Abstract
Various models have been used to demonstrate the pronounced effects of the microwave frequency range electromagnetic fields, as well as weak and very weak combined collinear magnetic fields (CMF) with static and variable components in the order of micro- and nano-tesla. One of such models, previously shown to be sensitive to variations in the parameters of applied magnetic fields, is the neutrophil respiratory burst. Using luminol-enhanced cell chemiluminescence assay, we studied the effects of the CMF exerted directly on neutrophil suspensions and, indirectly, through aqueous solutions. To experimentally create a uniform standard weak CMF with 60 µT static and 100 nT alternating magnetic field components, we engineered a shielded magnetic field induction device. CMF applied directly to neutrophils enhanced reactive oxygen species (ROS) production by more than 36%. The pronounced stimulating effect was observed only when using the signals that included the frequency of 12.6 Hz that corresponds to the ion cyclotron resonance (ICR) frequency of the hydrated hydronium ion. Similarly, to direct exposure, CMF pre-treatment of a water sample subsequently added to the neutrophil suspension increased ROS production by 66%. The effect of CMF pre-treatment was retained after a series of dilutions and mechanical treatment but disappeared in “magnetic vacuum” or without mechanical influence. Therefore, weak and super weak magnetic fields may indirectly, via water, activate ROS production by neutrophils, provided that modulation of super weak component of collinear field corresponds to the ICR frequency of the hydrated hydronium ion.
1. Introduction
The influence of physical factors, and in particular of the magnetic fields, on living organisms has become an increasingly relevant biophysical topic in recent years. While studies addressing interactions between high-intensity magnetic field and matter have been awarded a number of Nobel Prizes, effects of weak and super weak magnetic fields have become the subject of research only recently.
To most objectively elucidate the mechanisms underlying the biological action of weak and super weak combined (collinear static and alternating) magnetic fields (CMF), an experimental study of the effects associated with different CMF modes at a cellular level using well-studied standard models would be required. One of such models, previously shown to be sensitive to variations in the parameters of applied magnetic fields, is the neutrophil respiratory burst [1]. During phagocytosis and in response to soluble mediators (e.g., bacterial peptide N-formyl-Met-Leu-Phe (fMLF) or the phorbol ester phorbol 12-myristate 13-acetate (PMA)) neutrophils generate reactive oxygen species (ROS): hydrogen peroxide (H2O2), superoxide anion radical (O2−●), singlet oxygen (1O2), hydroxyl radical (OH●), and nitric oxide (NO). These molecules are involved in the destruction of phagocytosed objects. One of the most sensitive techniques that detects ROS is based on the measuring of chemiluminescence generated upon oxidation of luminol (luminol-enhanced chemiluminescence) [2].
We have previously detected a priming effect (pre-activation of the neutrophil respiratory burst) occurring with exposure to a weak combined collinear static (42 μT) and low-frequency alternating magnetic field (formed by a series of 1, 4.4 and 16.5 Hz sinusoidal signals with a total amplitude of 0.86 μT). This priming effect produced by the magnetic fields was evidenced by more pronounced enhancement of chemiluminescence in neutrophil suspensions (following 60-min exposure to CMF) in response to the administration of fMLF or PMA [1]. When selecting the parameters of the alternating low-frequency component of CMF for the respiratory burst triggering, we relied on the results obtained previously for the effects of magnetic field with similar characteristics on mice with transplanted Ehrlich ascites carcinoma [3,4,5]. In that study, as the algorithm to generate the alternating magnetic signal, we used the principle of adjusting the magnetic field to ICR frequencies of simple and complex ions: (NAD)+, the ionic form of glutamic acid and K+ ions (1.0, 4.4 and 16.5 Hz, respectively for these ions, using a static magnetic field of 42 μT) [2,4,5]. This combined triple-frequency signal had the most pronounced antitumor activity, as compared to each individual frequency [3,4,5]. When studying the molecular mechanism of the CMF activating effect on ROS production by neutrophils, we showed that it was less associated with lipid peroxidation. The lipid peroxidation inhibitor butyl hydroxytoluene (BHT) did not reduce the production of ROS [6]. However, the effect of CMF on calcium-dependent processes in neutrophils was confirmed using calcium chelator (BAPTA-AM), which dramatically reduced the CMF activating effect on ROS production [7].
Subsequent experiments demonstrated a relationship between pre-activation of the neutrophil respiratory burst by weak CMF and the concentration of atmospheric gases [8]. It was shown that preliminary mild partial deaeration of the neutrophil suspension at an atmospheric gas pressure of 640 mm Hg led to a substantial (4-fold) reduction in the response only when the CMF was applied. At the same time, the ability of the cells to generate the respiratory burst in response to a stimulus (fMLF) was nearly unchanged in the control assays, without the CMF applied [8]. This suggested that molecular oxygen could be one of the acceptors of a weak CMF within the test system (neutrophil suspension in an aqueous salt solution) and that the aqueous solution itself could consequently change its properties when exposed to the CMF. To experimentally test this possibility, we have conducted a number of studies, which will be described in this paper. Using luminol-enhanced cell chemiluminescence assay, we studied the effects of magnetic fields directly on neutrophil suspensions and indirectly, exerted through aqueous solutions pre-incubated in a shielding permalloy container used to reduce external magnetic fields to ~10 nT. These studies were combined with experiments aimed to evaluate the mechanical effects on the aqueous test solution. To confirm the physical origin of the CMF effect on the test water samples, the ultra-high dilution technology was applied. This technology has already proven itself not only in medicine and veterinary but also in engineering [9,10,11]. The samples prepared using this technology were exposed to standard magnetic fields, including weak CMF with a varying modulation of the alternating component using a standard sinusoidal signal with defined frequencies (12.6 or 48.5 Hz), as well as with an arbitrary waveform containing 12.6 Hz frequency band.
2. Materials and Methods
2.1. Reagents
The following reagents purchased from Sigma (St. Louis, MO, USA): Zymosan A from Saccharomyces cerevisiae, N-formyl-Met-Leu-Phe (fMLF); and luminol solution (Enzo Life Sciences, New York, NY, USA) were used in the study.
