenolics in Cajanus Cajan Leaves by Ultra Performance Liquid Chromatography. Sep. Purif. Technol. 2015, 149, 237–244. [Google Scholar] [CrossRef]
- Bakirtzi, C.; Triantafyllidou, K.; Makris, D.P. Novel Lactic Acid-Based Natural Deep Eutectic Solvents: Efficiency in the Ultrasound-Assisted Extraction of Antioxidant Polyphenols from Common Native Greek Medicinal Plants. J. Appl. Res. Med. Aromat. Plants 2016, 3, 120–127. [Google Scholar] [CrossRef]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural Deep Eutectic Solvents—Solvents for the 21st Century. Acs Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Huang, Y.; Feng, F.; Jiang, J.; Qiao, Y.; Wu, T.; Voglmeir, J.; Chen, Z.G. Green and Efficient Extraction of Rutin from Tartary Buckwheat Hull by Using Natural Deep Eutectic Solvents. Food Chem. 2017, 221, 1400–1405. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, L.A.; Pereira, C.V.; Leonardo, I.C.; Fernández, N.; Gaspar, F.B.; Silva, J.M.; Reis, R.L.; Duarte, A.R.C.; Paiva, A.; Matias, A.A. Terpene-Based Natural Deep Eutectic Systems as Efficient Solvents to Recover Astaxanthin from Brown Crab Shell Residues. Acs Sustain. Chem. Eng. 2020, 8, 2246–2259. [Google Scholar] [CrossRef]
- Zhao, B.Y.; Xu, P.; Yang, F.X.; Wu, H.; Zong, M.H.; Lou, W.Y. Biocompatible Deep Eutectic Solvents Based on ChCl: Characterization and Application to the Extraction of Rutin from Sophora Japonica. Acs Sustain. Chem. Eng. 2015, 3, 2746–2755. [Google Scholar] [CrossRef]
- Benlebna, M.; Ruesgas-Ramón, M.; Bonafos, B.; Fouret, G.; Casas, F.; Coudray, C.; Durand, E.; Cruz Figueroa-Espinoza, M.; Feillet-Coudray, C. Toxicity of Natural Deep Eutectic Solvent Betaine:Glycerol in Rats. J. Agric. Food Chem. 2018, 66, 6205–6212. [Google Scholar] [CrossRef]
- Volpi-Lagreca, G.; Duckett, S.K. Supplementation of Glycerol or Fructose via Drinking Water to Grazing Lambs on Tissue Glycogen Level and Lipogenesis. J. Anim. Sci. 2017, 95, 2558–2575. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Ping, K.; Jiang, Y.W.; Wang, L.T.; Niu, L.J.; Liu, Z.M.; Fu, Y.J. Natural Deep Eutectic Solvents Couple with Integrative Extraction Technique as an Effective Approach for Mulberry Anthocyanin Extraction. Food Chem. 2019, 296, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Duru, K.C.; Mukhlynina, E.A.; Moroz, G.A.; Gette, I.F.; Danilova, I.G.; Kovaleva, E.G. Anti-Diabetic Effect of Isoflavone Rich Kudzu Root Extract in Experimentally Induced Diabetic Rats. J. Funct. Foods 2020, 68, 103922. [Google Scholar] [CrossRef]
- Zhuang, B.; Dou, L.L.; Li, P.; Liu, E.H. Deep Eutectic Solvents as Green Media for Extraction of Flavonoid Glycosides and Aglycones from Platycladi Cacumen. J. Pharm. Biomed. Anal. 2017, 134, 214–219. [Google Scholar] [CrossRef]
