Abstract
The present study proposes microwave-assisted extraction as a sustainable technique for the biosynthesis of bioactive compounds from rice fermented with Aspergillus flavus (koji). First, fermentation conditions (i.e., pH from 3–12, five temperatures from 20–40 °C, and four culture-fermentation media viz. wheat, wheat bran, malt and rice) were optimized for producing microbial bioactive compounds. Microwave extraction was performed at 2450 MHz and 500 W for 20, 30, and 40 s with seven solvents (distilled water, ethyl acetate, hexane, ethanol, chloroform, diethyl ether, and methanol). The obtained results revealed that ethyl acetate is the most appropriate solvent for extraction. Effects of this ethyl acetate extract were compared with a commercial synthetic antioxidant. Antioxidant properties were enhanced by preventing the oxidation of the linoleic acid (C18H32O2) with an inhibition rate (antioxidant efficacy) of 73.13%. Notably, the ferrous ion binding ability was marginally lower when compared to the disodium salt of ethylenediaminetetraacetic acid (EDTA). Additionally, the obtained total content of phenolic compounds in the ethyl acetate extract of fermented rice (koji) by Aspergillus flavus was 232.11 mg based on gallic acid/mL. Antioxidant compounds in the ethyl acetate extract of fermented rice showed stability under neutral conditions, as well as at high temperatures reaching 185 °C during 2 h, but were unstable under acidic and alkaline conditions. The results demonstrate the efficacy of novel microwave-assisted extraction technique for accelerating antioxidant production during rice fermentation.
1. Introduction
Lipid oxidation may cause damage to food quality and human health, due to reactive oxygen and nitrogen species (RONS), which are present in food matrices and the human body [1]. These free radicals are often the result or end-product of normal aerobic biological metabolism, and can cause damage by oxidizing vital biomolecules such as carbohydrates, proteins, and lipids, leading to tissue damage, or even cell death [2]. Previous studies revealed that oxidation and/or oxidative stress are involved in several human diseases such as neurogenerative disease, ageing, cardiovascular disease, arteriosclerosis, cancer, rheumatism, and diabetes [3,4,5]. Adverse effects of RONS are counter balanced by antioxidants [6]. Therefore, it is necessary to consume foods containing antioxidants to help the human body reduce the damage of oxidation and delay the occurrence of several diseases. For this reason, industrial antioxidants such as butylated hydroxytoluene (BHT), butylated hydroxyanisole (BHA), propyl gallate (PG), and tertiary butylhydroquinone (TBHQ) have been used in diets, even though some side effects related to human health, associated with their decomposition, have been reported [7]. Some natural antioxidants, such as tocopherol and herbal extracts, have also been used on a limited basis due to their high costs, intense flavors, and colors.
Furthermore, plant sources require a large quantity of extract to obtain sufficient amounts of antioxidants [8,9]. In addition to the reported antioxidative properties, these bioactive compounds exhibit strong antimicrobial properties [10]. Previous research reported that these bioactive compounds are of immense value, especially in the management of human health [11].
A variety of techniques and methodologies have been developed and explored related to the production, purification, and extraction of various beneficial products such as antioxidants, pharmaceuticals (drugs), metabolites, and nutraceuticals from plant sources, whose antioxidant potential have been improved by fermentative processes [12,13]. Microbial antioxidants are the metabolic end-products of microorganisms, possessing high potential for capturing free radicals to inhibit lipoxygenase [14] or acting as bonding agents for metal ions.
Modern technologies are more economical, efficient, sustainable, and easily accessible [15,16]. Recently, the microwave-assisted extraction (MAE) method, also known as microwave-assisted solvent extraction (MASE), has been developed as a novel emerging technology for the extraction of several beneficial compounds such as flavonoids and bioactive compounds from different natural sources [17,18,19]. Moreover, MAE has significant benefits over traditional extraction techniques (such as solid-liquid extraction) including higher yield, better reproducibility, lower energy usage, and decreased extraction time [20]. The transfer of energy is the main feature that characterizes the microwave heating process. In the case of microwaves, the heated material receives the energy directly through the molecular interaction with the electromagnetic wave, which is then converted into thermal energy. Unlike conventional heating which happens from the outside to inside [21], the localized heating during microwaves is found more effective and efficient in enhancing the kinetics of various mass transfer processes including dehydration and extraction [22]. Microwave extraction is different from conventional extraction through electromagnetic waves caused by the microwave oven, which causes changes in the cell structure during the extraction procedure. Conventional/traditional extraction techniques such as solid-phase extraction (SPE), maceration extraction (ME), liquid-liquid extraction (LLE) and Soxhlet extraction (SE) are very costly and often impeded by low productivity or low recovery of compounds of interest or target components. In addition, conventional techniques have a longer operation time, higher demands, utilize a higher amount of organic solvent consumption, generate a large amount of thermal heat, which results in the degradation of thermolabile components, and do not offer a simultaneous analysis of wide varieties of samples. Both bipolar rotation, bipolar and ionic conduction through bipolar reflections and the movement of the charged ions found in the solute and solvent occur at the same time. In the electromagnetic field, a rapid movement of ions occurs, and the resistance of the solution to the ion movement results in friction [23]. MAE procedures can be used as a reliable technique for the laboratory, industry, as well as pilot-scale studies. There are various factors such as particle size, temperature, solvent type, time, and solvent to solid ratio that potentially affect the overall phenolic content, when MAE is used for antioxidant extraction. The 2,2-diphenyl-1-picrylhydrazyl (DPPH), ferric reducing antioxidant power (FRAP), and extraction yield were investigated in various antioxidant extraction methods from pistachio hull, showing the presence of high antioxidant activity [24]. Interestingly, during the last decade, green technologies such as MAE are claiming over 200 patents worldwide [25].
Koji rice is traditionally fermented rice produced with the use of solid state fermentation. Traditionally, this technique involved the use of Aspergillus oryzae species. A. oryzae is an aerobic fungus belonging to the Aspergillus section Flavi, previously known as the Aspergillus Flavus group, that contain industrially important A. oryzae and medically significant A. Flavus and A. parasiticus species known to produce aflatoxins [26]. These other strains are relatively less investigated in the literature, and it was hypothesized that using these alternate strains might open alternate pathways for the production of antioxidants from Koji fermented rice.
