Production of Microalgal Biomass in Photobioreactors as Feedstock for Bioenergy and Other Uses: A Techno-Economic Study of Harvesting Stage
Abstract
1. Introduction
2. Materials and Methods
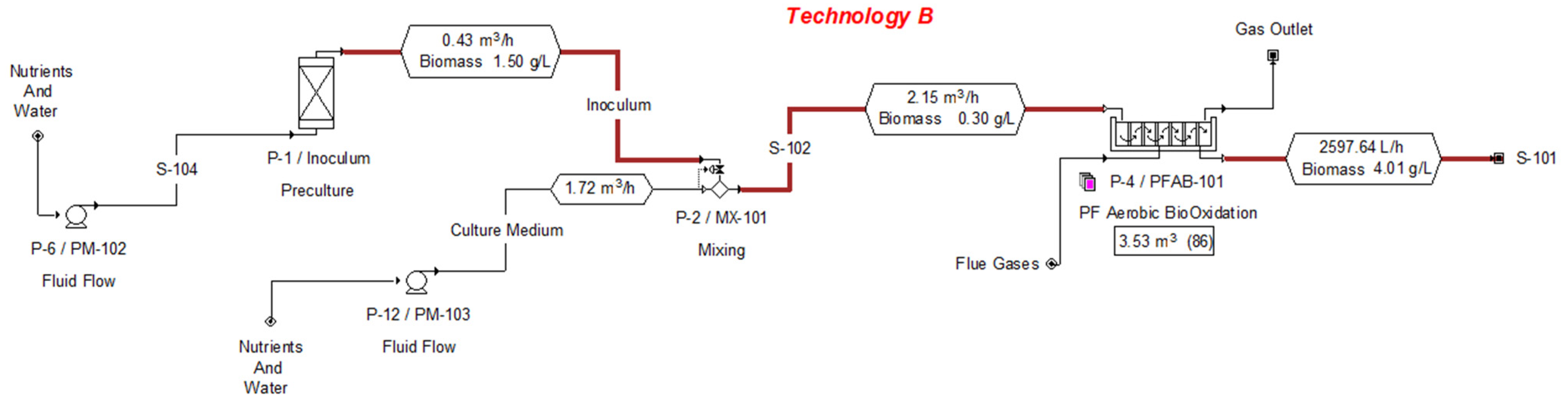
2.1. Data Collection for the Design of the Cultivation Stage
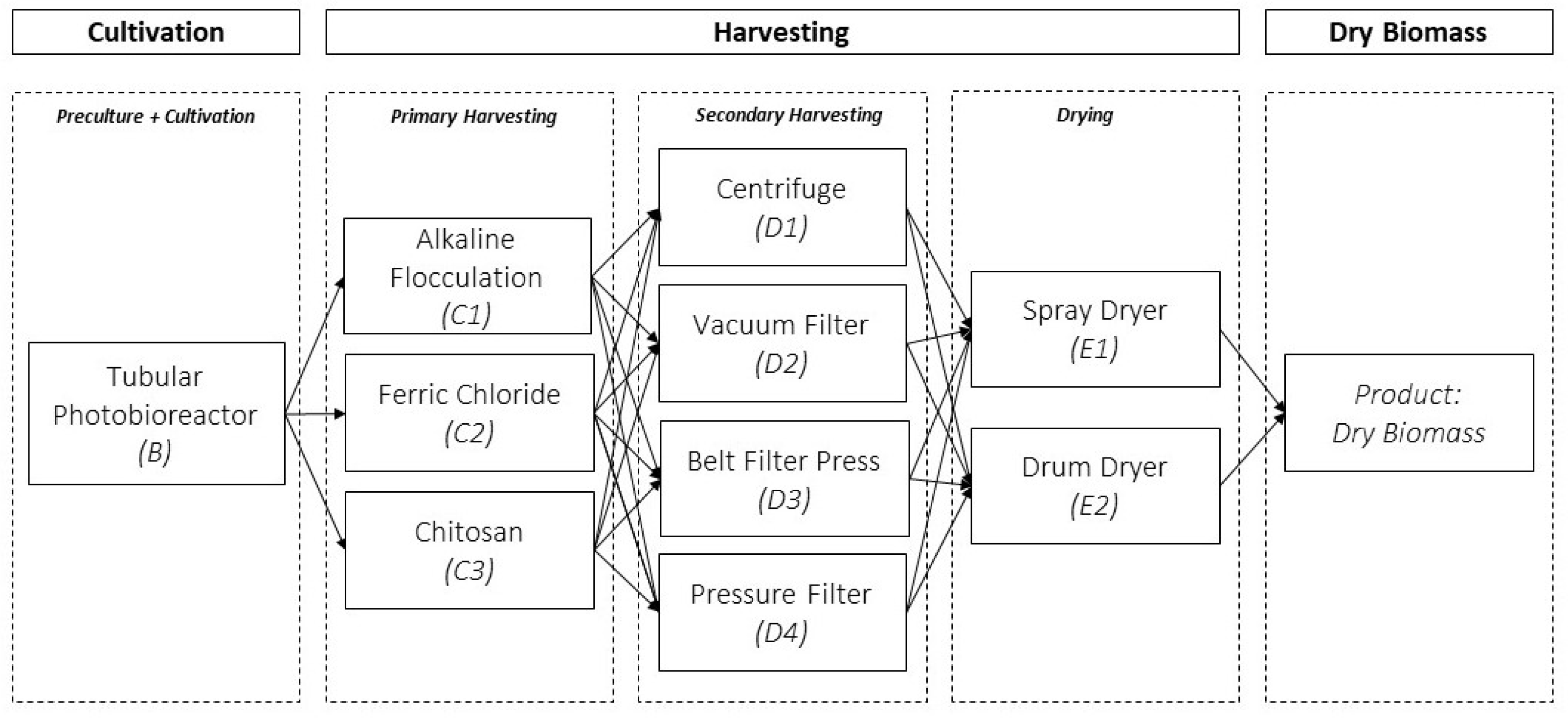
2.2. Data Collection for the Design of the Harvest Stage
2.3. Data Collection for the Design of the Drying Stage
2.4. Simulatión and Techno-Economic Analysis
3. Results and Discussion
3.1. Technical and Economic Evaluation by Process Stage
3.1.1. Technical and Economic Evaluation for the Cultivation Stage
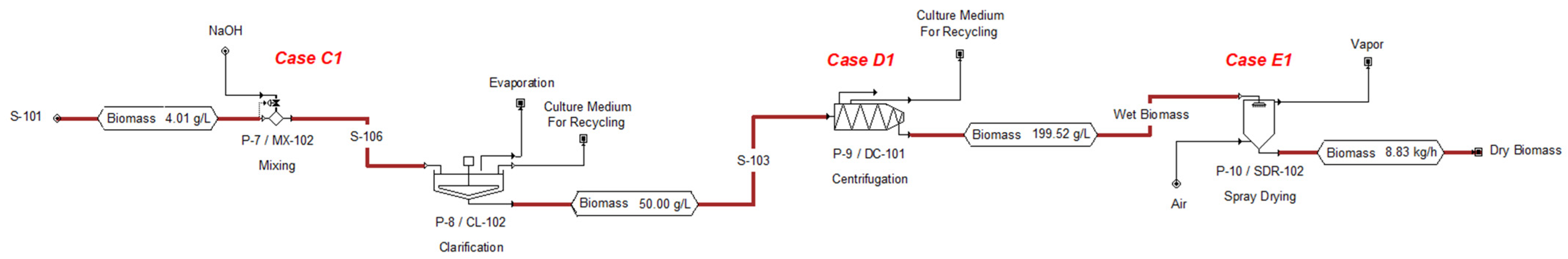
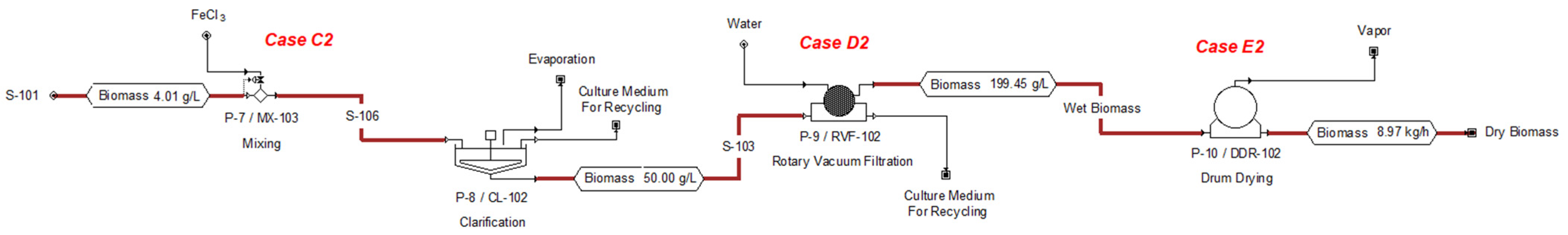
3.1.2. Technical and Economic Evaluation for the Primary and Secondary Harvest Stage
3.1.3. Technical and Economic Evaluation for the Drying Stage
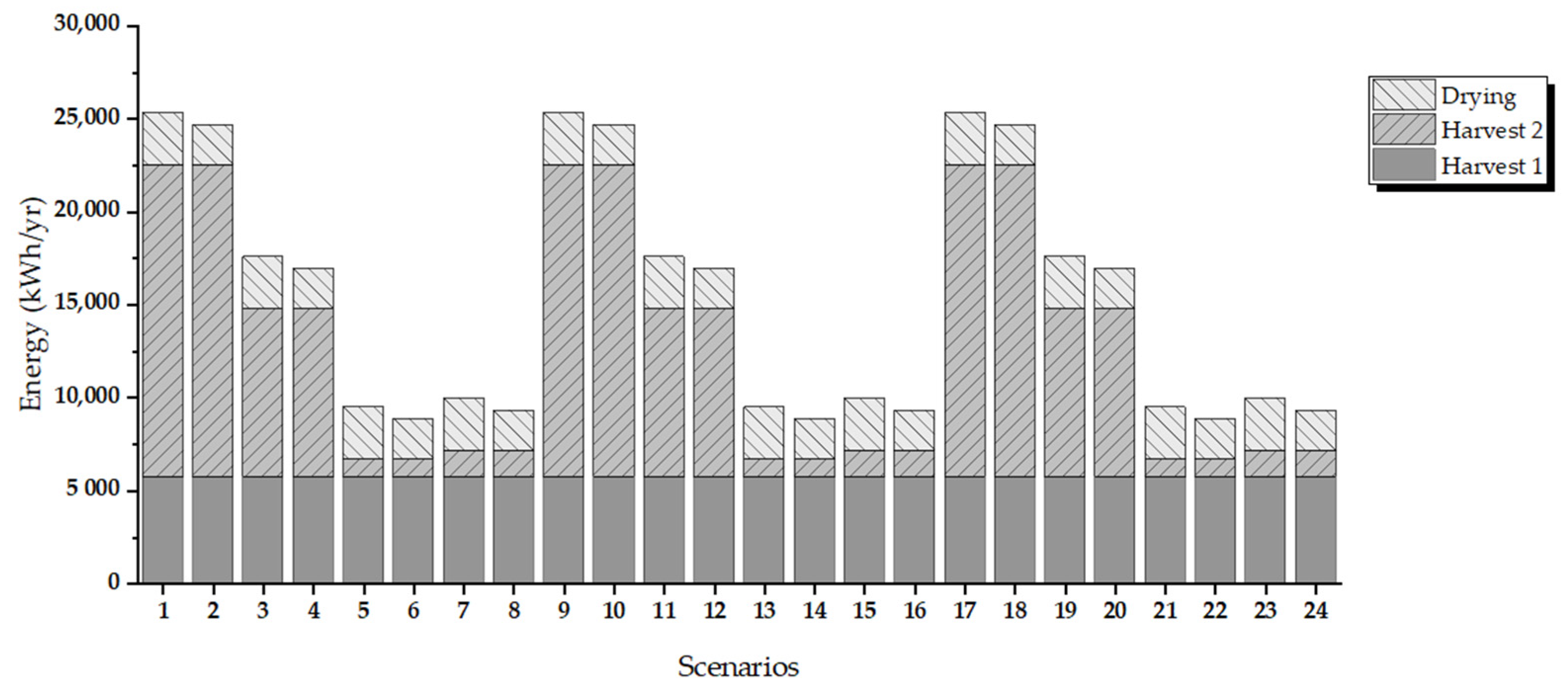
3.2. Energy Evaluation by Process Scenarios
3.3. Evaluation of Production Costs by Process Scenarios
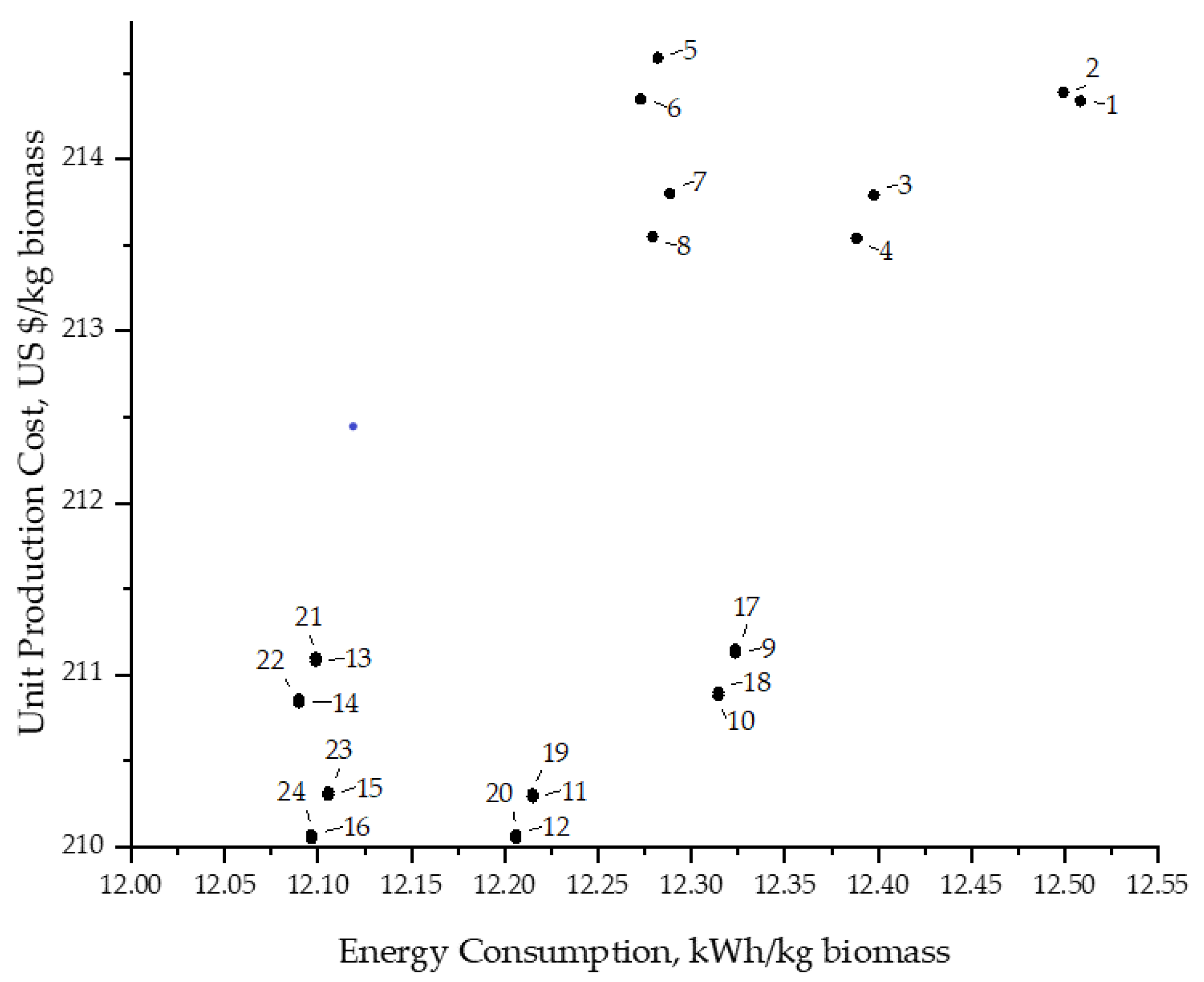
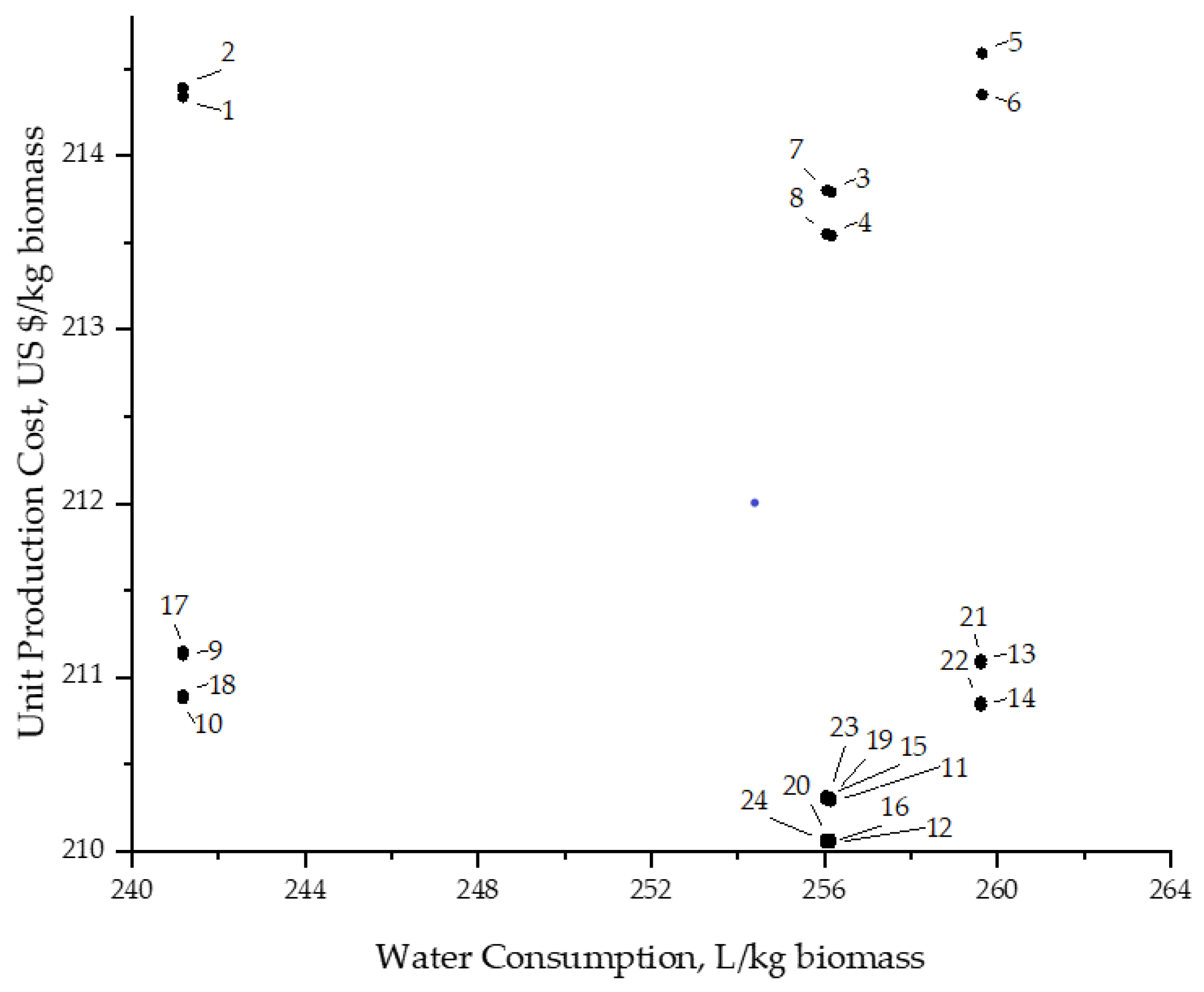
3.4. Comparison of Scenarios, Energy and Water Consumption vs. Production Cost per kg of Biomass Produced
3.5. Classification of the Scenarios Depending on the Use of Biomass
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, G.; Chen, F.; Wei, D.; Zhang, X.; Chen, G. Biodiesel production by microalgal biotechnology. Appl. Energy 2010, 87, 38–46. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Rajesh Banu, J.; Preethi; Kavitha, S.; Gunasekaran, M.; Kumar, G. Microalgae based biorefinery promoting circular bioeconomy-techno economic and life-cycle analysis. Bioresour. Technol. 2020, 302, 122822. [Google Scholar] [CrossRef] [PubMed]
- Matter, A.I.; Bui, K.V.; Jung, M.; Seo, Y.J.; Kim, Y.-E.; Lee, Y.-C.; Oh, Y.-K. Flocculation Harvesting Techniques for Microalgae: A Review. Appl. Sci. 2019, 9, 3069. [Google Scholar] [CrossRef]
- Raheem, A.; Prinsen, P.; Vuppaladadiyam, A.K.; Zhao, M.; Luque, R. A review on sustainable microalgae based biofuel and bioenergy production: Recent developments. J. Clean. Prod. 2018, 181, 42–59. [Google Scholar] [CrossRef]
