Assessment of Quality in Osteoporotic Human Trabecular Bone and Its Relationship to Mechanical Properties
Abstract
1. Introduction
2. Materials and Methods
- One bone sample from a 25 years old male donor, used as a reference for non-osteoporotic bone;
- Three bone samples from 45, 52, and 68 years-old female donors used for test of osteoporotic condition;
- Three bone samples from 50, 57, and 64 years-old male donors, also used for the examination of osteoporosis.
2.1. Machining Process
2.2. Bone Densitometry
2.3. Assessment of Bone Micro-Architecture
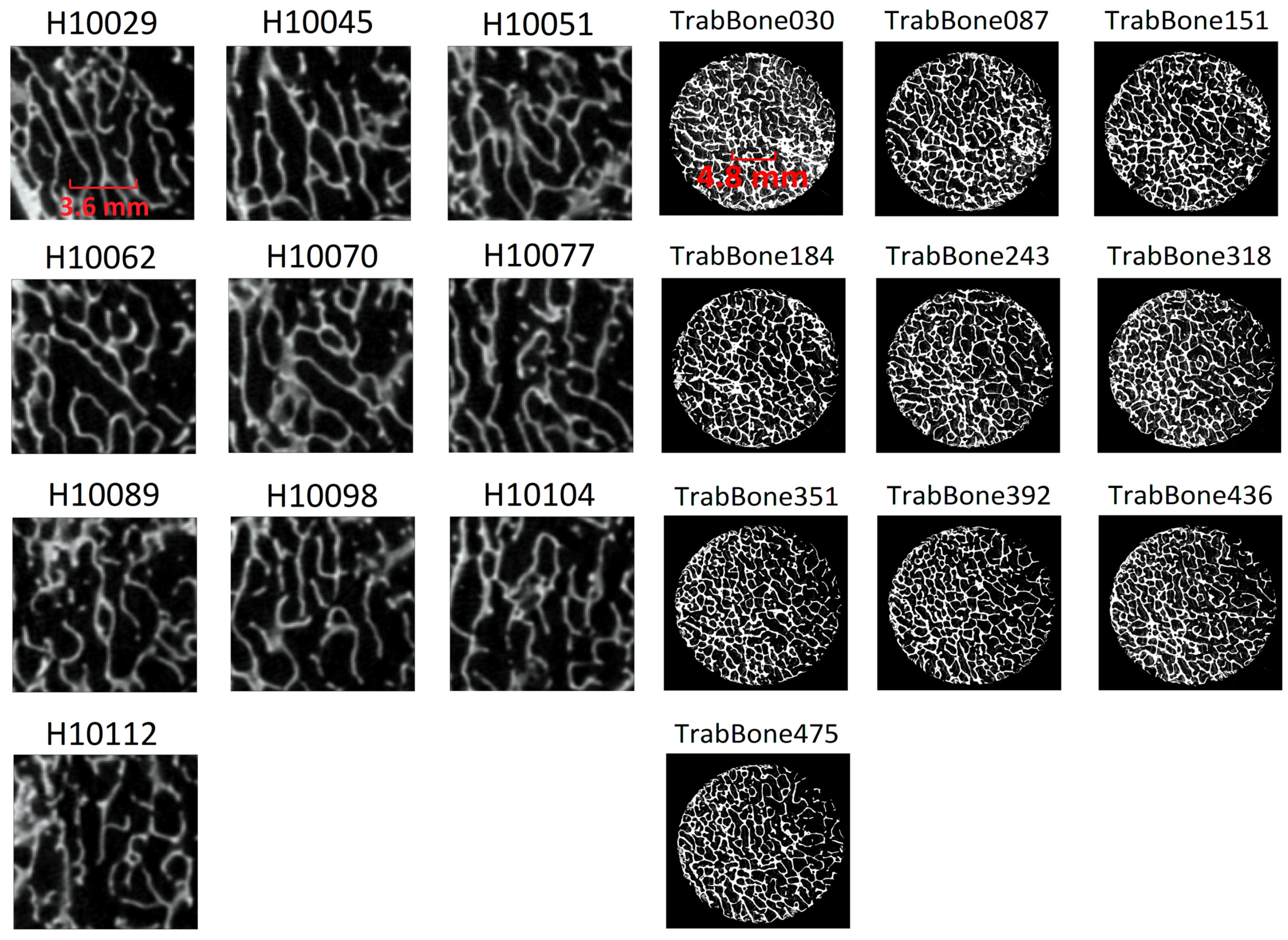
2.3.1. Micro-CT Analysis and 3D Estimation of Porosity
- The device at IF-UNAM [28] is an Oxford Instruments Apogee XTG5011 tungsten anode X-ray tube with a nominal focal spot size of 35 µm, coupled to a Rad-icon Shad-o-Box 2048 flat panel detector (Teledyne DALSA Incorporated, Waterloo, ON, Canada). The collection of the projection image data were at 50 kVp, 1 mA with an integration time of 500 ms per frame and 360 orbit in 1 steps.