The overall objective of the study was to investigate MAE as a technique to biosynthesize antioxidants from rice fermented with A. flavus strains. This was achieved in three sub-objectives, viz.: (1) Study the appropriate conditions related to the production of antioxidant compounds of fermented rice (koji), wheat, wheat bran and malt by A. flavus. (2) study the stability of antioxidants produced from solid state fermentation of rice (koji) with A. flavus, and (3) investigate the possibility of using microwave-assisted extraction technology for the extraction of antioxidants.
2. Materials and Methods
2.1. Materials
Whole and polished rice cultures were infested with fungi (Aspergillus flavus) obtained from the Laboratory of Life Sciences, Department of Marine Biology, Center of Marine Sciences, University of Basrah, Iraq. Malt extract agar (MEA) was purchased from Himedia (Mumbai, India). Hexane, ethanol, chloroform, methanol, DMSO, Folin-Ciocalteu, potassium ferricyanide, monosodium phosphate, and trichloroacetic acid (TCA) were obtained from Sigma-Aldrich (St. Louis, MO, USA).
2.2. Preparation of Inoculated Rice Koji
To create spore suspensions required for the inoculation of rice, A. flavus was incubated for 5 days on malt extract agar (MEA), then suspended spores were collected from the supernatant without centrifugation. To ensure the purity of spores, fungal isolates were examined for shape under the microscope by placing the fungus onto glass slides using cotton swabs soaked in a lactophenol solution containing blue cotton. After confirmation of the fungal identity, a suspension of A. flavus was prepared by activating the fungus through incubation of the MEA slant at 30 °C for 5 days. Then, 5 mL of the supernatant were collected. The number of spores was calculated using a hemocytometer. The solution was then diluted using distilled water to reach a final concentration of 1 × 107 spores/mL [27]. This solution was used to inoculate Koji.
Inoculated rice was prepared according to the Hoppe method [28]. Briefly, 50 g of Koji rice was soaked in distilled water for 1 h before being sterilized at 121.1 °C under 106 kPa steam pressure for 15 min. The boiled, sterile rice was then cooled to room temperature before being inoculated (10% v/w) with the 1 × 107 spores/mL solution by spraying the spore solution over the rice. The inoculated rice was then incubated at 30 °C for 15 days. An uninoculated rice sample was also prepared the same way for identifying any effects due to contamination.
2.3. Optimization of Fermentation Parameters
Fermentation parameters were optimized according to the method described in Yen and Lee [29]. The parameters optimized included pH, temperature, and media of fermentation using a full-factorial design (7 × 5 × 4). Four different fermentation media were used to study the optimum medium including rice, wheat, wheat bran, and malt as described earlier [29], whereas rice was found to be the most optimal media, and thereby used in further experiments. A pH range of 3 to 12 was investigated to optimize the production of antioxidants. Antioxidant efficacy was estimated by the thiocyanate method [30]. Different pH values were used for organic solutions including citrate (at pH 3 and 5) with a concentration of 0.2 M, and phosphate (regulated at pH 7, 9, 10, 11, and 12) with a concentration of 0.2 M to study the effect of pH on the stability of the antioxidative activity of A. flavus. The obtained data was compared to the control sample (containing BHT and alpha tocopherol) grown in the same pH. Different temperatures were used to optimize the production of antioxidants produced from rice koji inoculated with A. flavus. Five growth temperatures were explored; 20, 25, 30, 35, and 40 °C.
2.4. Microwave-Assisted Extraction
The primary extraction of antioxidants along with bioactive compounds involved mixing the fermented rice (koji) (obtained in Section 2.2.) in an electric blender with 100 mL of ethyl acetate. This mix was then filtered through Whatman no.1 filter paper and washed three times. The filtered organic extract was used for further extraction. An extraction method described earlier [31] using 90 °C for extraction with Teflon tubes (3 cm-diameter, 20 cm long, and 5 mm thickness) was used. Ten milliliters of the filtered organic extract was then taken and put in dark glass bottles as photodegradation of phenolics occurs due to light [32]. Next, 40 mL of different solvents (distilled water, ethyl acetate, hexane, ethanol, chloroform, diethyl ether, and methanol) were added. Each of the filtered organic extract were mixed with the solvent above using the magnetic plates for 30 min and then transferred to the Teflon tubes and sealed tightly and placed in the microwave device (capacity 2 L, MARS 6, CEM Corporation, Matthews, NC, USA) using a frequency of 2450 MHz and a power of 500 W for 20, 30, and 40 s. Next, centrifugation was performed in tubes at 600 g for 15 min. The filtrate was concentrated using the rotary vacuum evaporator at 40 °C to reduce the solvents to a final volume of 10 mL. The extracts were kept at −18 °C for further use.
2.5. Properties of Microbial Antioxidants
After the optimization of the fermentation parameters, antioxidants were then characterized as shown earlier [33].
2.5.1. Antioxidant Activity
The antioxidant activity was determined using the thiocyanate method [30]. Specifically, 4 mL each of 99.5% ethanol and 2.51% linoleic acid, and 8 mL of 0.02 M pH 7.0 phosphate buffer was added to 4 mg of the extract, along with 3.9 mL of distilled water. The solution was maintained at 40 °C in a covered test tube. Then, 0.1 mL of this reaction mixture was added to 9.7 mL of 75% aqueous ethanol and 0.1 mL each of 30% aqueous ammonium thiocyanate and 0.02 M ferrous chloride in 3.5% hydrochloric to generate a red color. The absorbance was measured at 500 nm in triplicate experiments. The inhibition of linoleic acid oxidation was calculated according to the Equation (1):
2.5.2. Determination of Phenolic Compounds
The method described in Wiktor et al. [32] was used to estimate the phenolic compounds in the ethyl acetate extract in fermented rice (koji) by Aspergillus flavus. Briefly, 0.5 mL of the extract was mixed with 7 mL of distilled water and 0.5 mL of Folin-Ciocalteu. After 3 min, 2 mL of 20% Na2CO3 was added and the mixture was heated to 100 °C in a water bath. After 1 min, the absorption of the extract solution was compared to a gallic acid standard by measuring the absorbances of the solution at 685 nm using a spectrophotometer. All results were expressed in mg gallic acid equivalent (GAE).
