Hydrogen Production from Offshore Wind Parks: Current Situation and Future Perspectives
Abstract
1. Introduction
2. System Components
2.1. Offshore Wind
2.2. Electrolyzer Technologies
2.2.1. Alkaline Electrolyzers
2.2.2. Proton Exchange Membrane Electrolyzers
2.2.3. Solid Oxide Electrolyzer
2.3. Hydrogen Storage
3. System Configurations
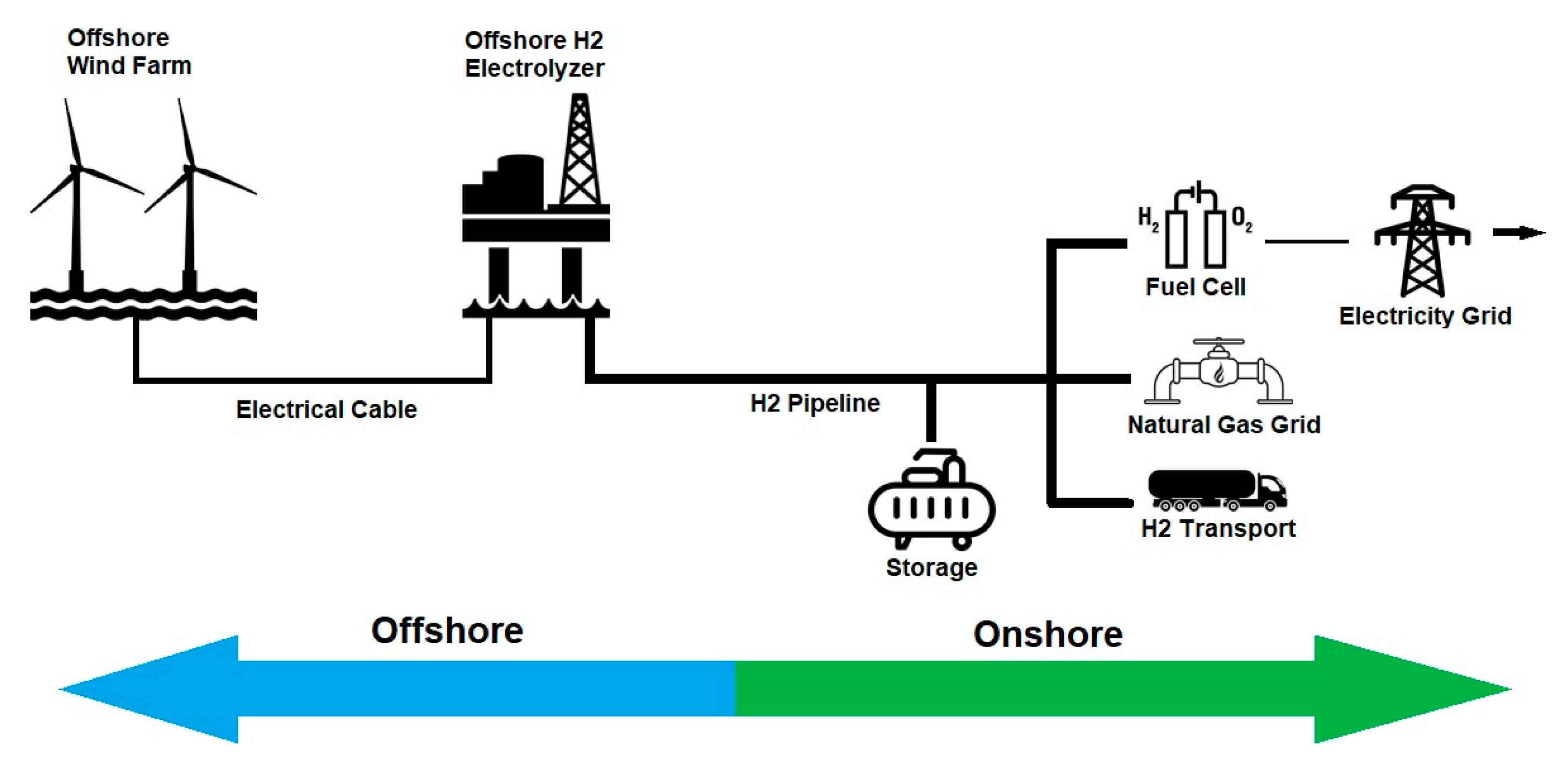
3.1. Offshore Electrolyzer Scenario
- PEMEL and the supporting electronics
- AC-DC rectifiers (possibly already included in electrolyzer)
- Desalination unit and reservoir for desalinated water
- Seawater pumps
- Export pipeline
- Backup power source
- Communication equipment
3.1.1. Centralized Electrolyzer
3.1.2. Individual Electrolyzers
3.2. Onshore Electrolyzer Scenario
4. Hydrogen Utilization
4.1. Generating Electricity
4.2. Power to Gas
4.3. Hydrogen as the End Product
5. State-of-the-Art Review
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| AEL | Alkaline Electrolyzer |
| AFC | Alkaline Fuel Cell |
| CHP | Combined Heat and Power |
| CSP | Concentrated Solar Power |
| DPB | Discounted Payback Period |
| FCR | Frequency Containment Reserve |
| HVAC | High Voltage Alternating Current |
| HVDC | High Voltage Direct Current |
| LCOE | Levelized Cost Of Energy |
| LCOH | Levelized Cost Of Hydrogen |
| LNG | Liquified Natural Gas |
| MCFC | Molten Carbonate Fuel Cell |
| NPV | Net Present Value |
| P2G | Power to Gas |
| P2M | Power to Mobility |
| P2P | Power to Power |
| PAFC | Phosphoric Acid Fuel Cell |
| PEMEL | Proton Exchange Membrane Electrolyzer |
| PEMFC | Proton Exchange Membrane Fuel Cell |
| PV | Photovoltaic |
| SDGs | United Nations Sustainable Development Goals |
| SMR | Steam Methane Reform |
| SOE | Solid Oxide Electrolyzer |
| SOFC | Solid Oxide Fuel Cell |
References
- IEA. Key World Energy Statistics; IEA: Paris, France, 2020; Available online: https://www.iea.org/reports/key-world-energy-statistics-2020 (accessed on 7 April 2021).