- TORATOM has a specific geometry movable to attain magnifications between 1.2 and 150 and comprises of two X-ray tube-detectors with accelerating voltage between 20 and 240 kV, maximum power of 260 W, and minimum spot size between 0.8 and 5 µm. TORATOM included as well one Dexela 1512CL X-ray detector (XWT-160-TCHR, X-ray WorX, Garbsen, Germany), with a sensitive area of 145.4 × 114.9 mm, and resolution of 1944 × 1536 mm pixels and pixel size of 74.8 µm; and a flat panel detector XRD 1622 AP 14 (Perkin Elmer, Waltham, MA, USA) with an active area of 409.6 × 409.6 mm, resolution of 2048 × 2048 pixels and pixel size of 200 µm.
2.3.2. 2D Imaging Estimation of Bone Structure
2.3.3. Comparing Porosity Values
2.4. Mechanical Testing
- Ultimate stress (), which is the maximum stress attained in the stress–strain diagram;
- Yield stress (), which is the amount of stress required towards the permanent deformation;
- Young’s modulus (E), which is the relationship between stress (force per unit area) and strain (proportional deformation) within the linear region in the uniaxial deformation.
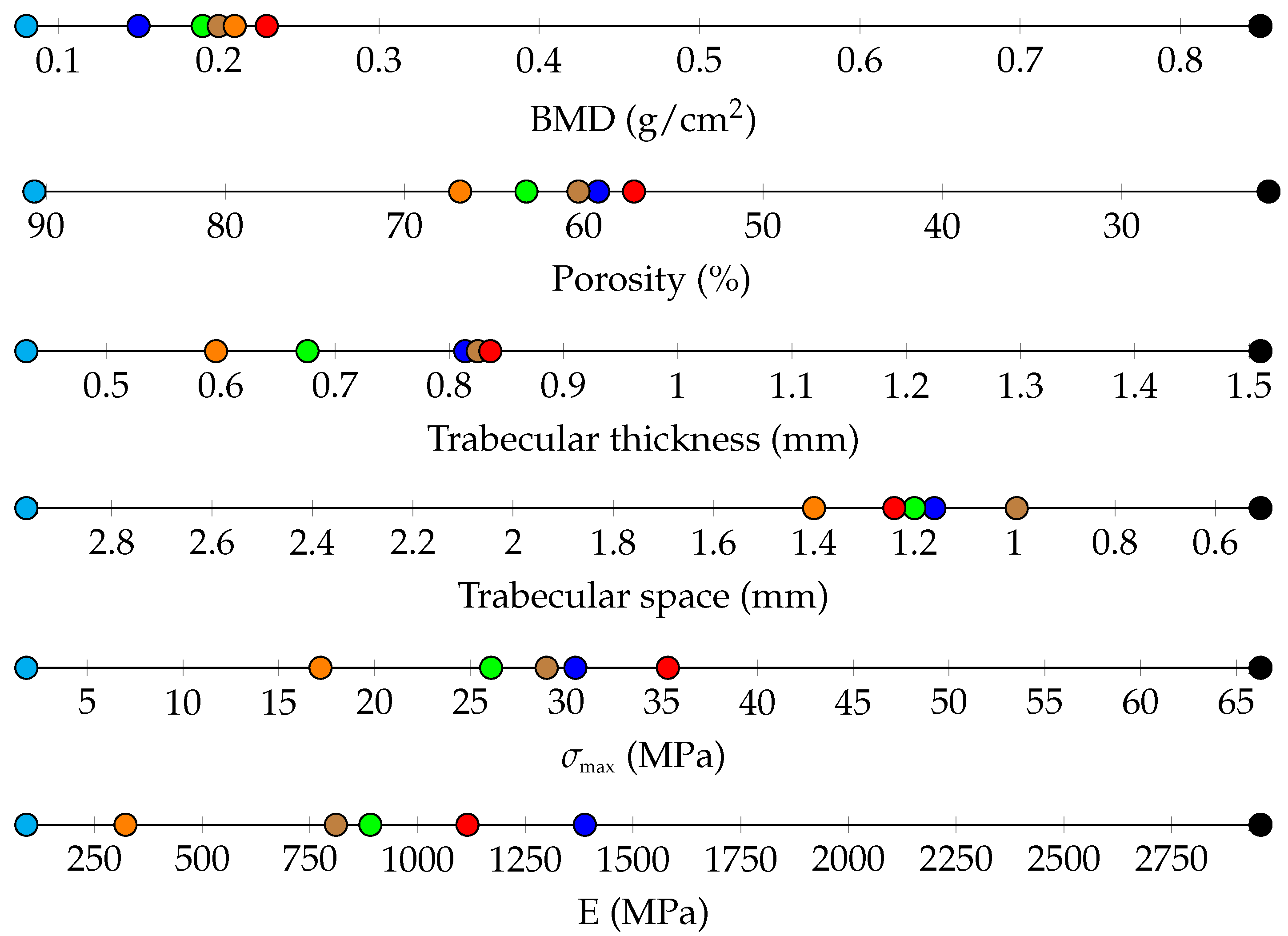
3. Results
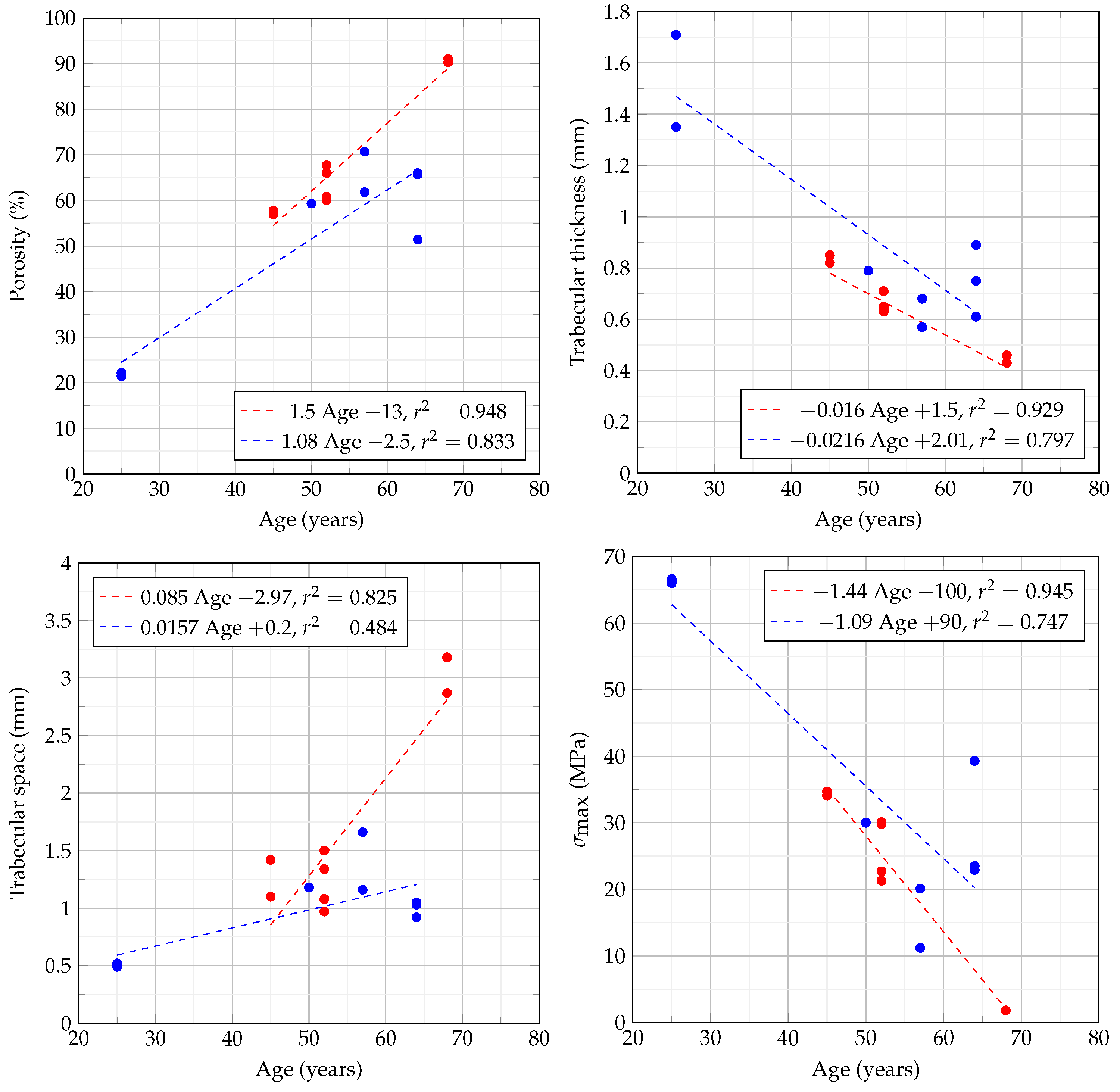
3.1. Porosity Assessment through Micro-CT
3.2. Comparison of Estimated Porosity against Gold-References
3.3. Percentage of Porosity in 2D
3.4. Bone Densitometry
3.5. Trabecular Thickness and Space
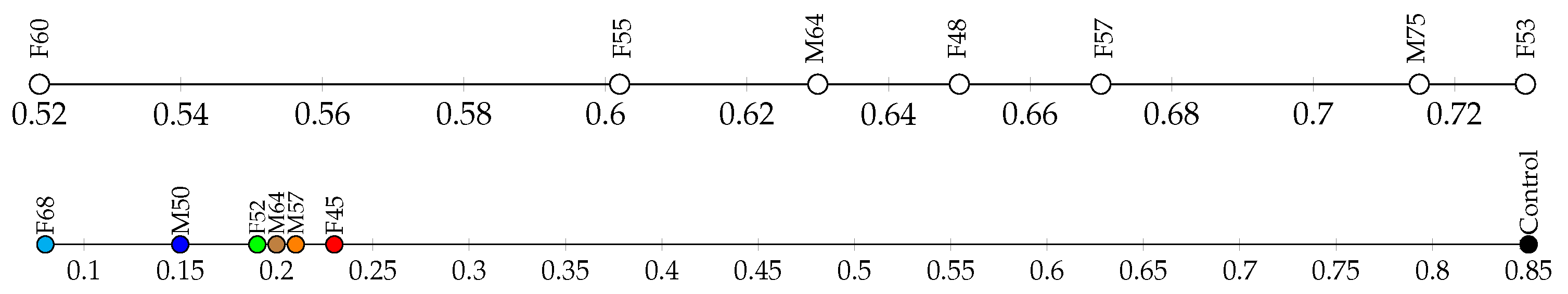
3.6. Compression Tests
4. Discussion
4.1. Bone Densitometry
4.2. Trabecular Thickness and Space
4.3. Comparing Clinical Versus Trabecular BMD
4.4. Comparison of Quality and Mechanical Tests
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cowin, S.C. Bone Mechanics Handbook; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Acevedo, C.; Stadelmann, V.A.; Pioletti, D.P.; Alliston, T.; Ritchie, R.O. Fatigue as the missing link between bone fragility and fracture. Nat. Biomed. Eng. 2018, 2, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.; Gluer, C.C. An update on the diagnosis and assessment of osteoporosis with densitometry. Osteoporos. Int. 2000, 11, 192–202. [Google Scholar] [CrossRef]
