CoNiZn and CoNiFe Nanoparticles: Synthesis, Physical Characterization, and In Vitro Cytotoxicity Evaluations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of NPs
2.3. Characterization
2.4. Cell Culture and Exposure of NPs
2.5. Cytotoxicity Evaluation and Morphological Observation
2.6. Lactate Dehydrogenase Leakage Assay
2.7. Statistical Analysis
3. Results and Discussion
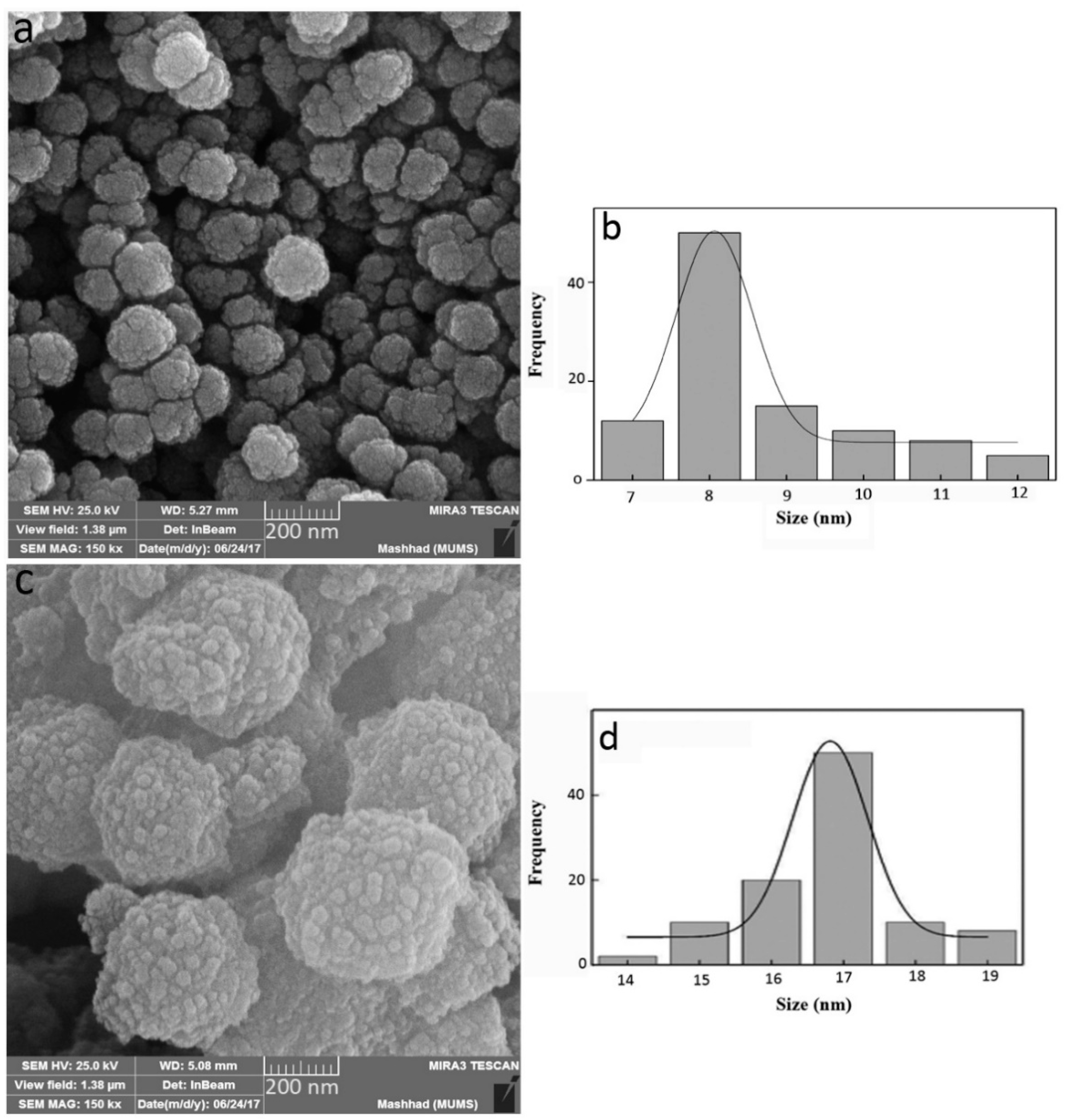
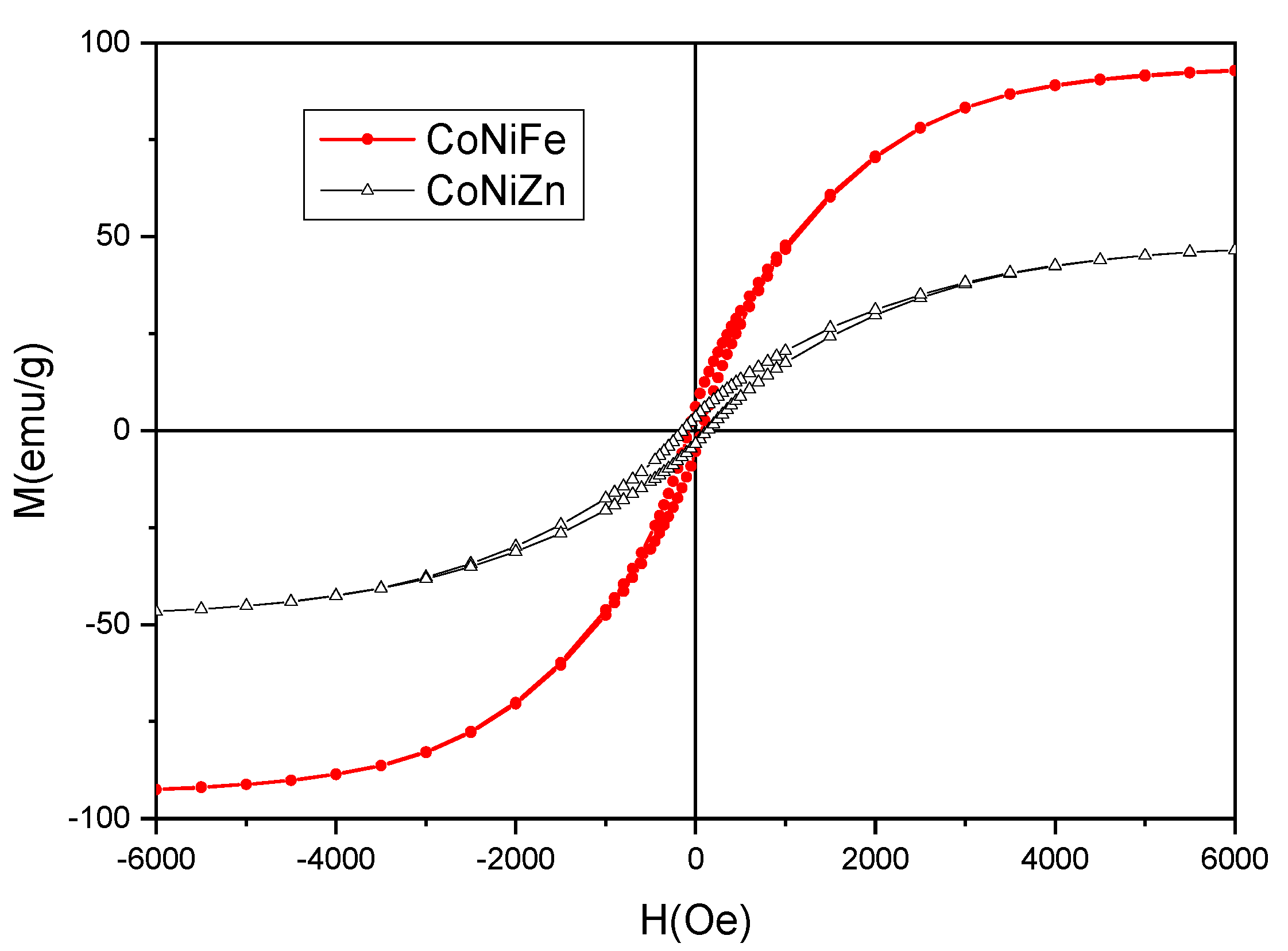
3.1. Characterization of NPs
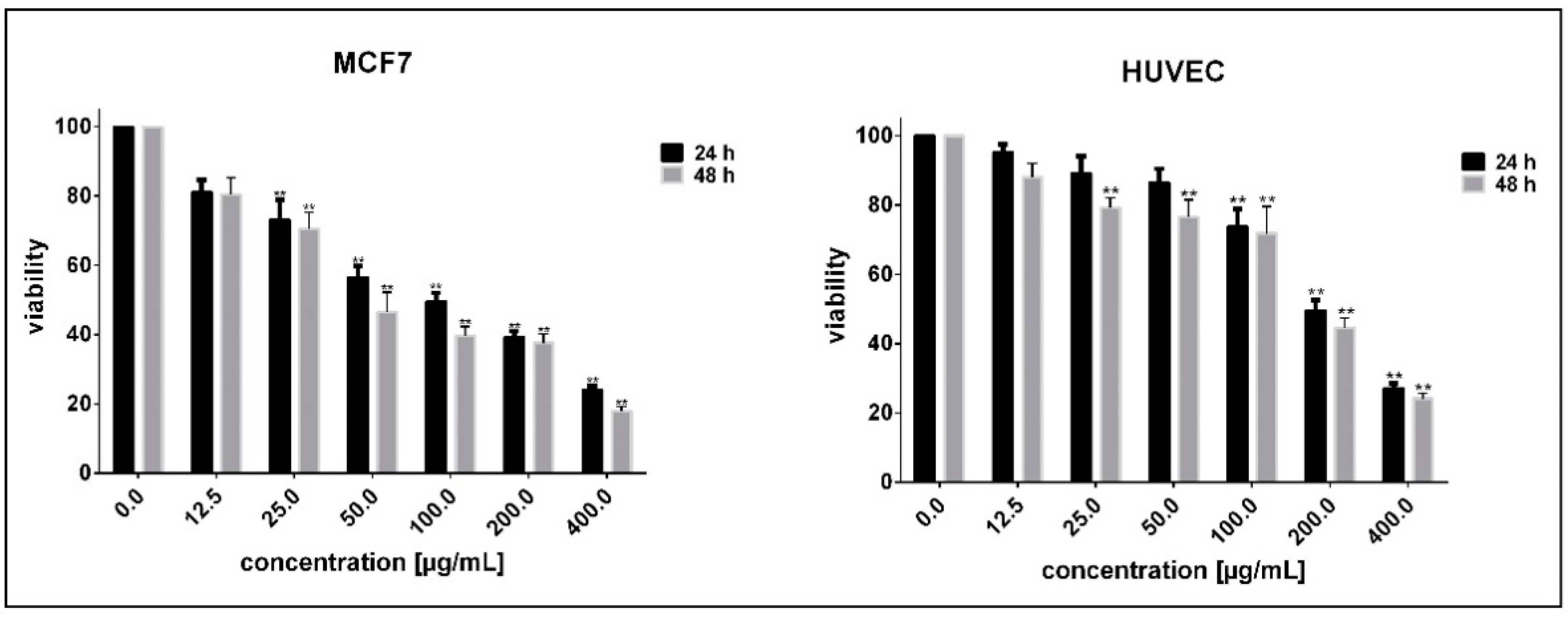
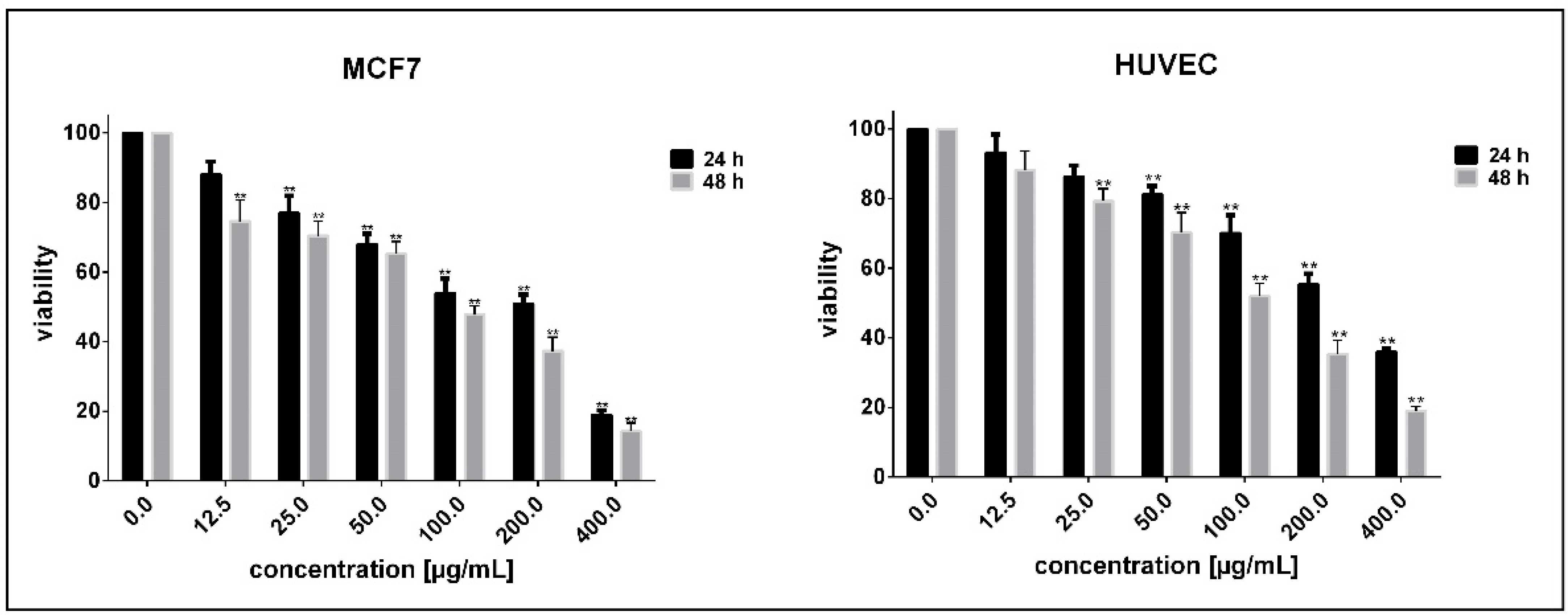
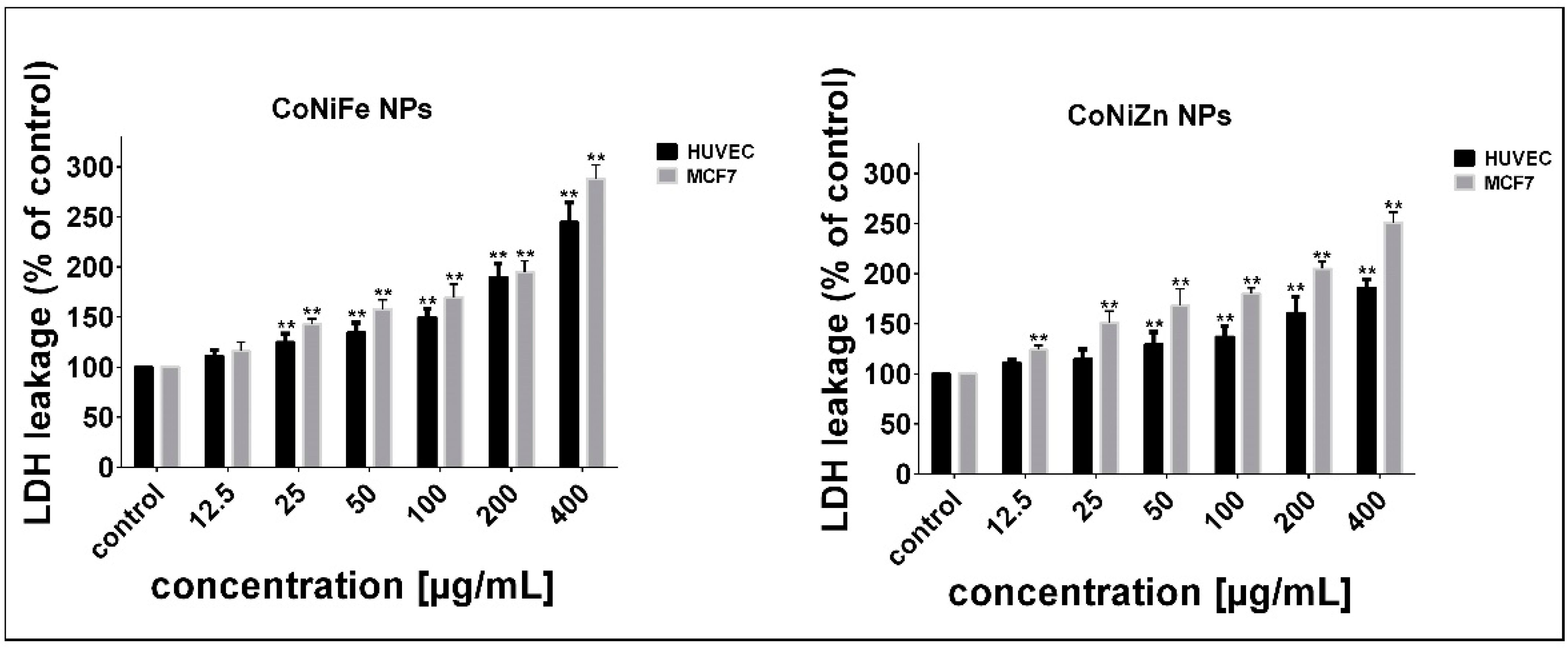
3.2. Determination of Cell Sensitivity to NPs
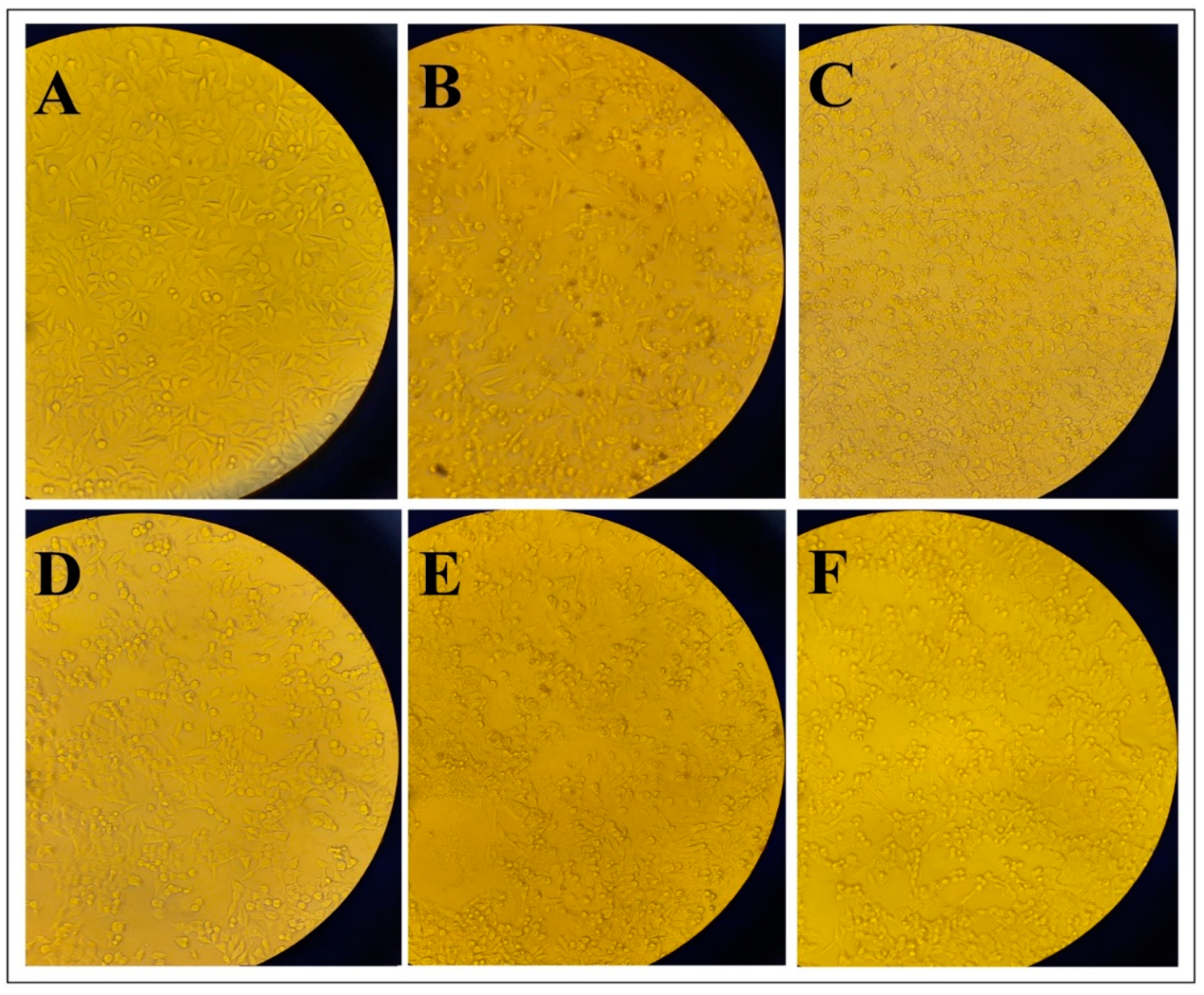
3.3. Morphological Changes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Madamsetty, V.S.; Mukherjee, A.; Mukherjee, S. Recent Trends of the Bio-Inspired Nanoparticles in Cancer Theranostics. Front. Pharmacol. 2019, 10. [Google Scholar] [CrossRef]
- Chen, G.; Qiu, H.; Prasad, P.N.; Chen, X. Upconversion nanoparticles: Design, nanochemistry, and applications in theranostics. Chem. Rev. 2014, 114, 5161–5214. [Google Scholar] [CrossRef]
- Gobbo, O.L.; Sjaastad, K.; Radomski, M.W.; Volkov, Y.; Prina-Mello, A. Magnetic nanoparticles in cancer theranostics. Theranostics 2015, 5, 1249. [Google Scholar] [CrossRef]
- Fernandes, N.; Rodrigues, C.F.; Moreira, A.F.; Correia, I.J. Overview of the application of inorganic nanomaterials in cancer photothermal therapy. Biomater. Sci. 2020, 8, 2990–3020. [Google Scholar] [CrossRef]
- Qin, X.; Kim, D.; Piao, Y. Metal-organic frameworks-derived novel nanostructured electrocatalysts for oxygen evolution reaction. Carbon Energy 2021, 3, 66–100. [Google Scholar] [CrossRef]