2.5.3. Free Radical Scavenging
The method described in Shimada et al. [34] was used with some modifications to estimate the scavenging of free radicals. Briefly, either 1 mL of the sample (extract or BHT) or 1 mL of water (control) was mixed with 4 mL of methanol and 1 mL of dimethyl sulfoxide (DMSO) solution instead of 2,2-diphenyl-1-picrylhydrazyl(DPPH). Absorbance was measured at 528 nm using a spectrophotometer. The scavenging efficiency was calculated according to the following Equation (2) [35]:
2.5.4. Ferrous Ion Chelating
To measure the ferrous ion, the method described by Decker and Welch [36] was used. Briefly, either 1 mL of the sample (extract or EDTA-2Na) or 1 mL of water (control) was mixed with 3.7 mL of methanol, 0.1 mL of iron(II) chloride, and 0.2 mL of 8-hydroxyquinoline rather than using Ferrozine 5 mM. The solution was mixed and left at room temperature for 10 min before the absorbance was measured at 562 nm using a spectrophotometer. The connectivity was calculated according to the following Equation (3):
2.5.5. Reducing Power Capacity
The reduction power of the samples was estimated according to Oyaizu [37]. Briefly, 0.3 mL of the extract was mixed with 0.3 mL of potassium ferricyanide and 0.3 mL of sodium phosphate buffer (pH 6.6, 0.2 mol/L). The solution was incubated for 20 min at 50 °C before 0.3 mL of 10% TCA was added. The solution was then centrifuged for 10 min at 4 °C and 6000 RPM using a centrifuge (LMC, 3000). Finally, 0.6 mL of the upper layer was removed and mixed with 0.12 mL of 0.1% ferric chloride and 0.6 mL of distilled water before being mixed for 10 min. The absorbance was measured at 700 nm using a spectrophotometer. Water was used as a control.
2.6. Stability of Antioxidant Compounds
In order to measure the efficiency of antioxidant compounds including the pH and temperature, the method described in Yen and Lee [29] was used. In brief, 1 mg of each sample was mixed with 0.5 mL of ethyl acetate, 2.5 mL of linoleic acid emulsion (0.02 M, pH 7.0), and 2 mL of phosphatase buffer (0.2 M, pH 7.0) and then placed in darkness at 37 °C. Absorbance at 500 nm was read in triplicate experiments with a spectrophotometer (Hitachi U-2000, Tokyo, Japan) after coloring with FeCl and thiocyanate.
2.6.1. Effect of pH
Different pH values were used for organic solutions including the solution of the regulated citrate (at pH 3 and 5) with a concentration of 0.2 molar, and the phosphate solution (regulated at pH 7 and 9) with a concentration of 0.2 molar to show the effect of pH on the stability of the antioxidant activity of antioxidant compounds. The obtained data were compared to the control sample grown in the same pH. Antioxidant efficacy was estimated by the method of thiocyanate [30] after 0, 20, 40, 60, and 80 h of incubation.
2.6.2. Effect of Exposure Time
Three milliliters of the antioxidant extract was placed into a 10 mL flask and heated at 185 °C for 0, 10, 30, 50, 70, 90, and 120 min, and then cooled at room temperature before adding 0.3 mL of ethyl acetate. The efficacy was measured according to the thiocyanate method [30].
2.7. Statistical Analysis
The Statistical Package for the Social Sciences (SPSS) (1998) was used for data analysis and an analysis of variance (ANOVA) was used followed by the least significant difference (LSD) at the probability level of 0.05. All experiments were performed in triplicate experiments. The mean comparison using Tukey’s test was performed with the STATISTICA 13 software (alpha = 0.05).
3. Results
Figure 1 shows the effect of the different (A) solvents, (B) pH values (3–12), (C) temperatures (20–40 °C), and (D) media (rice, malt, wheat, and fermented wheat bran) used to extract antioxidant compounds by Aspergillus flavus after 15 days of growth at 30 °C. Based on this, the fermentation of A. flavus on rice medium, with 15 days of incubation at 30 °C at pH 7 was chosen as the optimal procedure for the production of antioxidant compounds.
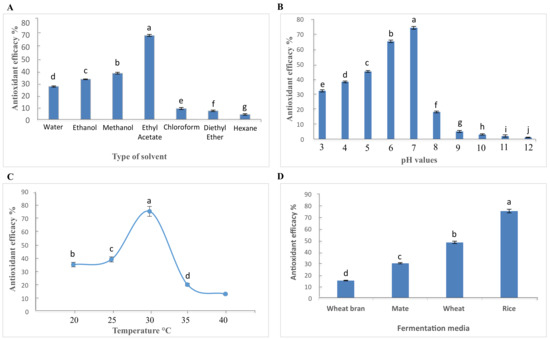
Figure 1.
(A) Effect of using different solvents on the effectiveness of antioxidant compounds of fermented rice (koji) inoculated with A. flavus following 15-days of incubation at 30 °C. (B) Effect of different pH values on the effectiveness of antioxidant compounds of fermented rice (koji) inoculated with A. flavus following 15-days of incubation at 30 °C. (C) Effect of different temperatures on the effectiveness of antioxidant compounds. Compounds of fermented rice (koji) inoculated with A. flavus following 15-days of incubation at pH 7. (D) Effect of different fermentation media on the effectiveness of antioxidant compounds of fermented rice (koji) inoculated with A. flavus following 15-days of incubation at 30 °C. * The different letters indicate significant differences at the probability level p < 0.05.
