Exploitation of Enzymes for the Production of Biofuels: Electrochemical Determination of Kinetic Parameters of LPMOs
Abstract
:1. Introduction
2. Materials and Methods
3. Results
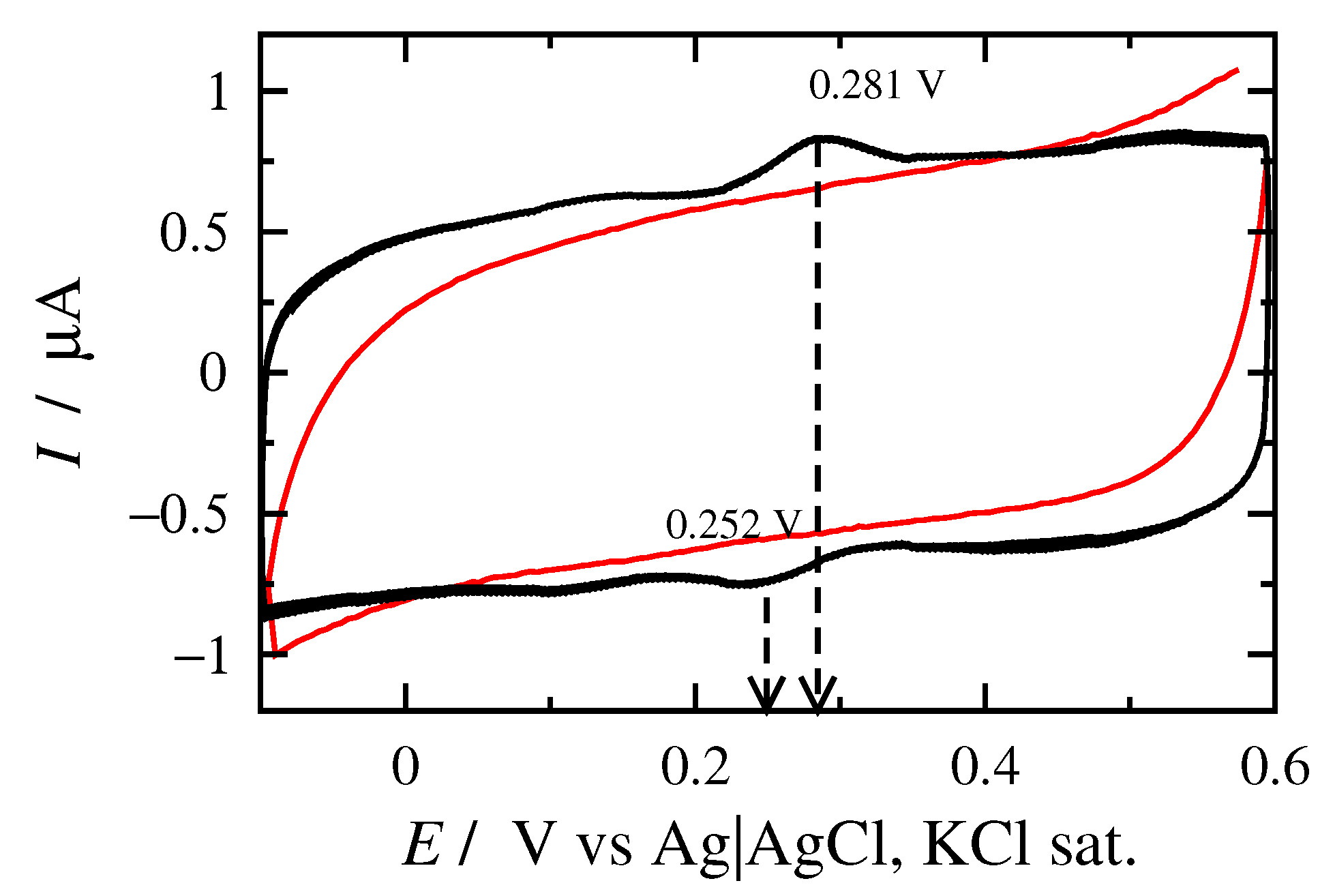
3.1. Cyclic Voltammetry
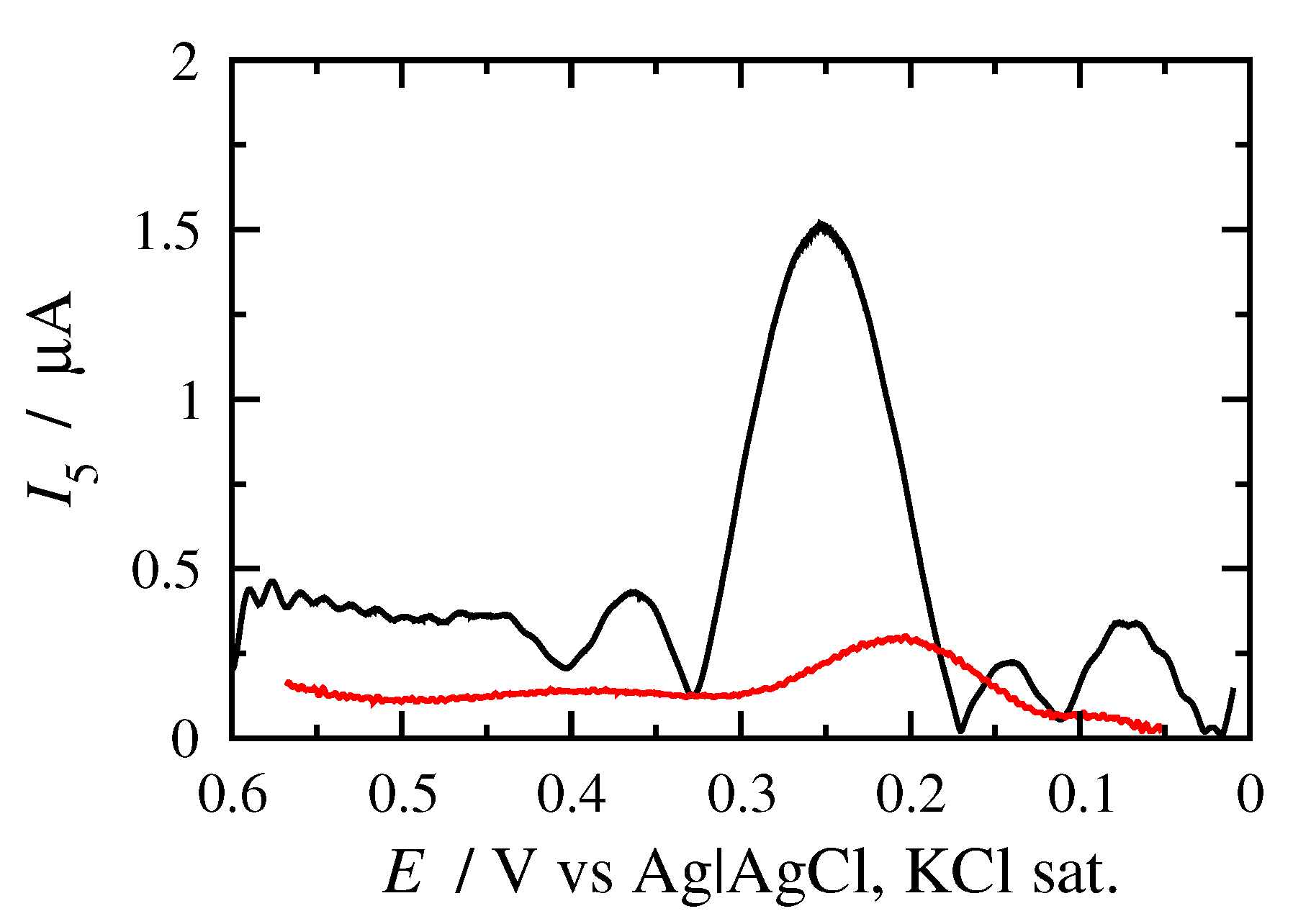
