Towards a Glass New World: The Role of Ion-Exchange in Modern Technology
Abstract
1. Introduction
2. Glass Strengthening: The Ion-Exchange Contribution
2.1. Thermal Strengthening
2.2. Chemical Strengthening
2.3. Chemical Strengthening: Applications
3. Noble Metal Ions/Nanoparticles by Ion-Exchange in Glass Substrates: Novel Platform for Photonic & Sensing Applications
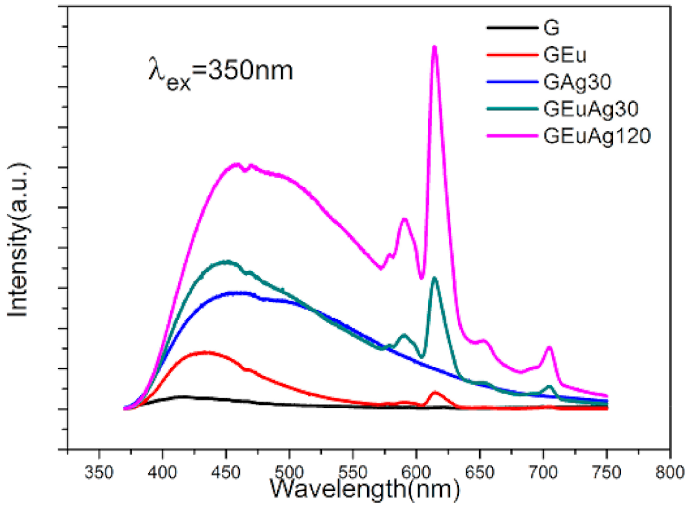
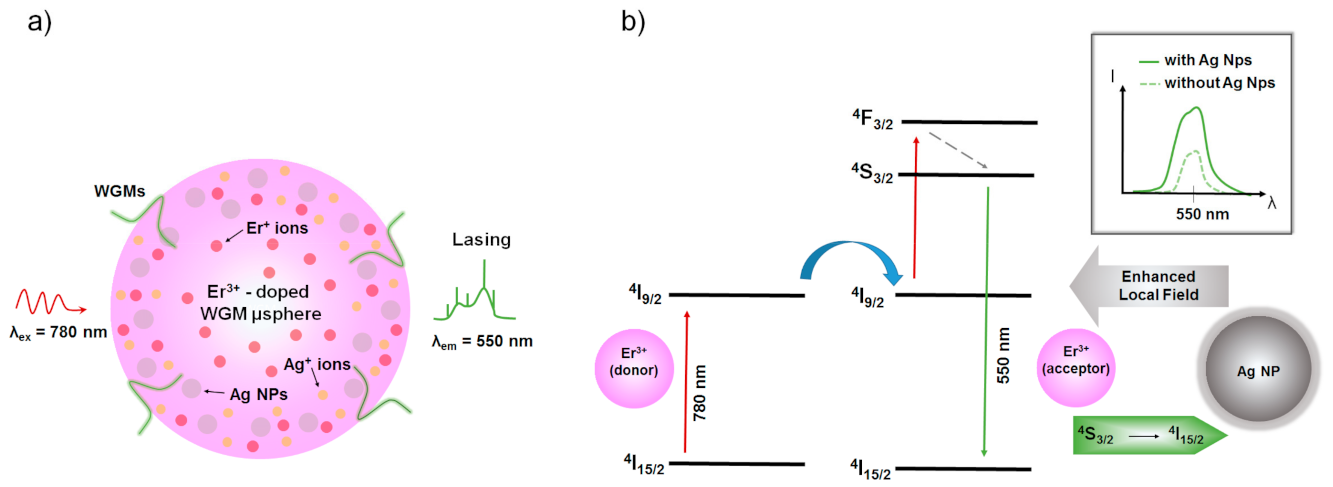
3.1. Ion-Exchanged Noble Metal Ions/Nanoparticles in Rare-Earth—Doped Glasses for Enhanced Light Sources and Photovoltaic Cells
3.2. Ion-Exchanged Noble Metal Nanoparticles in Glasses for Sers Applications
3.2.1. Glasses for SERS Substrate by Ion-Exchange Technique
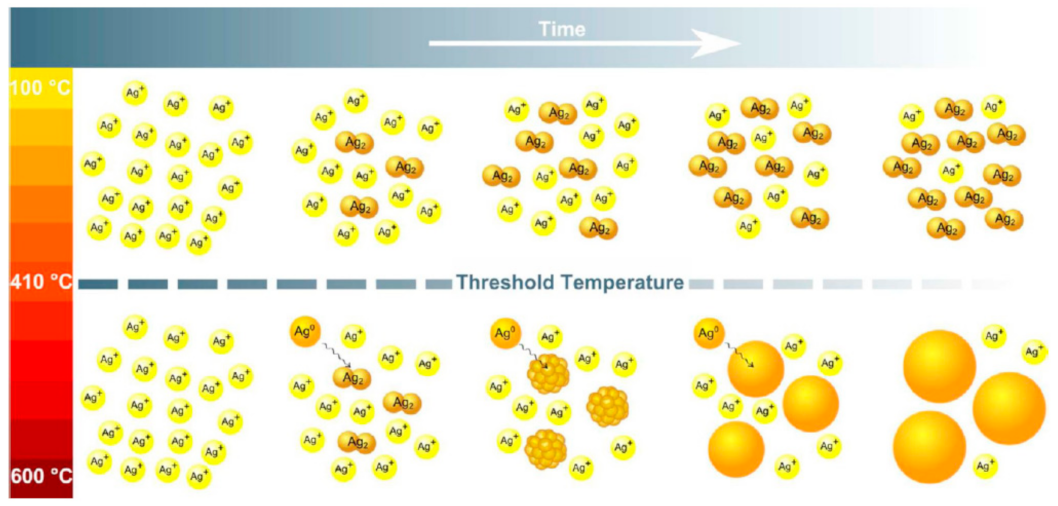
3.2.2. Thermal Annealing in Air (O2 Atmosphere)
3.2.3. Thermal Annealing in H2 Controlled Atmosphere
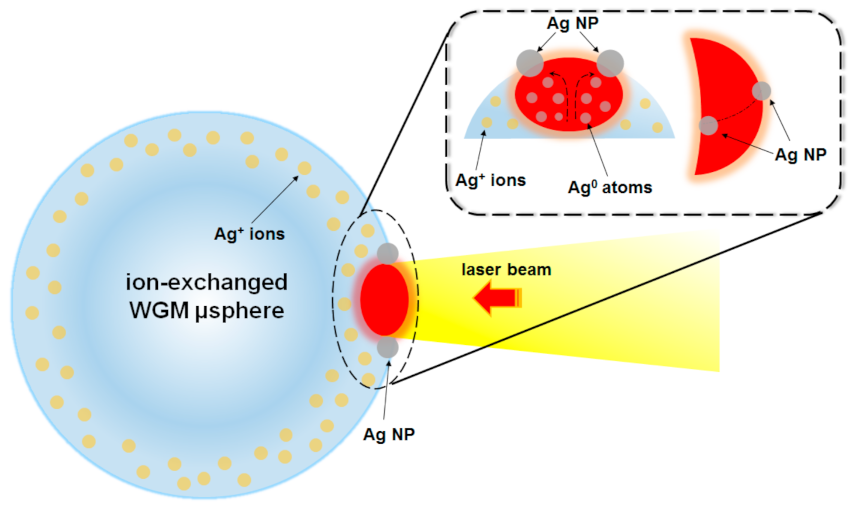
3.2.4. Laser Irradiation Techniques
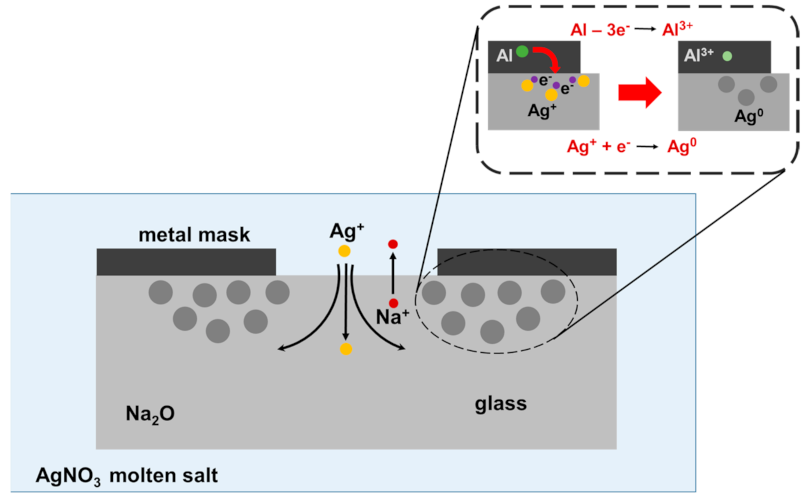
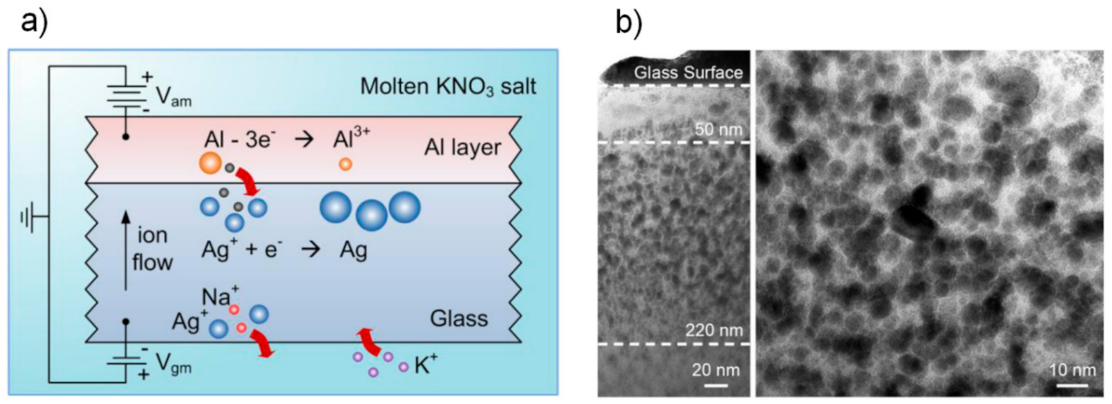
3.2.5. Metal Thin Film Deposition for Noble Metal Ions Reduction in Ion Exchange Process
4. Perspectives & Future Insights
4.1. All-Glass Flexible Photonics
4.2. New Insight towards the Development of Optical Devices Loaded with Noble Metal Ions/Nanoparticles by Ion-Exchange
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rehren, T.; Freestone, I.C. Ancient glass: From kaleidoscope to crystal ball. J. Archeol. Sci. 2015, 56, 233–241. [Google Scholar] [CrossRef]
- Scott, R.B.; Brems, D. The archaeometry of ancient glassmaking: Reconstructing ancient technology and the trade of raw materials. Perspective 2014, 2, 224–238. [Google Scholar] [CrossRef]
- Righini, G.C.; Laybourn, P.J.R. Integrated Optics. In Perspectives in Optoelectronics, 1st ed.; Jha, S.S., Ed.; World Scientific Publishing Co. Pte. Ltd.: Singapore; Hackensack, NJ, USA; London, UK; Hong Kong, China, 1995; Chapter 12; pp. 679–736. [Google Scholar]
- Righini, G.C.; Pelli, S. Ion-exchange in glass: A mature technology for photonics devices. In Proceedings of the International Symposium on Optical Science and Technology, San Diego, CA, USA, 29 July–3 August 2001; Volume 4453, pp. 93–99. [Google Scholar] [CrossRef]
- Pradell, T.; Molera, J.; Roque, J.; Vendrell-Saz, M.; Smith, A.D.; Pantos, E.; Crespo, D. Ionic-exchange mechanism in the formation of medieval luster decorations. J. Am. Ceram. Soc. 2005, 88, 1281–1289. [Google Scholar] [CrossRef]
- Mazzoldi, P.; Sada, C. A trip in the history and evolution of ion-exchange process. Mat. Sci. Eng. B 2008, 149, 112–117. [Google Scholar] [CrossRef]
- Mazzoldi, P.; Carturan, S.; Quaranta, A.; Sada, C.; Sglavo, V.M. Ion-exchange process: History, evolution and applications. Riv. Nuovo Cim. 2013, 36, 397–460. [Google Scholar] [CrossRef]
- Schulze, G. Versuche über die diffusion von silber in glas. Ann. Phys. 1913, 345, 335–367. [Google Scholar] [CrossRef]
- Kistler, S.S. Stresses in glass produced by nonuniform exchange of monovalent Ions. J. Am. Ceram. Soc. 1962, 45, 59–68. [Google Scholar] [CrossRef]
- Acloque, P.; Tochon, J. Measurement of Mechanical Resistance of Glass after Reinforcement. In Colloquium on Mechanical Strength of Glass and Ways of Improving It; Union Scientifique Continentale du Verre: Charleroi, Belgium, 1962; Volume 1044, pp. 687–704. [Google Scholar]
- Donald, I.W. Methods for improving the mechanical properties of oxide glasses. J. Mater. Sci. 1989, 24, 4177–4208. [Google Scholar] [CrossRef]
- Gy, R. Ion-exchange for glass strengthening. Mater. Sci. Eng. B 2008, 149, 159–165. [Google Scholar] [CrossRef]
- Karlsson, S.; Jonson, B. The technology of chemical glass strengthening—A review. Glass Technol. Eur. J. Glass Sci. Technol. A 2010, 51, 41–54. [Google Scholar]
- Agrawal, G.P. Optical Communication: Its History and Recent Progress. In Optics in Our Time; Al-Amri, M., El-Gomati, M., Zubairy, M., Eds.; Springer: Cham, Switzerland, 2016; Chapter 8; pp. 177–199. [Google Scholar] [CrossRef]
- Maurer, R.D.; Schultz, P.C. Fused Silica Optical Waveguide. US Patent 365995, 2 May 1972. [Google Scholar]
- Keck, D.B.; Schultz, P.C. Method of Producing Optical Waveguide Fibres. US Patent 3711262, 16 January 1973. [Google Scholar]
- Miller, S.E. Integrated Optics: An Introduction. Bell Syst. Tech. J. 1969, 48, 2059–2069. [Google Scholar] [CrossRef]
- Izawa, T.; Nakagome, H. Optical waveguide formed by electrically induced migration of ions in glass plates. Appl. Phys. Lett. 1972, 21, 584–586. [Google Scholar] [CrossRef]
- Giallorenzi, T.G.; West, E.J.; Kirk, R.D.; Ginther, R.J.; Andrews, R.A. Optical waveguides formed by thermal migration of ions in glass. Appl. Opt. 1973, 12, 1240–1245. [Google Scholar] [CrossRef] [PubMed]
- Righini, G.C.; Chiappini, A. Glass optical waveguides: A review of fabrication techniques. Opt. Eng. 2014, 53, 071819. [Google Scholar] [CrossRef]
- Thervonen, A.; West, B.R.; Honkanen, S. Ion-exchanged glass waveguide technology: A review. Opt. Eng. 2011, 50, 071107. [Google Scholar] [CrossRef]
- Ramaswamy, R.V.; Srivastava, R. Ion-exchanged glass waveguides: A review. IEEE J. Light. Technol. 1988, 6, 984–1000. [Google Scholar] [CrossRef]
- Honkanen, S.; West, B.R.; Yliniemi, S.; Madasamy, P.; Morrell, M.; Auxier, J.; Schülzgen, A.; Peyghambarian, N.; Carriere, J.; Frantz, J.; et al. Recent advances in ion-exchanged glass waveguides and devices. Phys. Chem. Glasses: Eur. J. Glass Sci. Technol. B 2006, 47, 110–120. [Google Scholar]
- Äyräs, P.; Nunzi Conti, G.; Honkanen, S.; Peyghambarian, N. Birefringence control for ion-exchanged channel glass waveguides. Appl. Opt. 1998, 37, 8400–8405. [Google Scholar] [CrossRef]
- Asquini, R.; D’Angelo, J.; d’Alessandro, A. A Switchable Optical Add-Drop Multiplexer using Ion-Exchange Waveguides and a POLICRYPS Grating Overlayer. Mol. Cryst. Liq. Cryst. 2006, 450, 203–214. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Donisi, D.; De Sio, L.; Beccherelli, R.; Asquini, R.; Caputo, R.; Umeton, C. Tunable integrated optical filter made of a glass ion-exchanged waveguide and an electro-optic composite holographic grating. Opt. Express 2008, 16, 9254–9260. [Google Scholar] [CrossRef]