- Jez, S.; Spinelli, D.; Fierro, A.; Dibenedetto, A.; Aresta, M.; Busi, E.; Basosi, R. Comparative life cycle assessment study on environmental impact of oil production from micro-algae and terrestrial oilseed crops. Bioresour. Technol. 2017, 239, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Khoo, K.S.; Chew, K.W.; Yew, G.Y.; Leong, W.H.; Chai, Y.H.; Show, P.L.; Chen, W.-H. Recent advances in downstream processing of microalgae lipid recovery for biofuel production. Bioresour. Technol. 2020, 304, 122996. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Ramaswamy, S. Dynamic process model and economic analysis of microalgae cultivation in open raceway ponds. Algal Res. 2017, 26, 330–340. [Google Scholar] [CrossRef]
- Rawat, I.; Ranjith Kumar, R.; Mutanda, T.; Bux, F. Biodiesel from microalgae: A critical evaluation from laboratory to large scale production. Appl. Energy 2013, 103, 444–467. [Google Scholar] [CrossRef]
- Brennan, L.; Owende, P. Biofuels from microalgae—A review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sustain. Energy Rev. 2010, 14, 557–577. [Google Scholar] [CrossRef]
- Schiano di Visconte, G.; Spicer, A.; Chuck, J.C.; Allen, J.M. The Microalgae Biorefinery: A Perspective on the Current Status and Future Opportunities Using Genetic Modification. Appl. Sci. 2019, 9, 4793. [Google Scholar] [CrossRef]
- Zhu, L. Biorefinery as a promising approach to promote microalgae industry: An innovative framework. Renew. Sustain. Energy Rev. 2015, 41, 1376–1384. [Google Scholar] [CrossRef]
- Baral, S.S.; Singh, K.; Sharma, P. The potential of sustainable algal biofuel production using CO2 from thermal power plant in India. Renew. Sustain. Energy Rev. 2015, 49, 1061–1074. [Google Scholar] [CrossRef]
- Khan, S.A.; Rashmi; Hussain, M.Z.; Prasad, S.; Banerjee, U.C. Prospects of biodiesel production from microalgae in India. Renew. Sustain. Energy Rev. 2009, 13, 2361–2372. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Rezvani, S.; Moheimani, N.R.; Bahri, P.A. Techno-economic assessment of CO2 bio-fixation using microalgae in connection with three different state-of-the-art power plants. Comput. Chem. Eng. 2016, 84, 290–301. [Google Scholar] [CrossRef]
- Choudhary, P.; Srivastava, R.K. Techno-economic case study: Bio-fixation of industrial emissions at an Indian oil and gas plant. J. Clean. Prod. 2020, 266, 121820. [Google Scholar] [CrossRef]
- Duarte, J.H.; Costa, J.A.V. Synechococcus nidulans from a thermoelectric coal power plant as a potential CO2 mitigation in culture medium containing flue gas wastes. Bioresour. Technol. 2017, 241, 21–24. [Google Scholar] [CrossRef]