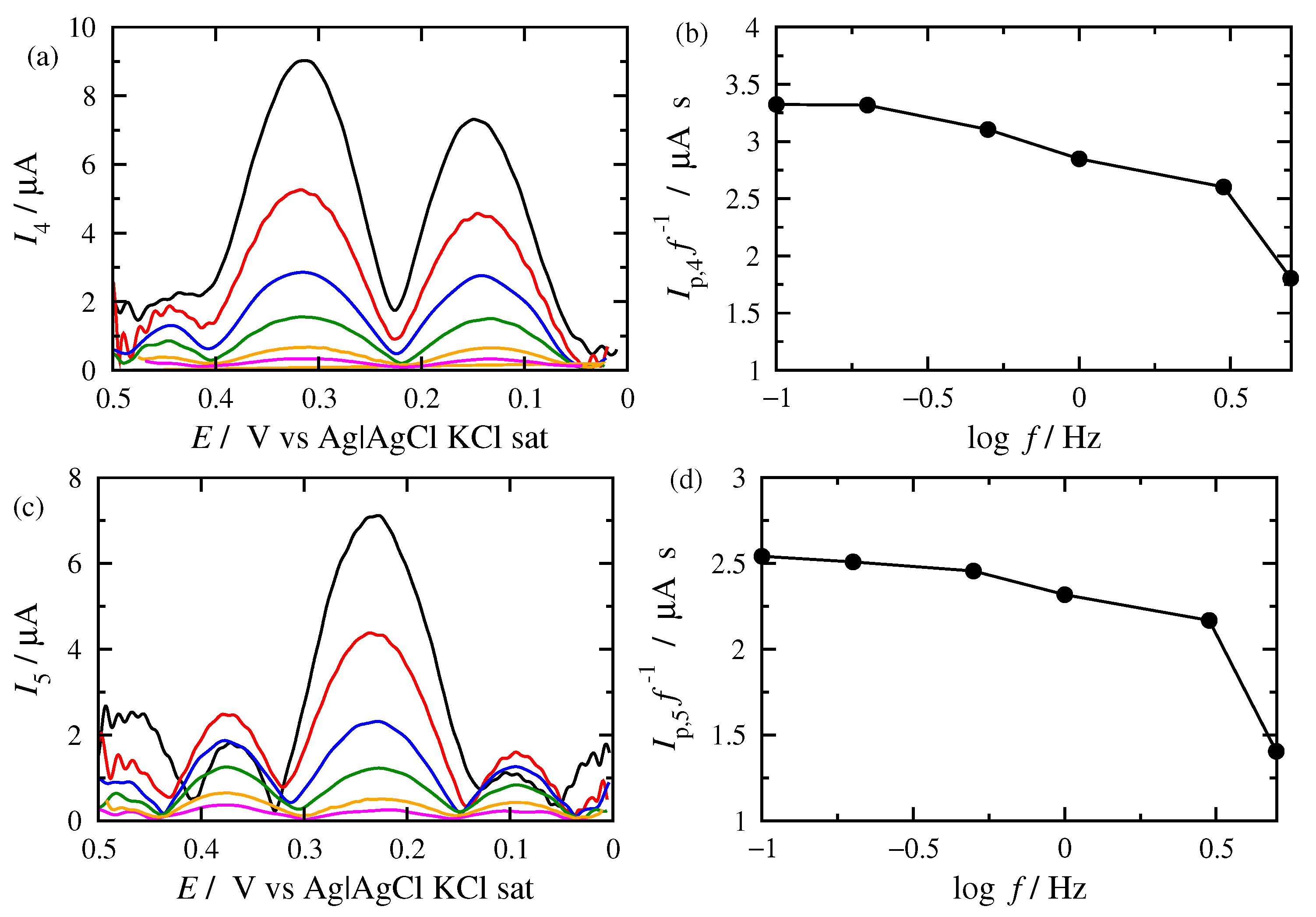
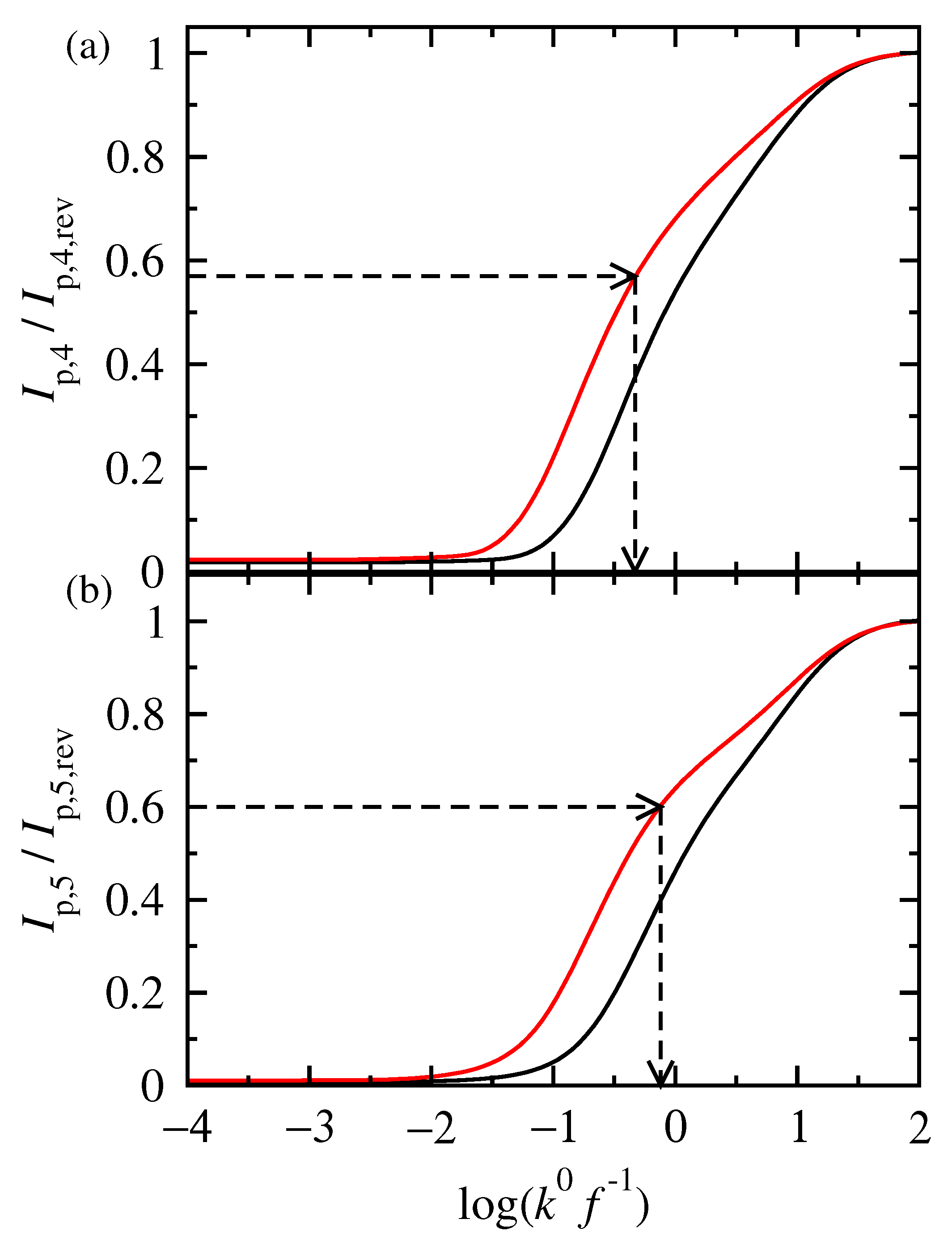
3.2. FT Alternating Current Voltammetry
- A harmonic h is chosen, being free of capacitance currents. This harmonic is recorded at a fixed amplitude and decreasing frequencies;
- The limiting value of the normalized peak height, , of the principal peak is determined. For this frequency, f, the reaction is at the reversible region;
- If the transfer coefficient is to be determined, the shift in the potential of the principal peak is plotted as a function of the logarithm of the inverse frequency, . Linear regression is performed in the region of high frequencies and the transfer coefficient is determined;
- The principal peak height of a quasi-reversible harmonic is determined and the value is calculated;
- The kinetic constant is determined from the graph representing the dependance of on .