- Lu, L. Nanoporous noble metal-based alloys: A review on synthesis and applications to electrocatalysis and electrochemical sensing. Microchim. Acta 2019, 186, 1–21. [Google Scholar] [CrossRef]
- Taylor, U.; Tiedemann, D.; Rehbock, C.; Kues, W.A.; Barcikowski, S.; Rath, D. Influence of gold, silver and gold–silver alloy nanoparticles on germ cell function and embryo development. Beilstein J. Nanotechnol. 2015, 6, 651–664. [Google Scholar] [CrossRef] [Green Version]
- Tiedemann, D.; Taylor, U.; Rehbock, C.; Jakobi, J.; Klein, S.; Kues, W.A.; Barcikowski, S.; Rath, D. Reprotoxicity of gold, silver, and gold–silver alloy nanoparticles on mammalian gametes. Analyst 2014, 139, 931–942. [Google Scholar] [CrossRef]
- Elegbede, J.A.; Lateef, A.; Azeez, M.A.; Asafa, T.B.; Yekeen, T.A.; Oladipo, I.C.; Hakeem, A.S.; Beukes, L.S.; Gueguim-Kana, E.B. Silver-gold alloy nanoparticles biofabricated by fungal xylanases exhibited potent biomedical and catalytic activities. Biotechnol. Prog. 2019, 35, e2829. [Google Scholar] [CrossRef]
- Akin, Y.; Obaidat, I.; Issa, B.; Haik, Y. Ni1-x Crx alloy for self controlled magnetic hyperthermia. Cryst. Res. Technol. J. Exp. Ind. Crystallogr. 2009, 44, 386–390. [Google Scholar] [CrossRef]
- Magaye, R.; Zhao, J.; Bowman, L.; Ding, M. Genotoxicity and carcinogenicity of cobalt-, nickel-and copper-based nanoparticles. Exp. Ther. Med. 2012, 4, 551–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stergar, J.; Ban, I.; Maver, U. The Potential Biomedical Application of NiCu Magnetic Nanoparticles. Magnetochemistry 2019, 5, 66. [Google Scholar] [CrossRef] [Green Version]