- Panfilov, M. 4—Underground and pipeline hydrogen storage. In Compendium of Hydrogen Energy, Woodhead Publishing Series in Energy; Gupta, R.B., Basile, A., Veziroglu, T.N., Eds.; Woodhead Publishing: Sawston, UK, 2016; pp. 91–115. [Google Scholar] [CrossRef]
- Miao, B.; Giordano, L.; Chan, S.H. Long-distance renewable hydrogen transmission via cables and pipelines. Int. J. Hydrogen Energy 2021, in press. [Google Scholar] [CrossRef]
- WindEurope. Offshore Wind in Europe: Key Trends and Statistics 2020. 2021. Available online: https://windeurope.org/intelligence-platform/product/offshore-wind-in-europe-key-trends-and-statistics-2020/ (accessed on 7 April 2021).
- The European Marine Observation and Data Network (EMODnet). Available online: https://portal.emodnet-bathymetry.eu/ (accessed on 7 April 2021).
- Offshore Renewable Energy (ORE) Catapult. Offshore Wind and Hydrogen: Solving the Integration Challenge. 2020. Available online: https://ore.catapult.org.uk/?orecatapultreports=offshore-wind-and-hydrogen-solving-the-integration-challenge (accessed on 7 April 2021).
- Airborne WindEurope. High-altitude Wind Energy Map Published. Available online: https://airbornewindeurope.org/resources/high-altitude-wind-energy-map-published-2/ (accessed on 7 April 2021).
- Equinor. Hywind Scotland. Available online: https://www.equinor.com/en/what-we-do/floating-wind/hywind-scotland.html (accessed on 7 April 2021).
- WindEurope. Offshore Wind in Europe: Key Trends and Statistics 2017. 2018. Available online: https://windeurope.org/intelligence-platform/product/the-european-offshore-wind-industry-key-trends-and-statistics-2017/ (accessed on 7 April 2021).
- ERM. Dolphyn Hydrogen Phase 1—Final Report. October 2019. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/866375/Phase_1_-_ERM_-_Dolphyn.pdf (accessed on 26 April 2021).
- IRENA. Hydrogen from Renewable Power: Technology Outlook for the Energy Transition. 2018. Available online: https://irena.org/publications/2018/Sep/Hydrogen-from-renewable-power (accessed on 7 April 2021).