- Kao, C.-Y.; Chen, T.-Y.; Chang, Y.-B.; Chiu, T.-W.; Lin, H.-Y.; Chen, C.-D.; Chang, J.-S.; Lin, C.-S. Utilization of carbon dioxide in industrial flue gases for the cultivation of microalga Chlorella sp. Bioresour. Technol. 2014, 166, 485–493. [Google Scholar] [CrossRef]
- Lu, W.; Asraful Alam, M.; Liu, S.; Xu, J.; Parra Saldivar, R. Critical processes and variables in microalgae biomass production coupled with bioremediation of nutrients and CO2 from livestock farms: A review. Sci. Total Environ. 2020, 716, 135247. [Google Scholar] [CrossRef] [PubMed]
- Thomassen, G.; Egiguren Vila, U.; Van Dael, M.; Lemmens, B.; Van Passel, S. A techno-economic assessment of an algal-based biorefinery. Clean Technol. Environ. Policy 2016, 18, 1849–1862. [Google Scholar] [CrossRef]
- Singh, J.; Dhar, D.W. Overview of Carbon Capture Technology: Microalgal Biorefinery Concept and State-of-the-Art. Front. Mar. Sci. 2019, 6, 29. [Google Scholar] [CrossRef]
- Norsker, N.H.; Barbosa, M.J.; Vermuë, M.H.; Wijffels, R.H. Microalgal production—A close look at the economics. Biotechnol. Adv. 2011, 29, 24–27. [Google Scholar] [CrossRef]
- Olivieri, G.; Salatino, P.; Marzocchella, A. Advances in photobioreactors for intensive microalgal production: Configurations, operating strategies and applications. J. Chem. Technol. Biotechnol. 2014, 89. [Google Scholar] [CrossRef]
- Suparmaniam, U.; Lam, M.K.; Uemura, Y.; Lim, J.W.; Lee, K.T.; Shuit, S.H. Insights into the microalgae cultivation technology and harvesting process for biofuel production: A review. Renew. Sustain. Energy Rev. 2019, 115, 109361. [Google Scholar] [CrossRef]
- Tan, X.B.; Lam, M.K.; Uemura, Y.; Lim, J.W.; Wong, C.Y.; Lee, K.T. Cultivation of microalgae for biodiesel production: A review on upstream and downstream processing. Chin. J. Chem. Eng. 2018, 26, 17–30. [Google Scholar] [CrossRef]
- Chew, K.W.; Yap, J.Y.; Show, P.L.; Suan, N.H.; Juan, J.C.; Ling, T.C.; Lee, D.-J.; Chang, J.-S. Microalgae biorefinery: High value products perspectives. Bioresour. Technol. 2017, 229, 53–62. [Google Scholar] [CrossRef]
- Hess Sandra, K.; Lepetit, B.; Kroth Peter, G.; Mecking, S. Production of chemicals from microalgae lipids—Status and perspectives. Eur. J. Lipid Sci. Technol. 2017, 120, 1700152. [Google Scholar] [CrossRef]