- Dang, W.; Li, T.; Li, B.; Ma, H.; Zhai, D.; Wang, X.; Chang, J.; Xiao, Y.; Wang, J.; Wu, C. A bifunctional scaffold with CuFeSe2 nanocrystals for tumor therapy and bone reconstruction. Biomaterials 2018, 160, 92–106. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Li, Y.; Zeng, M.; Gao, J.; Tang, Y.; Zeng, Z.; Zheng, Y. Design of chalcopyrite-type CuFeSe2 nanocrystals: Microstructure, magnetism, photoluminescence and sensing performances. J. Solid State Chem. 2019, 271, 292–297. [Google Scholar] [CrossRef]
- Pooresmaeil, M.; Namazi, H. pH-sensitive ternary Fe3O4/GQDs@ G hybrid microspheres; Synthesis, characterization and drug delivery application. J. Alloys Compd. 2020, 846, 156419. [Google Scholar] [CrossRef]
- Loh, A.; Li, X.; Taiwo, O.O.; Tariq, F.; Brandon, N.P.; Wang, P.; Xu, K.; Wang, B. Development of Ni–Fe based ternary metal hydroxides as highly efficient oxygen evolution catalysts in AEM water electrolysis for hydrogen production. Int. J. Hydrog. Energy 2020, 45, 24232–24247. [Google Scholar] [CrossRef]
- Kube, S.A.; Xing, W.; Kalidindi, A.; Sohn, S.; Datye, A.; Amram, D.; Schuh, C.A.; Schroers, J. Combinatorial study of thermal stability in ternary nanocrystalline alloys. Acta Mater. 2020, 188, 40–48. [Google Scholar] [CrossRef]
- Elsherif, S.A.; El Sawy, E.N.; Ghany, N.A.A. Polyol synthesized graphene/PtxNi100-x nanoparticles alloy for improved electrocatalytic oxidation of methanol in acidic and basic media. J. Electroanal. Chem. 2020, 856, 113601. [Google Scholar] [CrossRef]
- Dippong, T.; Cadar, O.; Levei, E.A.; Deac, I.G. Microstructure, porosity and magnetic properties of Zn0. 5Co0. 5Fe2O4/SiO2 nanocomposites prepared by sol-gel method using different polyols. J. Magn. Magn. Mater. 2020, 498, 166168. [Google Scholar] [CrossRef]