- Buttler, A.; Spliethoff, H. Current status of water electrolysis for energy storage, grid balancing and sector coupling via power-to-gas and power-to-liquids: A review. Renew. Sustain. Energy Rev. 2018, 82, 2440–2454. [Google Scholar] [CrossRef]
- Guo, Y.; Li, G.; Zhou, J.; Liu, Y. Comparison between hydrogen production by alkaline water electrolysis and hydrogen production by PEM electrolysis. IOP Conf. Ser. Earth Environ. Sci. 2019, 371, 042022. [Google Scholar] [CrossRef]
- McPhy. Augmented McLyzer. Available online: https://mcphy.com/en/equipment-services/electrolyzers/augmented/ (accessed on 7 April 2021).
- Andersson, J.; Grönkvist, S. Large-scale storage of hydrogen. Int. J. Hydrogen Energy 2019, 44, 11901–11919. [Google Scholar] [CrossRef]
- Dagdougui, H.; Sacile, R.; Bersani, C.; Ouammi, A. Chapter 4—hydrogen storage and distribution: Implementation scenarios. In Hydrogen Infrastructure for Energy Applications; Dagdougui, H., Sacile, R., Bersani, C., Ouammi, A., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 37–52. [Google Scholar] [CrossRef]
- Hydrogenics. Large Scale PEM Electrolysis: Technology Status and Upscaling Strategies. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwjz7KHVme3vAhVECxoKHSUdCCkQFjACegQIAxAD&url=http%3A%2F%2Fhybalance.eu%2Fwp-content%2Fuploads%2F2019%2F10%2FLarge-scale-PEM-electrolysis.pdf&usg=AOvVaw1YjdSYyby88zmNHVsqeAsz (accessed on 7 April 2021).
- Siemens. Decarbonizing Energy with Green Hydrogen: Technology Available and Proven in Production Today. Available online: https://assets.new.siemens.com/siemens/assets/api/uuid:390d0f48-499e-4451-a3c2-faa30c5bafe7/version:1598442587/power-to-x-technical-paper-siemens-short.pdf (accessed on 29 May 2021).
- Kruck, O.; Crotogino, F.; Prelicz, R.; Rudolph, T. Overview on all known underground storage technologies for hydrogen. In Project HyUnder–Assessment of the Potential, the Actors and Relevant Business Cases for Large Scale and Seasonal Storage of Renewable Electricity by Hydrogen Underground Storage in Europe. Report D. 2013, Volume 3. Available online: http://hyunder.eu/wp-content/uploads/2016/01/D3.1_Overview-of-all-known-underground-storage-technologies.pdf (accessed on 26 April 2021).
- Gallardo, F.I.; Ferrario, A.M.; Lamagna, M.; Bocci, E.; Garcia, D.A.; Baeza-Jeria, T.E. A techno-economic analysis of solar hydrogen production by electrolysis in the north of Chile and the case of exportation from Atacama desert to Japan. Int. J. Hydrogen Energy 2021, 46, 13709–13728. [Google Scholar] [CrossRef]
- Negra, N.B.; Todorovic, J.; Ackermann, T. Loss evaluation of HVAC and HVDC transmission solutions for large offshore wind farms. Electr. Power Syst. Res. 2006, 76, 916–927. [Google Scholar] [CrossRef]
- Papadopoulos, A.; Rodrigues, S.; Kontos, E.; Todorcevic, T.; Bauer, P.; Pinto, R.T. Collection and transmission losses of offshore wind farms for optimization purposes. In Proceedings of the 2015 IEEE Energy Conversion Congress and Exposition (ECCE), Montreal, QC, Canada, 20–24 September 2015; pp. 6724–6732. [Google Scholar] [CrossRef]
- North Sea Energy. A Vision on Hydrogen Potential from the North Sea. 2019. Available online: https://north-sea-energy.eu/static/29bef9235ee0548a2425dea4356a2f1e/NSE3-D1.6-D1.7-D1.8-Offshore-Hydrogen-Roadmap-linked-to-national-hydrogen-grid.pdf (accessed on 7 April 2021).
- Dinh, V.N.; Leahy, P.; McKeogh, E.; Murphy, J.; Cummins, V. Development of a viability assessment model for hydrogen production from dedicated offshore wind farms. Int. J. Hydrogen Energy 2020, in press. [Google Scholar] [CrossRef]
- General Electric. Switch It Up: This Tech Helps Take the World’s Largest Offshore Wind Turbine to a New Level. 2018. Available online: https://www.ge.com/news/reports/switch-tech-helps-take-worlds-largest-offshore-wind-turbine-new-level (accessed on 7 April 2021).