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eibinger, M.; Ganner, T.; Bubner, P.; Rošker, S.; Kracher, D.; Haltrich, D.; Ludwig, R.; Plank, H.; Nidetzky, B. Cellulose surface degradation by a lytic polysaccharide monooxygenase and its effect on cellulase hydrolytic efficiency. J. Biol. Chem. 2014, 289, 35929–35938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, R. Functional characterization of cellulose-degrading AA9 lytic polysaccharide monooxygenases and their potential exploitation. Appl. Microbiol. Biotechnol. 2020, 104, 3229–3243. [Google Scholar] [CrossRef]
- Moreau, C.; Tapin-Lingua, S.; Grisel, S.; Gimbert, I.; Le Gall, S.; Meyer, V.; Petit-Conil, M.; Berrin, J.-G.; Cathala, B.; Villares, A. Lytic polysaccharide monooxygenases (LPMOs) facilitate cellulose nanofibrils production. Biotechnol. Biofuels 2019, 12, 1–13. [Google Scholar] [CrossRef]
- Forsberg, Z.; Stepnov, A.A.; Kruge Nærdal, G.; Klinkenberg, G.; Eijsink, V.G.H. Engineering lytic polysaccharide monooxygenases (LPMOs). Method Enzymol. 2020, 644, 1–34. [Google Scholar]
- Hu, J.; Arantes, V.; Pribowo, A.; Saddler, J.N. The synergistic action of accessory enzymes enhances the hydrolytic potential of a “cellulase mixture” but is highly substrate specific. Biotechnol. Biofuels 2013, 6, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zerva, A.; Pentari, C.; Termentzi, A.; America, A.H.P.; Zouraris, D.; Bhattacharya, S.K.; Karantonis, A.; Zervakis, G.I.; Topakas, E. Discovery of two novel laccase-like multicopper oxidases from Pleurotus citrinopileatus and their application in phenolic oligomer synthesis. Biotechnol. Biofuels 2021, 14, 83. [Google Scholar] [CrossRef]
- Frandsen, K.E.H.; Lo Leggio, L. Lytic polysaccharide monooxygenases: A crystallographer’s view on a new class of biomass-degrading enzymes. IUCrJ 2016, 3, 448–467. [Google Scholar] [CrossRef] [Green Version]
- Bissaro, B.; Røhr, Å.K.; Müller, G.; Chylenski, P.; Skaugen, M.; Forsberg, Z.; Horn, S.J.; Vaaje-Kolstad, G.; Eijsink, V.G.H. Oxidative cleavage of polysaccharides by monocopper enzymes depends on H2O2. Nat. Chem. Biol. 2017, 13, 1123. [Google Scholar] [CrossRef]
- Vaaje-Kolstad, G.; Westereng, B.; Horn, S.J.; Liu, Z.; Zhai, H.; Sørlie, M.; Eijsink, V.G.H. An oxidative enzyme boosting the enzymatic conversion of recalcitrant polysaccharides. Science 2010, 330, 219–222. [Google Scholar] [CrossRef]
- Zerva, A.; Pentari, C.; Grisel, S.; Berrin, J.-G.; Topakas, E. A new synergistic relationship between xylan-active LPMO and xylobiohydrolase to tackle recalcitrant xylan. Biotechnol. Biofuels 2020, 13, 142. [Google Scholar] [CrossRef] [PubMed]
- Cannella, D.; Chia-wen, C.H.; Felby, C.; Jørgensen, H. Production and effect of aldonic acids during enzymatic hydrolysis of lignocellulose at high dry matter content. Biotechnol. Biofuels 2012, 5, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frommhagen, M.; Westphal, A.H.; Van Berkel, W.J.H.; Kabel, M.A. Distinct substrate specificities and electron-donating systems of fungal lytic polysaccharide monooxygenases. Front. Microbiol. 2018, 9, 1080. [Google Scholar] [CrossRef] [PubMed]