2.2. CMF Generation
The experiments were performed using relatively weak magnetic fields of magnitudes less or comparable to the magnitudes of the Earth’s magnetic field or geomagnetic field (GMF) (which normally fall within the range of 30 to 70 μT) and those within frequency ranges close to the industrial frequency present in laboratory facilities (50 Hz). Therefore, in order to standardize the measurement conditions, which should be minimally dependent on ambient conditions, specialized research equipment was used, i.e., a hypomagnetic environment device (Figure 1).
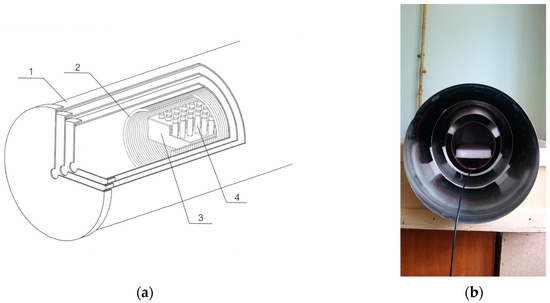
Figure 1.
Hypomagnetic environment device (magnetic field induction device): (a) cross-section diagram; (b) unit photograph. 1—magnetic shields; 2—solenoid; 3—thermostated rack; 4—experimental samples.
This device provided a high level of reduction in the external GMF—up to 10,000 times (with the residual static field not exceeding 10 nT), and substantially lowered the alternating technogenic noise (to a few nT, as confirmed by measuring). The device consisted of three cylindrical permalloy magnetic shields (with the 1 mm thick walls) fitted coaxially one inside the other and having closures with inlet ports to connect the device to measuring or temperature stabilizing equipment (the inner shield being 22 cm in diameter and 42 cm in length) (Figure 1). The residual fields inside the device were directly measured with a Mag-03MS100 fluxgate magnetometer (Bartington, Witney, UK). To experimentally create a uniform standard weak combined static and alternating magnetic field (CMF), a special electromagnetic induction coil (solenoid) was placed inside the device. The solenoid was connected to a source of direct current to generate a static field and to a source of low-frequency alternating signals to generate the alternating field component. The coil had the following characteristics: 18 cm diameter, 36 cm length (a 720-loop copper wire of 1 mm diameter), and 7.5 Ohm resistance. The solenoid was used to create an area within the shielding unit of a uniform weak CMF, in which test samples were incubated.
Two sets of experiments were performed using the magnetic field induction device described above. The first experiment was aimed at selecting the exact frequency of the CMF alternating component between 12.6 Hz or 48.5 Hz. The second experiment included exposure to a wide range of frequencies. As wide-range signal, an electroencephalogram (EEG) recording was used, which is a typical example of an electrophysiological signal with a power spectrum containing a large number of different frequencies, but most importantly, it contains the required frequency of 12.6 Hz.
2.2.1. Generation of the CMF with a Single Frequency Regime
The CMF alternating component frequencies were equivalent to the ICR frequencies of the hydronium ions H3O+ or complex ions within its hydrated form H3O+(3H2O) [12], and were calculated using the standard equation:
where is ion charge, is ion mass, and is induction of the CMF static component (a static magnetic field (SMF) of 60 μT). The frequencies obtained were 48.5 Hz (for H3O+) and 12.6 Hz (for H9O4+). An alternating magnetic field (AMF) of required frequency and amplitude was generated using a digital and analog transducer (DAT) based on an L-791 board (L-Card Company, Moscow, Russia). To generate the AMF, an alternating current of sinusoidal waveform, described by the equation below, was passed through the solenoid:
where is frequency in Hz.
Thus, the AMF with 100 nT induction was used.
2.2.2. Generation of the CMF with a Spectral Range Regime
To generate the CMF (in the device described above), the alternating component was formed using a signal which already contained a frequency spectrum comprising 12.6 Hz. This spectrum was obtained by averaging all channels from five EEG recordings from male and female volunteers aged between 25 and 54 years who signed informed consent for study participation. Each of the five EEGs was composed of 19 active channels, through which brain wave activity was recorded in accordance with the guidelines of the International Federation of Clinical Neurophysiology (IFCN) [13]. The resultant averaged signal was amplified proportionally to a maximum of 0.8 V (Figure 2). Each recording was performed for 5 min and repeated eight times to achieve the desired 40 min exposure to the CMF.
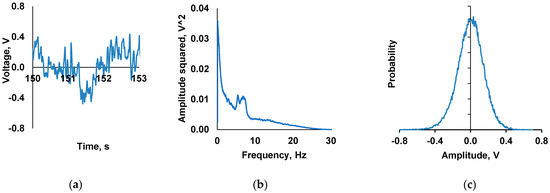
Figure 2.
Electrophysiological signal used to form the alternating component of the combined collinear magnetic fields (CMF): (a) fragment of averaged electroencephalogram (EEG) used for modulation of alternating component of the CMF; (b) power spectrum of averaged EEG shown in panel (a); (c) distribution histogram of averaged EEG shown in panel (a).
The signal shown in Figure 2a was used to form the CMF alternating component. The signal spectrum contained 12.6 Hz band (Figure 2b). The amplitude of the CMF alternating component was selected to be approximately 100 nT (this value roughly corresponds to half-width of histogram of EEG signal distribution shown in Figure 2c).