- Montero-Orille, C.; Moreno, V.; Prieto-Blanco, X.; Mateo, E.F.; Ip, E.; Crespo, J.; Liñares, J. Ion-exchanged glass binary phase plates for mode-division multiplexing. Appl. Opt. 2013, 52, 2332–2339. [Google Scholar] [CrossRef]
- Montero-Orille, C.; Prieto-Blanco, X.; González-Núñez, H.; Liñares, J. A Polygonal Model to Design and Fabricate Ion-Exchanged Diffraction Gratings. Appl. Sci. 2021, 11, 1500. [Google Scholar] [CrossRef]
- Bradley, J.D.B.; Pollnau, M. Erbium-doped integrated waveguide amplifiers and lasers. Laser Photonics Rev. 2011, 5, 368–403. [Google Scholar] [CrossRef]
- Righini, G.C.; Brenci, M.; Forastiere, M.A.; Pelli, S.; Ricci, R.; Nunzi Conti, G.; Peyghambarian, N.; Ferrari, M.; Montagna, M. Rare-earth-doped glasses and ion-exchanged integrated optical amplifiers and lasers. Phylos. Mag. 2002, 82, 721–734. [Google Scholar] [CrossRef]
- Pelli, S.; Bettinelli, M.; Brenci, M.; Calzolai, R.; Chiasera, A.; Ferrari, M.; Nunzi Conti, G.; Speghini, A.; Zampedri, L.; Zheng, J.; et al. Erbium-doped silicate glasses for integrated optical amplifiers and lasers. J. Non Cryst. Solids 2004, 345–346, 372–376. [Google Scholar] [CrossRef]
- Righini, G.C.; Arnaud, C.; Berneschi, S.; Bettinelli, M.; Brenci, M.; Chiasera, A.; Feron, P.; Ferrari, M.; Montagna, M.; Nunzi Conti, G.; et al. Integrated optical amplifiers and microspherical lasers based on erbium-doped oxide glasses. Opt. Mater. 2005, 27, 1711–1717. [Google Scholar] [CrossRef]
- Berneschi, S.; Bettinelli, M.; Brenci, M.; Dall’Igna, R.; Nunzi Conti, G.; Pelli, S.; Profilo, B.; Sebastiani, S.; Speghini, A.; Righini, G.C. Optical and spectroscopic properties of soda-lime alumino silicate glasses doped with Er3+ and/or Yb3+. Opt. Mater. 2006, 28, 1271–1275. [Google Scholar] [CrossRef]
- Ondráček, F.; Jágerská, J.; Salavcová, L.; Míka, M.; Špirková, J.; Čtyroký, J. Er–Yb Waveguide Amplifiers in Novel Silicate Glasses. IEEE J. Quantum Electron. 2008, 44, 536–541. [Google Scholar] [CrossRef]
- Laporta, P.; Taccheo, S.; Longhi, S.; Svelto, O.; Svelto, C. Erbium–ytterbium microlasers: Optical properties and lasing characteristics. Opt. Mater. 1999, 11, 269–288. [Google Scholar] [CrossRef]
- Della Valle, G.; Taccheo, S.; Sorbello, G.; Cianci, E.; Foglietti, V.; Laporta, P. Compact high gain erbium-ytterbium doped waveguide amplifier fabricated by Ag-Na ion-exchange. Electron. Lett. 2006, 42, 632–633. [Google Scholar] [CrossRef]
- Jaouën, Y.; du Mouza, L.; Barbier, D.; Delavaux, J.-M.; Bruno, P. Eight-Wavelength Er–Yb Doped Amplifier: Combiner/Splitter Planar Integrated Module. IEEE Photonics Technol. Lett. 1999, 11, 1105–1107. [Google Scholar] [CrossRef]
- Conzone, S.; Hayden, J.S.; Funk, D.S.; Roshko, A.; Veasey, D.L. Hybrid glass substrates for waveguide device manufacture. Opt. Lett. 2001, 26, 509–511. [Google Scholar] [CrossRef]
- Yliniemi, S.; Albert, J.; Wang, Q.; Honkanen, S. UV-exposed Bragg gratings for laser applications in silver-sodium phosphate glass waveguides. Opt. Express 2006, 14, 2898–2903. [Google Scholar] [CrossRef] [PubMed]
- Molina, G.; Murica, S.; Molera, J.; Roldan, C.; Crespo, D.; Pradell, T. Color and dichroism of silver-stained glasses. J. Nanopart. Res. 2013, 15, 1932–1937. [Google Scholar] [CrossRef]
- Puche-Roig, A.; Martín, V.P.; Murcia- Mascarós, S.; Ibáñez Puchades, R. Float glass colouring by ion-exchange. J. Cult. Herit. 2008, 9, e129–e133. [Google Scholar] [CrossRef]
- Berg, K.-J.; Berger, K.A.; Hofmeister, H. Small silver particles in glass surface layers produced by sodium-silver ion-exchange—their concentration and size depth profile. Z. Phys. D At. Mol. Clust. 1991, 20, 309–311. [Google Scholar] [CrossRef]
- Berger, A. Concentration and size depth profile of colloidal silver particles in glass surface produced by sodium-silver ion-exchange. J. Non Cryst. Solids 1992, 151, 88–94. [Google Scholar] [CrossRef]
- Manikandan, D.; Mohan, S.; Magudapathy, P.; Nair, K.G.M. Blue shift of plasmon resonance in Cu and Ag ion-exchanged and annealed soda-lime glass: An optical absorption study. Phys. B Condens. Matter. 2003, 325, 86–91. [Google Scholar] [CrossRef]
- Quaranta, A.; Rahman, A.; Mariotto, G.; Maurizio, C.; Trave, E.; Gonella, F.; Cattaruzza, E.; Gibaudo, E.; Broquin, J.E. Spectroscopic Investigation of Structural Rearrangements in Silver Ion-Exchanged Silicate Glasses. J. Phys. Chem. C 2012, 116, 3757–3764. [Google Scholar] [CrossRef]
- Mohapatra, S. Tunable surface plasmon resonance of silver nanoclusters in ion-exchanged soda lime glass. J. Alloys Compd. 2014, 598, 11–15. [Google Scholar] [CrossRef]
- Madrigal, J.B.; Tellez-Limon, R.; Gardillou, F.; Barbier, D.; Geng, W.; Couteau, C.; Salas-Montiel, R.; Blaize, S. Hybrid integrated optical waveguides in glass for enhanced visible photoluminescence of nanoemitters. Appl. Opt. 2016, 55, 10263–10268. [Google Scholar] [CrossRef] [PubMed]
- Ashley, P.R.; Bloemer, M.J.; Davis, J.H. Measurement of nonlinear properties in Ag-ion-exchange waveguides using degenerate four-wave mixing. Appl. Phys. Lett. 1990, 57, 1488–1490. [Google Scholar] [CrossRef]
- Faccio, D.; Trapani, D.P.; Borsella, E.; Gonella, F.; Mazzoldi, P.; Malvezzi, A.M. Measurement of the third-order nonlinear susceptibility of Ag nanoparticles in glass in a wide spectral range. Europhys. Lett. 1998, 43, 213–218. [Google Scholar] [CrossRef][Green Version]
- Tagantsev, D.K.; Kazansky, P.G.; Lipovskii, A.A.; Maluev, K.D. Ion-exchange-induced formation of glassy electrooptical and nonlinear optical nanomaterial. J. Non Cryst. Solids 2008, 354, 1369–1372. [Google Scholar] [CrossRef]
- Karvonen, L.; Rönn, J.; Kujala, S.; Chen, Y.; Säynätjoki, A.; Tervonen, A.; Svirko, Y.; Honkanen, S. High non-resonant third-order optical nonlinearity of Ag–glass nanocomposite fabricated by two-step ion-exchange. Opt. Mater. 2013, 36, 328–332. [Google Scholar] [CrossRef]
- Xiang, W.; Gao, H.; Ma, L.; Ma, X.; Huang, Y.; Pei, L.; Liang, X. Valence State Control and Third-Order Nonlinear Optical Properties of Copper Embedded in Sodium Borosilicate Glass. ACS Appl. Mater. Interfaces 2015, 7, 10162–10168. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Mathpal, M.C.; Hamad, S.; Rao, S.V.; Neethling, J.H.; Janse, A.; van Vuuren, A.J.; Njoroge, E.G.; Kroon, R.E.; Roos, W.D.; et al. Cu nanoclusters in ion-exchanged soda-lime glass: Study of SPR and nonlinear optical behavior for photonics. Appl. Mater. Today 2019, 15, 323–334. [Google Scholar] [CrossRef]