- Salati, A.; Ramazani, A.; Kashi, M.A. Tuning hyperthermia properties of FeNiCo ternary alloy nanoparticles by morphological and magnetic characteristics. J. Magn. Magn. Mater. 2020, 498, 166172. [Google Scholar] [CrossRef]
- Fotukian, S.M.; Barati, A.; Soleymani, M.; Alizadeh, A.M. Solvothermal synthesis of CuFe2O4 and Fe3O4 nanoparticles with high heating efficiency for magnetic hyperthermia application. J. Alloys Compd. 2020, 816, 152548. [Google Scholar] [CrossRef]
- El-Shahawy, A.A.; Moaty, S.A.; Zaki, A.; Mohamed, N.A.; GadelHak, Y.; Mahmoud, R.; Farghali, A. Prostate Cancer Cellular Uptake of Ternary Titanate Nanotubes/CuFe2O4/Zn-Fe Mixed Metal Oxides Nanocomposite. Int. J. Nanomed. 2020, 15, 619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, M.; Lu, F.; Zhou, T.; Zhao, J.; Ding, C.; Fakhri, A.; Gupta, V.K. Biosynthesis of nano bimetallic Ag/Pt alloy from Crocus sativus L. extract: Biological efficacy and catalytic activity. J. Photochem. Photobiol. B Biol. 2020, 212, 112025. [Google Scholar] [CrossRef]
- Camarillo, I.G.; Xiao, F.; Madhivanan, S.; Salameh, T.; Nichols, M.; Reece, L.M.; Leary, J.F.; Otto, K.; Natarajan, A.; Ramesh, A. Low and high voltage electrochemotherapy for breast cancer: An in vitro model study. Electroporation Based Ther. Cancer 2014, 55–102. [Google Scholar] [CrossRef]
- Chen, X.; Wang, X.; Wang, Y.; Yang, L.; Hu, J.; Xiao, W.; Fu, A.; Cai, L.; Li, X.; Ye, X. Improved tumor-targeting drug delivery and therapeutic efficacy by cationic liposome modified with truncated bFGF peptide. J. Control. Release 2010, 145, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Serda, R.E.; Gu, J.; Bhavane, R.C.; Liu, X.; Chiappini, C.; Decuzzi, P.; Ferrari, M. The association of silicon microparticles with endothelial cells in drug delivery to the vasculature. Biomaterials 2009, 30, 2440–2448. