High Resolution STEM Images of the Human Tooth Enamel Crystals
Abstract
1. Introduction
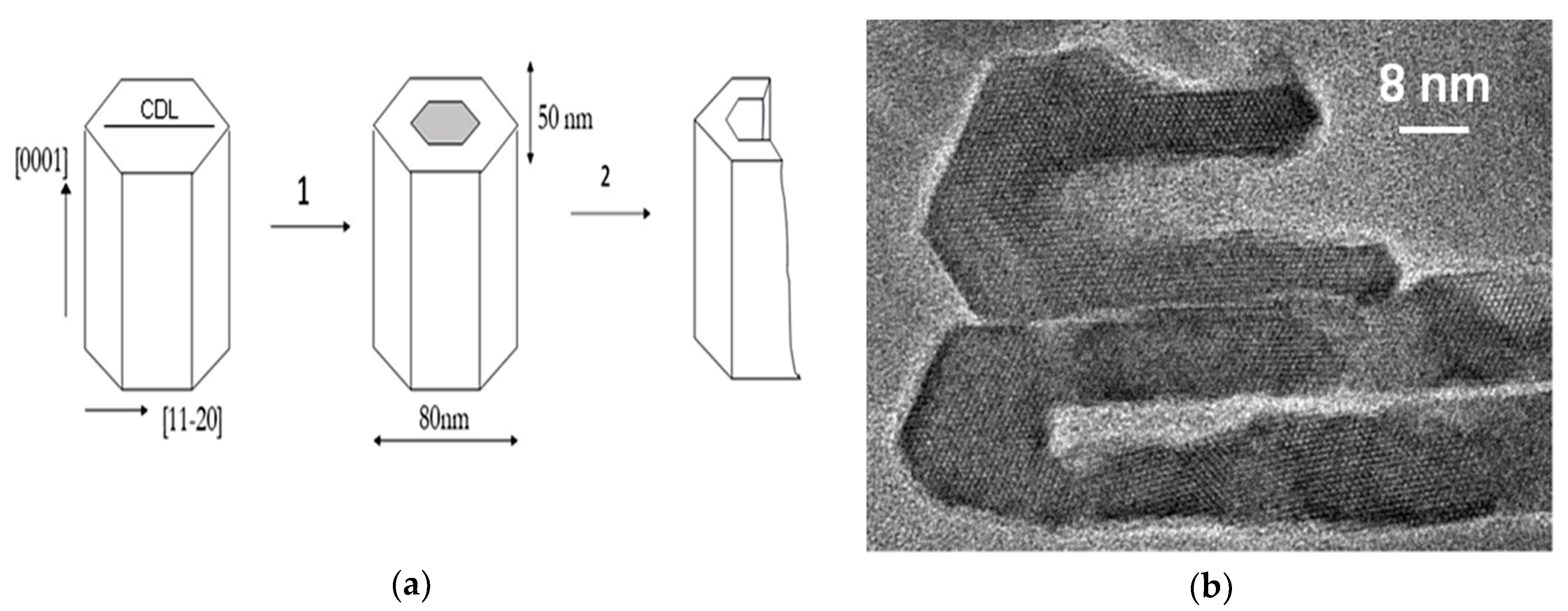
1.1. Human Tooth Structure
1.2. The Central Dark Line
1.3. Dental Caries and Defects at the Nanoscale
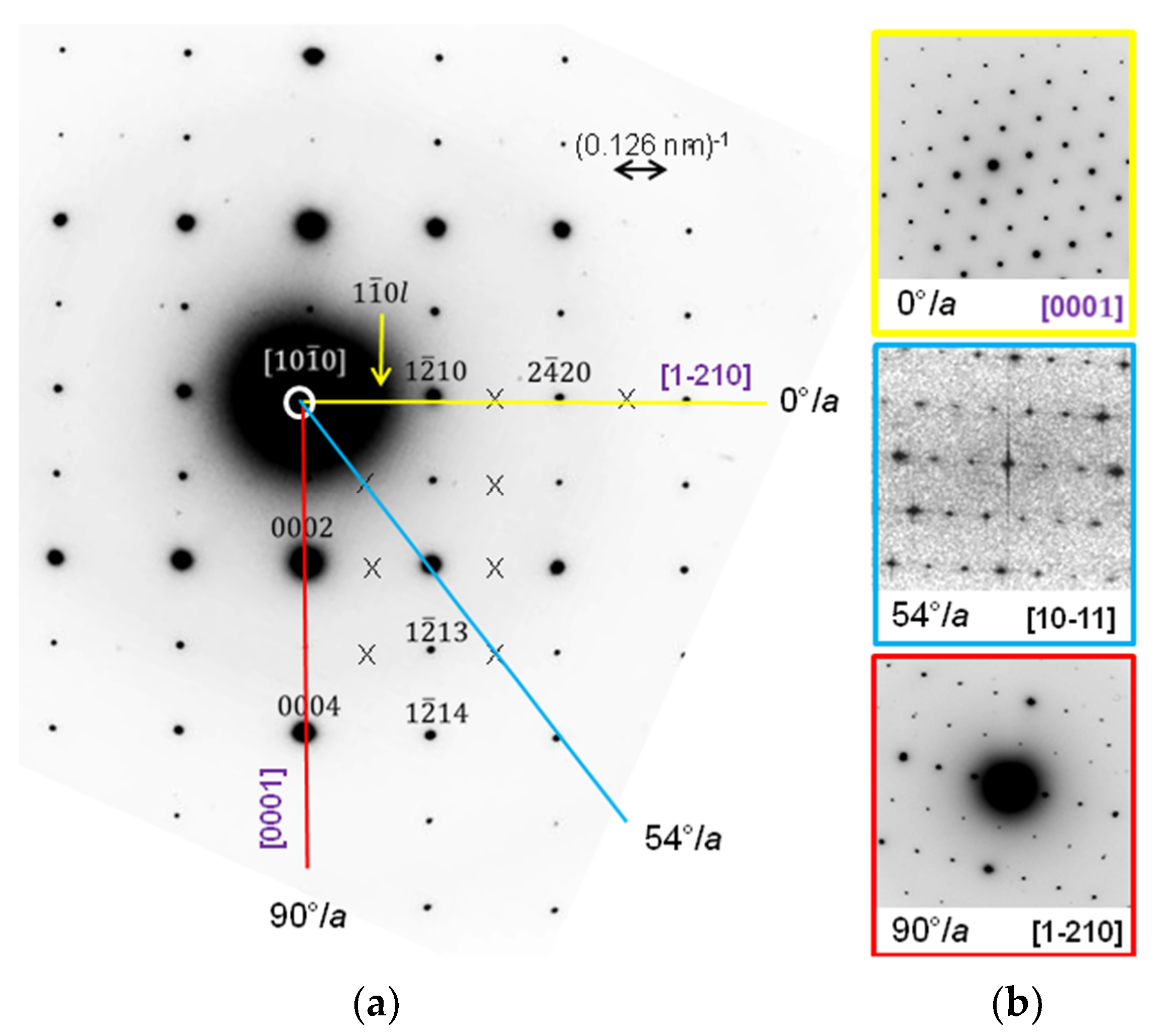
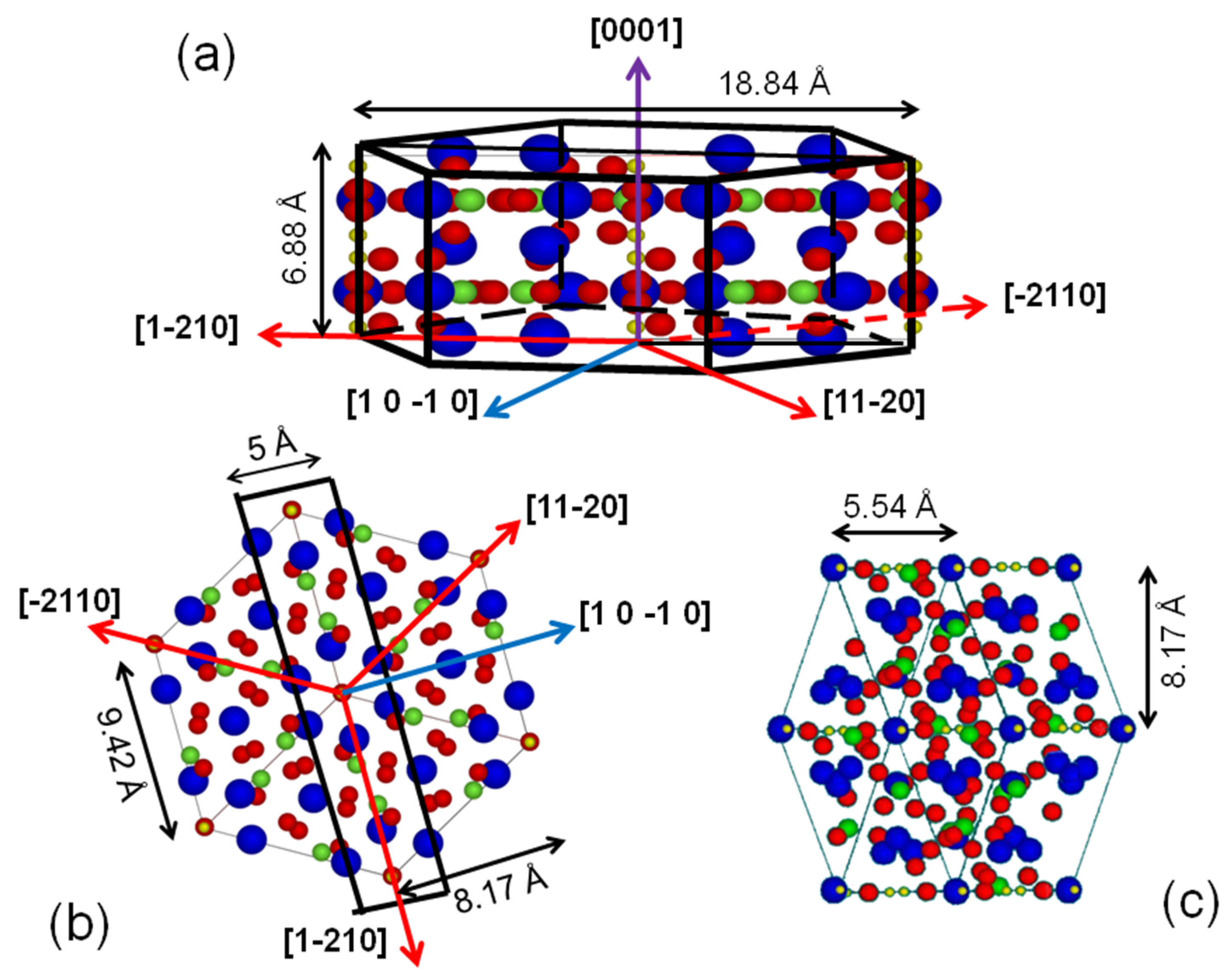
2. Materials and Methods
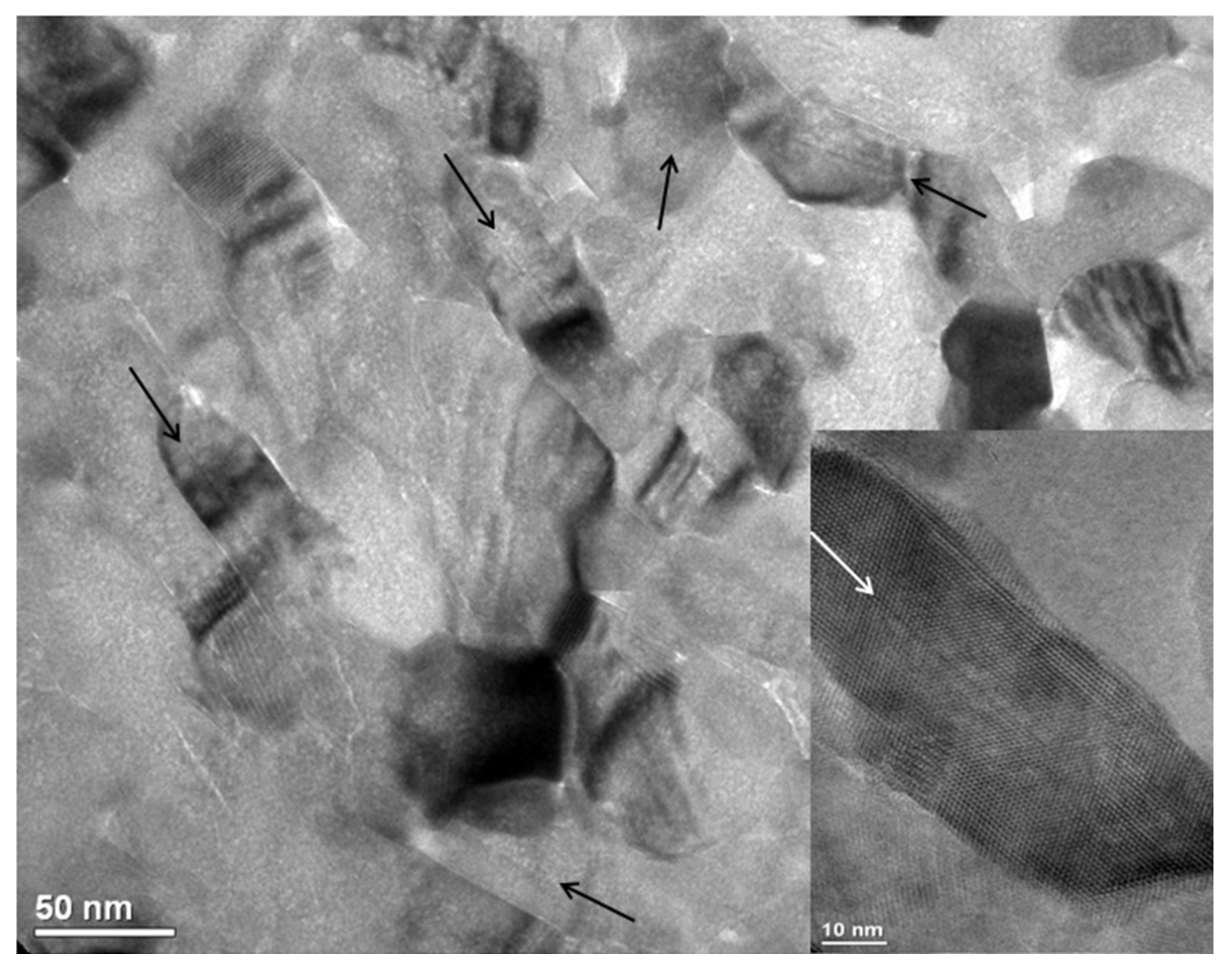
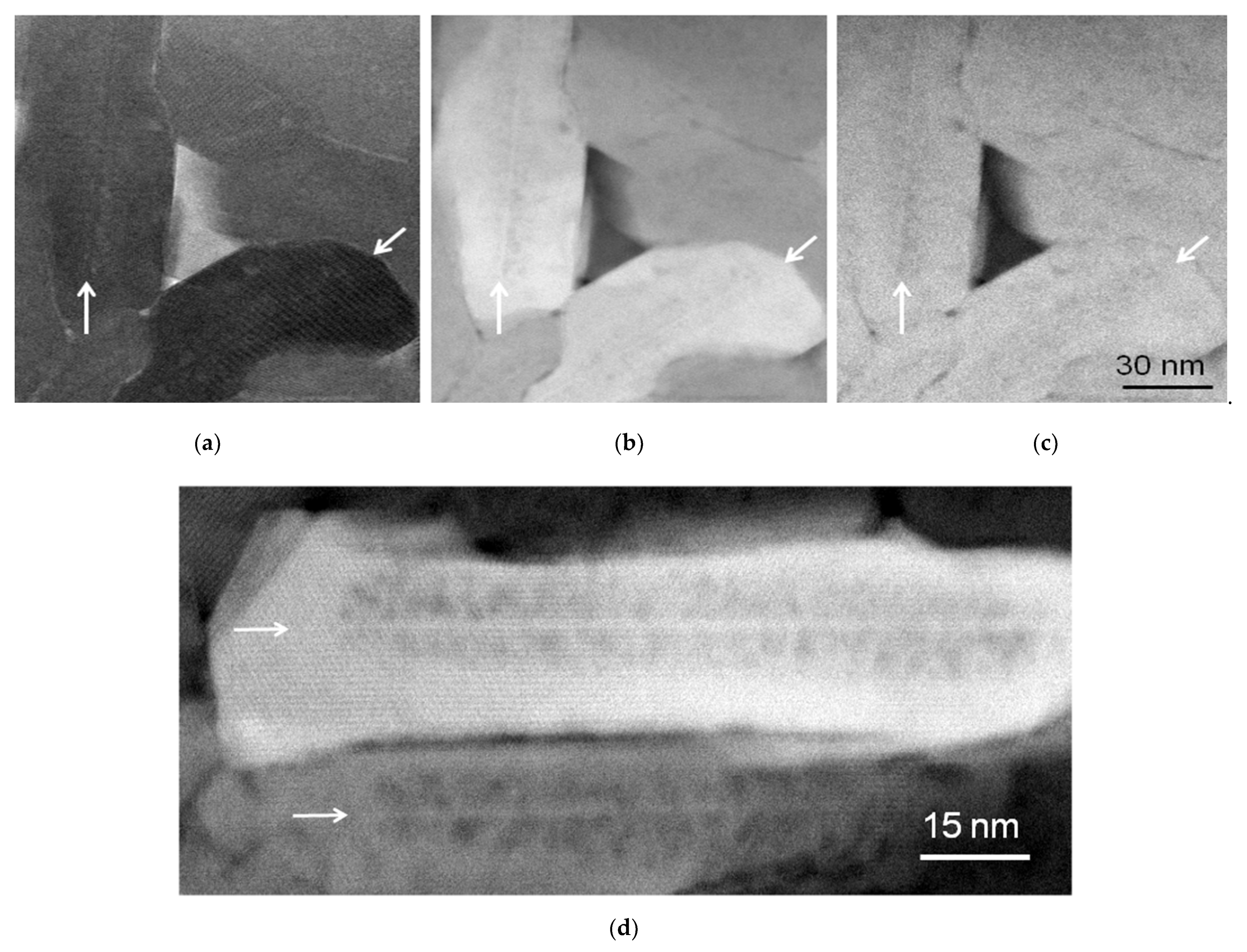
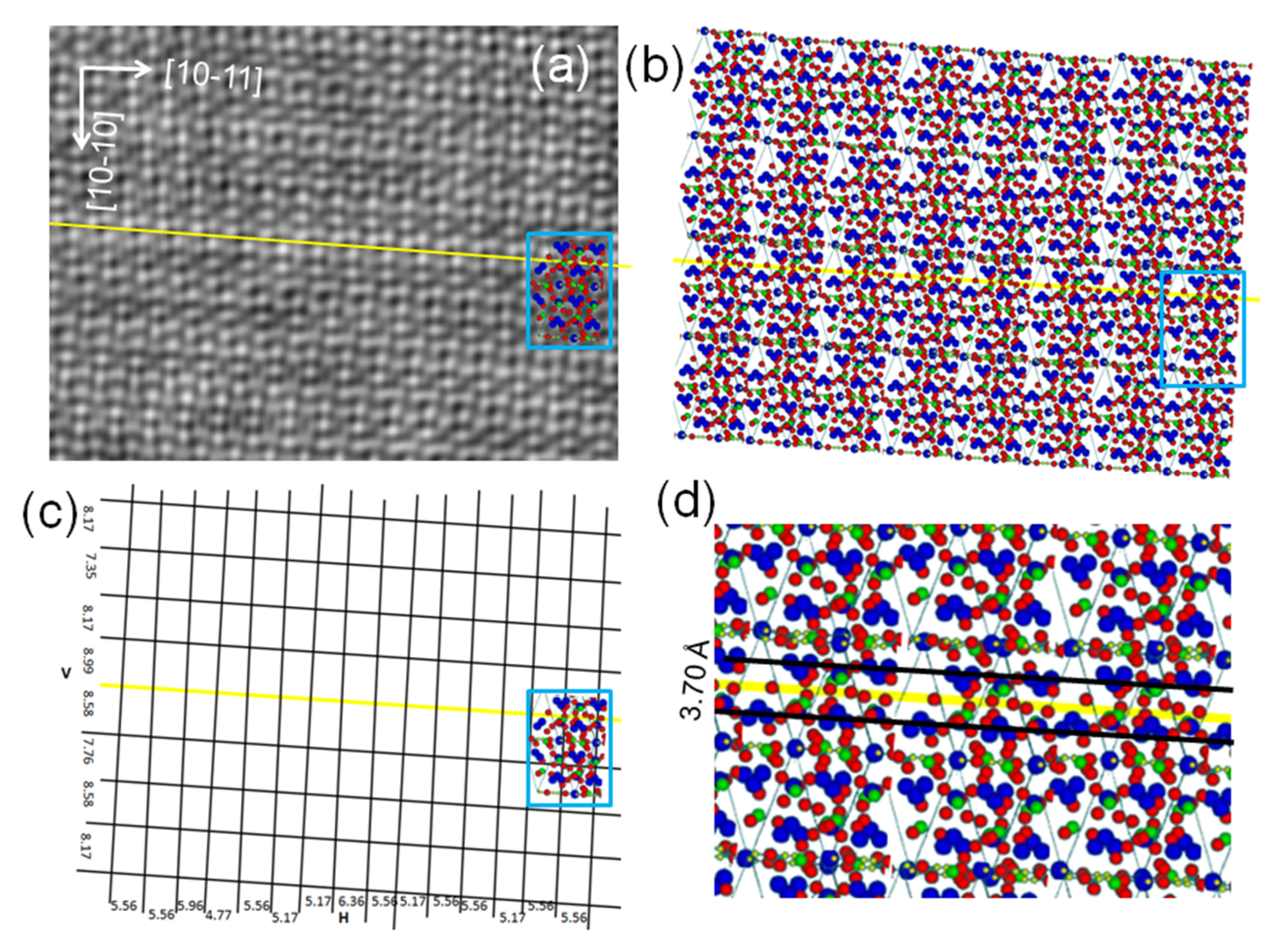
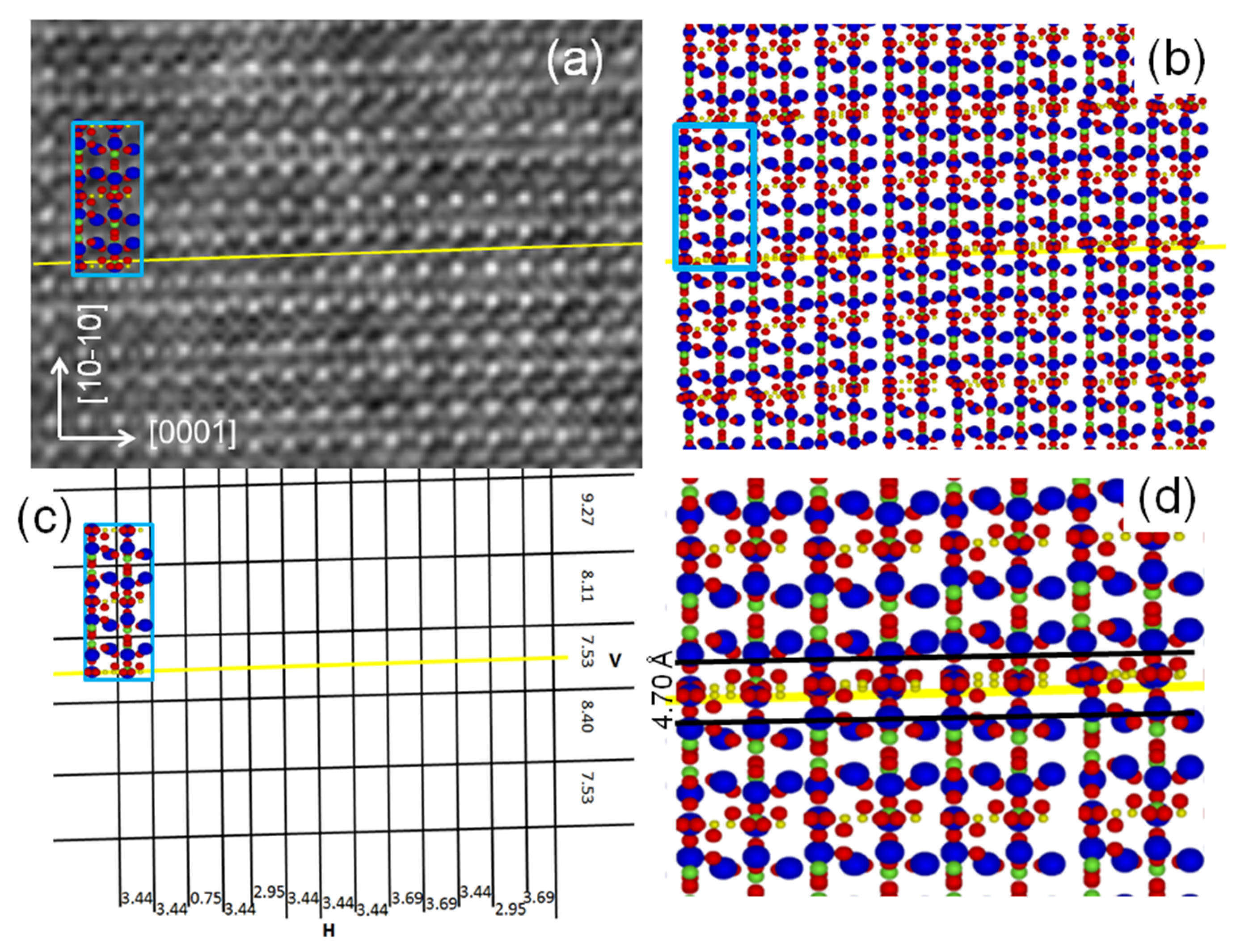
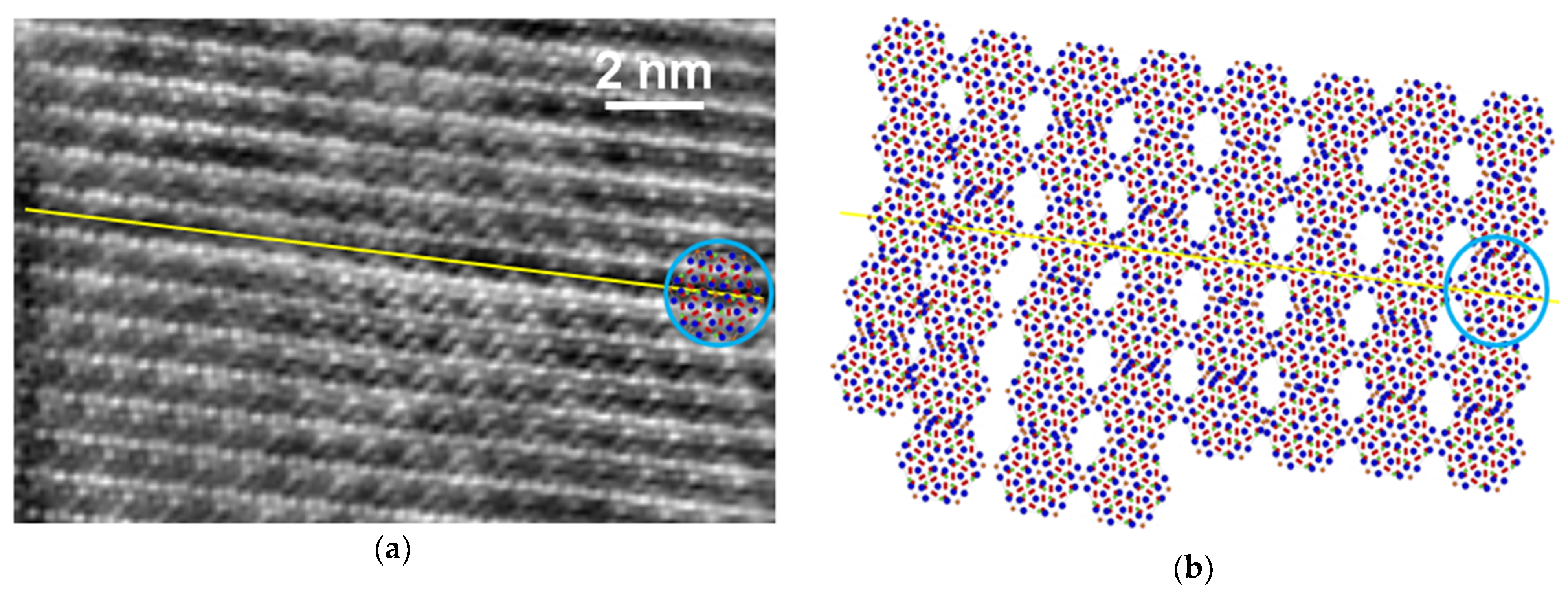
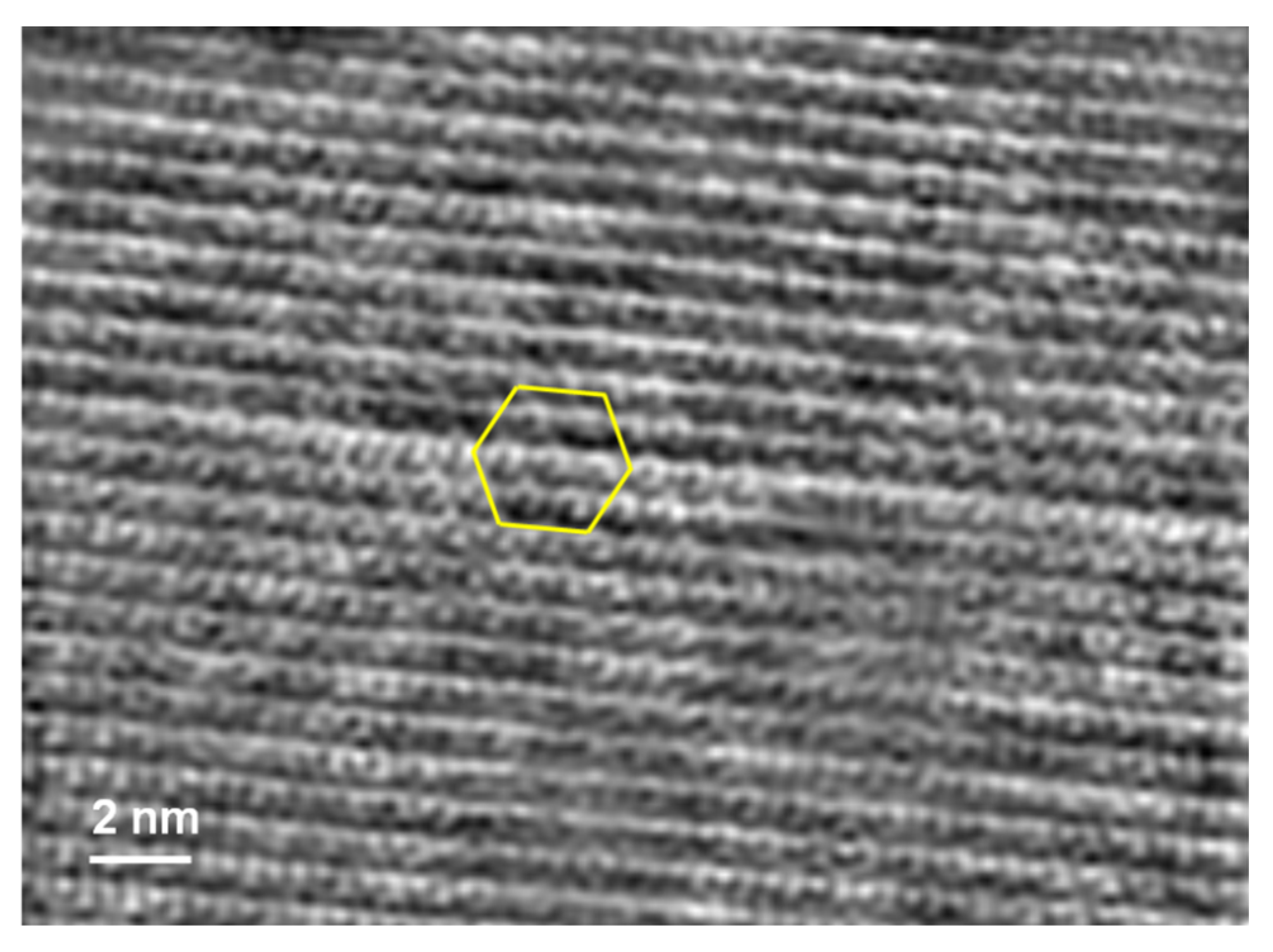
3. Results
4. Discussion
4.1. TEM and STEM Contrasts
4.2. Importance of Crystal Defects for the Properties of Enamel Crystals
4.3. Electron Radiation Damage
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- LeGeros, R.Z. Calcium Phosphates in Oral Biology and Medicine. Monogr. Oral Sci. 1991, 15. [Google Scholar] [CrossRef]