- Cheah, W.Y.; Show, P.L.; Chang, J.-S.; Ling, T.C.; Juan, J.C. Biosequestration of atmospheric CO2 and flue gas-containing CO2 by microalgae. Bioresour. Technol. 2015, 184, 190–201. [Google Scholar] [CrossRef]
- Gerardo, M.L.; Van Den Hende, S.; Vervaeren, H.; Coward, T.; Skill, S.C. Harvesting of microalgae within a biorefinery approach: A review of the developments and case studies from pilot-plants. Algal Res. 2015, 11, 248–262. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Matis, K.A. Electroflotation process: A review. J. Mol. Liq. 2016, 220, 657–664. [Google Scholar] [CrossRef]
- Rashid, N.; Ur Rehman, M.S.; Sadiq, M.; Mahmood, T.; Han, J.-I. Current status, issues and developments in microalgae derived biodiesel production. Renew. Sustain. Energy Rev. 2014, 40, 760–778. [Google Scholar] [CrossRef]
- Hosseinizand, H.; Lim, C.J.; Webb, E.; Sokhansanj, S. Economic analysis of drying microalgae Chlorella in a conveyor belt dryer with recycled heat from a power plant. Appl. Therm. Eng. 2017, 124, 525–532. [Google Scholar] [CrossRef]
- Show, K.-Y.; Lee, D.-J.; Chang, J.-S. Algal biomass dehydration. Bioresour. Technol. 2013, 135, 720–729. [Google Scholar] [CrossRef] [PubMed]
- Behera, B.; Aly, N.; Balasubramanian, P. Biophysical model and techno-economic assessment of carbon sequestration by microalgal ponds in Indian coal based power plants. J. Clean. Prod. 2019, 221, 587–597. [Google Scholar] [CrossRef]
- Juneja, A.; Murthy, G.S. Evaluating the potential of renewable diesel production from algae cultured on wastewater: Techno-economic analysis and life cycle assessment. AIMS Energy 2017, 5, 239–257. [Google Scholar] [CrossRef]
- Kumar, A.K.; Sharma, S.; Dixit, G.; Shah, E.; Patel, A. Techno-economic analysis of microalgae production with simultaneous dairy effluent treatment using a pilot-scale High Volume V-shape pond system. Renew. Energy 2020, 145, 1620–1632. [Google Scholar] [CrossRef]
- Musa, M.; Doshi, A.; Brown, R.; Rainey, T.J. Microalgae dewatering for biofuels: A comparative techno-economic assessment using single and two-stage technologies. J. Clean. Prod. 2019, 229, 325–336. [Google Scholar] [CrossRef]
- Deconinck, N.; Muylaert, K.; Ivens, W.; Vandamme, D. Innovative harvesting processes for microalgae biomass production: A perspective from patent literature. Algal Res. 2018, 31, 469–477. [Google Scholar] [CrossRef]