- Schmidt, F.N.; Zimmermann, E.A.; Walsh, F.; Plumeyer, C.; Schaible, E.; Fiedler, I.A.; Milovanovic, P.; Rößle, M.; Amling, M.; Blanchet, C.; et al. On the origins of fracture toughness in advanced teleosts: How the swordfish sword’s bone structure and composition allow for slashing under water to kill or stun prey. Adv. Sci. 2019, 6, 1900287. [Google Scholar] [CrossRef] [PubMed]
- Van Der Linden, J.C.; Verhaar, J.; Weinans, H. A three-dimensional simulation of age-related remodeling in trabecular bone. J. Bone Min. Res. 2001, 16, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Legrand, E.; Chappard, D.; Pascaretti, C.; Duquenne, M.; Krebs, S.; Rohmer, V.; Basle, M.F.; Audran, M. Trabecular bone microarchitecture, bone mineral density, and vertebral fractures in male osteoporosis. J. Bone Min. Res. 2000, 15, 13–19. [Google Scholar] [CrossRef]
- Montemurro, N.; Perrini, P.; Mangini, V.; Galli, M.; Papini, A. The Y-shaped trabecular bone structure in the odontoid process of the axis: A CT scan study in 54 healthy subjects and biomechanical considerations. J. Neurosurg. Spine 2019, 30, 585–592. [Google Scholar] [CrossRef]
- Genant, H.K.; Cooper, C.; Poor, G.; Reid, I.; Ehrlich, G.; Kanis, J.; Nordin, B.C.; Barrett-Connor, E.; Black, D.; Bonjour, J.; et al. Interim report and recommendations of the World Health Organization task-force for osteoporosis. Osteoporos. Int. 1999, 10, 259. [Google Scholar] [CrossRef]
- Blake, G.M.; Fogelman, I. The role of DXA bone density scans in the diagnosis and treatment of osteoporosis. Postgrad. Med. J. 2007, 83, 509–517. [Google Scholar] [CrossRef]
- Kanis, J.A. Diagnosis of osteoporosis and assessment of fracture risk. Lancet 2002, 359, 1929–1936. [Google Scholar] [CrossRef]