- Span, E.A.; Marletta, M.A. The framework of polysaccharide monooxygenase structure and chemistry. Curr. Opin. Struct. Biol. 2015, 35, 93–99. [Google Scholar] [CrossRef]
- Chen, H.; Dong, F.; Minteer, S.D. The progress and outlook of bioelectrocatalysis for the production of chemicals, fuels and materials. Nat. Catal. 2020, 3, 225–244. [Google Scholar] [CrossRef]
- Masa, J.; Schuhmann, W. Electrocatalysis and bioelectrocatalysis–Distinction without a difference. Nano Energy 2016, 29, 466–475. [Google Scholar] [CrossRef]
- Zouraris, D.; Karantonis, A. Large amplitude ac voltammetry: Chief observables for a reversible reaction of free electroactive species. J. Electroanal. Chem. 2019, 847, 113245. [Google Scholar] [CrossRef]
- Zouraris, D.; Karantonis, A. Determination of kinetic and thermodynamic parameters from large amplitude Fourier transform ac voltammetry of immobilised electroactive species. J. Electroanal. Chem. 2020, 876, 114729. [Google Scholar] [CrossRef]
- Engblom, S.O.; Myland, J.C.; Oldham, K.B.; Taylor, A.L. Large amplitude ac voltammetry—A comparison between theory and experiment. Electroanalysis 2001, 13, 626–630. [Google Scholar] [CrossRef]
- Bell, C.G.; Anastassiou, C.A.; O’Hare, D.; Parker, K.H.; Siggers, J.H. Large-amplitude ac voltammetry: Theory for reversible redox reactions in the “slow scan limit approximation”. EElectrochim. Acta 2011, 56, 6131–6141. [Google Scholar] [CrossRef]
- Zouraris, D.; Dimarogona, M.; Karnaouri, A.; Topakas, E.; Karantonis, A. Direct electron transfer of lytic polysaccharide monooxygenases (LPMOs) and determination of their formal potentials by large amplitude Fourier transform alternating current cyclic voltammetry. Bioelectrochemistry 2018, 124, 149–155. [Google Scholar] [CrossRef]
- Zouraris, D.; Kiafi, S.; Zerva, A.; Topakas, E.; Karantonis, A. FTacV study of electroactive immobilized enzyme/free substrate reactions: Enzymatic catalysis of epinephrine by a multicopper oxidase from Thermothelomyces thermophila. Bioelectrochemistry 2020, 134, 107538. [Google Scholar] [CrossRef] [PubMed]
- Karnaouri, A.; Muraleedharan, M.N.; Dimarogona, M.; Topakas, E.; Rova, U.; Sandgren, M.; Christakopoulos, P. Recombinant expression of thermostable processive MtEG5 endoglucanase and its synergism with MtLPMO from Myceliophthora thermophila during the hydrolysis of lignocellulosic substrates. Biotechnol. Biofuels 2017, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Savéant, J.-M.; Costentin, C. Elements of Molecular and Biomolecular Electrochemistry: An Electrochemical Approach to Electron Transfer Chemistry, 2nd ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2019. [Google Scholar]
- Zhang, Y.; Simonov, A.N.; Zhang, J.; Bond, A.M. Fourier transformed alternating current voltammetry in electromaterials research: Direct visualisation of important underlying electron transfer processes. Curr. Opin. Electrochem. 2018, 10, 72–81. [Google Scholar] [CrossRef]