- Malta, O.; Santa-Cruz, P.A.; de Sa´, G.F.; Auzel, F. Fluorescence enhancement induced by the presence of small silver particles in Eu3+ doped materials. J. Lumin. 1985, 33, 261–272. [Google Scholar] [CrossRef]
- Hayakawa, T.; Selvan, S.T.; Nogami, M. Field enhancement effect of small Ag particles on the fluorescence from Eu3+-doped SiO2 glass. Appl. Phys. Lett. 1999, 74, 1513–1515. [Google Scholar] [CrossRef]
- Strohhöfer, C.; Polman, A. Silver as a sensitizer for erbium. Appl. Phys. Lett. 2002, 81, 1414–1416. [Google Scholar] [CrossRef]
- Chiasera, A.; Ferrari, M.; Mattarelli, M.; Montagna, M.; Pelli, S.; Portales, H.; Zheng, J.; Righini, G.C. Assessment of spectroscopic properties of erbium ions in a soda-lime silicate glass after silver–sodium exchange. Opt. Mater. 2005, 27, 1743–1747. [Google Scholar] [CrossRef]
- Mattarelli, M.; Montagna, M.; Vishnubhatla, K.; Chiasera, A.; Ferrari, M.; Righini, G.C. Mechanisms of Ag to Er energy transfer in silicate glasses: A photoluminescence study. Phys. Rev. B 2007, 75, 125102. [Google Scholar] [CrossRef]
- Li, L.; Yang, Y.; Zhou, D.; Yang, Z.; Xu, X.; Qiu, J. Investigation of the role of silver species on spectroscopic features of Sm3+-activated sodium aluminosilicate glasses via Ag+-Na+ ion-exchange. J. Appl. Phys. 2013, 113, 193103. [Google Scholar] [CrossRef]
- Li, L.; Yang, Y.; Zhou, D.; Yang, Z.; Xu, X.; Qiu, J. Investigation of the interaction between different types of Ag species and europium ions in Ag+-Na+ ion-exchange glass. Opt. Mater. Express 2013, 3, 806–812. [Google Scholar] [CrossRef]
- Gonella, F. Silver doping glasses. Ceram. Int. 2015, 41, 6693–6701. [Google Scholar] [CrossRef]
- Stanek, S.; Nekvindova, P.; Svecova, B.; Vytykacova, S.; Mika, M.; Oswald, J.; Barkman, O.; Spirkova, J. The influence of silver ion-exchange on the luminescence properties of Er-Yb silicate glasses. Opt. Mater. 2017, 72, 183–189. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, Z.; Yu, C.; Qiu, J.; Song, Z. Preparation of ultra-small molecule-like Ag nano-clusters in silicate glass based on ion-exchange process: Energy transfer investigation from molecule-like Ag nano-clusters to Eu3+ ions. Chem. Eng. J. 2018, 341, 175–186. [Google Scholar] [CrossRef]
- Zhao, J.; Zhu, J.; Yang, Z.; Jiao, Q.; Yu, C.; Qiu, J.; Song, Z. Selective preparation of Ag species on photoluminescence of Sm3+ in borosilicate glass via Ag+-Na+ ion-exchange. J. Am. Ceram. Soc. 2019, 103, 955–964. [Google Scholar] [CrossRef]
- Mironov, L.Y.; Sgibnev, Y.M.; Kolesnikov, I.E.; Nikonorov, N.V. Luminescence and energy transfer mechanisms in photo-thermo-refractive glasses co-doped with silver molecular clusters and Eu3+. Phys. Chem. Chem. Phys. 2020, 22, 23342–23350. [Google Scholar] [CrossRef]
- Sgibnev, Y.; Asamoah, B.; Nikonorov, N.; Honkanen, S. Tunable photoluminescence of silver molecular clusters formed in Na+-Ag+ ion-exchanged antimony-doped photo-thermo-refractive glass matrix. J. Lumin. 2020, 226, 117411. [Google Scholar] [CrossRef]
- Allsopp, B.L.; Orman, R.; Johnson, S.R.; Baistow, I.; Sanderson, G.; Sundberg, P.; Stålhandske, C.; Grund, L.; Andersson, A.; Booth, J.; et al. Towards improved cover glasses for photovoltaic devices. Prog. Photovolt. Res. Appl. 2020, 28, 1187–1206. [Google Scholar] [CrossRef]
- Huang, X.; Han, S.; Huang, W.; Liu, X. Enhancing solar cell efficiency: The search for luminescent materials as spectral converters. Chem. Soc. Rev. 2013, 42, 173–201. [Google Scholar] [CrossRef] [PubMed]
- Cattaruzza, E.; Mardegan, M.; Pregnolato, T.; Ungaretti, G.; Aquilanti, G.; Quaranta, A.; Battaglin, G.; Trave, E. Ion-exchange doping of solar cell coverglass for sunlight down-shifting. Sol. Energy Mater. Sol. Cells 2014, 130, 272–280. [Google Scholar] [CrossRef]
- Cattaruzza, E.; Caselli, V.M.; Mardegan, M.; Gonella, F.; Bottaro, G.; Quaranta, A.; Valotto, G.; Enrichi, F. Ag+↔Na+ ion-exchanged silicate glasses for solar cells covering: Down-shifting properties. Ceram. Int. 2015, 41, 7221–7226. [Google Scholar] [CrossRef]
- Mardegan, M.; Cattaruzza, E. Cu-doped photovoltaic glasses by ion-exchange for sunlight down–shifting. Opt. Mater. 2016, 61, 105–110. [Google Scholar] [CrossRef]
- Li, Y.; Chen, F.; Liu, C.; Han, J.; Zhao, X. UV–Visible spectral conversion of silver ion-exchanged aluminosilicate glasses. J. Non-Cryst. Solids 2017, 471, 82–90. [Google Scholar] [CrossRef]
- Enrichi, F.; Armellini, C.; Battaglin, G.; Belluomo, F.; Belmokhtar, S.; Bouajaj, A.; Cattaruzza, E.; Ferrari, M.; Gonella, F.; Lukowiak, A.; et al. Silver doping of silica-hafnia waveguides containing Tb3+/Yb3+ rare earths for down conversion in PV solar cells. Opt. Mater. 2016, 60, 264–269. [Google Scholar] [CrossRef]
- Sgibnev, Y.; Cattaruzza, E.; Dubrovin, V.; Vasilyev, V.; Nikonorov, N. Photo-Thermo-Refractive Glasses Doped with Silver Molecular Clusters as Luminescence Downshifting Material for Photovoltaic Applications. Part. Part. Syst. Charact. 2018, 35, 1800141. [Google Scholar] [CrossRef]
- Bubli, I.; Ali, S.; Ali, M.; Hayat, K.; Iqbal, Y.; Zulfiqar, S.; ul Haq, A.; Cattaruzza, E. Enhancement of solar cell efficiency via luminescent downshifting by an optimized coverglass. Ceram. Int. 2020, 46, 2110–2115. [Google Scholar] [CrossRef]
- Enrichi, F.; Belmokhtar, S.; Benedetti, A.; Bouajaj, A.; Cattaruzza, E.; Coccetti, F.; Colusso, E.; Ferrari, M.; Ghamgosar, P.; Gonella, F.; et al. Ag nanoaggregates as efficient broadband sensitizers for Tb3+ ions in silica-zirconia ion-exchanged sol-gel glasses and glass-ceramics. Opt. Mater. 2018, 84, 668–674. [Google Scholar] [CrossRef]
- Tresnakova, P.; Malichova, H.; Spirkova, J.; Mika, M. Fabrication of optical layers containing Er(III) and Cu(I) in silicate glasses. J. Phys. Chem. Solids 2007, 68, 1276–1279. [Google Scholar] [CrossRef]
- Yu, C.; Zhao, J.; Zhu, J.; Huang, A.; Qiu, J.; Song, Z.; Zhou, D. Luminescence enhancement and white light generation of Eu3+ and Dy3+ single-doped and co-doped tellurite glasses by Ag nanoparticles based on Ag+-Na+ ion-exchange. J. Alloys Compd. 2018, 748, 717–729. [Google Scholar] [CrossRef]