- Schott, A.; Cormier, C.; Hans, D.; Favier, F.; Hausherr, E.; Dargent-Molina, P.; Delmas, P.; Ribot, C.; Sebert, J.; Breart, G.; et al. How hip and whole-body bone mineral density predict hip fracture in elderly women: The EPIDOS Prospective Study. Osteoporos. Int. 1998, 8, 247–254. [Google Scholar] [CrossRef]
- Leali, P.T.; Muresu, F.; Melis, A.; Ruggiu, A.; Zachos, A.; Doria, C. Skeletal fragility definition. Clin. Cases Min. Bone Metab. 2011, 8, 11. [Google Scholar]
- Sornay-Rendu, E.; Munoz, F.; Garnero, P.; Duboeuf, F.; Delmas, P.D. Identification of osteopenic women at high risk of fracture: The OFELY study. J. Bone Min. Res. 2005, 20, 1813–1819. [Google Scholar] [CrossRef]
- Liu, Z.; Piao, J.; Pang, L.; Qing, X.; Nan, S.; Guo, Y.; Wang, X.; Li, F.; Liu, J.; Cheng, X.; et al. The diagnostic criteria for primary osteoporosis and the incidence of osteoporosis in China. J. Bone Min. Metab. 2002, 20, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.; Johnell, O.; Oden, A.; De Laet, C.; Mellstrom, D. Diagnosis of osteoporosis and fracture threshold in men. Calcif. Tissue Int. 2001, 69, 218–221. [Google Scholar] [CrossRef]
- Kazakia, G.J.; Burghardt, A.J.; Link, T.M.; Majumdar, S. Variations in morphological and biomechanical indices at the distal radius in subjects with identical BMD. J. Biomech. 2011, 44, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Bouxsein, M.L. Mechanisms of osteoporosis therapy: A bone strength perspective. Clin. Cornerstone 2003, 5, S13–S21. [Google Scholar] [CrossRef]
- Ammann, P.; Rizzoli, R. Bone strength and its determinants. Osteoporos. Int. 2003, 14, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Qiu, Y.; Yeung, H.Y.; Lee, K.M.; Cheng, C.y.J. Trabecular bone micro-architecture and bone mineral density in adolescent idiopathic and congenital scoliosis. Orthop. Surg. 2009, 1, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Ginini, J.G.; Maor, G.; Emodi, O.; Shilo, D.; Gabet, Y.; Aizenbud, D.; Rachmiel, A. Effects of extracorporeal shock wave therapy on distraction osteogenesis in rat mandible. Plast. Reconstr. Surg. 2018, 142, 1501–1509. [Google Scholar] [CrossRef]