- Alassi, A.; Bañales, S.; Ellabban, O.; Adam, G.; MacIver, C. HVDC transmission: Technology review, market trends and future outlook. Renew. Sustain. Energy Rev. 2019, 112, 530–554. [Google Scholar] [CrossRef]
- IRENA. Green Hydrogen Cost Reduction: Scaling Up Electrolyzers to Meet the 1.5 °C Climate Goal. 2020. Available online: https://www.irena.org/publications/2020/Dec/Green-hydrogen-cost-reduction (accessed on 7 April 2021).
- Toyota. 2021 Toyota Mirai. Available online: https://www.toyota.com/mirai/ (accessed on 29 May 2021).
- Wood, A.; He, H.; Joia, T.; Brown, C.C. Reversible solid oxide fuel cell development at versa power systems. ECS Trans. 2015, 66, 23. [Google Scholar] [CrossRef]
- US. Department of Energy. Comparison of Fuel Cell Technologies. 2016. Available online: https://www.energy.gov/eere/fuelcells/comparison-fuel-cell-technologies (accessed on 7 April 2021).
- Weidner, E.; Ortiz Cebolla, R.; Davies, J. Global Deployment of Large Capacity Stationary Fuel Cells–Drivers of, and Barriers to, Stationary Fuel Cell Deployment; JRC115923; EUR 29693 EN; Publications Office of the European Union: Luxembourg, 2019; ISBN 978-92-76-00841-5. [Google Scholar] [CrossRef]
- Doosan starts installation of hydrogen-fueled 50 MW fuel cell power plant in South Korea. Fuel Cells Bull. 2018, 2018, 1. [CrossRef]
- Fuel Cells Works. Daesan Hydrogen Fuel Cell Power Plant Completed with Help of Doosan Fuel Cells. Available online: https://fuelcellsworks.com/news/daesan-hydrogen-fuel-cell-power-plant-completed-with-help-of-doosan-fuel-cells/ (accessed on 26 April 2021).
- HyDeploy. Available online: https://hydeploy.co.uk/ (accessed on 7 April 2021).
- Melaina, M.W.; Antonia, O.; Penev, M. Blending hydrogen into natural gas pipeline networks: A review of key issues. Tech. Rep. 2013. [Google Scholar] [CrossRef]
- Quarton, C.J.; Samsatli, S. Power-to-gas for injection into the gas grid: What can we learn from real-life projects, economic assessments and systems modelling? Renew. Sustain. Energy Rev. 2018, 98, 302–316. [Google Scholar] [CrossRef]
- EDP. FLEXnCONFU: Power-to-X to Increase the Flexibility of Thermal Plants. Available online: https://www.edp.com/en/innovation/flexnconfu-power-to-increase-the-flexibility-of-thermal-plants (accessed on 26 April 2021).
- FLEXnCONFU. Available online: https://flexnconfu.eu/demonstration/ (accessed on 29 May 2021).
- Kakoulaki, G.; Kougias, I.; Taylor, N.; Dolci, F.; Moya, J.; Jager-Waldau, A. Green hydrogen in Europe—a regional assessment: Substituting existing production with electrolysis powered by renewables. Energy Convers. Manag. 2021, 228, 113649. [Google Scholar] [CrossRef]
- Gusain, D.; Cvetković, M.; Bentvelsen, R.; Palensky, P. Technical assessment of large-scale PEM electrolyzers as flexibility service providers. In Proceedings of the 2020 IEEE 29th International Symposium on Industrial Electronics (ISIE), Delft, The Netherlands, 24 September 2020; pp. 1074–1078. [Google Scholar] [CrossRef]
- CIGRE. Available online: https://www.cigre.org/ (accessed on 26 April 2021).