The antioxidant activity of the optimized ethyl acetate extract of fermented rice (koji) inoculated with A. flavus was compared to the synthetic antioxidant BHT and the natural antioxidant α-tocopherol at concentrations of 200 μg/mL, as shown in Table 1. The antioxidant efficacy was comparable to that of commercial extracts.

Table 1.
Antioxidant activity of the ethyl acetate extract of fermented rice (koji) using A. flavus.
The free radical scavenging activity, Fe2+ binding ability, and Fe3+ reducing ability of fermented rice (koji) extract inoculated with A. flavus are reported in Figure 2. The free radical scavenging activity was marginally less than that of commercial antioxidant BHT, which also follows from their antioxidant efficacy reported in Table 1. The Fe2+ binding power of A. flavus extracts were also slightly less than that of EDTA-2 Na. Similarly, as compared to BHT, the Fe3+ reducing power was a little lower for the A. flavus extract.
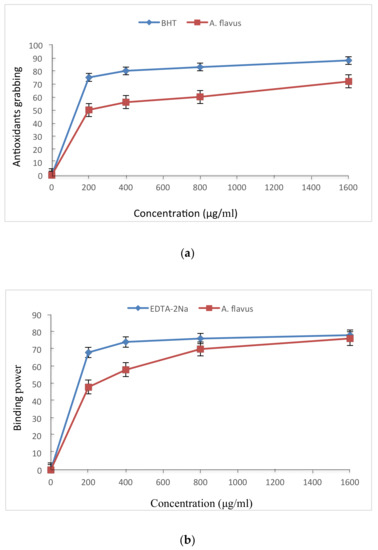
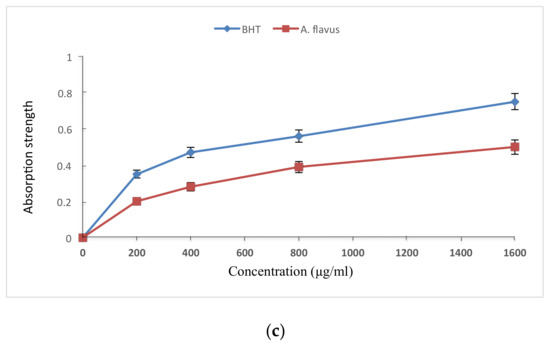
Figure 2.
Antioxidant properties of fermented rice (koji) by A. flavus: (a) Free radical scavenging activity compared to BHT for free radical scavenger DMSO at different concentrations; (b) ferrous ion chelating power compared to EDTA-2Na at different concentrations; and (c) strength of reduction of antioxidant compounds compared to BHT at different concentrations.
The effect of pH on the ability of these fermented rice (koji) extracts to inhibit the oxidation of linoleic acid at 40 °C incubation for 80 h is reported in Table 2, wherein biosynthesized antioxidants were found to be most stable under neutral pH, with increasing activity up to 80 h of incubation, whereas the stability was lower under acidic and basic pH.

Table 2.
Effect of pH on the absorption of fermented rice (koji) extract by A. flavus in different incubation periods at 40 °C.
The effect of thermal treatment on the effectiveness of antioxidant compounds of fermented rice (koji) extracts on heating at 185 °C at different time intervals are reported in Table 3. It was seen that the heating at 185 °C did not reduce the antioxidant efficacy significantly for up to 10 min, and started decreasing afterwards. Even after 120 min of heating, up to 92.7% of antioxidants were retained, demonstrating good stability of the biosynthesized antioxidants.

Table 3.
Effect of exposure time to 185 °C on the effectiveness of antioxidant compounds of fermented rice (koji) by A. flavus.
4. Discussions
4.1. Production of Effective Antioxidant Compounds
The fungus began to grow after 2 days on the surface of the rice in a visible and clear manner after the inoculation of rice with Aspergillus flavus. Growth of the mold became abundant as the incubation period progressed, with the black A. flavus spores appearing clearly on the surface of the inoculated rice, whereas the uninoculated rice samples showed no growth ruling out possibilities of contamination and confirming the spore germination as an effect of the inoculation with A. flavus spores. After 15 days of incubation, the surface of inoculated samples was covered entirely with A. flavus mold spores.
It is known that molds produce secondary metabolic products during fermentation, some of which, similar to aflatoxins from A. flavus, may also be toxic to human health. The extract was tested for toxicity according to Shotwell [38], with known quantities of standard aflatoxins on the same plate, and no toxicity was found. Other secondary metabolites produced can have an antioxidant effect that increases during the fermentation process [39] showing that molds have the ability to produce antioxidants on solid culture during the fermentation process. Furthermore, it has been reported that antioxidant efficacy is related to the number of hydrogen atoms [34]. The effectiveness of antioxidants to inhibit the oxidation of linoleic acid is due to different mechanisms, including the disruption of the chain of the initiation reaction, bonding of iron ions, destruction of peroxides, prevention of hydrogen removal, reducibility, and free radicals [40].
4.2. Optimization of the Fermentation Process
Fermentation media can affect the production of antioxidants [39]. Rice was chosen as the fermentation media, based on results shown in Figure 1D, which exhibited significant differences at the probability level of p < 0.05 in the effectiveness of antioxidant compounds of different media. The results revealed that the antioxidant activity of A. flavus grown on rice media had an efficacy rate of 74.9%, which was considerably higher than the other substrates tested, making it the most suitable media for fermentation. In contrast, the isolation revealed a significant decrease in the effectiveness of antioxidant compounds on the media of wheat bran with an efficacy rate of only 15.2%. The obtained results were in agreement with Miyake et al. [39], in which rice media was used to produce antioxidant compounds from molds. This also suggests the possibility of using other plant-based media such as peas for exploring the possibility of producing antioxidants, given that pea protein has increased antioxidant activity of pH-adjusted canola oil nanoemulsions [41].
The pH value is known to have a significant effect on antioxidants produced during fermentation. Figure 1B shows significant differences (p < 0.05) in the production of antioxidant compounds for all the pH values investigated. The highest effectiveness was obtained at pH 7, with an efficacy rate of 74.1%. The lowest value was observed at pH 12, with an efficacy rate of only 1.1%. This low efficacy rate was due to a lack of growth of the fungus at this high pH and thus reduced the antioxidant efficiency of the ethyl acetate extract. Since pH 7 showed the highest efficacy rate, it was used to further investigate the effect of temperature on the production of antioxidants in fermented rice koji inoculated with A. flavus.