- Ostertag, A.; Papadakis, G.E.; Collet, C.; Trabado, S.; Maione, L.; Pitteloud, N.; Bouligand, J.; De Vernejoul, M.C.; Cohen-Solal, M.; Young, J. Compromised Volumetric Bone Density and Microarchitecture in men with Congenital Hypogonadotropic Hypogonadism. J. Clin. Endocrinol. Metab. 2021. [Google Scholar] [CrossRef]
- Brennan, T.A.; Egan, K.P.; Lindborg, C.M.; Chen, Q.; Sweetwyne, M.T.; Hankenson, K.D.; Xie, S.X.; Johnson, F.B.; Pignolo, R.J. Mouse models of telomere dysfunction phenocopy skeletal changes found in human age-related osteoporosis. Dis. Model. Mech. 2014, 7, 583–592. [Google Scholar] [CrossRef]
- Thomas, C.D.L.; Feik, S.A.; Clement, J.G. Increase in pore area, and not pore density, is the main determinant in the development of porosity in human cortical bone. J. Anat. 2006, 209, 219–230. [Google Scholar] [CrossRef]
- Ahmed, L.A.; Shigdel, R.; Joakimsen, R.M.; Eldevik, O.P.; Eriksen, E.F.; Ghasem-Zadeh, A.; Bala, Y.; Zebaze, R.; Seeman, E.; Bjørnerem, Å. Measurement of cortical porosity of the proximal femur improves identification of women with nonvertebral fragility fractures. Osteoporos. Int. 2015, 26, 2137–2146. [Google Scholar] [CrossRef]
- Krause, M.; Hubert, J.; Deymann, S.; Hapfelmeier, A.; Wulff, B.; Petersik, A.; Püschel, K.; Amling, M.; Hawellek, T.; Frosch, K.H. Bone microarchitecture of the tibial plateau in skeletal health and osteoporosis. Knee 2018, 25, 559–567. [Google Scholar] [CrossRef]
- Bala, Y.; Zebaze, R.; Seeman, E. Role of cortical bone in bone fragility. Curr. Opin. Rheumatol. 2015, 27, 406–413. [Google Scholar] [CrossRef]