- DeRocher, K.A.; Smeets, P.J.M.; Goodge, B.H.; Zachman, M.J.; Balachandran, P.V.; Stegbauer, L.; Cohen, M.J.; Gordon, L.M.; Rondinelli, J.M.; Kourkoutis, L.F.; et al. Chemical gradients in human enamel crystallites. Nature 2020, 583, 66. [Google Scholar] [CrossRef]
- Yun, F.; Swain, M.V.; Chen, H.; Cairney, J.; Qu, J.; Sha, G.; Liu, H.; Ringer, S.P.; Han, Y.; Liu, L.; et al. Nanoscale pathways for human tooth decay—Central planar defect, organic rich precipitate and high-angle grain boundary. Biomaterials 2020, 235, 119748. [Google Scholar] [CrossRef]
- Marshall, A.F.; Lawless, K.R. TEM study of the central dark line in enamel crystallites. J. Dent. Res. 1981, 60, 1773–1782. [Google Scholar] [CrossRef]
- Reyes-Gasga, J.; Hémmerlé, J.; Brès, E.F. Aberration-corrected transmission electron microscopic study of the central dark line defect in human tooth enamel crystals. Microsc. Microanal. 2016, 22, 1047–1055. [Google Scholar] [CrossRef]
- Brès, E.F.; Barry, J.C.; Hutchison, J.L. A structural basis for the carious dissolution of the apatite crystals of human tooth enamel. Ultramicroscopy 1983, 12, 367–372. [Google Scholar] [CrossRef]
- Voegel, J.C.; Frank, R.M. Stages in the dissolution of human enamel crystals in dental caries. Calcif. Tissue. Res. 1977, 24, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Gasga, J.; Arellano-Jiménez, M.J.; Garcia-Garcia, R. On the observation of the HAP-OCP interface by electron microscopy. Acta Microsc. 2010, 19, 279–284. [Google Scholar]
- Reyes Gasga, J.; Carbajal-de-la-Torre, G.; Bres, E.; Gil-Chavarria, I.M.; Rodrıguez-Hernandez, A.G.; Garcia-Garcia, R. STEM-HAADF electron microscopy analysis of the central dark line defect of human tooth enamel crystallites. J. Mater. Sci. Mater. Med. 2008, 19, 877–882. [Google Scholar] [CrossRef]