- Tang, L.; Chen, N. White light emitting YVO4:Eu3+,Tm3+,Dy3+ nanometer- and submicrometer-sized particles prepared by an ion-exchange method. Ceram. Int. 2016, 42, 302–309. [Google Scholar] [CrossRef]
- Sgibnev, Y.M.; Nikonorov, N.V.; Ignatiev, A.I. Luminescence of silver clusters in ion-exchanged cerium-doped photo-thermo-refractive glasses. J. Lumin. 2016, 176, 292–297. [Google Scholar] [CrossRef]
- Sgibnev, Y.M.; Nikonorov, N.V.; Ignatiev, A.I. High efficient luminescence of silver clusters in ion-exchanged antimony doped photo-thermo-refractive glasses: Influence of antimony content and heat treatment parameters. J. Lumin. 2017, 188, 172–179. [Google Scholar] [CrossRef]
- Zhou, Y.; Laybourn, P.J.R.; Magill, J.V.; De La Rue, R.M. An evanescent fluorescence biosensor using ion-exchanged buried waveguides and the enhancement of peak fluorescence. Biosens. Bioelectron. 1991, 6, 595–607. [Google Scholar] [CrossRef]
- Zhou, Y.; Magill, J.V.; De La Rue, R.M.; Laybourn, P.J.R. Evanescent fluorescence immunoassays performed with a disposable ion-exchanged patterned waveguide. Sens. Actuators B Chem. 1993, 11, 245–250. [Google Scholar] [CrossRef]
- Srivastava, R.; Bao, C.; Gómez-Reino, C. Planar-surface waveguide evanescent-wave chemical sensors. Sens. Actuators A Phys. 1996, 51, 165–171. [Google Scholar] [CrossRef]
- Hassanzadeh, A.; Nitsche, M.; Mittler, S.; Armstrong, S.; Dixon, S.J.; Langbein, U. Waveguide evanescent field fluorescence microscopy: Thin film fluorescence intensities and its application in cell biology. Appl. Phys. Lett. 2008, 92, 233503. [Google Scholar] [CrossRef]
- Hassanzadeh, A.; Nitsche, M.; Armstrong, S.; Nabavi, N.; Harrison, R.; Dixon, S.J.; Langbein, U. Optical waveguides formed by silver ion-exchange in Schott SG11 glass for waveguide evanescent field fluorescence microscopy: Evanescent images of HEK293 cells. J. Biomed. Opt. 2010, 15, 036018. [Google Scholar] [CrossRef]
- Araci, I.E.; Mendes, S.B.; Yurt, N.; Honkanen, S.; Peyghambarian, N. Highly sensitive spectroscopic detection of heme-protein sub-monolayer films by channel integrated optical waveguide. Opt. Express 2007, 15, 5595–5603. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Karabchevsky, A.; Kavokin, A.V. Giant absorption of light by molecular vibrations on a chip. Sci. Rep. 2016, 6, 21201. [Google Scholar] [CrossRef]
- Zhu, M.; Kari, N.; Yan, Y.; Yimit, A. The fabrication and gas sensing application of a fast-responding m-CP-PVP composite film/potassium ion-exchanged glass optical waveguide. Anal. Methods 2017, 9, 5494–5501. [Google Scholar] [CrossRef]
- Du, B.; Tong, Z.; Mu, X.; Xu, J.; Liu, S.; Liu, Z.; Cao, W.; Qi, Z.-M. A Potassium Ion-Exchanged Glass Optical Waveguide Sensor Locally Coated with a Crystal Violet-SiO2 Gel Film for Real-Time Detection of Organophosphorus Pesticides Simulant. Sensors 2019, 19, 4219. [Google Scholar] [CrossRef] [PubMed]
- Homola, J.; Čtyroký, J.; Skalský, M.; Hradilová, J.; Kolářová, J.P. A surface plasmon resonance based integrated optical sensor. Sens. Actuators B Chem. 1997, 39, 286–290. [Google Scholar] [CrossRef]
- Dostálek, J.; Čtyroký, J.; Homola, J.; Brynda, E.; Skalský, M.; Nekvindová, P.; Špirková, J.; Škvor, J.; Schröfel, J. Surface plasmon resonance biosensor based on integrated optical waveguide. Sens. Actuators B Chem. 2001, 76, 8–12. [Google Scholar] [CrossRef]
- De Bonnault, S.; Bucci, D.; Zermatten, P.J.; Charette, P.G.; Broquin, J.E. Hybrid metallic ion-exchanged waveguides for SPR biological sensing. In Proceedings of the SPIE, San Francisco, CA, USA, 7–12 February 2015; Volume 9365, p. 93650. [Google Scholar] [CrossRef]
- Tellez-Limon, R.; Gardillou, F.; Coello, V.; Salas-Montiel, R. Coupled localized surface plasmon resonances in periodic arrays of gold nanowires on ion-exchange waveguide technology. J. Opt. 2021, 23, 25801. [Google Scholar] [CrossRef]
- Nashchekin, A.V.; Nevedomskiy, V.N.; Obraztsov, P.A.; Stepanenko, O.V.; Sidorov, A.I.; Usov, O.A.; Turoverov, K.K.; Konnikov, S.G. Waveguide-type localized plasmon resonance biosensor for noninvasive glucose concentration detection. In Proceedings of the SPIE, Brussels, Belgium, 16–19 April 2012; Volume 8427, p. 842739. [Google Scholar] [CrossRef]
- Pilot, R.; Signorini, R.; Durante, C.; Orian, L.; Bhamidipati, M.; Fabris, L. A Review on Surface-Enhanced Raman Scattering. Biosensors 2019, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- Mosier-Boss, P.A. Review of SERS Substrates for Chemical Sensing. Nanomaterials 2017, 7, 142. [Google Scholar] [CrossRef]
- Zhang, J.; Dong, W.; Sheng, J.; Zheng, J.; Li, J.; Qiao, L.; Jiang, L. Silver nanoclusters formation in ion-exchanged glasses by thermal annealing, UV-laser and X-ray irradiation. J. Non Cryst. Solids 2008, 310, 234–239. [Google Scholar] [CrossRef]
- Kolobkova, E.; Sergeevna Kuznetsova, M.; Nikonorov, N. Ag/Na Ion-exchange in Fluorophosphate Glasses and Formation of Ag Nanoparticles in the Bulk and on the Surface of the Glass. ACS Appl. Nano Mater. 2019, 2, 6928–6938. [Google Scholar] [CrossRef]
- Karvonen, L.; Chen, Y.; Säynätjoki, A.; Taiviola, K.; Tervonen, A.; Honkanen, S. SERS-active silver nanoparticle aggregates produced in high-iron float glass by ion-exchange process. Opt. Mater. 2011, 34, 1–5. [Google Scholar] [CrossRef]
- Babich, E.; Kaasik, V.; Redkov, A.; Maurer, T.; Lipovskii, A. SERS-Active Pattern in Silver-Ion-Exchange Glass Drawn by Infrared Nanosecond Laser. Nanomaterials 2020, 10, 1849. [Google Scholar] [CrossRef] [PubMed]
- Tite, T.; Ollier, N.; Sow, M.C.; Vocanson, F.; Goutaland, F. Ag nanoparticles in soda-lime glass grown by continuous wave laser irradiation as an efficient SERS platform for pesticides detection. Sens. Actuators B Chem. 2017, 242, 127–131. [Google Scholar] [CrossRef]
- Chen, Y.; Jaakola, J.; Ge, Y.; Säynätjoki, A.; Tervonen, A.; Hannula, S.-P.; Honkanen, S. In situ fabrication of waveguide-compatible glass-embedded silver nanoparticle patterns by masked ion-exchange process. J. Non Cryst. Solids 2009, 355, 2224–2227. [Google Scholar] [CrossRef]
- Chen, Y.; Jaakola, J.; Säynätjoki, A.; Tervonen, A.; Honkanen, S. Glass-embedded silver nanoparticle patterns by masked ion-exchange process for surface-enhanced Raman scattering. J. Raman Spectrosc. 2011, 42, 936–940. [Google Scholar] [CrossRef]