- Fasaei, F.; Bitter, J.H.; Slegers, P.M.; van Boxtel, A.J.B. Techno-economic evaluation of microalgae harvesting and dewatering systems. Algal Res. 2018, 31, 347–362. [Google Scholar] [CrossRef]
- Weschler, M.K.; Barr, W.J.; Harper, W.F.; Landis, A.E. Process energy comparison for the production and harvesting of algal biomass as a biofuel feedstock. Bioresour. Technol. 2014, 153, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Jorquera, O.; Kiperstok, A.; Sales, E.A.; Embiruçu, M.; Ghirardi, M.L. Comparative energy life-cycle analyses of microalgal biomass production in open ponds and photobioreactors. Bioresour. Technol. 2010, 101, 1406–1413. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.C.; Davis, R. The potentials and challenges of algae based biofuels: A review of the techno-economic, life cycle, and resource assessment modeling. Bioresour. Technol. 2015, 184, 444–452. [Google Scholar] [CrossRef]
- Rouf, S.A.; Douglas, P.L.; Moo-Young, M.; Scharer, J.M. Computer simulation for large scale bioprocess design. Biochem. Eng. J. 2001, 8, 229–234. [Google Scholar] [CrossRef]
- Duarte, J.H.; Fanka, L.S.; Costa, J.A.V. Utilization of simulated flue gas containing CO2, SO2, NO and ash for Chlorella fusca cultivation. Bioresour. Technol. 2016, 214, 159–165. [Google Scholar] [CrossRef]
- Acién, F.G.; Fernández, J.M.; Magán, J.J.; Molina, E. Production cost of a real microalgae production plant and strategies to reduce it. Biotechnol. Adv. 2012, 30, 1344–1353. [Google Scholar] [CrossRef]
- Kuo, C.-M.; Chen, T.-Y.; Lin, T.-H.; Kao, C.-Y.; Lai, J.-T.; Chang, J.-S.; Lin, C.-S. Cultivation of Chlorella sp. GD using piggery wastewater for biomass and lipid production. Bioresour. Technol. 2015, 194, 326–333. [Google Scholar] [CrossRef]
- Ummalyma, S.B.; Gnansounou, E.; Sukumaran, R.K.; Sindhu, R.; Pandey, A.; Sahoo, D. Bioflocculation: An alternative strategy for harvesting of microalgae—An overview. Bioresour. Technol. 2017, 242, 227–235. [Google Scholar] [CrossRef]
- Pragya, N.; Pandey, K.K.; Sahoo, P.K. A review on harvesting, oil extraction and biofuels production technologies from microalgae. Renew. Sustain. Energy Rev. 2013, 24, 159–171. [Google Scholar] [CrossRef]
- Vandamme, D.; Foubert, I.; Muylaert, K. Flocculation as a low-cost method for harvesting microalgae for bulk biomass production. Trends Biotechnol. 2013, 31, 233–239. [Google Scholar] [CrossRef]