- Yang, M.; Cao, J.; Cao, F.; Lu, C.; Su, E. Efficient Extraction of Bioactive Flavonoids from Ginkgo Biloba Leaves Using Deep Eutectic Solvent/Water Mixture as Green Media. Chem. Biochem. Eng. Q. 2018, 32, 315–324. [Google Scholar] [CrossRef]
- Guo, H.; Liu, S.; Li, S.; Feng, Q.; Ma, C.; Zhao, J.; Xiong, Z. Deep Eutectic Solvent Combined with Ultrasound-Assisted Extraction as High Efficient Extractive Media for Extraction and Quality Evaluation of Herba Epimedii. J. Pharm. Biomed. Anal. 2020, 185, 113228. [Google Scholar] [CrossRef]
- Torres-Vega, J.; Gomez-Alonso, S.; Perez-Navarro, J.; Pastene-navarrete, E. Green Extraction of Alkaloids and Polyphenols from Peumus Boldus Leaves with Natural Deep Eutectic. Plants 2020, 9, 242. [Google Scholar] [CrossRef] [Green Version]
- Nam, M.W.; Zhao, J.; Lee, M.S.; Jeong, J.H.; Lee, J. Enhanced Extraction of Bioactive Natural Products Using Tailor-Made Deep Eutectic Solvents: Application to Flavonoid Extraction from Flos Sophorae. Green Chem. 2015, 17, 1718–1727. [Google Scholar] [CrossRef]
- Ali, M.C.; Chen, J.; Zhang, H.; Li, Z.; Zhao, L.; Qiu, H. Effective Extraction of Flavonoids from Lycium Barbarum L. Fruits by Deep Eutectic Solvents-Based Ultrasound-Assisted Extraction. Talanta 2019, 203, 16–22. [Google Scholar] [CrossRef]
- Dai, Y.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural Deep Eutectic Solvents as a New Extraction Media for Phenolic Metabolites in Carthamus Tinctorius L. Anal. Chem. 2013, 85, 6272–6278. [Google Scholar] [CrossRef]
- Dai, Y.; Rozema, E.; Verpoorte, R.; Choi, Y.H. Application of Natural Deep Eutectic Solvents to the Extraction of Anthocyanins from Catharanthus Roseus with High Extractability and Stability Replacing Conventional Organic Solvents. J. Chromatogr. A 2016, 1434, 50–56. [Google Scholar] [CrossRef]
- Barbieri, J.B.; Goltz, C.; Batistão Cavalheiro, F.; Theodoro Toci, A.; Igarashi-Mafra, L.; Mafra, M.R. Deep Eutectic Solvents Applied in the Extraction and Stabilization of Rosemary (Rosmarinus officinalis L.) Phenolic Compounds. Ind. Crops Prod. 2020, 144, 112049. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Ravi, H.K.; Khadhraoui, B.; Hilali, S.; Perino, S.; Tixier, A.S.F. Review of Alternative Solvents for Green Extraction of Food and Natural Products: Panorama, Principles, Applications and Prospects. Molecules 2019, 24, 3007. [Google Scholar] [CrossRef] [Green Version]
- Chanioti, S.; Tzia, C. Extraction of Phenolic Compounds from Olive Pomace by Using Natural Deep Eutectic Solvents and Innovative Extraction Techniques. Innov. Food Sci. Emerg. Technol. 2018, 48, 228–239. [Google Scholar] [CrossRef]
- Alañón, M.E.; Ivanović, M.; Gómez-Caravaca, A.M.; Arráez-Román, D.; Segura-Carretero, A. ChCl Derivative-Based Deep Eutectic Liquids as Novel Green Alternative Solvents for Extraction of Phenolic Compounds from Olive Leaf. Arab. J. Chem. 2020, 13, 1685–1701. [Google Scholar] [CrossRef]