Another factor known to have a significant impact on the production of antioxidants is temperature. Figure 1C shows significant differences (p < 0.05) in the production of antioxidant compounds at different temperatures. The lowest efficacy rate was observed at an incubation temperature of 20 °C (35.2%), while the antioxidant activity was highest at an incubation temperature of 30 °C (75.1%). The effectiveness of the antioxidant compounds decreased significantly at 40 °C, with an efficacy rate of 13.2%. These observations are in agreement with Lin et al. [42]. It has been reported that molds had the ability to produce antioxidant compounds at 30 °C [43], while the antioxidant activity decreased at both lower and higher temperatures. Moreover, future optimization of fermentation will be performed at pH 7 at 30 °C.
Furthermore, the following optimized growth conditions were chosen to further characterize antioxidants produced from rice koji inoculated with A. flavus: Incubation for 15 days at 30 °C grown on rice medium at pH 7. The overall optimized methodology is summarized in Figure 3.
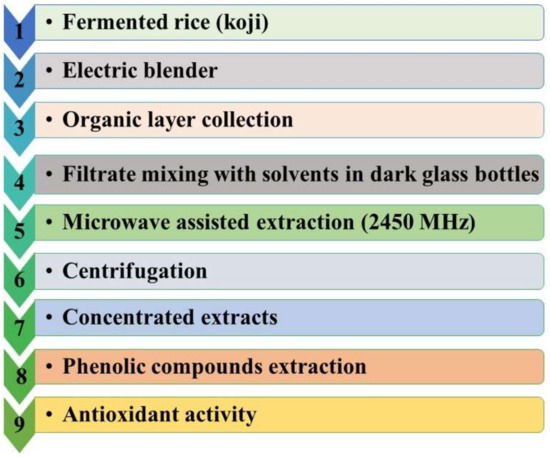
Figure 3.
Diagram showing a summary of the proposed methodology used to extract phenolic compounds and antioxidant activity measurement.
4.3. Microwave Extraction of Antioxidant Compounds
As shown in Figure 1A, a significant difference in the antioxidant efficacy (p < 0.05) was observed between all extraction solvents used with ethyl acetate showing the highest efficacy at 69.4%. The effectiveness of the antioxidant compounds was significantly lower when the extraction was carried out using hexane, with an efficacy rate of 4.7%. Microwave extraction for 40 s produced the highest concentration of antioxidant compounds using the ethyl acetate solvent. These results are in agreement with others [44], who demonstrated that the ethyl acetate solvent is highly efficient in extracting antioxidant compounds from A. flavus when compared to other solvents. The results showed differences in the effectiveness of antioxidant compounds when using different solvents to extract antioxidants. This is the result of a range of polarities observed within various solvents tested, as well as the chemical nature of the active compounds. As a result, antioxidant compounds such as phenolic compounds are preferably dissolved into polar solvents as ethyl acetate, which has a similar polarity to the extracted antioxidant compounds [45]. The obtained results were in agreement with another research [46], in which ethyl acetate was used to extract antioxidant compounds from Mortierella sp. In addition, bioactive compounds such as 3.3-dihydroxyterphenyllin, 3-hydroxyterphenyllin, and candidus were isolated from A. candidus using an acetylene solvent [47]. Furthermore, the previous study demonstrates that the extraction of phenolic compounds, which also have an antioxidant activity, strongly depends on the solvent used [48].
4.4. The Properties of Effective Antioxidants Biosynthesized Using A. Flavus
The total phenolic content in the ethyl acetate extract of fermented rice (koji) by Aspergillus flavus was 0.23 g GAE/mL. Previous research reported that the total phenolic content in the extract increased after the fermentation process, indicating the presence of larger quantities of phenolic compounds produced after the fermentation process [49]. Furthermore, these compounds corresponding to secondary metabolites are present in plants and are linked through hydroxyl groups with glucose in the form of glucosides [50].
Table 1 shows that BHT had a significantly higher (p < 0.05) antioxidant efficacy (85.2%) as compared to α-tocopherol (82.0%) and A. flavus (73.1%). The obtained results exhibited a significant difference (at p < 0.05) in the antioxidant effects of the three antioxidants. The effectiveness of antioxidants increases as the extract contains large quantities of antioxidant compounds [39]. Therefore, the effectiveness of antioxidant compounds increased by plant isoflavones, which were released during the fermentation process [50]. As shown in Figure 2, at all concentrations, the A. flavus extracts were almost as strong (only marginally inferior) than controls BHT and EDTA-2Na in terms of free radical scavenging activity, iron-ions binding efficiency, and reducing power capacity, when compared to the extract obtained from fermented rice koji inoculated with A. flavus. In each of the above three parameters tested, a logarithmic increase was observed that proportionally increased with the increasing concentration. This is in agreement with previous studies [51,52,53] which confirmed that antioxidant compounds of fermented rice (koji) using A. flavus have the ability to donate a hydrogen atom and thereby prohibit free radicals. This is also in agreement with previous studies [42,43,54], which demonstrate that the compounds of microbial antioxidants increased logarithmically with the concentration. A significant increase (p < 0.05) in the reduction power was observed while increasing the concentration, which is consistent with the reported observations [55]. The presence of compounds may explain the observed reduction strength of the extract which are formed during the fermentation process and can interact with free radicals to be converted into more stable products and therefore terminate the free radical reaction chain [56], while the reductase compounds interact with the peroxides and inhibit their formation.