- Simo, A.; Joseph, V.; Fenger, R.; Kneipp, J.; Rademann, K. Long-Term Stable Silver Subsurface Ion-Exchanged Glasses for SERS Applications. Chem. Phys. Chem. 2011, 12, 1683–1688. [Google Scholar] [CrossRef]
- Simo, A.; Polte, J.; Pfänder, N.; Vainio, U.; Emmerling, F.; Rademann, K. Formation Mechanism of Silver Nanoparticles Stabilized in Glassy Matrices. J. Am. Chem. Soc. 2012, 134, 18824–18833. [Google Scholar] [CrossRef]
- Hofmeister, H.; Tan, G.L.; Dubiel, M. Shape and internal structure of silver nanoparticles embedded in glass. J. Mater. Res. 2005, 20, 1551–1562. [Google Scholar] [CrossRef]
- Williams, T. The Glass Menagerie, 1st ed.; Random House Publisher: New York, NY, USA, 1945. [Google Scholar]
- Chesterton, G.K. Orthodoxy, 1st ed.; John Lane/Bodley Head Publisher: London, UK, 1909. [Google Scholar]
- Varshneya, A.K. Chemical Strengthening of Glass: Lessons Learned and Yet to Be Learned. Int. J. Appl. Glass Sci. 2010, 1, 131–142. [Google Scholar] [CrossRef]
- Romaniuk, R.S. Tensile strength of tailored optical fibres. Opto Electron. Rev. 2000, 8, 101–116. [Google Scholar]
- Wisitsorasak, A.; Wolynes, P. On the strength of glasses. PNAS 2012, 109, 16068–16072. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.H.; Bjarrum, M. Deformations and strain energy in fragments of tempered glass: Experimental and numerical investigation. Glass Struct. Eng. 2017, 2, 133–146. [Google Scholar] [CrossRef]
- Datsiou, K.C.; Overend, M. The strength of aged glass. Glass Struct. Eng. 2017, 2, 105–120. [Google Scholar] [CrossRef]
- Gardon, R. Thermal tempering of glass. In Glass Science and Technology. Elasticity and Strength in Glasses; Uhlmann, D.R., Kreidl, N.J., Eds.; Academic Press: New York, NY, USA, 1980; Volume 5, pp. 145–216. [Google Scholar] [CrossRef]
- Sglavo, V.M.; Quaranta, A.; Allodi, V.; Mariotto, G. Analysis of surface structure of soda lime silicate glasses after chemical strengthening in different KNO3 salt bath. J. Non Cryst. Solids 2014, 401, 105–109. [Google Scholar] [CrossRef]
- Mognato, E.; Schiavonato, M.; Barbieri, A.; Pittoni, M. Process influences on mechanical strength of chemical strengthened glass. Glass Struct. Eng. 2016, 1, 247–260. [Google Scholar] [CrossRef][Green Version]
- Rogoziński, R. Stresses Produced in the BK7 Glass by the K+ –Na+ Ion-exchange: Real-Time Process Control Method. Appl. Sci. 2019, 9, 2548. [Google Scholar] [CrossRef]
- Cooper, A.R.; Krohn, D.A. Strengthening of glass fibres II: Ion-exchange. J. Am. Ceram. Soc. 1969, 52, 665–669. [Google Scholar] [CrossRef]
- Dugnani, R. Residual stress in ion-exchanged silicate glass: An analytical solution. J. Non Cryst. Solids 2017, 471, 368–378. [Google Scholar] [CrossRef]
- Varshneya, A.K. The physics of chemical strengthening of glass: Room for a new view. J. Non Cryst. Solids 2010, 356, 2289–2294. [Google Scholar] [CrossRef]
- Macrelli, G. Chemically strengthened glass by ion-exchange: Strength evaluation. Int. J. Appl. Glass Sci. 2017, 9, 156–166. [Google Scholar] [CrossRef]
- Macrelli, G.; Varshneya, A.K.; Mauro, J.C. Simulation of glass network evolution during chemical strengthening: Resolution of the subsurface compression maximum anomaly. J. Non Cryst. Solids 2019, 552, 119457. [Google Scholar] [CrossRef]
- Macrelli, G.; Özben, N.; Kayaalp, A.C.; Ersundu, M.C.; Sökmen, I. Stress in ion-exchanged soda-lime silicate and sodium aluminosilicate glasses: Experimental and theoretical comparison. Int. J. Appl. Glass Sci. 2020, 11, 730–742. [Google Scholar] [CrossRef]
- Terakado, N.; Sasaki, R.; Takahashi, Y.; Fujiwara, T.; Orihara, S.; Orihara, Y. A novel method for stress evaluation in chemically strengthened glass based on micro-Raman spectroscopy. Commun. Phys. 2020, 3, 1–7. [Google Scholar] [CrossRef]
- Erdem, I.; Guldiren, D.; Aydin, S. Chemical tempering of soda lime silicate glasses by ion-exchange process for the improvement of surface and bulk mechanical strength. J. Non Cryst. Solids 2017, 473, 170–178. [Google Scholar] [CrossRef]
- Aaldenberg, E.M.; Lezzi, P.J.; Seaman, J.H.; Blanchet, T.A.; Tomozawa, M. Ion-Exchanged Lithium Aluminosilicate Glass: Strength and Dynamic Fatigue. J. Am. Ceram. Soc. 2016, 99, 2645–2654. [Google Scholar] [CrossRef]
- Li, X.; Jiang, L.; Wang, Y.; Mohagheghian, I.; Dear, J.P.; Ki, L.; Yan, Y. Correlation between K+-Na+ diffusion coefficient and flexural strength of chemically tempered aluminosilicate glass. J. Non Cryst. Solids 2017, 47, 72–81. [Google Scholar] [CrossRef]
- Ragoen, C.; Sen, S.; Lambricht, T.; Godet, S. Effect of Al2O3 content on the mechanical and interdiffusional properties of ion-exchanged Na-aluminosilicate glasses. J. Non Cryst. Solids 2017, 458, 129–136. [Google Scholar] [CrossRef]
- Available online: https://www.corning.com/microsites/csm/gorillaglass/PI_Sheets/2020/Corning%20Gorilla%20Glass%206_PI%20Sheet.pdf (accessed on 17 May 2021).
- Available online: https://photonicsolutioncenter.com/media/companies/133/products/439/documents/agc-dragontrail-chemically.pdf (accessed on 17 May 2021).
- Available online: https://www.schott.com/d/xensation/7d59e8ca-a167-4d23-b375-6a8040cc9eb6/1.0/schott-xensation-up-datasheet-english-row-20052019.pdf (accessed on 17 May 2021).
- Available online: https://abrisatechnologies.com/specs/SCHOTT%20Xensation%20Spec%20Sheet.pdf#zoom=100 (accessed on 17 May 2021).
- Wilson, J.R.; Jones, C.D.; Bartlow, C.C.; Doan, A.R. Patterned Asymmetric Chemical Strengthening. US Patent 20200017406A1, 16 January 2020. [Google Scholar]
- Luzzato, V.; Prest, C.D.; Memering, D.N.; Rogers, M.S. Asymmetric Chemical Strengthening. US Patent 20170334769A1, 23 November 2017. [Google Scholar]
- Rogers, M.S.; Memering, D.N.; Prest, C.D. Asymmetric Chemical Strengthening. US Patent 20150274585A1, 1 October 2015. [Google Scholar]
- Available online: https://www.corning.com/microsites/csm/gorillaglass/PI_Sheets/2020/Corning%20Gorilla%20Glass%20Victus_PI%20Sheet.pdf (accessed on 17 May 2021).
- Available online: https://www.apple.com/iphone-12/ (accessed on 17 May 2021).
- Available online: https://media.ford.com/content/fordmedia/fna/us/en/news/2015/12/15/industry-first-gorilla-glass-hybrid-windshield-on-all-new-ford-GT.html (accessed on 17 May 2021).