- Osorio, L.L.D.R.; Flórez-López, E.; Grande-Tovar, C.D. The Potential of Selected Agri-Food Loss and Waste to Contribute to a Circular Economy: Applications in the Food, Cosmetic and Pharmaceutical Industries. Molecules 2021, 26, 515. [Google Scholar] [CrossRef] [PubMed]
- Putnik, P.; Bursać Kovačević, D.; Režek Jambrak, A.; Barba, F.J.; Cravotto, G.; Binello, A.; Lorenzo, J.M.; Shpigelman, A. Innovative “Green” and Novel Strategies for the Extraction of Bioactive Added Value Compounds from Citruswastes—A Review. Molecules 2017, 22, 680. [Google Scholar] [CrossRef] [Green Version]
- Gullón, B.; Muñiz-Mouro, A.; Lú-Chau, T.A.; Moreira, M.T.; Lema, J.M.; Eibes, G. Green Approaches for the Extraction of Antioxidants from Eucalyptus Leaves. Ind. Crops Prod. 2019, 138, 111473. [Google Scholar] [CrossRef]
- Hernández-Corroto, E.; Plaza, M.; Marina, M.L.; García, M.C. Sustainable Extraction of Proteins and Bioactive Substances from Pomegranate Peel (Punica granatum L.) Using Pressurized Liquids and Deep Eutectic Solvents. Innov. Food Sci. Emerg. Technol. 2020, 60, 102314. [Google Scholar] [CrossRef]
- Saha, S.K.; Chakraborty, R. Effect of Deep Eutectic Solvent’s Characteristics on Extraction and Bioactivity of Polyphenols from Sapodilla Pulp. Chem. Pap. 2021, 75, 691–702. [Google Scholar] [CrossRef]
- Saha, S.K.; Dey, S.; Chakraborty, R. Effect of ChCl-Oxalic Acid Based Deep Eutectic Solvent on the Ultrasonic Assisted Extraction of Polyphenols from Aegle Marmelos. J. Mol. Liq. 2019, 287, 110956. [Google Scholar] [CrossRef]
- Panić, M.; Radić Stojković, M.; Kraljić, K.; Škevin, D.; Radojčić Redovniković, I.; Gaurina Srček, V.; Radošević, K. Ready-to-Use Green Polyphenolic Extracts from Food by-Products. Food Chem. 2019, 283, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M. de los Á.; Espino, M.; Gomez, F.J.V.; Silva, M.F. Novel Approaches Mediated by Tailor-Made Green Solvents for the Extraction of Phenolic Compounds from Agro-Food Industrial by-Products. Food Chem. 2018, 239, 671–678. [Google Scholar] [CrossRef] [PubMed]
- El Kantar, S.; Rajha, H.N.; Boussetta, N.; Vorobiev, E.; Maroun, R.G.; Louka, N. Green Extraction of Polyphenols from Grapefruit Peels Using High Voltage Electrical Discharges, Deep Eutectic Solvents and Aqueous Glycerol. Food Chem. 2019, 295, 165–171. [Google Scholar] [CrossRef]
- Gao, C.; Cai, C.; Liu, J.; Wang, Y.; Chen, Y.; Wang, L.; Tan, Z. Extraction and Preliminary Purification of Polysaccharides from Camellia Oleifera Abel. Seed Cake Using a Thermoseparating Aqueous Two-Phase System Based on EOPO Copolymer and Deep Eutectic Solvents. Food Chem. 2020, 313, 126164. [Google Scholar] [CrossRef] [PubMed]