- Siris, E.S.; Miller, P.D.; Barrett-Connor, E.; Faulkner, K.G.; Wehren, L.E.; Abbott, T.A.; Berger, M.L.; Santora, A.C.; Sherwood, L.M. Identification and fracture outcomes of undiagnosed low bone mineral density in postmenopausal women: Results from the National Osteoporosis Risk Assessment. JAMA 2001, 286, 2815–2822. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Dávalos, A. An Overview of the Development and Evaluation of a Benchtop microCT Scanner. AIP Conf. Proc. 2010, 1310, 7–11. [Google Scholar]
- Doktor, T.; Kumpová, I.; Wroński, S.; Śniechowski, M.; Tarasiuk, J.; Forte, G.; Kytýř, D. Influence of printing and loading direction on mechanical response in 3d printed models of human trabecular bone. Acta Polytech. CTU Proc. 2018, 18, 24–27. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef] [PubMed]
- Brun, F.; Mancini, L.; Kasae, P.; Favretto, S.; Dreossi, D.; Tromba, G. Pore3D: A software library for quantitative analysis of porous media. Nucl. Instrum. Meth. A 2010, 615, 326–332. [Google Scholar] [CrossRef]
- Hildebrand, T.; Ruegsegger, P. A new method for the model-independent assessment of thickness in three-dimensional images. J. Microsc. 1997, 185, 67–75. [Google Scholar] [CrossRef]
- Frost, H.M. Wolff’s Law and bone’s structural adaptations to mechanical usage: An overview for clinicians. Angle Orthod. 1994, 64, 175–188. [Google Scholar] [PubMed]
- Martin, R.B. Porosity and specific surface of bone. Crit. Rev. Biomed. Eng. 1984, 10, 179–222. [Google Scholar] [PubMed]
- Berli, M.; Borau, C.; Decco, O.; Adams, G.; Cook, R.B.; Garcia Aznar, J.M.; Zioupos, P. Localized tissue mineralization regulated by bone remodelling: A computational approach. PLoS ONE 2017, 12, e0173228. [Google Scholar] [CrossRef]
- Lerebours, C.; Thomas, C.; Clement, J.; Buenzli, P.; Pivonka, P. The relationship between porosity and specific surface in human cortical bone is subject specific. Bone 2015, 72, 109–117. [Google Scholar] [CrossRef]