2.3. Obtaining Neutrophil Suspension
The study was carried out on peritoneal mouse neutrophils. To obtain peritoneal neutrophils for multiple experiments, 54 laboratory CD-1 male mice weighing 22–25 g were used. The animals were received from the breeding facility branch of the Shemyakin–Ovchinnikov Institute of Bioorganic Chemistry, Russian Academy of Sciences. Mice were injected peritoneally with 150 µL of suspension of mouse serum-opsonized zymosan (Zymozan A from Saccharomyces cerevisiae, Sigma, USA) at a concentration of 5 mg/mL. After 12 h, the animals were sacrificed by cervical dislocation, and their abdominal cavities were rinsed with 4 mL of chilled calcium-free Hanks solution. Peritoneal washout was collected and centrifuged for 5 min at 600× g. The supernatant was discarded, and pellet was resuspended in 4 mL of calcium-free Hanks solution and left for 60 min at 4 °C. The total number of isolated cells was counted by a hemocytometer (Goryayev chamber). Cell viability was assessed by trypan blue exclusion. The proportion of viable cells was at least 96%. To prepare neutrophil suspension for chemiluminescence measurements with a concentration of 106 cells/mL, cells were diluted with Hanks balanced salt solution prepared in-house (138 mM NaCl, 6 mM KCl, 1 mM MgSO4, 1 mM Na2HPO4, 5 mM NaHCO3, 5.5 mM glucose, 1 mM CaCl2, 10 mM HEPES, pH 7,4).
The animal studies were conducted in accordance with the Guidelines for Ethical Conduct in the Care and Use of Animals and approved by the institutional animal care and use committee (protocol number 57.30.12.2011) at the Institute of Cell Biophysics.
2.4. Neutrophil Suspension Exposure to the Combined Magnetic Field
Neutrophil suspension is suitable for measurements only on the day of its isolation. Therefore, the variety of control and experimental conditions applied in this study required re-isolation of fresh neutrophils for each of the experiments (i.e., different mice were used on different days). The results of each measurement were normalized to control, which was included in each experiment. As a control, we used neutrophil suspension incubated similarly to the study samples but outside the magnetic field induction device (i.e., at the geomagnetic field (GMF)).
Neutrophils were incubated at 37.0 ± 0.2 °C for 40 min at concentration of 106 cells/mL in 0.25 mL aliquots in 5 mL round-bottomed polystyrene tubes (Sarstedt, Germany) further used to measure chemiluminescence. The temperature was maintained by an UH4 circulating bath (MLW, Germany).
In the first experiment, experimental conditions for control samples were selected. Suspension of neutrophils in the first group (“GMF (Control)”) was incubated at the GMF (outside the device) with static component of ~44 µT and a level of AMF of 15–50 nT (with the frequency of technogenic background of power lines—50 Hz). The second group (“SMF (60 µT) “sham control”) was incubated in the magnetic field induction device, at the SMF with static component intensity of 60 µT, though with not set parameters of amplitude and frequency of alternating component of the field. The temperature was the same for both groups. The results of these measurements are presented in Table 1.

Table 1.
Comparison of intensity of chemiluminescence of neutrophil suspension after incubation under geomagnetic field conditions (“GMF (Control)”), and inside the device, static magnetic field (“SMF (60 µT)”), with static component of 60 µT, but without alternating component of CMF).
In the second experiment, two pairs of neutrophil suspensions were investigated. The first sample in each pair was a control that was incubated in the local GMF. The second sample in each pair was incubated in the magnetic field induction device at the CMF with static component of 60 µT and alternating component of 100 nT. However, the alternating component of magnetic field for the second sample in the first pair was changed according to the sinusoidal regime with 12.6 Hz (designated as “12.6 Hz, 100 nT”), and for the second pair of suspensions (designated as “48.5 Hz, 100 nT”)—with frequency of 48.5 Hz. Results of this experiment are presented in Table 2.

Table 2.
Intensity of chemiluminescence (maximum value) of neutrophil suspension after direct exposure to CMF (with static component of 60 µT and varying conditions of alternating magnetic field (AMF)).
In both experiments, the samples were exposed at the particular magnetic field settings for 40 min. The experiments were repeated 5–6 times.
2.5. CMF Water Treatment with a Series of Dilutions and Vigorous Shaking
In addition to the experiments with direct exposure of neutrophil suspension to the CMF (described in the subsection “Neutrophil suspension exposure to the combined magnetic field”), we performed studies in which water samples used for the subsequent preparation of neutrophil suspensions were similarly exposed to the natural (GMF) or various artificial (CMF, “magnetic vacuum”) magnetic fields. More detailed description of the magnetic fields, at which water exposure was carried out, are presented below in the relevant subsections.
Water sample exposure to the CMF was performed in 18 mL optic glass spectrophotometric cubic cuvettes (Hellma Analytics, Germany, Cat. No. 704-001-30-10) and lasted for 40 min in the magnetic field induction device (described in “CMF generation”) at room temperature (23–24 °C). Moreover, 20 h prior to the CMF exposure, water samples were purified to high degree (specific resistance of 18.2 MOhm·cm at 25 °C, at the time of purification).
The exposed water samples were then added to the suspension of intact neutrophils under the GMF, and the intensity of cell chemiluminescence was measured (using the procedure described in the Materials and Methods subsection “Measurement of luminol-enhanced cell chemiluminescence”).
2.5.1. Sample Preparation for Investigation of Water-Mediated Activation of Neutrophils. The Role of Dilutions, Magnetic Vacuum, and Methods of Mechanical Treatment
The experiment included five groups of water samples, some of which were exposed to both magnetic field and the serial dilution process.
“Control” sample was purified water incubated outside the magnetic field induction device (under the GMF conditions). The results of all other measurements were normalized according to the results of these sample measurements.
“Experimental” sample was a water sample similar to “Control” sample, but incubated under the CMF conditions with static component of 60 µT and alternating component of 100 nT formed according to the sinusoidal regime with 12.6 Hz frequency.
The resulting “Experimental” sample was divided into four parts:
- Homonymous “Experimental” sample measured without any additional exposure.
- “GMF, dilution factor 1099”—the sample was subjected to 50 serial centesimal dilutions with vigorous shaking between dilution steps. Both dilution and shaking procedures were performed under the GMF conditions (outside the magnetic field induction device).
- “Magnetic vacuum”, dilution factor 1099”—the sample was subjected to 50 serial centesimal dilutions with vigorous shaking between dilution steps. However, dilutions were made under the GMF conditions and each shaking was performed inside the magnetic field induction device with disconnected power supply and with residual magnetic field intensity ~10 nT. Noteworthy, “GMF, dilution factor 1099” and “Magnetic vacuum” samples were subjected to shaking identically due to the fact that they were interconnected by a rigid nonmagnetic bar at the time of shaking (Figure 3).