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xue, H.; Gao, C.; Carr, L.; Wang, J.; Chu, B.; Jiang, S. Imaging and cell targeting characteristics of magnetic nanoparticles modified by a functionalizable zwitterionic polymer with adhesive 3, 4-dihydroxyphenyl-l-alanine linkages. Biomaterials 2010, 31, 6582–6588. [Google Scholar] [CrossRef]
- Mokarian, M.H.; Almasi-kashi, M.; Alikhanzadeh-Arani, S.; Ramazani, A. The fcc/bcc phase transition in FexNi100−x nanoparticles resolved by first-order reversal curves. J. Mater. Sci. 2017, 52, 7831–7842. [Google Scholar] [CrossRef]
- Mashayekhi, S.; Rasoulpoor, S.; Shabani, S.; Esmaeilizadeh, N.; Serati-Nouri, H.; Sheervalilou, R.; Pilehvar-Soltanahmadi, Y. Curcumin-loaded mesoporous silica nanoparticles/nanofiber composites for supporting long-term proliferation and stemness preservation of adipose-derived stem cells. Int. J. Pharm. 2020, 587, 119656. [Google Scholar] [CrossRef] [PubMed]
- Resali, N.A.; Hyie, K.M.; Berhan, M.; Mardziah, C. Investigation of Heat Treated Electrodeposited CoNiFe on Microstructure and Hardness. In Advanced Materials Research; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2015; pp. 56–61. [Google Scholar]
- Koay, M.H.; Salleh, Z.; Mardziah, C.; Mohd Masdek, N.R.N.; Resali, N.A. Corrosion Behavior of Heat Treated Nanocrystalline Co Ni Fe Coating on Stainless Steel/Koay Mei Hyie…[et al.]. J. Mech. Eng. 2018, 5, 191–203. [Google Scholar]
- Fatima, H.; Charinpanitkul, T.; Kim, K.-S. Fundamentals to Apply Magnetic Nanoparticles for Hyperthermia Therapy. Nanomaterials 2021, 11, 1203. [Google Scholar] [CrossRef] [PubMed]