- Lama, S.; Muylaert, K.; Karki, T.B.; Foubert, I.; Henderson, R.K.; Vandamme, D. Flocculation properties of several microalgae and a cyanobacterium species during ferric chloride, chitosan and alkaline flocculation. Bioresour. Technol. 2016, 220, 464–470. [Google Scholar] [CrossRef]
- Barros, A.I.; Gonçalves, A.L.; Simões, M.; Pires, J.C.M. Harvesting techniques applied to microalgae: A review. Renew. Sustain. Energy Rev. 2015, 41, 1489–1500. [Google Scholar] [CrossRef]
- Silva, C.; Soliman, E.; Cameron, G.; Fabiano, L.A.; Seider, W.D.; Dunlop, E.H.; Coaldrake, A.K. Commercial-Scale Biodiesel Production from Algae. Ind. Eng. Chem. Res. 2014, 53, 5311–5324. [Google Scholar] [CrossRef]
- Christenson, L.; Sims, R. Production and harvesting of microalgae for wastewater treatment, biofuels, and bioproducts. Biotechnol. Adv. 2011, 29, 686–702. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, J.R.; McAloon, A.J.; Taylor, F.; Johnston, D.B. Modeling the process and costs of fuel ethanol production by the corn dry-grind process. Ind. Crops Prod. 2006, 23, 288–296. [Google Scholar] [CrossRef]
- Valdovinos-García, E.M.; Barajas-Fernández, J.; Olán-Acosta, M.D.; Petriz-Prieto, M.A.; Guzmán-López, A.; Bravo-Sánchez, M.G. Techno-Economic Study of CO2 Capture of a Thermoelectric Plant Using Microalgae (Chlorella vulgaris) for Production of Feedstock for Bioenergy. Energies 2020, 13, 413. [Google Scholar] [CrossRef]
- Razzak, S.A.; Ali, S.A.M.; Hossain, M.M.; deLasa, H. Biological CO2 fixation with production of microalgae in wastewater—A review. Renew. Sustain. Energy Rev. 2017, 76, 379–390. [Google Scholar] [CrossRef]
- Hapońska, M.; Clavero, E.; Salvadó, J.; Farriol, X.; Torras, C. Pilot scale dewatering of Chlorella sorokiniana and Dunaliella tertiolecta by sedimentation followed by dynamic filtration. Algal Res. 2018, 33, 118–124. [Google Scholar] [CrossRef]
- Vandamme, D.; Foubert, I.; Fraeye, I.; Meesschaert, B.; Muylaert, K. Flocculation of Chlorella vulgaris induced by high pH: Role of magnesium and calcium and practical implications. Bioresour. Technol. 2012, 105, 114–119. [Google Scholar] [CrossRef]