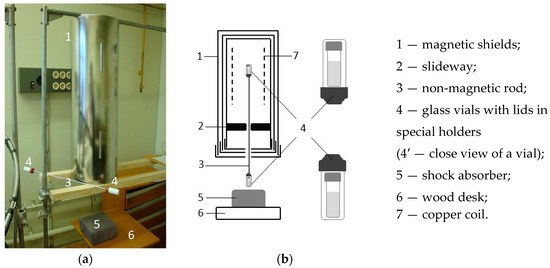 Figure 3. Unit for vigorous shaking of water under various magnetic conditions (GMF, “magnetic vacuum”, CMF): (a) general view; (b) schematic drawing.
Figure 3. Unit for vigorous shaking of water under various magnetic conditions (GMF, “magnetic vacuum”, CMF): (a) general view; (b) schematic drawing. - “Dilution without shaking, dilution factor 1099”—the sample was subjected to 50 serial centesimal dilutions, but without vigorous shaking under the GMF conditions.
Five more groups of water samples were analyzed additionally. Their preparation was the same as described above; however, “Experimental” sample was incubated under CMF conditions with static component of 60 µT and alternating component of 100 nT, which was formed according to the sinusoidal regime with 48.5 Hz. This sample was further divided into four parts which were treated the same way in terms of magnetic field and dilution conditions as those described above.
The results of the experiment are presented in Table 3.

Table 3.
Intensity of chemiluminescence (maximum value) of neutrophil suspension after addition of water samples pre-exposed to the CMF as well as dilution and vigorous shaking under geomagnetic field (GMF) and “magnetic vacuum” (~10 nT) conditions.
2.5.2. Preparation of Samples to Investigate Water-Mediated Activation of Neutrophils. The Role of Single-Frequency Combined Magnetic Field at Dilution
The experiment included four groups of water samples, some of which were exposed to magnetic field and serial dilution.
“Control” sample was purified water incubated outside the magnetic field induction device (under the GMF conditions). The results of all other measurements were normalized according to the results of these sample measurements.
“Experimental” sample was water sample similar to “Control” sample, but incubated under the CMF conditions with static component of 60 µT and alternating component of 100 nT, which was formed according to the sinusoidal regime with 12.6 Hz.
The resulting “Experimental” sample was divided into three parts:
- Homonymous “Experimental” sample measured without any additional exposure.
- “in GMF, dilution factor 1099”—the sample was subjected to 50 serial centesimal dilutions with vigorous shaking between dilution steps. Both dilution and shaking procedures were performed under the GMF conditions (outside the magnetic field induction device).
- “in CMF, dilution factor 1099”—the sample was subjected to 50 serial centesimal dilutions with vigorous shaking between dilution steps. The dilutions were made under the GMF conditions and shaking—inside the magnetic field induction device (CMF) with static component of 60 µT and alternating component of 100 nT, which was formed according to the sinusoidal regime with 12.6 Hz. Noteworthy, “GMF, dilution factor 1099” and “Magnetic vacuum” samples were subjected to shaking identically due to the fact that they were interconnected by a rigid nonmagnetic bar at the time of shaking (Figure 3).
Four more groups of water samples were analyzed additionally. Their preparation was similar to the described above; however, “Experimental” sample was incubated under CMF conditions with static component of 60 µT and alternating component of 100 nT, which was formed according to the sinusoidal regime with 48.5 Hz. This sample was further divided into three parts which were treated in the same way in terms of magnetic field and dilution conditions as those described above.
The results of the experiment are presented in Table 4.

Table 4.
Intensity of chemiluminescence (maximum value) of neutrophil suspension after addition of water samples (“control”), water pre-exposed to CMF (“experimental” sample) and the same samples exposed to dilution process and vigorous shaking under the geomagnetic field (GMF) or combined collinear magnetic field (CMF).
2.5.3. Preparation of Samples to Investigate Water-Mediated Activation of Neutrophils. The Role of Mechanical Treatment Under Geomagnetic Field Conditions
The experiment included three groups of water samples exposed only to the GMF and serial dilution process.
“Control” sample was purified water incubated outside the magnetic field induction device (under the GMF conditions). The results of all other measurements were normalized according to the results of these sample measurements.
“Control” sample was divided into three parts:
- Homonymous “Control” sample measured without any additional exposure.
- “in GMF” (without shaking), dilution factor 1099”—the sample was subjected to 50 serial centesimal dilutions under the GMF conditions, but without vigorous shaking between dilution steps.
- “in GMF, dilution factor 1099”—the sample was subject to 50 serial centesimal dilutions with vigorous shaking between dilution steps. Both dilution and shaking were performed under the GMF conditions (outside the magnetic field induction device).
The results of the experiment are presented in Table 5.

Table 5.
Intensity of chemiluminescence (maximum value) of neutrophil suspension after addition of control water samples to culture medium as well as control water samples pre-exposed to dilution process and vigorous shaking.
2.5.4. Preparation of Samples to Investigate Water-Mediated Activation of Neutrophils. The Role of the CMF Formed with Spectral Signal and Dilution Method
The experiment included three groups of water samples, some of which were exposed to magnetic field and serial dilution.
“Control” sample was purified water incubated outside the magnetic field induction device (under the GMF conditions). The results of all other measurements were normalized according to the results of these sample measurements.
“Experimental” sample was a water sample similar to “Control” sample, but incubated under CMF conditions with static component of 60 µT and alternating component of ~100 nT, which was formed by a random signal in the form of a signal of electrical brain activity derived as described in the subsection “Generation of the CMF with a spectral range regime”. Power spectrum of the signal was within the frequency interval between 2 to 30 Hz (Figure 2a,b).
“Experimental” sample was divided into two parts:
- Homonymous “Experimental” sample measured without any additional exposure.