- Giustini, A.J.; Petryk, A.A.; Cassim, S.M.; Tate, J.A.; Baker, I.; Hoopes, P.J. Magnetic nanoparticle hyperthermia in cancer treatment. Nano Life 2010, 1, 17–32. [Google Scholar] [CrossRef]
- Bui, T.Q.; Ngo, H.T.M.; Tran, H.T. Surface-protective assistance of ultrasound in synthesis of superparamagnetic magnetite nanoparticles and in preparation of mono-core magnetite-silica nanocomposites. J. Sci. Adv. Mater. Devices 2018, 3, 323–330. [Google Scholar] [CrossRef]
- Shakeri-Zadeh, A.; Zareyi, H.; Sheervalilou, R.; Laurent, S.; Ghaznavi, H.; Samadian, H. Gold nanoparticle-mediated bubbles in cancer nanotechnology. J. Control. Release 2020. [Google Scholar] [CrossRef]
- Barani, M.; Mukhtar, M.; Rahdar, A.; Sargazi, G.; Thysiadou, A.; Kyzas, G.Z. Progress in the Application of Nanoparticles and Graphene as Drug Carriers and on the Diagnosis of Brain Infections. Molecules 2021, 26, 186. [Google Scholar] [CrossRef]
- Tsai, T.-L.; Lai, Y.-H.; Chen, H.H.; Su, W.-C. Overcoming Radiation Resistance by Iron-Platinum Metal Alloy Nanoparticles in Human Copper Transport 1-Overexpressing Cancer Cells via Mitochondrial Disturbance. Int. J. Nanomed. 2021, 16, 2071. [Google Scholar] [CrossRef]
- Godipurge, S.; Yallappa, S.; Biradar, N.J.; Biradar, J.; Dhananjaya, B.; Hegde, G.; Jagadish, K.; Hegde, G. A facile and green strategy for the synthesis of Au, Ag and Au–Ag alloy nanoparticles using aerial parts of R. hypocrateriformis extract and their biological evaluation. Enzym. Microb. Technol. 2016, 95, 174–184. [Google Scholar] [CrossRef]