- Bosiljkov, T.; Dujmić, F.; Cvjetko Bubalo, M.; Hribar, J.; Vidrih, R.; Brnčić, M.; Zlatic, E.; Radojčić Redovniković, I.; Jokić, S. Natural Deep Eutectic Solvents and Ultrasound-Assisted Extraction: Green Approaches for Extraction of Wine Lees Anthocyanins. Food Bioprod. Process. 2017, 102, 195–203. [Google Scholar] [CrossRef]
- Lin, Z.; Jiao, G.; Zhang, J.; Celli, G.B.; Brooks, M.S.L. Optimization of Protein Extraction from Bamboo Shoots and Processing Wastes Using Deep Eutectic Solvents in a Biorefinery Approach. Biomass Convers. Biorefinery 2020, in press. [Google Scholar] [CrossRef]
- Rodríguez-Juan, E.; Rodríguez-Romero, C.; Fernández-Bolaños, J.; Florido, M.C.; Garcia-Borrego, A. Phenolic Compounds from Virgin Olive Oil Obtained by Natural Deep Eutectic Solvent (NADES): Effect of the Extraction and Recovery Conditions. J. Food Sci. Technol. 2021, 58, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jia, W.; Lai, G.; Wang, L.; del Mar Contreras, M.; Yang, D. Extraction for Profiling Free and Bound Phenolic Compounds in Tea Seed Oil by Deep Eutectic Solvents. J. Food Sci. 2020, 85, 1450–1461. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Wu, M.; Cheng, H.; Chen, L.; Qi, Z. Deep Deterpenation of Citrus Essential Oils Intensified by in Situ Formation of a Deep Eutectic Solvent in Associative Extraction. Ind. Eng. Chem. Res. 2020, 59, 9223–9232. [Google Scholar] [CrossRef]
- García, A.; Rodríguez-Juan, E.; Rodríguez-Gutiérrez, G.; Rios, J.J.; Fernández-Bolaños, J. Extraction of Phenolic Compounds from Virgin Olive Oil by Deep Eutectic Solvents (DESs). Food Chem. 2016, 197, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, B.; Esteban, J.; Gonzalez-Miquel, M. Deterpenation of Citrus Essential Oils Using Glycerol-Based Deep Eutectic Solvents. J. Chem. Eng. Data 2018, 63, 2384–2393. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, K.; Yang, G.; Yu, J. A Highly Efficient Microextraction Technique Based on Deep Eutectic Solvent Formed by ChCl and P-Cresol for Simultaneous Determination of Lignans in Sesame Oils. Food Chem. 2019, 281, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Abdul Hadi, N.; Ng, M.H.; Choo, Y.M.; Hashim, M.A.; Jayakumar, N.S. Performance of Choline-Based Deep Eutectic Solvents in the Extraction of Tocols from Crude Palm Oil. JAOCS J. Am. Oil Chem. Soc. 2015, 92, 1709–1716. [Google Scholar] [CrossRef]
- Faraji, M.; Mahmoodi-Maymand, M.; Dastmalchi, F. Green, Fast and Simple Dispersive Liquid-Liquid Microextraction Method by Using Hydrophobic Deep Eutectic Solvent for Analysis of Folic Acid in Fortified Flour Samples before Liquid Chromatography Determination. Food Chem. 2020, 320, 126486. [Google Scholar] [CrossRef]
- Balaraman, H.B.; Rathnasamy, S.K. High Selective Purification of IgY from Quail Egg: Process Design and Quantification of Deep Eutectic Solvent Based Ultrasound Assisted Liquid Phase Microextraction Coupled with Preparative Chromatography. Int. J. Biol. Macromol. 2020, 146, 253–262. [Google Scholar] [CrossRef] [PubMed]