- “in GMF, dilution factor 1099”—the sample was subjected to 50 serial centesimal dilutions with vigorous shaking between dilution steps. Both dilutions and shaking were performed under CMF conditions with static component of 60 µT and alternating component of 100 nT, which was formed by a random signal (described in the subsection “Generation of the CMF with a spectral range regime”).
The results of the experiment are presented in Table 6.

Table 6.
Intensity of chemiluminescence (maximum value) of neutrophil suspension after supplementation of culture medium with water samples pre-exposed to CMF formed with electroencephalogram signal and subjected to dilution and vigorous shaking.
For all the experiments described above, the samples were prepared with the procedure of serial consecutive dilutions with shaking at each dilution step. Highly diluted samples were prepared in 20 mL glass vials with lids (Glastechnik Grafenroda, Germany). To prepare the first dilution, two vials were filled with 4.5 mL of water. After that, 0.5 mL of the sample previously incubated in the appropriate magnetic field was added to each vial (1:10 dilution). Then the vials were closed with lids and shaken intensively under various magnetic fields (according to the corresponding experimental model). The shaking procedure took about 10 s. The second and subsequent dilutions were prepared identically except that 50 µL of the sample from the previous dilution was added to the vial with 4.95 mL of water (1:100 dilution). This procedure was repeated 49 times. All subsequent dilutions of each sample were made in the same vial.
Vigorous shaking of the vials was carried out simultaneously under two conditions: outside the magnetic field induction device (corresponding to the GMF conditions) and inside the device under conditions of SMF, which varied for different samples from the so-called “magnetic vacuum” (corresponding to ~10 nT) to conditions of CMF (with static component of 60 µT but without alternating component). This procedure was performed using a special device similar to the one described above (Figure 1) but positioned upright (Figure 3). For simultaneous shaking under various magnetic field conditions (i.e., simultaneously inside and outside the device), two vials were rigidly interconnected by the material insensitive to magnetic field (duraluminum) at the distance of 56 cm (Figure 3, #3). Similarly, to the device shown in Figure 1, an electromagnetic induction coil (solenoid) was placed inside the device shown in Figure 3 to generate the CMF. All the manipulations related to disassembling and assembling the structure that combined two vials on non-magnetic rod took about 1 min. The total duration of the dilution and shaking process was about 40 min.
These water samples were tested for the ability to pre-activate (prime) or deactivate neutrophils. Incubation of neutrophil suspension with the water samples was performed before administration of luminol and fMLF.
For neutrophil culturing, eight volume parts of experimental water samples pretreated under conditions of various magnetic fields and mechanical treatment between dilutions (as described above) were mixed with 1 volume part of concentrated Hanks balanced salt solution. The resulting solution was added to neutrophil suspension to obtain the final concentration of 1 million cells/mL in 0.25 mL for the subsequent measurement of chemiluminescence. Neutrophils in the medium containing the investigated experimental water samples were incubated under the GMF conditions at 37 ± 0.1 °C for 40 min. After incubation, the intensity of the cell chemiluminescence was measured after addition of luminol solution and inducer of ROS generation—fMLF—using the procedure described in the subsection “Measurement of luminol-enhanced cell chemiluminescence”.
2.6. Measurement of Luminol-Enhanced Cell Chemiluminescence
After incubation of neutrophil suspension under the conditions with selected parameters of static and alternating magnetic field, the intensity of chemiluminescence was measured in control and experimental groups of samples. Immediately before measurement, luminol solution (Enzo Life Sciences, USA) at the final concentration of 0.35 mM, as well as an inducer of ROS generation by neutrophils—chemotactic formylated peptide N-formyl-Met-Leu-Phe (fMLF) (Sigma, USA) at the final concentration of 1 µM were added to all samples. A Lum-1200 chemiluminometer (DISoft LLC, Moscow, Russia) was used for measurements. PowerGraph software (DISoft LLC, Russia) was used to analyze the results of chemiluminescence measurements. Intensity values at the maximum of the chemiluminescence kinetics curve were recorded. Results in Table 1, Table 2, Table 3, Table 4, Table 5 and Table 6 are presented in absolute values and as relative to that in the control, which was set to 100%. This normalization is necessary for an adequate comparison of the results of experiments conducted on neutrophils obtained from different animals.
2.7. Statistical Analysis
Data were analyzed using the non-parametric Mann–Whitney test with the continuity correction (for the data presented in Table 1 and Table 2) and Benjamini–Hochberg’s correction for the multiplicity of comparisons (for the data presented in Table 3, Table 4, Table 5 and Table 6). p-Values < 0.05 were considered significant. All values were expressed as means ± SD. The data were processed using MS Office Excel.
3. Results
3.1. Effect of Artificial and Geomagnetic Field on Neutrophil Activity
For experimental selection of correct conditions for magnetic exposure of neutrophil suspension and choosing suitable control group, measurements between two groups were compared. The first group of samples was incubated under the GMF conditions with estimated static component of ~44 µT and in the presence of technogenic magnetic field from centralized electrical supply network, which corresponded to intensity of alternating magnetic field of 15–50 nT at 50 Hz at the experimental device. The second group of samples was placed inside the magnetic field induction device with switched off source of alternating magnetic field generating static magnetic field (SMF) of 60 µT. The results of measurement of cell chemiluminescence for these two groups are presented in Table 1.
As shown in Table 1, comparison of chemiluminescence intensity of neutrophils incubated both under the GMF and under artificial magnetic field with static component of 60 µT (SMF) did not reveal any differences in respiratory burst between these two groups. For this reason, as well as due to the convenience of simultaneous incubation with experimental samples, the samples exposed to the GMF were used as control in all further experiments.
3.2. Effect of Combined Magnetic Fields on ROS Generation by Neutrophils
3.2.1. Direct Neutrophil Exposure to the Magnetic Field
According to the results described in the above section, the GMF and SMF with 60 µT induction demonstrated comparable effect on chemiluminescence of neutrophils.