- Schlesinger, A.; Eisenstadt, D.; Bar-Gil, A.; Carmely, H.; Einbinder, S.; Gressel, J. Inexpensive non-toxic flocculation of microalgae contradicts theories; overcoming a major hurdle to bulk algal production. Biotechnol. Adv. 2012, 30, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Show, K.-Y.; Yan, Y.-G.; Lee, D.-J. Chapter 7—Algal biomass harvesting and drying. In Biofuels from Algae, 2nd ed.; Pandey, A., Chang, J.-S., Soccol, C.R., Lee, D.-J., Chisti, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 135–166. ISBN 978-0-444-64192-2. [Google Scholar]
- Hoffman, J.; Pate, R.C.; Drennen, T.; Quinn, J.C. Techno-economic assessment of open microalgae production systems. Algal Res. 2017, 23, 51–57. [Google Scholar] [CrossRef]
- Davis, R.; Aden, A.; Pienkos, P.T. Techno-economic analysis of autotrophic microalgae for fuel production. Appl. Energy 2011, 88, 3524–3531. [Google Scholar] [CrossRef]
- Divakaran, R.; Sivasankara Pillai, V.N. Flocculation of algae using chitosan. J. Appl. Phycol. 2002, 14, 419–422. [Google Scholar] [CrossRef]
- Udom, I.; Zaribaf, B.H.; Halfhide, T.; Gillie, B.; Dalrymple, O.; Zhang, Q.; Ergas, S.J. Harvesting microalgae grown on wastewater. Bioresour. Technol. 2013, 139, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Goswami, R.K.; Mehariya, S.; Verma, P.; Lavecchia, R.; Zuorro, A. Microalgae-based biorefineries for sustainable resource recovery from wastewater. J. Water Process Eng. 2021, 40, 101747. [Google Scholar] [CrossRef]
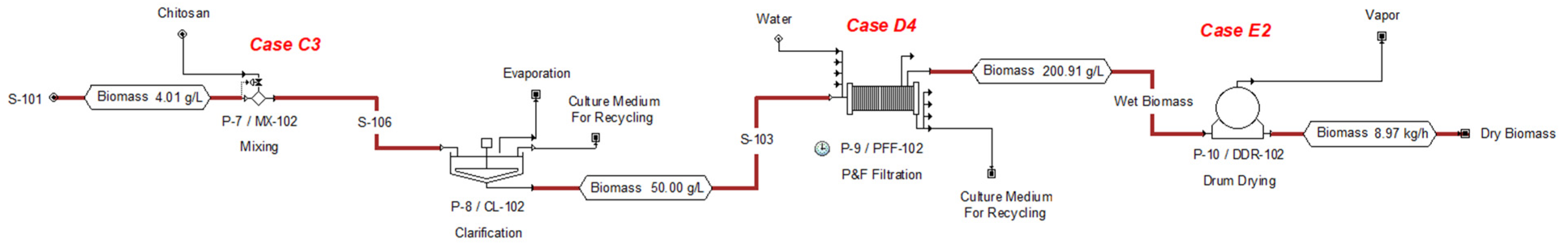


| Technology (Case) | Energy Consumption Considered (kWh/m3 Feed) | Energy Consumption Reference (kWh/m3 Feed) |
|---|---|---|
| Centrifugation (D1) | 8.70 | 3.3 [39], 3.6–10.8 [40], 0.7–1.30 [41] |
| Vacuum filter (D2) | 5.80 | 0.1–5.9 [40], 1.22–5.9 [41] |
| Belt filter press (D3) | 0.88 | 0.054–0.384 [39], 0.16–0.88 [40] |
| Pressure filter (D4) | 0.90 | 0.88 [40], 0.5–0.9 [41] |
| No. | Scenario | No. | Scenario | No. | Scenario |
|---|---|---|---|---|---|
| 1 | B-C1-D1-E1 | 9 | B-C2-D1-E1 | 17 | B-C3-D1-E1 |
| 2 | B-C1-D1-E2 | 10 | B-C2-D1-E2 | 18 | B-C3-D1-E2 |
| 3 | B-C1-D2-E1 | 11 | B-C2-D2-E1 | 19 | B-C3-D2-E1 |
| 4 | B-C1-D2-E2 | 12 | B-C2-D2-E2 | 20 | B-C3-D2-E2 |
| 5 | B-C1-D3-E1 | 13 | B-C2-D3-E1 | 21 | B-C3-D3-E1 |
| 6 | B-C1-D3-E2 | 14 | B-C2-D3-E2 | 22 | B-C3-D3-E2 |
| 7 | B-C1-D4-E1 | 15 | B-C2-D4-E1 | 23 | B-C3-D4-E1 |
| 8 | B-C1-D4-E2 | 16 | B-C2-D4-E2 | 24 | B-C3-D4-E2 |
| Variable | Case C1 | Case C2 | Case C3 |
|---|---|---|---|
| Biomass Production (tons/year) | 77.73 | 78.91 | 78.91 |