4.5. Stability of Antioxidant Compounds
A significant difference (p < 0.05) in the absorption of the extract obtained from koji inoculated with A. flavus was found in all the pH values tested (Table 2). The obtained results show the effect of acidic pH on the absorption of fermented rice (koji) by A. flavus, in addition to a significant decrease in the absorption of the extract with a gradually increasing incubation time at acidic pH when a maximum time is reached (after 80 h). The results also exhibit the significant effect of pH on the absorption of the extract from fermented rice (koji) inoculated with A. flavus (p > 0.05). A slight reduction in the antioxidant activity was observed, which was consistent with Yen and Lee [29]. The rate of absorption value was significantly reduced in basal pH. The presence of a significant variation in the absorption of the control sample was also observed as it began to decline rapidly after 45 h, which could be explained by the breakdown of peroxides formed during the oxidation process of linoleic acid.
The effectiveness of the remaining antioxidant compounds was estimated after the heating process. The results in Table 3 revealed significant differences (p < 0.05) in the effectiveness of antioxidant compounds for the heating periods from 30 to 120 min except for the early heating period from 0 to 10 min. No significant differences (p > 0.05) were observed in the antioxidant activity, except for a slight decrease in the antioxidant activity when the heating time reached 10 min. The antioxidant activity of fermented rice (koji) by A. flavus decreased significantly when the extraction heating time increased to 120 min with an inhibition ratio of 70.11%. This result could be explained by the adverse effects of exposure to high temperatures for a long time, thereby causing degradation of the bioactive compounds. This result was in agreement with Hamama and Nawar [57], which reported that industrial antioxidants BHA, BHT, PG, and TBHQ lose their effectiveness at 185 °C heating for 1 h, with a damping rate of 42.8, 20.4, 37.1, and 47.7%, respectively.
5. Conclusions
The ever-increasing demand to extract a plethora of compounds from various sources provides a continuous demand for the most convenient method of extraction and production of antioxidants. The present study demonstrated a significant increase in the antioxidant efficacy when antioxidants were biosynthesized from the microbial community of A. flavus grown on the rice media with an inhibitory rate of 74.89%. The antioxidant strength of A. flavus extracts was only marginally less than the antioxidant BHT. The current study revealed that the antioxidant compounds from the extract of fermented rice (koji) by A. flavus were stable under neutral conditions, but less stable under acidic and alkaline conditions, thus reducing the antioxidant effect of the compounds formed. The antioxidant effect of fermented rice (koji) by A. flavus was considerably preserved at increased exposure times to high temperatures. Although multiple approaches for antioxidant biosynthesis are available, this study, on the use of novel species A. flavus for the production of antioxidants from rice, will open new avenues for the production of potential bioactive compounds from microbial communities.
Author Contributions
Conceptualization, A.A., A.P.-S., and N.L.; methodology, H.H.M.A.R., A.A., and A.K.J.A.R.; validation, H.H.M.A.R., A.A., and A.K.J.A.R.; investigation, A.A., N.L., and A.P.-S.; writing—original draft preparation, H.H.M.A.R., A.A., and A.K.J.A.R.; writing—review and editing, A.A., N.L., and A.P.-S.; funding acquisition, A.P.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science and Engineering Research Council of Canada (NSERC) Discovery Grant no. RGPIN-2018-04735 to Anubhav Pratap-Singh.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to intellectual property issues.
Acknowledgments
The authors would like to thank the Food Science Department, Agriculture College, University of Basrah for providing the facilities and equipment.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pratap-Singh, A.; Fathordoobady, F.; Guo, Y.; Singh, A.; Kitts, D.D. Antioxidants help favorably regulate the kinetics of lipid peroxidation, polyunsaturated fatty acids degradation and acidic cannabinoids decarboxylation in hempseed oil. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kitts, D.D.; Singh, A.; Fathordoobady, F.; Doi, B.; Pratap-Singh, A. Plant Extracts Inhibit the Formation of Hydroperoxides and Help Maintain Vitamin E Levels and Omega-3 Fatty Acids During High Temperature Processing and Storage of Hempseed and Soybean Oils. J. Food Sci. 2019, 84, 3147–3155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tsao, R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Losada-Barreiro, S.; Bravo-Díaz, C. Free radicals and polyphenols: The redox chemistry of neurodegenerative diseases. Eur. J. Med. Chem. 2017, 133, 379–402. [Google Scholar] [CrossRef] [PubMed]
- Matschke, V.; Theiss, C.; Matschke, J. Oxidative stress: The lowest common denominator of multiple diseases. Neural Regen. Res. 2019, 14, 238–241. [Google Scholar] [CrossRef] [PubMed]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Novel antioxidants in food quality preservation and health promotion. Eur. J. Lipid Sci. Technol. 2010, 112, 930–940. [Google Scholar] [CrossRef]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of Natural Plant Origins: From Sources to Food Industry Applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef]