- Song, P.; Ma, H.; Meng, L.; Wang, Y.; Nguyen, H.V.; Lawrence, N.S.; Fisher, A.C. Fourier transform large amplitude alternating current voltammetry investigations of the split wave phenomenon in electrocatalytic mechanisms. Phys. Chem. Chem. Phys. 2017, 19, 24304–24315. [Google Scholar] [CrossRef] [PubMed]
- Léger, C.; Jones, A.K.; Albracht, S.P.J. Armstrong, F.A. Effect of a dispersion of interfacial electron transfer rates on steady state catalytic electron transport in [NiFe]-hydrogenase and other enzymes. J. Phys. Chem. B 2002, 106, 13058–13063. [Google Scholar] [CrossRef]
- Robinson, M.; Ounnunkad, K.; Zhang, J.; Gavaghan, D.; Bond, A.M. Models and their limitations in the voltammetric parameterization of the six-electron surface-confined reduction of [PMo12O40]-3 at glassy carbon and boron-doped diamond electrodes. ChemElectrochem 2019, 6, 5499–5510. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.A.; Guo, S.-X.; Laurans, M.; Izzet, G.; Proust, A.; Bond, A.M.; Zhang, J. Thermodynamics, electrode kinetics, and mechanistic nuances associated with the voltammetric reduction of dissolved [n-Bu4N]4[PW11O39{Sn(C6H4)C≡C(C6H4)(N3C4H10)}] and a surface-confined diazonium derivative. ACS Appl. Energy Mater. 2020, 3, 13991–14006. [Google Scholar] [CrossRef]
- Morris, G.P.; Baker, R.E.; Gillow, K.; Davis, J.J.; Gavaghan, D.J.; Bond, A.M. Theoretical analysis of the relative significance of thermodynamic and kinetic dispersion in the dc and ac voltammetry of surface-confined molecules. Langmuir 2015, 31, 4996–5004. [Google Scholar] [CrossRef]
- Stevenson, G.P.; Baker, R.E.; Kennedy, G.F.; Bond, A.M.; Gavaghan, D.J.; Gillow, K. Access to enhanced differences in Marcus-Hush and Butler-Volmer electron transfer theories by systematic analysis of higher order AC harmonics. Phys. Chem. Chem. Phys. 2013, 15, 2210–2221. [Google Scholar] [CrossRef]



| /s | /V |
|---|---|
| 100 | 0.000 |
| 20 | 0.008 |
| 10 | 0.014 |
| 5 | 0.026 |
| 2 | 0.059 |
| 1 | 0.098 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zouraris, D.; Karnaouri, A.; Xydou, R.; Topakas, E.; Karantonis, A. Exploitation of Enzymes for the Production of Biofuels: Electrochemical Determination of Kinetic Parameters of LPMOs. Appl. Sci. 2021, 11, 4715. https://doi.org/10.3390/app11114715
Zouraris D, Karnaouri A, Xydou R, Topakas E, Karantonis A. Exploitation of Enzymes for the Production of Biofuels: Electrochemical Determination of Kinetic Parameters of LPMOs. Applied Sciences. 2021; 11(11):4715. https://doi.org/10.3390/app11114715
Chicago/Turabian StyleZouraris, Dimitrios, Anthi Karnaouri, Raphaela Xydou, Evangelos Topakas, and Antonis Karantonis. 2021. "Exploitation of Enzymes for the Production of Biofuels: Electrochemical Determination of Kinetic Parameters of LPMOs" Applied Sciences 11, no. 11: 4715. https://doi.org/10.3390/app11114715
APA StyleZouraris, D., Karnaouri, A., Xydou, R., Topakas, E., & Karantonis, A. (2021). Exploitation of Enzymes for the Production of Biofuels: Electrochemical Determination of Kinetic Parameters of LPMOs. Applied Sciences, 11(11), 4715. https://doi.org/10.3390/app11114715