- Haider, M.; Rose, H.; Uhlemann, S.; Schwan, E.; Kabius, B.; Urban, K. A spherical-aberration-corrected 200kV transmission electron microscope. Ultramicroscopy 1998, 75, 53–60. [Google Scholar] [CrossRef]
- Grieb, T.; Muller, K.; Fritz, R.; Schowalter, M.; Neugebohrn, N.; Knaub, N.; Volz, K.; Rosenauer, A. Determination of the chemical composition of GaNAs using STEM-HAADF imaging and STEM strain state analysis. Ultramicroscopy 2012, 117, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Loesche, W.J. Role of streptococcus mutans in human dental decay. Microbiol. Rev. 1986, 50, 353–380. [Google Scholar] [CrossRef] [PubMed]
- Arends, J. Dislocations and dissolution of enamel. Caries Res. 1973, 7, 261–268. [Google Scholar] [CrossRef]
- Kwon, K.Y.; Wang, E.; Chung, A.; Chang, N.; Saiz, E.; Choe, U.J.; Koobatian, M.; Lee, S.W. Defect induced asymmetric pit formation on hydroxyapatite. Langmuir 2008, 24, 11063–11066. [Google Scholar] [CrossRef]
- Arends, J.; Jongebloed, W.L. Dislocations and dissolution in apatites: Theoretical considerations. Caries Res. 1977, 11, 186–188. [Google Scholar] [CrossRef]
- Gordon, L.M.; Cohen, M.J.; MacRenaris, K.W.; Pasteris, J.D.; Seda, T.; Joester, D. Amorphous intergranular phases control the properties of rodent tooth enamel. Science 2015, 347, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Gasga, J.; García-García, R. Analysis of the electron beam radiation damage of TEM samples in the accelerating energy range from 0.1 MeV using the standard theory for fast electrons. Radiat. Phys. Chem. 2002, 64, 359–367. [Google Scholar] [CrossRef]