| Annual Flocculant Consumption (tons/year) | 2.59 | 0.63 | 0.754 |
| Material Cost (US $/year) | 1191 | 544 | 1579 |
| Energy Consumption (kWh/year) | 5720 | 5720 | 5721 |
| Operating Cost (US $/year) | 7000 | 6000 | 7000 |
| Unit Production Cost (US $/kg) | 0.09 | 0.07 | 0.09 |
| Variable | Case D1 | Case D2 | Case D3 | Case D4 |
|---|---|---|---|---|
| Biomass Production (tons/year) | 69.957 | 69.957 | 69.957 | 69.957 |
| Energy Consumption (kWh/year) | 16,777 | 9027 | 941 | 1399 |
| Operating Cost (US $/year) | 76,000 | 15,000 | 71,000 | 15,000 |
| Unit Production Cost (US $/kg) | 1.08 | 0.21 | 1.01 | 0.21 |
| Variable | Case E1 | Case E2 |
|---|---|---|
| Biomass Production (tons/year) | 69.957 | 69.957 |
| Energy Consumption (kWh/year) | 2,816 | 2,177 |
| Operating Cost (US $/year) | 37,000 | 19,000 |
| Unit Production Cost (US $/kg) | 0.53 | 0.28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valdovinos-García, E.M.; Petriz-Prieto, M.A.; Olán-Acosta, M.d.l.Á.; Barajas-Fernández, J.; Guzmán-López, A.; Bravo-Sánchez, M.G. Production of Microalgal Biomass in Photobioreactors as Feedstock for Bioenergy and Other Uses: A Techno-Economic Study of Harvesting Stage. Appl. Sci. 2021, 11, 4386. https://doi.org/10.3390/app11104386
Valdovinos-García EM, Petriz-Prieto MA, Olán-Acosta MdlÁ, Barajas-Fernández J, Guzmán-López A, Bravo-Sánchez MG. Production of Microalgal Biomass in Photobioreactors as Feedstock for Bioenergy and Other Uses: A Techno-Economic Study of Harvesting Stage. Applied Sciences. 2021; 11(10):4386. https://doi.org/10.3390/app11104386
Chicago/Turabian StyleValdovinos-García, Esveidi Montserrat, Moisés Abraham Petriz-Prieto, María de los Ángeles Olán-Acosta, Juan Barajas-Fernández, Adriana Guzmán-López, and Micael Gerardo Bravo-Sánchez. 2021. "Production of Microalgal Biomass in Photobioreactors as Feedstock for Bioenergy and Other Uses: A Techno-Economic Study of Harvesting Stage" Applied Sciences 11, no. 10: 4386. https://doi.org/10.3390/app11104386
APA StyleValdovinos-García, E. M., Petriz-Prieto, M. A., Olán-Acosta, M. d. l. Á., Barajas-Fernández, J., Guzmán-López, A., & Bravo-Sánchez, M. G. (2021). Production of Microalgal Biomass in Photobioreactors as Feedstock for Bioenergy and Other Uses: A Techno-Economic Study of Harvesting Stage. Applied Sciences, 11(10), 4386. https://doi.org/10.3390/app11104386