- López, R.; D’Amato, R.; Trabalza-Marinucci, M.; Regni, L.; Proetti, P.; Maratta, A.; Cerutti, S.; Pacheco, P. Green and Simple Extraction of Free Seleno-Amino Acids from Powdered and Lyophilized Milk Samples with Natural Deep Eutectic Solvents. Food Chem. 2020, 326, 126965. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Wang, D.; Yang, F.; Zhang, L.; Ji, S.; Wang, S. Enhanced Extraction of Hydroxyapatite from Bighead Carp (Aristichthys Nobilis) Scales Using Deep Eutectic Solvent. J. Food Sci. 2020, 85, 150–156. [Google Scholar] [CrossRef]
| Sample | Bioactive Compounds | DES (Molar Ratio; Amount; Water Content) | Extraction Procedure | Analytical Technique | Comments | Ref. |
|---|---|---|---|---|---|---|
| Pueraria lobata Kudzu root | Puerarin, daidzein, genistein, vitexin, 4-hydroxyflavone | ChCl: citric acid (−) | UAE | HPLC-UV |
| [15] |
| Platycladi Cacumen | Flavonoid glycosides (micricitrin and quercitrin) and biflavone aglycones (amentoflavone and hinokiflavone) | ChCl:laevulinic acid (1:2; -; -) | UAE | HPLC-UV |
| [16] |
| Ginkgo biloba leaves | Quercetin, kaempferol, and isorhamnetin | ChCl/levulinic acid (1:2; -; 40% (w/w) water) | SLE and UAE | HPLC |
| [17] |
| Herba Epimedii | Flavonoids: Icarrin, IcarisdII, Epidimcdin A, Epimcdin B, and Epimcdin C | L-proline: ethylene glycol (1:4; -;-) | UAE | HPLC-UV |
| [18] |
| Peumus boldus leaves | Alkaloids and phenolic compounds | ChCl-lactic acid and proline:oxalic acid(−) | UAE | HPLC-PDA-ESI-IT/MS HPLC-QTOF-MS/MS |
| [19] |
| Sophora japonica L. Flos sophorae—dried flowers of Sophora japonica L. | Quercetin, kaempferol and isorhamnetin glucoside | L-proline:glycerol (2:5; -: -) | SLE and UAE | LC-UV UHPLC-QTOF-MS |
| [20] |
| Lycium barbarum L. fruits | Chlorogenic acid, morine, luteolin, coumaric acid, ferulic acid, hyperoside, rutin, myricetin, quercitrin, apigenin | ChCl:p-toluene sulfonic acid (1:2; -) | SLE and UAE | HPLC-UV |
| [21] |
| Carthamus tinctorius L. | Yellow hydroxysafflor, cartormin, carthamin, tripcoumaroylspermidines | 75% (v/v) proline:malic acid (-;-; 75% (w/w) water); sucrose: ChCl (-;-; 75% (w/w) water) | SLE | HPLC, NMR, and MS Analysis |
| [22] |
| Catharanthus roseus | Anthocyanins | Lactic acid:glucose, and 1,2-propanediol:ChCl(−) | UAE and UAEH | HPLC-DAD, UHPLC-TOF-MS |
| [23] |
| Rosmarinus officinalis L. | Rosmarinic acid, caffeic acid, 7- ethylrosmanol, rutin, naringin, ferulic acid | Glycerol:ChCl (1:2; -; 10% (w/w) water); lactic acid:ChCl (1:3; -; 10% (w/w) water); 1,2-propanediol:ChCl (1:2; -; 10% (w/w) water); oxalic acid:ChCl (1:1; -; 10% (w/w) water) | UAE | HPLC-DAD |
| [24] |