The purpose of this section was to study the influence of the variable component of the magnetic field, which was used in addition to the static component. The combination of static (60 µT) and alternating magnetic field (100 nT), which is formed according to the sinusoidal regime at 12.6 or 48.5 Hz, generates combined collinear magnetic field (CMF).
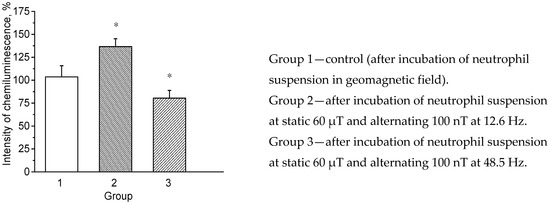
Figure 4.
Effect of combined magnetic fields with static component of 60 µT on intensity of neutrophil chemiluminescence. On Y-axis—intensity of chemiluminescence (maximum value) in %, relative to control. Data is presented as M ± SD. *—p < 0.05 vs. control group.
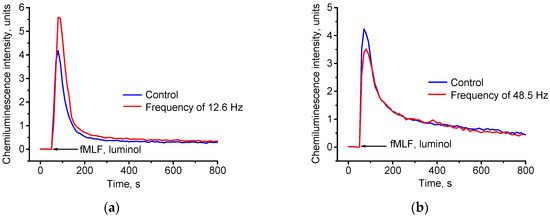
Figure 5.
Effect of combined magnetic fields on kinetics and intensity of chemiluminescence of neutrophils incubated under CMF with static component of 60 µT and alternating component of 100 nT with sinusoidal signal at: (a) frequency of 12.6 Hz; (b) frequency of 48.5 Hz.
According to the results presented in Table 2, pre-incubation of neutrophil suspension in the CMF with alternating component of 12.6 Hz induced significant activation of respiratory burst in neutrophils compared to control. Intensity of chemiluminescence of neutrophil suspension after such exposure increased by more than 36%. In contrast, at 48.5 Hz, statistically significant inhibitory effect of the CMF on neutrophil suspension was observed compared to control (reduced cell chemiluminescence by almost 20%).
3.2.2. Water-Mediated Effect of Magnetic Fields on Neutrophils
Results presented in the sections above clearly demonstrated the effect of combined static and alternating magnetic fields on ROS generation by peritoneal neutrophils. However, it remains unclear whether the CMF act directly on the molecular mechanisms responsible for the production of ROS, or indirectly through water. Similar water-mediated effect of physical exposures in the form of microwaves at 36 GHz and 42 GHz has already been investigated in our laboratory both using biological and engineering models (capacitor discharge) [14,15].
To check the ability of aqueous medium to mediate the effect of CMF on the ROS generation by neutrophils, we carried out a number of studies using similar cell chemiluminescence method. Water samples were first exposed to the CMF then they were added to neutrophil suspension, and luminol-enhanced chemiluminescence was measured after stimulation with fMLF.
The settings of the magnetic field induction device for water CMF exposure were similar to the described in the previous sections and corresponded to static component of 60 µT and alternating component of 100 nT with sinusoidal modulation of 12.6 or 48.5 Hz.
Table 3 shows that the exposure of “Experimental” sample to the CMF with alternating component of 12.6 Hz caused an increase of the cell chemiluminescence intensity by approximately 65% relative to “Control” (water sample incubated outside the magnetic field induction device in the GMF) (p = 0.002167). Meanwhile, no significant differences from “Control” sample were observed for the treatment with the CMF with alternating component of 48.5 Hz (p = 0.9372).
Noteworthy, samples obtained after addition of water pre-exposed to the CMF with alternating component of 12.6 Hz demonstrated more pronounced stimulating effect on the intensity of chemiluminescence (water-mediated effect) compared to direct neutrophil suspension exposure to the CMF with the same frequency (Table 2). An increase in chemiluminescence amounted to 66% with the indirect effect of the CMF versus a 36% increase with the direct effect of the CMF on neutrophil suspension.
3.2.3. Water-Mediated Activation of Neutrophils with Samples Exposed to Magnetic Fields, Dilution, and Intensive Mechanical Treatment
In this part of the study, “Experimental” sample (pre-incubated under CMF at 12.6 Hz or 48.5 Hz) was subjected to serial dilutions and shaking under the GMF or “magnetic vacuum”. The CMF exposure was performed in the magnetic field induction device with the settings identical to the ones described in the previous sections and corresponding to magnetic field with static component of 60 µT and alternating component of 100 nT. Alternating component corresponded to sinusoidal signal of 12.6 Hz or 48.5 Hz. To confirm the relevance of individual procedures of the serial dilution method, vigorous shaking was carried out in three versions (detailed protocol is described in the relevant section of “Materials and Methods”) and the results are shown in Table 3:
- “GMF at 1099 dilution”—dilution and shaking were performed under the GMF (outside the magnetic field induction device).
- “Magnetic vacuum” at 1099 dilution”—dilutions were made under the GMF (outside the magnetic field induction device), while shaking was made inside the device under the so-called “magnetic vacuum” (~10 nT).
- “Experimental sample dilution to 1099 without shaking”—dilutions were made under the GMF (outside the magnetic field induction device), but without shaking between dilution steps.
As can be seen in Table 3, the addition of sample “GMF at 1099 dilution” that was pre-exposed to the CMF with the frequency of alternating component of 12.6 Hz to neutrophil suspension increased the intensity of cell chemiluminescence by more than 44%. However, the effect of serial dilution and shaking under the GMF conditions caused only insignificant reduction in stimulating effect of water pre-exposed to the CMF (“Experimental” sample): the effect decreased insignificantly approximately from 65% to 44% (p = 0.09307).
However, if shaking between dilution steps was performed under “magnetic vacuum” (~10 nT) conditions (Sample “Magnetic vacuum” at 1099”), the ability of such solution to stimulate cell chemiluminescence was significantly lower than that of “Experimental” sample (p = 0.00325) or “GMF at 1099 dilution” (p = 0.00433). The same differences were observed for the group “Experimental sample dilution to 1099 without shaking” which were lower than for “Experimental” sample (p = 0.00325) or “GMF at 1099 dilution” (p = 0.00433). To the former sample only dilutions with delicate mixing under the GMF conditions were applied, but without vigorous shaking between the steps. Therefore, both presence of non-zero magnetic field and shaking process are important for the ROS production increase effect implementation.