- Song, X.R.; Yu, S.X.; Jin, G.X.; Wang, X.; Chen, J.; Li, J.; Liu, G.; Yang, H.H. Plant Polyphenol-Assisted Green Synthesis of Hollow CoPt Alloy Nanoparticles for Dual-Modality Imaging Guided Photothermal Therapy. Small 2016, 12, 1506–1513. [Google Scholar] [CrossRef] [PubMed]
- Patskovsky, S.; Bergeron, E.; Rioux, D.; Simard, M.; Meunier, M. Hyperspectral reflected light microscopy of plasmonic Au/Ag alloy nanoparticles incubated as multiplex chromatic biomarkers with cancer cells. Analyst 2014, 139, 5247–5253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karthika, V.; Arumugam, A.; Gopinath, K.; Kaleeswarran, P.; Govindarajan, M.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Benelli, G. Guazuma ulmifolia bark-synthesized Ag, Au and Ag/Au alloy nanoparticles: Photocatalytic potential, DNA/protein interactions, anticancer activity and toxicity against 14 species of microbial pathogens. J. Photochem. Photobiol. B Biol. 2017, 167, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wei, J.; Aifantis, K.E.; Fan, Y.; Feng, Q.; Cui, F.Z.; Watari, F. Current investigations into magnetic nanoparticles for biomedical applications. J. Biomed. Mater. Res. Part A 2016, 104, 1285–1296. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, X.L.; Zhang, Y.F.; Gao, F.; Li, G.L.; He, Y.; Peng, M.L.; Fan, H.M. Magnetic nanoparticles based cancer therapy: Current status and applications. Sci. China Life Sci. 2018, 61, 400–414. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Han, X.; He, L.; Deng, L.; Yu, K.; Jiang, H.; Wu, C.; Jia, Q.; Shan, S. Synthesis and characterization of magnetic dextran nanogel doped with iron oxide nanoparticles as magnetic resonance imaging probe. Int. J. Biol. Macromol. 2019, 128, 768–774. [Google Scholar] [CrossRef]