| Fructus Mori Fresh mulberry (Fructus Mori) | Vitamins, minerals, phenolic acids, flavanols and anthocyanins: cyanidin-3-O-glucoside and cyanidin-3-O-rutinoside | ChCl:citric acid:glucose (1:1:1; -; 10% (w/w) water) | HSH-CBE | HPLC-UV |
| [14] |
| Leaves of Cajanus cajan | Polar, polar-weak phenolic compounds | ChCl:maltose (1:2; -; 20% (w/w) water) | MAE | UHPLC-UV |
| [6] |
| Sophora japonica flower buds | Routin | ChCl:triethylene glycol (1:4; -; -) | SLE | HPLC-UV |
| [11] |
| Sample (Amount) | Bioactive Compound | DES (Molar Ratio; Amount; Water Content) | Extraction Procedure | Analytical Technique | Extraction Efficiency | Comments | Ref. |
|---|---|---|---|---|---|---|---|
| Aegle marmelos Bael pulp | 10 phenolic acids, 6 flavonoids and cinnamic acid | ChCl:oxalic acid (1:1; 25 mL; 25% water (v/v)) | UAE | HPLC-DAD | >60 (b) |
| [33] |
| Bamboo shoots tip, basal and sheath bamboo shoots | Proteins | ChCl:levulinic acid (1:6; 40% (w/w) water; -) | SLE | Spectrophotometry | 39 (a) |
| [39] |
| Camellia oleifera Abel. seed cake | Polysaccharides | ChCl:EtGly (1:2; 3 mL; 30% (w/w) water) | UAE | Spectrophotometry | 85 (b) |
| [37] |
| Eucalyptus leaves | 26 phenolic compounds (tentatively identified) | ChCl:EtGly (1:2; 20 mL; 20% water (v/v)) | SLE | UHPLC-(ToF)-MS/MS | - |
| [30] |
| Grape and olive pomace | 2 phenolic acids, 2 phenolic alcohols, vanillin (phenolic aldehyde), 11 flavonoids and pinoresinol | ChCl:citric acid (2:1; 10 mL; 30% water (v/v)) | UAE/MAE | HPLC-DAD | - |
| [34] |
| Hojiblanca olive leaves Hojiblanca cultivar (by-products) | 48 phenolic compounds | ChCl:EtGly (1:2; 1.5 mL; 43% (w/w) water) | MAE | HPLC-DAD-(ToF)-MS |
| [28] | |
| Manilkara zapota Sapodilla pulp | 7 phenolic acids and 4 flavonoids | ChCl based-DESs (1:1; 7 mL; 25% water (v/v)) | UAE | HPLC-UV | 71–86 (b) |
| [32] |
| Olive, onion, tomato, and pear food by-products | 4 phenolic acids, 2 phenolic alcohols, 6 flavonoids, oleuropein (phenolic secoiridoids) and cinnamic acid | Lactic acid:glucose (5:1; -; 15% water (v/v)) | UAE | HPLC-DAD | 82–110 (b) |
| [35] |
| Punica granatum pomegranate peel | Proteins and other phenolic compounds | ChCl:acetic acid:water (1:1:10; 5 mL; -) | UAE | HPLC-(Q-ToF)-MS | - |
| [31] |
| Vitis vinifera Grapefruit peels | Polyphenols | Lactic acid:glucose (5:1; -;-) | HVED, SLE | HPLC-DAD |
| [36] | |
| Wine lees | Anthocyanins | ChCl:malic acid (1:1; -; 35.4% (w/w) water) | UAE | HPLC-DAD |
| [38] |
| Sample (Amount) | Bioactive Compound | DES (Molar Ratio; Amount; Water Content) | Extraction Procedure | Analytical Technique | Extraction Efficiency | Comments | Ref. |
|---|---|---|---|---|---|---|---|
| Brown crab and shrimp shells and H. pluvialis | Astaxanthin | Menthol:myristic acid (8:1; 2.5 g; -) | SLE | HPLC-UV |
| [10] | |
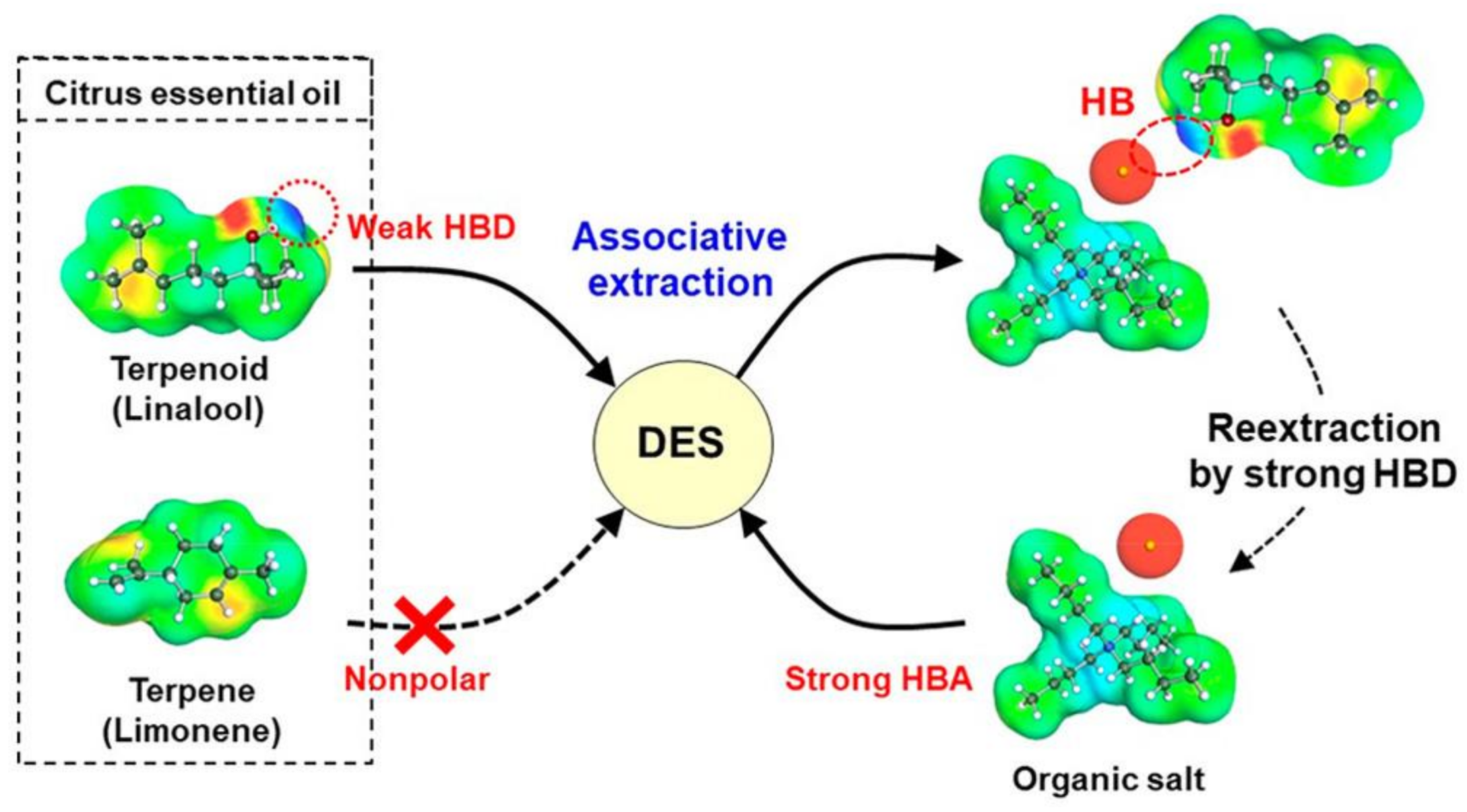