- Available online: https://phys.org/news/2015-12-gorilla-glass-cell-cars.html (accessed on 17 May 2021).
- Wang, H.F.; Xing, G.Z.; Wang, X.Y.; Zhang, L.L.; Zhang, L.; Li, S. Chemically strengthened protection glasses for the applications of space solar cells. AIP Adv. 2014, 4, 47133. [Google Scholar] [CrossRef]
- Kambe, M.; Hara, K.; Mitarai, K.; Takeda, S.; Fukawa, M.; Ishimaru, N.; Kondo, M. Chemically strengthened cover glass for preventing potential induced degradation of crystalline silicon solar cells. In Proceedings of the 39th Photovoltaic Specialists Conference, Tampa Bay, FL, USA, 16–21 June 2013. [Google Scholar] [CrossRef]
- Li, J.; Wei, R.; Liu, X.; Guo, H. Enhanced luminescence via energy transfer from Ag+ to RE ions (Dy3+, Sm3+, Tb3+) in glasses. Opt. Express 2012, 20, 10122–10127. [Google Scholar] [CrossRef]
- Jiao, Q.; Wang, X.; Qiu, J.; Zhou, D. Effect of silver ions and clusters on the luminescence properties of Eu-doped borate glasses. Mater. Res. Bull. 2015, 72, 264–268. [Google Scholar] [CrossRef]
- Li, L.; Yang, Y.; Zhou, D.; Xu, X.; Qiu, J. The influence of Ag species on spectroscopic features of Tb3+-activated sodium–aluminosilicate glasses via Ag+–Na+ ion exchange. J. Non Cryst. Solids 2014, 385, 95–99. [Google Scholar] [CrossRef]
- Bozelli, J.C.; de Oliveira Nunes, L.A.; Sigoli, F.A.; Mazali, I.O. Erbium and Ytterbium Codoped Titanoniobophosphate Glasses for Ion-Exchange-Based Planar Waveguides. J. Am. Ceram. Soc. 2010, 93, 2689–2692. [Google Scholar] [CrossRef]
- Vařák, P.; Vytykáčová, S.; Nekvindová, P.; Michalcová, A.; Malinský, P. The influence of copper and silver in various oxidation states on the photoluminescence of Ho3+/Yb3+ doped zinc-silicate glasses. Opt. Mater. 2019, 91, 253–260. [Google Scholar] [CrossRef]
- He, X.; Xu, X.; Shi, Y.; Qiu, J. Effective enhancement of Bi near-infrared luminescence in silicogermanate glasses via silver–sodium ion exchange. J. Non Cryst. Solids 2015, 409, 178–182. [Google Scholar] [CrossRef]
- Paje, S.E.; García, M.A.; Villegas, M.A.; Llopis, J. Cerium doped soda-lime-silicate glasses: Effects of silver ion-exchange on optical properties. Opt. Mater. 2001, 17, 459–469. [Google Scholar] [CrossRef]
- Grand, J.; Lamy de la Chapelle, M.; Bijeon, J.-L.; Adam, P.-M.; Vial, A.; Royer, P. Role of localized surface plasmons in surface-enhanced Raman scattering of shape-controlled metallic particles in regular arrays. Phys. Rev. B 2005, 72, 033407. [Google Scholar] [CrossRef]
- Li, P.; Long, F.; Chen, W.; Chen, J.; Chu, P.K.; Wang, H. Fundamentals and applications of surface-enhanced Raman spectroscopy-based biosensors. Curr. Opin. Biomed. Eng. 2020, 13, 51–59. [Google Scholar] [CrossRef]
- Betz, J.F.; Yu, W.W.; Cheng, Y.; White, I.M.; Rubloff, G.W. Simple SERS substrates: Powerful, portable, and full of potential. PCCP 2014, 16, 2224–2239. [Google Scholar] [CrossRef]
- Fan, M.; Andrade, G.F.S.; Brolo, A.G. A review on the fabrication of substrates for surface enhanced Raman spectroscopy and their applications in analytical chemistry. Anal. Chim. Acta 2011, 693, 7–25. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Lin, J.; Zhang, W.; Wei, H.; Chen, Y. SERS-active Ag nanoparticles embedded in glass prepared by a two-step electric field-assisted diffusion. Opt. Mater. 2014, 39, 97–102. [Google Scholar] [CrossRef]
- Chen, Y.; Jaakola, J.; Säynätjoki, A.; Tervonen, A.; Honkanen, S. SERS-active silver nanoparticles in ion-exchanged glass. J. Nonlinear Opt. Phys. 2010, 19, 527–533. [Google Scholar] [CrossRef]
- Manikandan, P.; Manikandan, D.; Manikandan, E.; Christy Ferdinand, A. Surface enhanced Raman scattering (SERS) of silver ions embedded nanocomposite glass. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 124, 203–207. [Google Scholar] [CrossRef]
- Manikandan, P.; Manikandan, D.; Manikandan, E.; Christy Ferdinand, A. Structural, Optical and Micro-Raman Scattering Studies of Nanosized Copper Ion (Cu+) Exchanged Soda Lime Glasses. Plasmonics 2014, 9, 637–643. [Google Scholar] [CrossRef]
- Quaranta, A.; Cattaruzza, E.; Gonella, F.; Rahman, A.; Mariotto, G. Cross-sectional Raman micro-spectroscopy study of silver nanoparticles in soda–lime glasses. J. Non Cryst. Solids 2014, 401, 219–223. [Google Scholar] [CrossRef]
- Chen, Y.; Karvonen, L.; Säynätjoki, A.; Ye, C.; Tervonen, A.; Honkanen, S. Ag nanoparticles embedded in glass by two-step ion exchange and their SERS application. Opt. Mater. Express 2011, 1, 164–172. [Google Scholar] [CrossRef]
- Manzani, D.; Franco, D.F.; Afonso, C.R.M.; Sant’Ana, A.C.; Nalih, M.; Ribeiro, S.J.L. A new SERS substrate based on niobium lead-pyrophosphate glasses obtained by Ag+/Na+ ion exchange. Sens. Actuators B Chem. 2018, 277, 347–352. [Google Scholar] [CrossRef]
- De Marchi, G.; Caccavale, F.; Gonella, F.; Mattei, G.; Mazzoldi, P.; Battaglin, G.; Quaranta, A. Silver nanoclusters formation in ion-exchanged waveguides by annealing in hydrogen atmosphere. Appl. Phys. A 1996, 63, 403–407. [Google Scholar] [CrossRef]
- Redkov, A.V.; Zhurikhina, V.V.; Lipovskii, A.A. Formation and self-arrangement of silver nanoparticles in glass via annealing in hydrogen: The model. J. Non Cryst. Solids 2013, 376, 152–157. [Google Scholar] [CrossRef]
- Chervinskii, S.; Sevriuk, V.; Reduto, I.; Lipovskii, A. Formation and 2D-patterning of silver nanoisland film using thermal poling and out-diffusion from glass. J. Appl. Phys. 2013, 114, 224301. [Google Scholar] [CrossRef]
- Redkov, A.; Chervinskii, S.; Baklanov, A.; Reduto, I.; Zhurikhina, V.; Lipovskii, A. Plasmonic molecules via glass annealing in hydrogen. Nanoscale Res. Lett. 2014, 9, 606. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Goutaland, F.; Sow, M.; Ollier, N.; Vacanson, F. Growth of highly concentrated silver nanoparticles and nanoholes in silver-exchanged glass by ultraviolet continuous wave laser exposure. Opt. Mater. Express 2012, 2, 350–357. [Google Scholar] [CrossRef]
- Goutaland, F.; Colombier, J.-P.; Cherif Sow, M.; Ollier, N.; Vocanson, F. Laser-induced periodic alignment of Ag nanoparticles in soda-lime glass. Opt. Express 2013, 21, 31789–31799. [Google Scholar] [CrossRef]
- Niry, M.D.; Mostafavi-Amjad, J.; Khalesifard, H.R.; Ahangary, A.; Azizian-Kalandaragh, Y. Formation of silver nanoparticles inside a soda-lime glass matrix in the presence of a high intensity Ar+ laser beam. J. Appl. Phys. 2012, 111, 033111. [Google Scholar] [CrossRef]
- Miotello, A.; Bonelli, M.; De Marchi, G.; Mattei, G.; Mazzoldi, P.; Sada, C.; Gonella, F. Formation of silver nanoclusters by excimer–laser interaction in silver-exchanged soda-lime glass. Appl. Phys. Lett. 2001, 79, 2456–2458. [Google Scholar] [CrossRef]
- Sheng, J.; Zheng, J.; Zhang, J.; Zhou, C.; Jiang, L. UV-laser-induced nanoclusters in silver ion-exchanged soda-lime silicate glass. Physica B 2007, 387, 32–35. [Google Scholar] [CrossRef]
- Wackerow, S.; Abdolvand, A. Laser-assisted one-step fabrication of homogeneous glass–silver composite. Appl. Phys. A 2012, 109, 45–49. [Google Scholar] [CrossRef]
- Wackerow, S.; Abdolvand, A. Generation of silver nanoparticles with controlled size and spatial distribution by pulsed laser irradiation of silver ion-doped glass. Opt. Express 2014, 22, 5076–5085. [Google Scholar] [CrossRef]