- Pivonka, P.; Buenzli, P.R.; Scheiner, S.; Hellmich, C.; Dunstan, C.R. The influence of bone surface availability in bone remodelling—A mathematical model including coupled geometrical and biomechanical regulations of bone cells. Eng. Struct. 2013, 47, 134–147. [Google Scholar] [CrossRef]
- Rouhi, G.; Epstein, M.; Sudak, L.; Herzog, W. Free surface density and microdamage in the bone remodeling equation: Theoretical considerations. Int. J. Eng. Sci. 2006, 44, 456–469. [Google Scholar] [CrossRef]
- Ciarallo, A.; Barralet, J.; Tanzer, M.; Kremer, R. An approach to compare the quality of cancellous bone from the femoral necks of healthy and osteoporotic patients through compression testing and microcomputed tomography imaging. McGill J. Med. MJM 2006, 9, 102. [Google Scholar]
- Le Corroller, T.; Pithioux, M.; Chaari, F.; Rosa, B.; Parratte, S.; Maurel, B.; Argenson, J.N.; Champsaur, P.; Chabrand, P. Bone texture analysis is correlated with three-dimensional microarchitecture and mechanical properties of trabecular bone in osteoporotic femurs. J. Bone Miner. Metab. 2013, 31, 82–88. [Google Scholar] [CrossRef]
- Souzanchi, M.F.; Palacio-Mancheno, P.; Borisov, Y.A.; Cardoso, L.; Cowin, S.C. Microarchitecture and bone quality in the human calcaneus: Local variations of fabric anisotropy. J. Bone Miner. Res. 2012, 27, 2562–2572. [Google Scholar] [CrossRef] [PubMed]
- Presbítero, G.; Hernandez-Rodríguez, M.A.; Contreras-Hernandez, G.R.; Vilchez, J.F.; Susarrey, O.; Gutiérrez, D. Microdamage distribution in fatigue fractures of bone allografts following gamma-ray exposure. Acta Bioeng. Biomech. 2017, 19, 43–53. [Google Scholar]
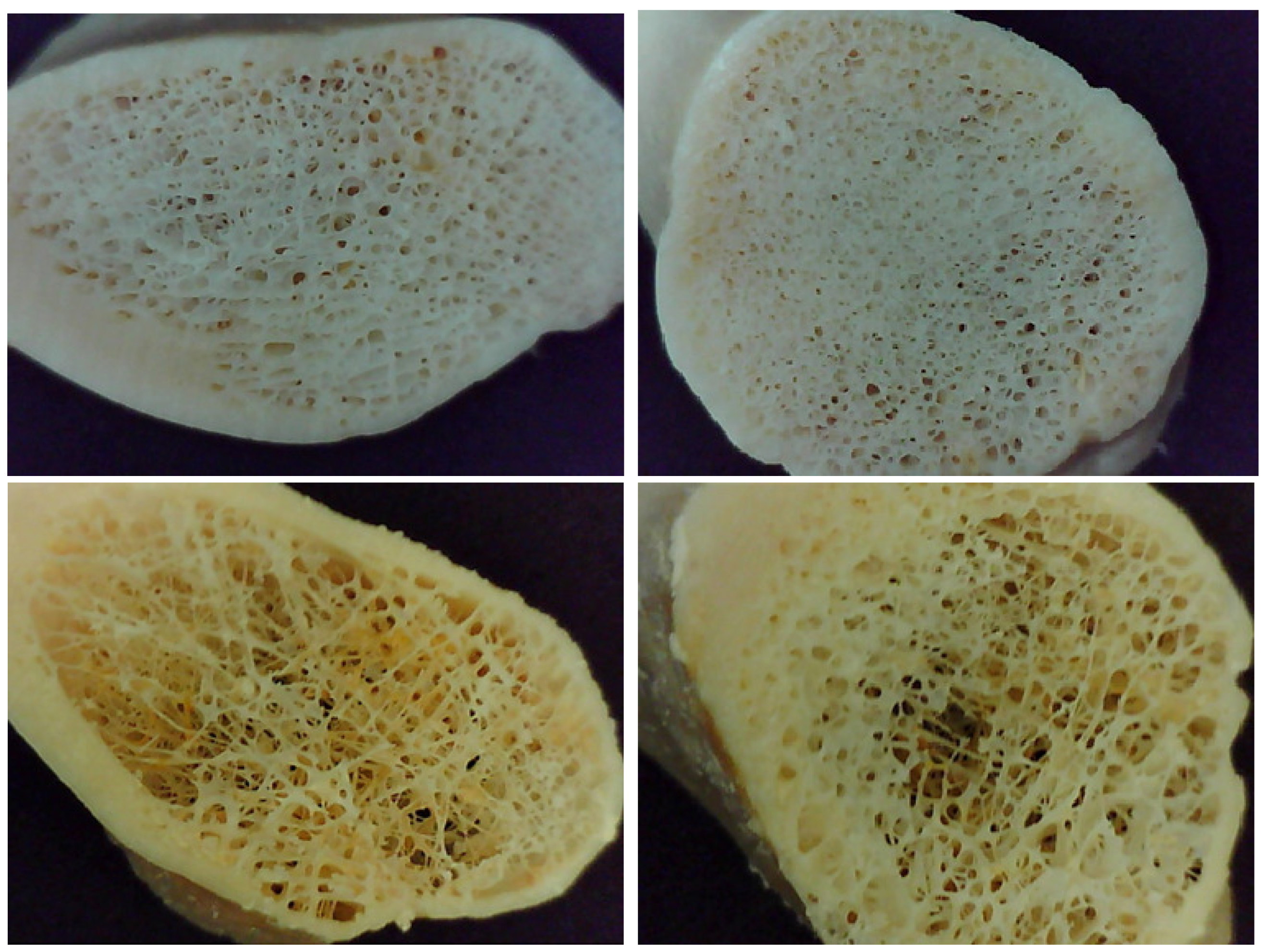
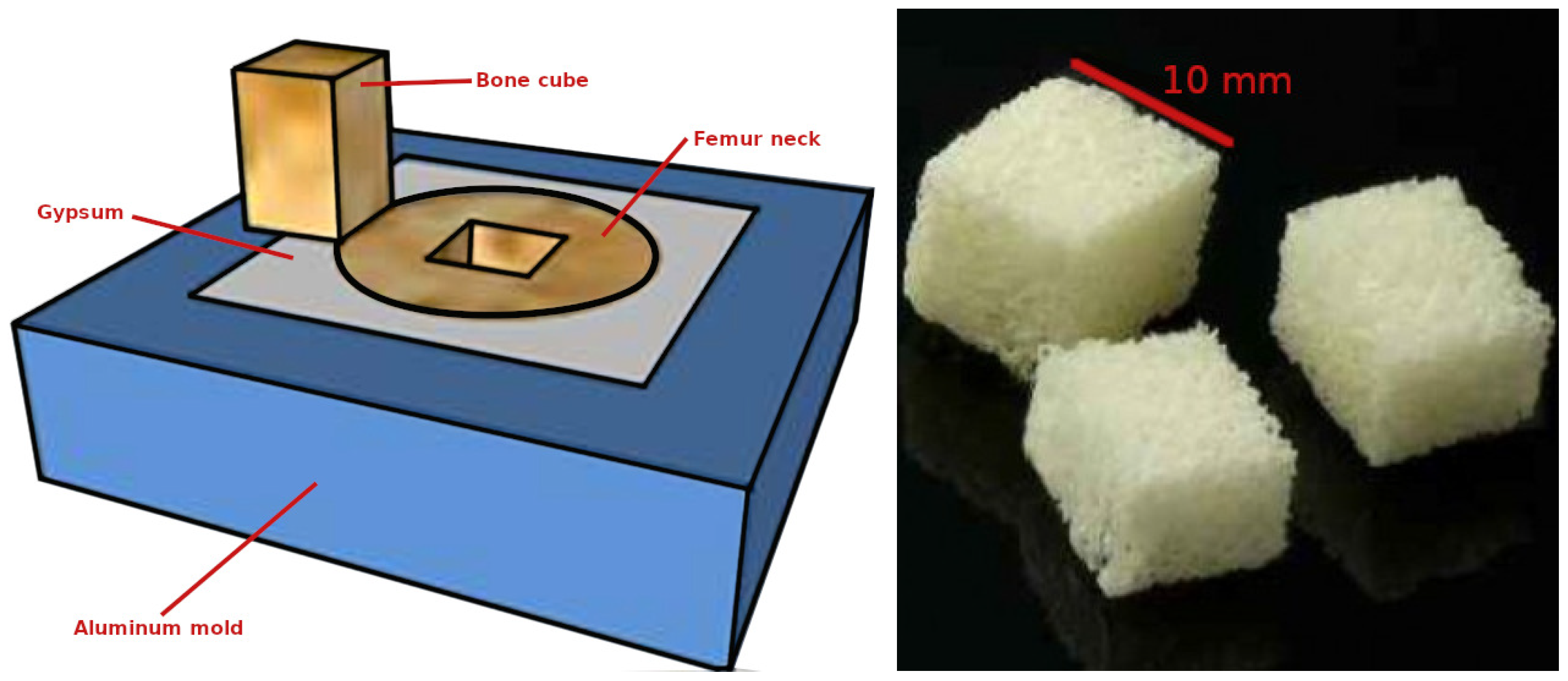





| Donor | Gender | Age (Years) | Sample Cube(s) | Porosity (%) |
|---|---|---|---|---|
| Control | Male | 25 | # 1 # 2 | n/a n/a |
| 1 | Female | 45 | # 1 # 2 | n/a n/a |
| 2 | Male | 50 | # 1 | n/a |
| 3 | Female | 52 | # 1 (left) # 2 (left) # 1 (right) # 2 (right) | 71.6 n/a n/a n/a |