| Citrus essential oil | Linalool | TBAC:analyte (20:1; -) | Heating and stirring | GC | 99 (b) |
| [42] |
| Citrus essential oil | Linalool | ChCl:glycerol (1:2; -) | LLE | GC-(Q)-MS | - |
| [44] |
| Crude palm oil | Tocopherols and tocotrienols | ChCl:malonic acid (1:1; 30 g) | LLE | HPLC-PDA | 1.08·10−2 (a) |
| [46] |
| Fish scales | Hydroxyapatite | ChCl:glycerol (1:2; 4.5 g;-) | SLE | FTIR, XRD, EDS, ICP-OES, SEM, TG and particle size distribution analysis | 48 | - Other HBDs were evaluated: citric and acetic acids. | [50] |
| Olive oil | 2 phenolic alcohols, 2 flavonoids and 6 phenolic metabolites | ChCl:xylitol:water (2:1:3; 14 g) | LLE | HPLC-DAD | - | - DES was removed using a resin prior to analysis. | [40] |
| Olive oil | 4 phenolic acids and alcohols, 2 flavonoids, 6 phenolic metabolites and enolic acid | ChCl-based DESs (-; 14 g) | LLE | HPLC-DAD and HPLC-(Q-ToF)-MS | - |
| [25] |
| Powdered and lyophilized milk | 3 seleno amino acids | Lactic acid:glucose (5:1; 3.09 mL; 25% (v/v) water) | UAE | HPLC-ICP-MS | 90–109 (b) | - Citric acid: glucose and fructose:citric acid were evaluated. | [49] |
| Quail egg | Immunoglobulins | BTBAC: glycerol (1:2; 500 µL) | UA-LLME-preparative HPLC | SEC-UV | 85 (b) |
| [48] |
| Sesame oil | 3 lignans | ChCl:p-cresol (1:2; 400 µL) | UA-LLME | HPLC-UV | 97–120 (b) |
| [45] |
| Tea seed oil | 31 phenolic compounds identified, 25 phenolic compounds quantified | ChCl:glycerol (1:2; 6 g) | LLE | UHPLC-(QqQ)-MS/MS; UHPLC-Q-ToF-MS/MS | 8.4–2.7·10−5 (a) |
| [41] |
| Wheat flour | Folic acid (vitamin B9) | TOMAC:isoamyl alcohol (1:4; 150 µL) | SLE, VA-DLLME | HPLC-UV | 92–100 (b) |
| [47] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Socas-Rodríguez, B.; Torres-Cornejo, M.V.; Álvarez-Rivera, G.; Mendiola, J.A. Deep Eutectic Solvents for the Extraction of Bioactive Compounds from Natural Sources and Agricultural By-Products. Appl. Sci. 2021, 11, 4897. https://doi.org/10.3390/app11114897
Socas-Rodríguez B, Torres-Cornejo MV, Álvarez-Rivera G, Mendiola JA. Deep Eutectic Solvents for the Extraction of Bioactive Compounds from Natural Sources and Agricultural By-Products. Applied Sciences. 2021; 11(11):4897. https://doi.org/10.3390/app11114897
Chicago/Turabian StyleSocas-Rodríguez, Bárbara, Monica Vanessa Torres-Cornejo, Gerardo Álvarez-Rivera, and Jose A. Mendiola. 2021. "Deep Eutectic Solvents for the Extraction of Bioactive Compounds from Natural Sources and Agricultural By-Products" Applied Sciences 11, no. 11: 4897. https://doi.org/10.3390/app11114897
APA StyleSocas-Rodríguez, B., Torres-Cornejo, M. V., Álvarez-Rivera, G., & Mendiola, J. A. (2021). Deep Eutectic Solvents for the Extraction of Bioactive Compounds from Natural Sources and Agricultural By-Products. Applied Sciences, 11(11), 4897. https://doi.org/10.3390/app11114897