- Schmidl, G.; Dellith, J.; Schneidewind, H.; Zopf, D.; Stranik, O.; Gawlik, A.; Anders, S.; Tympel, V.; Katzer, C.; Schmidl, F.; et al. Formation and characterization of silver nanoparticles embedded in optical transparent materials for plasmonic sensor surfaces. Mater. Sci. Eng. B 2015, 193, 207–216. [Google Scholar] [CrossRef]
- Chervinskii, S.; Matikainen, A.; Dergachev, A.; Lipovskii, A.A.; Honkanen, S. Out-diffused silver island films for surface-enhanced Raman scattering protected with TiO2 films using atomic layer deposition. Nanoscale Res. Lett. 2014, 9, 398. [Google Scholar] [CrossRef][Green Version]
- Inácio, P.L.; Barreto, B.J.; Horowitz, F.; Correia, R.R.B.; Pereira, M.B. Silver migration at the surface of ion-exchange waveguides: A plasmonic template. Opt. Mater. Express 2013, 3, 390–399. [Google Scholar] [CrossRef]
- Hu, J.; Li, L.; Lin, H.; Zhang, P.; Zhou, W.; Ma, Z. Flexible integrated photonics: Where materials, mechanics and optics meet. Opt. Mater. Express 2013, 3, 1313–1331. [Google Scholar] [CrossRef]
- Geiger, S.; Michon, J.; Liu, S.; Qin, J.; Ni, J.; Hu, J.; Gu, T.; Lu, N. Flexible and stretchable Photonics: The Next Stretch of Opportunities. ACS Photonics 2020, 7, 2618–2635. [Google Scholar] [CrossRef]
- Righini, G.C.; Szczurek, A.; Krzak, J.; Lukowiak, A.; Ferrari, M.; Varas, S.; Chiasera, A. Flexible Photonics: Where Are We Now? In Proceedings of the 22nd International Conference on Transparent Optical Networks (ICTON), Bari, Italy, 19–23 July 2020; pp. 1–4. [Google Scholar] [CrossRef]
- Righini, G.C.; Krzak, J.; Lukowiak, A.; Macrelli, G.; Varas, S.; Ferrari, M. From flexible electronics to flexible photonics: A brief overview. Opt. Mater. 2021, 115, 111011. [Google Scholar] [CrossRef]
- Sayginer, O.; Iacob, E.; Varas, S.; Szczurek, A.; Ferrari, M.; Lukowiak, A.; Righini, G.C.; Bursi, O.S.; Chiasera, A. Design, fabrication and assessment of an optomechanical sensor for pressure and vibration detection using flexible glass multilayers. Opt. Mater. 2021, 115, 111023. [Google Scholar] [CrossRef]
- Chen, Y.; Li, H.; Li, M. Flexible and tunable silicon photonic circuits on plastic substrates. Sci. Rep. 2012, 2, 622. [Google Scholar] [CrossRef]
- Fan, L.; Varghese, L.T.; Xuan, Y.; Wang, J.; Niu, B.; Qi, M. Direct fabrication of silicon photonic devices on a flexible platform and its application for strain sensing. Opt. Express 2012, 20, 20564–20575. [Google Scholar] [CrossRef]
- Chiasera, A.; Sayginer, O.; Iacob, E.; Szczurek, A.; Varas, S.; Krzak, J.; Bursi, O.S.; Zonta, D.; Lukowiak, A.; Righini, G.C.; et al. Flexible photonics: RF-sputtering fabrication of glass-based systems operating under mechanical deformation conditions. In Proceedings of the SPIE, Strasbourg, France, 29 March–2 April 2020; Volume 11357, p. 1135705. [Google Scholar] [CrossRef]
- Li, L.; Lin, H.; Qiao, S.; Zou, Y.; Danto, S.; Richardson, K.; Musgraves, J.D.; Lu, N.; Hu, J. Integrated flexible chalcogenide glass photonic devices. Nature Photon. 2014, 8, 643–649. [Google Scholar] [CrossRef]
- Li, L.; Lin, H.; Qiao, S.; Huang, Y.-H.; Li, J.-Y.; Michon, J.; Gu, T.; Alosno-Ramos, C.; Vivien, L.; Yadav, A.; et al. Monolithically integrated stretchable photonics. Light Sci. Appl. 2018, 7, 17138. [Google Scholar] [CrossRef]
- Li, L.; Lin, H.; Michon, J.; Huang, Y.; Li, J.; Du, Q.; Yadav, A.; Richardson, K.; Gu, T.; Hu, J. A new twist on glass: A brittle material enabling flexible integrated photonics. Int. J. Appl. Glass Sci. 2017, 8, 61–68. [Google Scholar] [CrossRef]
- Lapointe, J.; Ledemi, Y.; Loranger, S.; Iezzi, V.L.; De Lima Filho, E.S.; Parent, F.; Morency, S.; Messaddeq, Y.; Kashyap, R. Fabrication of ultrafast laser written low-loss waveguides in flexible As2S3 chalcogenide glass tape. Opt. Lett. 2016, 41, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.corning.com/worldwide/en/innovation/corning-emerging-innovations/corning-willow-glass.html (accessed on 17 May 2021).
- Available online: https://www.schott.com/en-gb/products/af-32-eco (accessed on 17 May 2021).
- Hou, Y.; Cheng, J.; Kang, J.; Cui, J. The forming simulation of flexible glass with silt down draw method. IOP Conf. Ser.: Mater. Sci. Eng. 2018, 324, 12020. [Google Scholar] [CrossRef]
- Garner, S.; Glaesemann, S.; Li, X. Ultra-slim flexible glass for roll-to-roll electronic device fabrication. Appl. Phys. A 2014, 116, 403–407. [Google Scholar] [CrossRef]
- Huang, S.; Li, M.; Garner, S.M.; Li, M.-J.; Chen, K.P. Flexible photonic components in glass substrates. Opt. Express 2015, 23, 22532–22543. [Google Scholar] [CrossRef] [PubMed]
- Ellison, A.; Cornejo, I.A. Glass Substrates for Liquid Crystal Displays. Int. J. Appl. Glass Sci. 2010, 1, 87–103. [Google Scholar] [CrossRef]
- Mauro, J.C.; Truesdale, C.M.; Adib, K.; Whittier, A.M.; Martin, A.W. Chemical Strengthening of Alkali-Free Glass via Pressure Vessel Ion Exchange. Int. J. Appl. Glass Sci. 2016, 7, 446–451. [Google Scholar] [CrossRef]
- Martin, A.W.; Mauro, J.C.; Truesdale, C.M. Strengthened Glass, Glass-Ceramic, and Ceramic Articles and Methods of Making the Same through Pressurized Ion Exchange. U.S. Patent Application 2016/0145152 A1, 2 June 2016. [Google Scholar]
- Macrelli, G.; Varshneya, A.K.; Mauro, J.C. Ultra-thin glass as a substrate for flexible photonics. Opt. Mater. 2020, 106, 109994. [Google Scholar] [CrossRef]
- Macrelli, G. Strengthened glass by ion exchange, mechanical and optical properties: Perspectives and limits of glass as a substrate for flexible photonics. In Proceedings of the SPIE Photonics Europe, Strasbourg, France, 29 March–2 April 2020; Volume 11357, p. 1135707. [Google Scholar] [CrossRef]
- Chiasera, A.; Dumeige, Y.; Féron, P.; Ferrari, M.; Jestin, Y.; Nunzi Conti, G.; Pelli, S.; Soria, S.; Righini, G.C. Spherical whispering-gallery-mode microresonators. Laser Photonics Rev. 2010, 4, 457–482. [Google Scholar] [CrossRef]
- Ward, J.; Benson, O. WGM microresonators: Sensing, lasing and fundamental optics with microspheres. Laser Photonics Rev. 2011, 5, 553–570. [Google Scholar] [CrossRef]
- Strekalov, D.V.; Marquardt, C.; Matsko, A.B.; Schwefel, H.G.L.; Leuchs, G. Nonlinear and quantum optics with whispering gallery resonators. J. Opt. 2016, 18, 18–123002. [Google Scholar] [CrossRef]
- Gorodetsky, M.L.; Savchenkov, A.A.; Ilchenko, V.S. Ultimate Q of optical microsphere resonators. Opt. Lett. 1996, 21, 453–455. [Google Scholar] [CrossRef]
- Ristić, D.; Berneschi, S.; Camerini, M.; Farnesi, D.; Pelli, S.; Trono, C.; Chiappini, A.; Chiasera, A.; Ferrari, M.; Lukowiak, A.; et al. Photoluminescence and lasing in whispering gallery mode glass microspherical resonators. J. Lumin. 2016, 170, 755–760. [Google Scholar] [CrossRef]
- Ward, J.M.; Yang, Y.; Nic Chormaic, S. Glass-on-glass fabrication of bottle-shaped tunable microlasers and their applications. Sci. Rep. 2016, 6, 25152. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Lewis, E.; Farrell, G.; Wang, P. Compound glass microsphere resonator devices. Micromachines 2018, 9, 356. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zohrabi, M.; Bae, K.; Horning, T.M.; Grayson, M.B.; Park, W.; Gopinath, J.T. Nonlinear characterization of silica and chalcogenide microresonators. Optica 2019, 6, 716–722. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, J.; Wang, R.; LI, A.; Zhang, M.; Wang, S.; Wang, P.; Ward, J.M.; Nic Chormaic, S. A tellurite glass optical microbubble resonator. Opt. Express 2020, 28, 32858–32868. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, H.; Sasaki, K. Up-conversion lasing of a thulium-ion-doped fluorozirconate glass microsphere. J. Appl. Phys. 1999, 86, 2385–2388. [Google Scholar] [CrossRef]