- Stadelmann, P.A. EMS-a software package for electron diffraction analysis and HREM image simulation in materials science. Ultramicroscopy 1987, 21, 131–145. Available online: http://cimewww.epfl.ch/stadelmann/jemsWebSite/jems.html (accessed on 10 May 2021). [CrossRef]
- Ruhle, M.; Sass, L. The detection f the change in mean inner potential at dislocations in grain boundaries in NiO. Phil. Mag. A 1984, 49, 759–782. [Google Scholar] [CrossRef]
- Brès, E.F.; Reyes-Gasga, J.; Rey, C.; Michel, J. Probe size study of apatite irradiation in STEM. Eur. Phys. J. Appl. Phys. 2014, 67, 20401. [Google Scholar] [CrossRef][Green Version]
- Frank, F.C. The influence of dislocations on crystal growth. Discuss. Faraday Soc. 1949, 5, 48–66. [Google Scholar] [CrossRef]
- Henricks, S.P. Screw dislocation and charge balance as a factor of crystal growth. J. Am. Miner. Soc. 1955, 40, 139–146. [Google Scholar]
- Slifkin, L. Surface and Dislocation Effects on Diffusion in Ionic Crystals. In Diffusion in Materials; Laskar, A.L., Bocquet, J.L., Brebec, G., Monty, C., Eds.; NATO ASI Series (Series E: Applied Sciences); Springer: Dordrecht, The Netherlands, 1990; Volume 179. [Google Scholar] [CrossRef]
- Amelinckx, S. Dislocations in particular structures. In Dislocations in Solids Volume 2 Dislocations in Crystals; Nabarro, F.R.N., Ed.; North Holland Publ., Co.: Amsterdam, The Netherlands, 1979; pp. 377–398. [Google Scholar] [CrossRef]
- Shtukenberg, A.G.; Ward, M.D.; Kahr, B. Crystal growth with macromolecular additives. Chem. Rev. 2017, 117, 14042–14090. [Google Scholar] [CrossRef] [PubMed]

| Image | TEM | HTEM | STEM | |||
| Contrast | Amplitude (1-beam) | Phase (N-beams) | Amplitude (N-beams) | |||
| Mode | BF | DF | BF | BF | ADF | HAADF |
| Contrast color | Dark or white (defocusing function) | Dark | Dark | White | Dark (but weak) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reyes-Gasga, J.; Brès, E.F. High Resolution STEM Images of the Human Tooth Enamel Crystals. Appl. Sci. 2021, 11, 7477. https://doi.org/10.3390/app11167477
Reyes-Gasga J, Brès EF. High Resolution STEM Images of the Human Tooth Enamel Crystals. Applied Sciences. 2021; 11(16):7477. https://doi.org/10.3390/app11167477
Chicago/Turabian StyleReyes-Gasga, José, and Etienne F. Brès. 2021. "High Resolution STEM Images of the Human Tooth Enamel Crystals" Applied Sciences 11, no. 16: 7477. https://doi.org/10.3390/app11167477
APA StyleReyes-Gasga, J., & Brès, E. F. (2021). High Resolution STEM Images of the Human Tooth Enamel Crystals. Applied Sciences, 11(16), 7477. https://doi.org/10.3390/app11167477