- Clúa, J.G.G.; Mantz, R.J.; de Battista, H. Optimal sizing of a grid-assisted wind-hydrogen system. Energy Convers. Manag. 2018, 166, 402–408. [Google Scholar] [CrossRef]
- Nguyen, T.; Abdin, Z.; Holm, T.; Me´rida, W. Grid-connected hydrogen production via large-scale water electrolysis. Energy Convers. Manag. 2019, 200, 112108. [Google Scholar] [CrossRef]
- Heuser, P.-M.; Ryberg, D.S.; Grube, T.; Robinius, M.; Stolten, D. Techno-economic analysis of a potential energy trading link between Patagonia and Japan based on CO2 free hydrogen. Int. J. Hydrogen Energy 2019, 44, 12733–12747. [Google Scholar] [CrossRef]
- McDonagh, S.; Ahmed, S.; Desmond, C.; Murphy, J.D. Hydrogen from offshore wind: Investor perspective on the profitability of a hybrid system including for curtailment. Appl. Energy 2020, 265, 114732. [Google Scholar] [CrossRef]
- Xiao, P.; Hu, W.; Xu, X.; Liu, W.; Huang, Q.; Chen, Z. Optimal operation of a wind-electrolytic hydrogen storage system in the electricity/hydrogen markets. Int. J. Hydrogen Energy 2020, 45, 24412–24423. [Google Scholar] [CrossRef]
- Hou, P.; Enevoldsen, P.; Eichman, J.; Hu, W.; Jacobson, M.Z.; Chen, Z. Optimizing investments in coupled offshore wind -electrolytic hydrogen storage systems in Denmark. J. Power Sources 2017, 359, 186–197. Available online: http://dx.doi.org/10.1016/j.jpowsour.2017.05.048 (accessed on 26 April 2021). [CrossRef]
- Schnuelle, C.; Wassermann, T.; Fuhrlaender, D.; Zondervan, E. Dynamic hydrogen production from PV & wind direct electricity supply—modeling and techno-economic assessment. Int. J. Hydrogen Energy 2020, 45, 29938–29952. [Google Scholar] [CrossRef]
- Touili, S.; Merrouni, A.A.; El Hassouani, Y.; Amrani, A.i.; Rachidi, S. Analysis of the yield and production cost of large-scale electrolytic hydrogen from different solar technologies and under several Moroccan climate zones. Int. J. Hydrogen Energy 2020, 45, 26785–26799. [Google Scholar] [CrossRef]
- Loisel, R.; Baranger, L.; Chemouri, N.; Spinu, S.; Pardo, S. Economic evaluation of hybrid off-shore wind power and hydrogen storage system. Int. J. Hydrogen Energy 2015, 40, 6727–6739. [Google Scholar] [CrossRef]