Noteworthy, in the similar experiment performed at 48.5 Hz, no effect of dilution process was detected. Significant differences were detected neither for samples compared to “Experimental” sample (p = 0.6991 for each comparison) nor compared to “GMF at 1099 dilution” (p = 0.2403 for each comparison).
At the next step, we evaluated whether effects of the samples shaken under the GMF conditions are different from those shaken under the CMF conditions. The experiment included both samples obtained under CMF at 12.6 Hz and at 48.5 Hz. We demonstrated (Table 4) that the effect of “Experimental” sample was still significantly higher than that of Control (p = 0.00217) at alternating component frequency of 12.6 Hz. Moreover, if shaking process was performed under CMF conditions at alternating component frequency of 12.6 Hz, the resulting sample increased neutrophil chemiluminescence (approximately by 36%) similarly to the sample incubated under the GMF conditions (an increase of about 43%) (p = 0.1797). However, none of such effects manifested at 48.5 Hz: neither “Experimental” sample differed from the Control (p = 0.4848), nor the CMF or GMF shaking conditions affected the neutrophil respiratory burst (p = 0.132) (Table 4).
Meanwhile, lack of water pretreatment with the CMF (i.e., exclusion of preparation stage of “Experimental” sample) did not enable samples to stimulate cell chemiluminescence even after dilution (p = 0.3095 vs. Control) and vigorous shaking (p = 0.9307 vs. “GMF without shaking”) (Table 5).
3.2.4. Water-Mediated Activation of Neutrophils Exposed to the Spectral-Formed CMF with Intensive Mechanical Exposure and Dilution
According to the results presented in the previous sections, activating effect on production of ROS by neutrophils is exerted by water pre-exposed to the CMF, the parameters of which are comparable to the GMF intensity. An important role for the preparation of samples that are able to increase cell chemiluminescence when added to neutrophils belongs to both magnetic field intensity and frequency of its alternating component, where the most impressive results were obtained at the frequency corresponding to ICR of the hydrated form of hydronium ions (12.6 Hz) but not at H3O+ frequency (48.5 Hz).
We hypothesized that activating effect on neutrophils mediated by water pre-exposed to the CMF may be exerted not only at a specific frequency (12.6 Hz) but also after water exposure to the spectrum of frequencies including the 12.6 Hz. To test the hypothesis of spectral effect of magnetic fields on water samples, the signal of human encephalogram was used where all the channels of multichannel EEG were averaged by each time point until a single averaged channel was obtained (Figure 2a in “Materials and Methods: Generation of the CMF with a spectral range regime”). The most pronounced part of spectrum of EEG signal is approximately between the frequencies from near zero to 20–25 Hz, which also include the 12.6 Hz band. This band, according to the results of the experiments shown in Table 3 and Table 4, has stimulating activity on the cell chemiluminescence.
The results describing the effect of water samples pre-exposed to CMF with static component of 60 µT and alternating component formed proportionally to EEG signal with an amplitude of approximately 100 nT are presented in Table 6. Unlike harmonic signal where the amplitude may be defined, EEG signal is variable and is characterized by a specific distribution histogram presented in Figure 2c in the section “Materials and Methods: Generation of the CMF with a spectral range regime”). Moreover, 100 nT value approximately corresponds to the amplitude of the distribution half-width (Figure 2c).
The results in Table 6 demonstrate that addition to neutrophil suspension of water samples incubated under the CMF conditions with alternating component formed with a random signal (EEG signal) similarly to the previously described experiments, increased chemiluminescence (p = 0.02597 vs. Control). In other words, obtained values of “Experimental’ samples in Table 3, Table 4 and Table 6 are comparable and equal to 66%, 52%, and 56%, respectively.
Meanwhile, the results presented in Table 4 and Table 6 show that the addition of the CMF pre-exposed samples as well as same samples but after serial dilution under conditions similarly decreased the maximum neutrophil chemiluminescence by approximately 16% and 23%, respectively. Therefore, the CMF, which were formed using a signal proportional to electroencephalogram recording, as well as single-frequency 12.6 Hz band signal, may result in biological cell response: rapid increase in ROS generation.
4. Discussion
Our current study was designed to investigate the role of water in biological effects of the CMF. Selection of parameters (frequencies and amplitude) of magnetic alternating component was carried out taking into account the latest experimental data on effects taking place in pure water after exposure to the CMF corresponding to the ICR frequency [16,17] of hydronium H3O+ ions (protonated water) and its hydrated forms [12,18]. These studies demonstrated that stimulation of water with super weak magnetic field results in transient change in refraction index, conductivity, and potential for hydrogen (pH). In addition, such water starts to emit stable transitional magnetic signal at 48.5 Hz, for at least 60 min after the end of the CMF exposure in the absence of any other measurable field. In turn, these experiments were carried out based on the results of our previous research [19,20,21,22] showing that conductivity of protonated form of glutamic acid (GluH+) in aqueous solution transiently increases when exposed to the CMF under the ICR conditions, including in combination with vanishingly small value of alternating magnetic field (~50 nT).
Our experiments with direct exposure of neutrophil suspension to the CMF demonstrated that pre-treatment with the CMF set for ICR for hydronium ion (48.5 Hz) inhibited respiratory burst, while the CMF set for ICR for hydrated form of this complex ion (12.6 Hz), on the contrary, activated it. Such result may be explained by proximity of the ICR frequency of hydronium ion (48.5 Hz) and ferric ion (Fe3+)—49.5 Hz (using static magnetic field of 60 µT), in which we previously detected significant (~60%) reduction in respiratory burst intensity in neutrophil suspension in response to fMLF, which was also measured using luminol-enhanced chemiluminescence [23].