- Von Klitzing, W.; Jahier, E.; Long, R.; Lissillour, F.; Lefèvre-Seguin, V.; Hare, J.; Raimond, J.-M.; Haroche, S. Very low threshold green lasing in microspheres by up-conversion of IR photons. J. Opt. B Quantum Semiclass. Opt. 2000, 2, 204–206. [Google Scholar] [CrossRef]
- Wu, Y.; Ward, J.M.; Nic Chormaic, S. Ultralow threshold green lasing and optical bistability in ZBNA (ZrF4–BaF2–NaF–AlF3) microspheres. J. Appl. Phys. 2010, 33103. [Google Scholar] [CrossRef]
- Wang, X.; Yu, Y.; Wang, S.; Ward, J.M.; Nic Chormaic, S.; Wang, P. Single mode green lasing and multicolour luminescent emission from an Er3+-Yb3+ co-doped compound fluorosilicate glass microsphere resonator. OSA Contin. 2018, 1, 261–273. [Google Scholar] [CrossRef]
- Huang, Y.; Zhuang, S.; Peng, L.; Wu, J.; Liao, T.; Xu, C.; Duan, Y. Efficiency improvement of up-conversion luminescence of Yb3+/Tm3+ co-doped tellurite glass microsphere. J. Alloys Compd. 2018, 748, 93–99. [Google Scholar] [CrossRef]
- Singh, S.K.; Giri, N.K.; Rai, D.K.; Rai, S.B. Enhanced up-conversion emission in Er3+-doped tellurite glass containing silver nanoparticles. Solid State Sci. 2010, 12, 1480–1483. [Google Scholar] [CrossRef]
- Reza Dousti, M. Efficient infrared-to-visible up-conversion emission in Nd3+-doped PbO-TeO2 glass containing silver nanoparticles. J. Appl. Phys. 2013, 114, 113105. [Google Scholar] [CrossRef]
- Soltani, I.; Hraiech, S.; Horchani-Naifer, K.; Férid, M. Effects of silver nanoparticles on the enhancement of up-conversion and infrared emission in Er3+/Yb3+ co-doped phosphate glasses. Opt. Mater. 2018, 77, 161–169. [Google Scholar] [CrossRef]
- Milenko, K.; Konidakis, I.; Pissadakis, S. Silver iodide phosphate glass microsphere resonator integrated on an optical fiber taper. Opt. Lett. 2016, 41, 2185–2188. [Google Scholar] [CrossRef]
- Dong, C.H.; Yang, Y.; Shen, Y.L.; Zou, C.L.; Sun, F.W.; Ming, H.; Guo, G.C.; Han, Z.F. Observation of microlaser with Er-doped phosphate glass coated microsphere pumped by 780 nm. Opt. Commun. 2010, 283, 5117–5120. [Google Scholar] [CrossRef]
- Mai, H.H.; Kaydashev, V.E.; Tikhomirov, V.K.; Janssens, E.; Shestakov, M.V.; Meledina, M.; Turner, S.; Van Tendeloo, G.; Moshchalkov, V.V.; Lievens, P. Nonlinear Optical Properties of Ag Nanoclusters and Nanoparticles Dispersed in a Glass Host. J. Phys. Chem. C 2014, 118, 15995–16002. [Google Scholar] [CrossRef]
- Adamiv, V.T.; Bolesta, Y.V.; Gamernyk, R.V.; Karbovnyk, I.D.; Kolych, I.I.; Kovalchuk, M.G.; Kushnir, O.O.; Periv, M.V.; Teslyuk, I.M. Nonlinear optical properties of silver nanoparticles prepared in Ag doped borate glasses. Physica B 2014, 449, 31–35. [Google Scholar] [CrossRef]
- Ferrari, P.; Upadhyay, S.; Shestakov, M.V.; Vanbuel, J.; De Roo, B.; Kuang, Y.; Di Vece, M.; Moshchalkov, V.V.; Locquet, J.-P.; Lievens, P.; et al. Wavelength-Dependent Nonlinear Optical Properties of Ag Nanoparticles Dispersed in a Glass Host. J. Phys. Chem. C 2017, 121, 27580–27589. [Google Scholar] [CrossRef]
- Chen, F.; Cheng, J.; Dai, S.; Xu, Z.; Ji, W.; Tan, R.; Zhang, Q. Third-order optical nonlinearity at 800 and 1300 nm in bismuthate glasses doped with silver nanoparticles. Opt. Express 2014, 22, 13438–13447. [Google Scholar] [CrossRef]
- Amjad, R.J.; Sahar, M.R.; Ghoshal, S.K.; Dousti, M.R.; Riaz, S.; Tahir, B.A. Enhanced infrared to visible upconversion emission in Er3+ doped phosphate glass: Role of silver nanoparticles. J. Lumin. 2012, 132, 2714–2718. [Google Scholar] [CrossRef]
- Konidakis, I.; Zito, G. Pissadakis, S. Silver plasmon resonance effects in AgPO3/silica photonic bandgap fiber. Opt. Lett. 2014, 39, 3374–3377. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.A.; Argyros, A.; Sorin, F. Hybrid Optical Fibers—An Innovative Platform for In-Fiber Photonic Devices. Adv. Optical Mater. 2016, 4, 13–36. [Google Scholar] [CrossRef]
- Jain, C.; Rodrigues, B.P.; Wieduwilt, T.; Kobelke, J.; Wondraczek, L.; Schmidt, M.A. Silver metaphosphate glass wires inside silica fibers—a new approach for hybrid optical fibers. Opt. Express 2016, 24, 3258–3267. [Google Scholar] [CrossRef] [PubMed]
- Ballato, J.; Peacock, A.C. Perspective: Molten core optical fiber fabrication—A route to new materials and applications. APL Photonics 2018, 3, 120903. [Google Scholar] [CrossRef]
- Kang, S.; Dong, G.; Qiu, J.; Yang, Z. Hybrid glass optical fibers-novel fiber materials for optoelectronic application. Opt. Mater. X 2020, 6, 100051. [Google Scholar] [CrossRef]
- Ballato, J.; Dragic, P.D. Glass: The carrier of light—Part II—A brief look into the future of optical fiber. Int. J. Appl. Glass Sci. 2021, 12, 3–24. [Google Scholar] [CrossRef]
- Pisco, M.; Cusano, A. Lab-On-Fiber Technology: A Roadmap toward Multifunctional Plug and Play Platforms. Sensors 2020, 20, 4705. [Google Scholar] [CrossRef]
- Kostovski, G.; White, D.J.; Mitchell, A.; Austin, M.W.; Stoddart, P.R. Nanoimprinted optical fibres: Biotemplated nanostructures for SERS sensing. Biosens. Bioelectron. 2009, 24, 1531–1535. [Google Scholar] [CrossRef]
- Barucci, A.; Cosi, F.; Giannetti, A.; Pelli, S.; Griffini, D.; Insinna, M.; Salvadori, S.; Tiribilli, B.; Righini, G.C. Optical fibre nanotips fabricated by a dynamic chemical etching for sensing applications. J. Appl. Phys. 2015, 117, 053104. [Google Scholar] [CrossRef]
- Hutter, T.; Elliott, S.R.; Mahajan, S. Optical fibre-tip probes for SERS: Numerical study for design considerations. Opt. Express 2018, 26, 15539–15550. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Huang, T.; Han, L.; Su, H.; Wang, H.; Liu, M.; Zhang, W.; Wang, X.; Mei, T. Tip-Enhanced Raman Spectroscopy with High-Order Fiber Vector Beam Excitation. Sensors 2018, 18, 3841. [Google Scholar] [CrossRef] [PubMed]
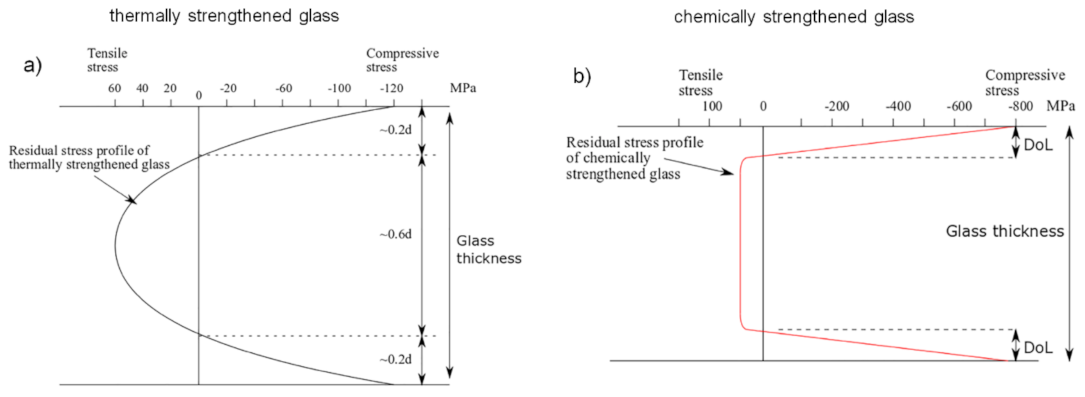




| Parameter | Thermal Strengthening | Chemical Strengthening |
|---|---|---|
| Strength (max. value range) | ~(200–400) MPa | ~(800–1000) MPa |
| Surface compression layer | Thick | Thin |
| Stress distribution profile | Parabolic | More flat & square |
| Minimum sample thickness | 2–3 mm | <1 mm |
| Process Time | Short (mins) | Long (hours) |
| Process Cost | Cheap | Expensive |
| Glass Composition | No relevant | Alkaline glass (i.e., soda-lime or aluminosilicate) |
| Product Shape | Simple geometries | Unusual shape |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berneschi, S.; Righini, G.C.; Pelli, S. Towards a Glass New World: The Role of Ion-Exchange in Modern Technology. Appl. Sci. 2021, 11, 4610. https://doi.org/10.3390/app11104610
Berneschi S, Righini GC, Pelli S. Towards a Glass New World: The Role of Ion-Exchange in Modern Technology. Applied Sciences. 2021; 11(10):4610. https://doi.org/10.3390/app11104610
Chicago/Turabian StyleBerneschi, Simone, Giancarlo C. Righini, and Stefano Pelli. 2021. "Towards a Glass New World: The Role of Ion-Exchange in Modern Technology" Applied Sciences 11, no. 10: 4610. https://doi.org/10.3390/app11104610
APA StyleBerneschi, S., Righini, G. C., & Pelli, S. (2021). Towards a Glass New World: The Role of Ion-Exchange in Modern Technology. Applied Sciences, 11(10), 4610. https://doi.org/10.3390/app11104610