- Guaitolini, S.V.M.; Yahyaoui, I.; Fardin, J.F.; Encarnac¸ao, L.F.; Tadeo, F. A review of fuel cell and energy cogeneration technologies. In Proceedings of the 2018 9th International Renewable Energy Congress (IREC), Hammamet, Tunisia, 20–22 March 2018; pp. 1–6. [Google Scholar] [CrossRef]
- Alshehri, F.; Torres, J.R.; Perilla, A.; Tuinema, B.; van der Meijden, M.; Palensky, P.; Gonzalez-Longatt, F. Generic model of PEM fuel cells and performance analysis in frequency containment period in systems with decreased inertia. In Proceedings of the 2019 IEEE 28th International Symposium on Industrial Electronics (ISIE), Vancouver, BC, Canada, 12–14 June 2019; pp. 1810–1815. [Google Scholar] [CrossRef]
- Bernstein, P.A.; Heuer, M.; Wenske, M. Fuel cell system as a part of the smart grid. In Proceedings of the 2013 IEEE Grenoble Conference, Grenoble, France, 16–20 June 2013; pp. 1–4. [Google Scholar] [CrossRef]
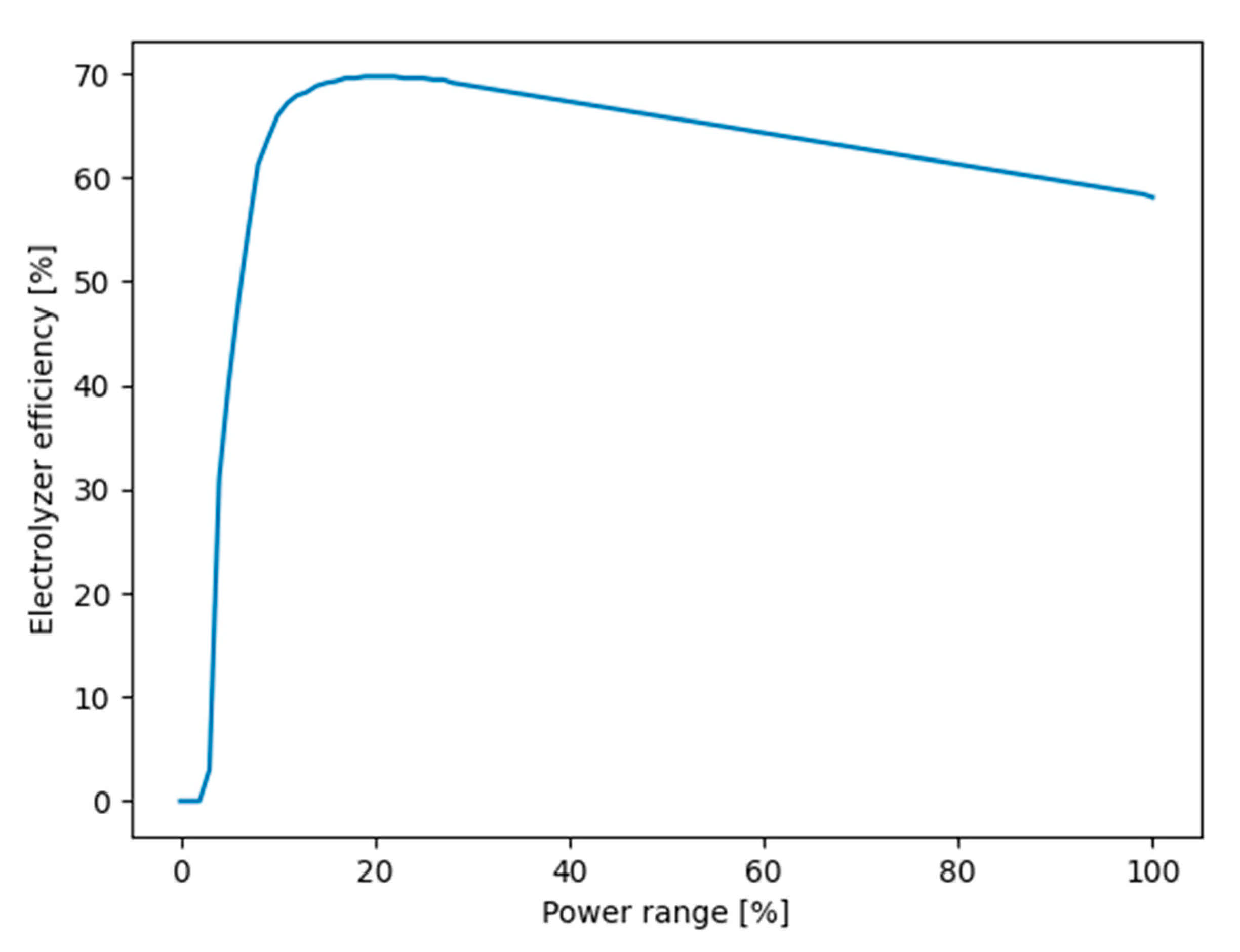

| Power (MW) | Flow Rate (Nm3/h) | Hydrogen Mass (ton/day) | Hydrogen Volume (m3/day) |
|---|---|---|---|
| 25 | 5000 | 10.8 | 1386 |
| 100 | 20,000 | 43.2 | 5544 |
| 500 | 100,000 | 216 | 27,720 |
| 1000 | 200,000 | 432 | 55,440 |
| Power (MW) | Hydrogen Volume (m3/day) | Cylinder Radius (m) | Cylinder Height (m) |
|---|---|---|---|
| 25 | 1386 | 9 | 6 |
| 100 | 5544 | 13 | 11 |
| 500 | 27,720 | 25 | 15 |
| 1000 | 55,440 | 30 | 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calado, G.; Castro, R. Hydrogen Production from Offshore Wind Parks: Current Situation and Future Perspectives. Appl. Sci. 2021, 11, 5561. https://doi.org/10.3390/app11125561
Calado G, Castro R. Hydrogen Production from Offshore Wind Parks: Current Situation and Future Perspectives. Applied Sciences. 2021; 11(12):5561. https://doi.org/10.3390/app11125561
Chicago/Turabian StyleCalado, Gonçalo, and Rui Castro. 2021. "Hydrogen Production from Offshore Wind Parks: Current Situation and Future Perspectives" Applied Sciences 11, no. 12: 5561. https://doi.org/10.3390/app11125561
APA StyleCalado, G., & Castro, R. (2021). Hydrogen Production from Offshore Wind Parks: Current Situation and Future Perspectives. Applied Sciences, 11(12), 5561. https://doi.org/10.3390/app11125561