- Salar, R.K.; Purewal, S.S.; Sandhu, K.S. Bioactive profile, free-radical scavenging potential, DNA damage protection activity, and mycochemicals in Aspergillus awamori (MTCC 548) extracts: A novel report on filamentous fungi. 3 Biotech 2017, 7, 164. [Google Scholar] [CrossRef]
- Govindan, L.; Anbazhagan, S.; Altemimi, A.B.; Lakshminarayanan, K.; Kuppan, S.; Pratap-Singh, A.; Kandasamy, M. Efficacy of Antimicrobial and Larvicidal Activities of Green Synthesized Silver Nanoparticles Using Leaf Extract of Plumbago auriculata Lam. Plants 2020, 9, 1577. [Google Scholar] [CrossRef]
- Sun-Waterhouse, D. The development of fruit-based functional foods targeting the health and wellness market: A review. Int. J. Food Sci. Technol. 2011, 46, 899–920. [Google Scholar] [CrossRef]
- Tabernero, M.; de Cedrón, M.G. Microbial metabolites derived from colonic fermentation of non-digestible compounds. Curr. Opin. Food Sci. 2017, 13, 91–96. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Munekata, P.E.; Gómez, B.; Barba, F.J.; Mora, L.; Pérez-Santaescolástica, C.; Toldrá, F. Bioactive peptides as natural antioxidants in food products—A review. Trends Food Sci. Technol. 2018, 79, 136–147. [Google Scholar] [CrossRef]
- Staerck, C.; Gastebois, A.; Vandeputte, P.; Calenda, A.; Larcher, G.; Gillmann, L.; Papon, N.; Bouchara, J.-P.; Fleury, M.J.J. Microbial antioxidant defense enzymes. Microb. Pathog. 2017, 110, 56–65. [Google Scholar] [CrossRef]
- Pratap-Singh, A.; Yen, P.P.-L.; Ramaswamy, H.S.; Singh, A. Recent advances in agitation thermal processing. Curr. Opin. Food Sci. 2018, 23, 90–96. [Google Scholar] [CrossRef]
- Pratap-Singh, A.; Mandal, R.; Shojaei, M.; Singh, A.; Kowalczewski, P.; Ligaj, M.; Pawlicz, J.; Jarzębski, M. Novel Drying Methods for Sustainable Upcycling of Brewers’ Spent Grains as a Plant Protein Source. Sustainability 2020, 12, 3660. [Google Scholar] [CrossRef]
- Ekezie, F.-G.C.; Sun, D.-W.; Cheng, J.-H. Acceleration of microwave-assisted extraction processes of food components by integrating technologies and applying emerging solvents: A review of latest developments. Trends Food Sci. Technol. 2017, 67, 160–172. [Google Scholar] [CrossRef]
- Altemimi, A.B.; Mohammed, M.J.; Yi-Chen, L.; Watson, D.G.; Lakhssassi, N.; Cacciola, F.; Ibrahim, S.A. Optimization of Ultrasonicated Kaempferol Extraction from Ocimum basilicum Using a Box–Behnken Design and Its Densitometric Validation. Foods 2020, 9, 1379. [Google Scholar] [CrossRef]
- Rodsamran, P.; Sothornvit, R. Extraction of phenolic compounds from lime peel waste using ultrasonic-assisted and microwave-assisted extractions. Food Biosci. 2019, 28, 66–73. [Google Scholar]
- Putnik, P.; Lorenzo, J.; Barba, F.J.; Roohinejad, S.; Jambrak, A.R.; Granato, D.; Montesano, D.; Kovačević, D.B. Novel Food Processing and Extraction Technologies of High-Added Value Compounds from Plant Materials. Foods 2018, 7, 106. [Google Scholar] [CrossRef]
- Pratap-Singh, A.; Singh, A.; Ramaswamy, H.S. Effect of reciprocating agitation thermal processing (RA-TP) on quality of canned tomato (Solanum lycopersicum) puree. J. Sci. Food Agric. 2017, 97, 2411–2418. [Google Scholar] [CrossRef] [PubMed]
- Yen, P.P.; Pratap-Singh, A. Vacuum microwave dehydration decreases volatile concentration and soluble protein content of pea (Pisum sativum L.) protein. J. Sci. Food Agric. 2021, 101, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Routray, W.; Orsat, V. Microwave-Assisted Extraction of Flavonoids: A Review. Food Bioprocess Technol. 2012, 5, 409–424. [Google Scholar] [CrossRef]
- Tabaraki, R.; Ghadiri, F. Comparative study of extraction methods for pistachio hull antioxidants by multiple assays. J. Appl. Chem. 2016, 37, 19–29. [Google Scholar]
- Chemat, F.; Vian, M.A.; Fabiano-Tixier, A.-S.; Nutrizio, M.; Jambrak, A.R.; Munekata, P.E.S.; Lorenzo, J.; Barba, F.J.; Binello, A.; Cravotto, G. A review of sustainable and intensified techniques for extraction of food and natural products. Green Chem. 2020, 22, 2325–2353. [Google Scholar] [CrossRef]
- Gomi, K. ASPERGILLUS | Aspergillus oryzae. In Encyclopedia of Food Microbiology; Elsevier BV: Amsterdam, The Netherlands, 2014; pp. 92–96. [Google Scholar]
- Yen, G.-C.; Chang, Y.-C.; Su, S.-W. Antioxidant activity and active compounds of rice koji fermented with Aspergillus candidus. Food Chem. 2003, 83, 49–54. [Google Scholar] [CrossRef]
- Hoppe, M.B.; Jha, H.C.; Egge, H. Structure of an antioxidant from fermented soybeans (tempeh). J. Am. Oil Chem. Soc. 1997, 74, 477–479. [Google Scholar] [CrossRef]
- Yen, G.C.; Lee, C.E. Antioxidative properties of extracts from Aspergillus candidus broth filtrate. J. Sci. Food Agric. 1997, 75, 326–332. [Google Scholar] [CrossRef]
- Kikuzaki, H.; Usuguchi, J.; Nakatani, N. Constituents of Zingiberaceae. I. Diarylheptanoids from the rhizomes of ginger (Zingiber officinale Roscoe). Chem. Pharm. Bull. 1991, 39, 120–122. [Google Scholar] [CrossRef]
- Oufnac, D.S. Determination of Antioxidant Capacity in Corn Germ, Wheat Germ and Wheat Bran using Solvent and Microwave-Assisted Solvent Extraction. Ph.D. Thesis, Louisiana State University and Agricultural and Mechanical College, Baton Rouge, LA, USA, 2006; pp. 1–68. [Google Scholar]