| 4 | Male | 57 | # 1 (left) # 1 (right) | 64.4 75.8 |
| 5 | Male | 64 | # 1 (left) # 2 (left) #1 (right) | 70.1 n/a n/a |
| 6 | Female | 68 | # 1 # 2 | n/a n/a |
| Sample | (MPa) | (MPa) | E (MPa) |
|---|---|---|---|
| M25 | 66.6 | 57.9 | 2994.9 |
| 66 | 48.1 | 2918.5 | |
| F45 | 34.7 | 24.2 | 1764.3 |
| 34.1 | 26.5 | 557.1 | |
| M50 | 30 | 21.6 | 1391.4 |
| F52 | 29.8 | 23.3 | 1052.2 |
| 22.7 | 21.4 | 282.7 | |
| 21.3 | 16.5 | 865.1 | |
| 30.2 | 27.1 | 1444 | |
| M57 | 11.2 | 9.6 | 174.4 |
| 20.1 | 15.6 | 541 | |
| M64 | 23.5 | 20.1 | 460.7 |
| 22.9 | 19.5 | 762.7 | |
| 39.3 | 27.4 | 1429.6 | |
| F68 | 1.8 | 1.4 | 127.6 |
| 1.8 | 1.4 | 56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Presbítero, G.; Gutiérrez, D.; Lemus-Martínez, W.R.; Vilchez, J.F.; García, P.; Arizmendi-Morquecho, A. Assessment of Quality in Osteoporotic Human Trabecular Bone and Its Relationship to Mechanical Properties. Appl. Sci. 2021, 11, 5479. https://doi.org/10.3390/app11125479
Presbítero G, Gutiérrez D, Lemus-Martínez WR, Vilchez JF, García P, Arizmendi-Morquecho A. Assessment of Quality in Osteoporotic Human Trabecular Bone and Its Relationship to Mechanical Properties. Applied Sciences. 2021; 11(12):5479. https://doi.org/10.3390/app11125479
Chicago/Turabian StylePresbítero, Gerardo, David Gutiérrez, Wendy Ruth Lemus-Martínez, José Félix Vilchez, Pedro García, and Ana Arizmendi-Morquecho. 2021. "Assessment of Quality in Osteoporotic Human Trabecular Bone and Its Relationship to Mechanical Properties" Applied Sciences 11, no. 12: 5479. https://doi.org/10.3390/app11125479
APA StylePresbítero, G., Gutiérrez, D., Lemus-Martínez, W. R., Vilchez, J. F., García, P., & Arizmendi-Morquecho, A. (2021). Assessment of Quality in Osteoporotic Human Trabecular Bone and Its Relationship to Mechanical Properties. Applied Sciences, 11(12), 5479. https://doi.org/10.3390/app11125479