- Peymani-Motlagh, S.M.; Sobhani-Nasab, A.; Rostami, M.; Sobati, H.; Eghbali-Arani, M.; Fasihi-Ramandi, M.; Ganjali, M.R.; Rahimi-Nasrabadi, M. Assessing the magnetic, cytotoxic and photocatalytic influence of incorporating Yb 3+ or Pr 3+ ions in cobalt–nickel ferrite. J. Mater. Sci. Mater. Electron. 2019, 30, 6902–6909. [Google Scholar] [CrossRef]
- Martínez-Rodríguez, N.L.; Tavárez, S.; González-Sánchez, Z.I. In vitro toxicity assessment of zinc and nickel ferrite nanoparticles in human erythrocytes and peripheral blood mononuclear cell. Toxicol. In Vitro 2019, 57, 54–61. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alikhanzadeh-Arani, S.; Almasi-Kashi, M.; Sargazi, S.; Rahdar, A.; Arshad, R.; Baino, F. CoNiZn and CoNiFe Nanoparticles: Synthesis, Physical Characterization, and In Vitro Cytotoxicity Evaluations. Appl. Sci. 2021, 11, 5339. https://doi.org/10.3390/app11125339
Alikhanzadeh-Arani S, Almasi-Kashi M, Sargazi S, Rahdar A, Arshad R, Baino F. CoNiZn and CoNiFe Nanoparticles: Synthesis, Physical Characterization, and In Vitro Cytotoxicity Evaluations. Applied Sciences. 2021; 11(12):5339. https://doi.org/10.3390/app11125339
Chicago/Turabian StyleAlikhanzadeh-Arani, Sima, Mohammad Almasi-Kashi, Saman Sargazi, Abbas Rahdar, Rabia Arshad, and Francesco Baino. 2021. "CoNiZn and CoNiFe Nanoparticles: Synthesis, Physical Characterization, and In Vitro Cytotoxicity Evaluations" Applied Sciences 11, no. 12: 5339. https://doi.org/10.3390/app11125339
APA StyleAlikhanzadeh-Arani, S., Almasi-Kashi, M., Sargazi, S., Rahdar, A., Arshad, R., & Baino, F. (2021). CoNiZn and CoNiFe Nanoparticles: Synthesis, Physical Characterization, and In Vitro Cytotoxicity Evaluations. Applied Sciences, 11(12), 5339. https://doi.org/10.3390/app11125339