- Wiktor, A.; Mandal, R.; Singh, P.; Singh, A.; Pratap-Singh, A. Pulsed Light treatment below a Critical Fluence (3.82 J/cm2) minimizes photo-degradation and browning of a model Phenolic (Gallic Acid) Solution. Foods 2019, 8, 380. [Google Scholar] [CrossRef]
- Coelho, E.; Vilanova, M.; Genisheva, Z.; Oliveira, J.; Coimbra, M.A.; Domingues, L. Systematic approach for the development of fruit wines from industrially processed fruit concentrates, including optimization of fermentation parameters, chemical characterization and sensory evaluation. LWT 2015, 62, 1043–1052. [Google Scholar] [CrossRef]
- Shimada, K.; Fujikawa, K.; Yahara, K.; Nakamura, T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J. Agric. Food Chem. 1992, 40, 945–948. [Google Scholar] [CrossRef]
- Amiri, A.; Mousakhani-Ganjeh, A.; Amiri, Z.; Guo, Y.-G.; Singh, A.P.; Kenari, R.E. Fabrication of cumin loaded-chitosan particles: Characterized by molecular, morphological, thermal, antioxidant and anticancer properties as well as its utilization in food system. Food Chem. 2020, 310, 125821. [Google Scholar] [CrossRef] [PubMed]
- Decker, E.A.; Welch, B. Role of ferritin as a lipid oxidation catalyst in muscle food. J. Agric. Food Chem. 1990, 38, 674–677. [Google Scholar] [CrossRef]
- Oyaizu, M. Antioxidative activities of browning reaction prepared from glucosamine. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Shotwell, O.L.; Hesseltine, C.W.; Stubblefield, R.D.; Sorenson, W.G. Production of aflatoxin on rice. Appl. Microbiol. 1966, 14, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Miyake, Y.; Ito, C.; Itoigawa, M.; Osawa, T. Isolation of the Antioxidant Pyranonigrin-A from Rice Mold Starters Used in the Manufacturing Process of Fermented Foods. Biosci. Biotechnol. Biochem. 2007, 71, 2515–2521. [Google Scholar] [CrossRef]
- Yıldırım, A.; Mavi, A.; Kara, A.A. Determination of Antioxidant and Antimicrobial Activities ofRumex crispusL. Extracts. J. Agric. Food Chem. 2001, 49, 4083–4089. [Google Scholar] [CrossRef]
- Jarzębski, M.; Fathordoobady, F.; Guo, Y.; Xu, M.; Singh, A.; Kitts, D.D.; Kowalczewski, P.Ł.; Jeżowski, P.; Pratap-Singh, A. Pea Protein for Hempseed Oil Nanoemulsion Stabilization. Molecules 2019, 24, 4288. [Google Scholar] [CrossRef]
- Lin, C.-H.; Wei, Y.-T.; Chou, C.-C. Enhanced antioxidative activity of soybean koji prepared with various filamentous fungi. Food Microbiol. 2006, 23, 628–633. [Google Scholar] [CrossRef]
- Lin, C.-H.; Wei, Y.-T.; Yu, R.-C.; Chou, C.-C. Cultivation temperature and length affect the antioxidant activity and total phenolic content of soybean Koji prepared with Aspergillus awamori. J. Food Drug Anal. 2020, 14, 6. [Google Scholar] [CrossRef]
- Esaki, H.; Onozaki, H.; Kawakishi, S.; Osawa, T. New Antioxidant Isolated from Tempeh. J. Agric. Food Chem. 1996, 44, 696–700. [Google Scholar] [CrossRef]
- Jiménez, M.S.; Velarte, R.; Castillo, J.R. Direct determination of phenolic compounds and phospholipids in virgin olive oil by micellar liquid chromatography. Food Chem. 2007, 100, 8–14. [Google Scholar] [CrossRef]
- Hirota, A.; Morimitsu, Y.; Hojo, H. New antioxidative indophenol-reducing phenol compounds isolated from the Mortierella sp. fungus. Biosci. Biotechnol. Biochem. 1997, 61, 647–650. [Google Scholar] [CrossRef]
- Yen, G.-C.; Chang, Y.-C.; Sheu, F.; Chiang, H.-C. Isolation and characterization of antioxidant compounds from Aspergillus candidus broth filtrate. J. Agric. Food Chem. 2001, 49, 1426–1431. [Google Scholar] [CrossRef] [PubMed]
- Romero, A.M.; Doval, M.M.; Sturla, M.A.; Judis, M.A. Antioxidant properties of polyphenol-containing extract from soybean fermented withSaccharomyces cerevisiae. Eur. J. Lipid Sci. Technol. 2004, 106, 424–431. [Google Scholar] [CrossRef]
- Randhir, R.; Vattem, D.; Shetty, K. Solid-state bioconversion of fava bean by Rhizopus oligosporus for enrichment of phenolic antioxidants and l-DOPA. Innov. Food Sci. Emerg. Technol. 2004, 5, 235–244. [Google Scholar] [CrossRef]
- Robbins, R.C. Medical and Nutritional Aspects of Citrus Bioflavonoids; American Chemical Society (ACS): Washington, DC, USA, 1980; pp. 43–59. [Google Scholar]
- Chung, Y.-C.; Chang, C.-T.; Chao, W.-W.; Lin, C.-F.; Chou, S.-T. Antioxidative Activity and Safety of the 50 Ethanolic Extract from Red Bean Fermented byBacillus subtilisIMR-NK1. J. Agric. Food Chem. 2002, 50, 2454–2458. [Google Scholar] [CrossRef]
- Farhoosh, R.; Golmovahhed, G.A.; Khodaparast, M.H. Antioxidant activity of various extracts of old tea leaves and black tea wastes (Camellia sinensis L.). Food Chem. 2007, 100, 231–236. [Google Scholar] [CrossRef]
- Wu, H.-C.; Chen, H.-M.; Shiau, C.-Y. Free amino acids and peptides as related to antioxidant properties in protein hydrolysates of mackerel (Scomber austriasicus). Food Res. Int. 2003, 36, 949–957. [Google Scholar] [CrossRef]
- Chou, S.-T.; Chang, C.-T.; Chao, W.-W.; Chung, Y.-C. Evaluation of Antioxidative and Mutagenic Properties of 50% Ethanolic Extract from Red Beans Fermented by Aspergillus oryzae. J. Food Prot. 2002, 65, 1463–1469. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-H.; Mau, J.-L.; Ko, P.-T.; Huang, L.-C. Antioxidant properties of fermented soybean broth. Food Chem. 2000, 71, 249–254. [Google Scholar] [CrossRef]
- Yang, J.-H.; Lin, H.-C.; Mau, J.-L. Antioxidant properties of several commercial mushrooms. Food Chem. 2002, 77, 229–235. [Google Scholar] [CrossRef]
- Hamama, A.A.; Nawar, W.W. Thermal decomposition of some phenolic antioxidants. J. Agric. Food Chem. 1991, 39, 1063–1069. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).