Lack of the effect of the CMF at 48.5 Hz on pure water detected in the current study may result from the influence of external magnetic fields on internal processes of generation of electromagnetic fluctuations in water at the same frequency (48.5 Hz) as described previously [18]. Regardless of interpretation, our experiments demonstrated that the stimulating effect on intensity of respiratory burst in neutrophil suspension was caused by pre-treatment using the CMF set specifically for the hydrated hydronium ion H9O4+ (frequency 12.6 Hz) [23]. This stimulating effect of the CMF is passed via water (when water is pre-exposed to the CMF and subsequently added to suspension of neutrophils) as similarly shown in a number of our own experiments [24,25,26,27,28] as well as in numerous reports from other authors [29,30,31,32].
Of special interest is the fact that water after magnetic exposure retains its stimulating properties during the serial dilution process accompanied by vigorous shaking under the GMF conditions. Usually, serial dilution and vigorous shaking procedures after each dilution step are used for investigation of properties of highly diluted aqueous solutions of certain chemical compounds [33], but our experiments demonstrated that modifying effect on water may be also generated as a result of magnetic exposure (CMF). Our results clearly demonstrate magnetic properties of water samples after shaking procedure that was performed under the CMF conditions. Along with that, it was demonstrated that shaking of water in a device shielded from the GMF (with permalloy shield) did not result in retention of its properties of stimulating ROS generation by neutrophils. Furthermore, administration of the CMF source into the GMF-shielded device, in which sample shaking had been carried out, caused effect compatible with the GMF. These data correlate well with the results of the experiments investigating the effect of magnetic conditions on the processes of structure generation in highly diluted aqueous solutions [34] showing an absence of water associates detectable by dynamic light scattering in hypomagnetic conditions.
Currently, the ability of liquid water to form structures at ambient temperature cannot be considered well studied. Numerous difficulties related to mathematical modeling of water structure do not yet allow development of a model simultaneously describing all its anomalous properties [35]. This suggests that consideration of simple short-range electrostatic interactions is insufficient for description of water physics. Therefore, longer-range interactions seem to be more significant than anticipated.
To date, the data on long-term electromagnetic fluctuations in water are considered promising for practical use. These include previously mentioned magnetic fluctuations at 48.5 Hz after water exposure under ICR conditions [18], and the earlier data obtained after water exposure at 36 GHz [14]. In this study [14], characteristics of fading voltage fluctuations on capacitor plates filled with water were measured during discharge. Computer analysis of low-frequency fluctuations revealed two well-distinguished peaks in fluctuation spectrum close to 5 and 47 Hz. Cell exposure for several minutes to microwave radiation field at 36 GHz inhibited 47 Hz peak. Such water state was retained after switching off microwave field for several minutes or hours depending on radiation power. It is noteworthy that weak radiation of 50 µWt/cm2 exhibited much more of a pronounced effect compared to that of a hundred times more powerful radiation. Another study [15] demonstrated that aqueous solution used to measure the activity of calcium-dependent potassium channels remembers the effect of microwave radiation. Therefore, the previous data obtained both in physical (characteristics of voltage fluctuations on capacitor plates) and biological systems (activity of calcium-dependent potassium channels) are in line with the results of the current study suggesting that water is capable of modifying its properties after not only microwave exposure, but also after the CMF exposure.
The current study demonstrated that the magnetic and mechanical (serial dilution and shaking) treatment of water alters its ability to cause biological effects. Our results may indicate a change in the properties of water under the influence of applied magnetic and mechanical treatments, which may be responsible for a more pronounced neutrophil respiratory burst and, subsequently, for functional, organizational and structural changes of tissues and organs. These data can be important to consider when preparing samples of substances for the study of their physico-chemical and biological properties. A potential medical application may be of interest, in which the exposure of water to magnetic field with a certain frequency will lead to the desired physiological result. Further studies of the demonstrated effects in the animal models will be required for identifying their potential applications in medicine.
5. Conclusions
- (1)
- The results of our experiments demonstrate that water exposure to a physical effect in the form of CMF with alternating component, which changes by sinusoidal signal at 12.6 Hz, modifies its properties (66% increase in intensity of neutrophil suspension chemiluminescence relative to control samples).
- (2)
- These properties are retained during serial dilution and vigorous shaking of the exposed water (44% increase in chemiluminescence of neutrophil suspension relative to control samples).
- (3)
- Similar but less pronounced indirect effect of the CMF pre-exposed water is observed when using the CMF formed by a random signal proportional to electroencephalogram recording (which also includes 12.6 Hz band). Chemiluminescence intensity increased both after adding to the cells the CMF pre-exposed water (56% increase) and after adding the same CMF pre-exposed water but mechanically treated between serial dilutions (33% increase).
Author Contributions
Conceptualization, V.V.N. and E.E.F.; methodology, V.V.N. and E.V.Y.; data analysis, V.V.N. and E.V.Y.; writing, V.V.N. and E.E.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
Authors express their sincere gratitude to all the staff at the Laboratory of reception mechanisms, and to Nikolay E. Shvirst and Vasily M. Vershinin for engineering assistance in completing this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Novikov, V.V.; Yablokova, E.V.; Fesenko, E.E. Priming of the respiratory burst in neutrophils exposed to a combination of weak constant and alternating low-frequency magnetic fields in vitro. Biophysics 2016, 61, 429–434. [Google Scholar] [CrossRef]
- Dahlgren, C.; Karlsson, A. Respiratory burst in human neutrophils. J. Immunol. Methods 1999, 232, 3–14. [Google Scholar] [CrossRef]
- Novikov, G.V.; Novikov, V.V.; Fesenko, E.E. Effect of weak combined static and low-frequency alternating magnetic fields on the Ehrlich ascites carcinoma in mice. Biophysics 2009, 54, 741–747. [Google Scholar] [CrossRef]
- Novikov, V.V.; Novikov, G.V.; Fesenko, E.E. Effect of weak combined static and extremely low-frequency alternating magnetic fields on tumor growth in mice inoculated with the Ehrlich ascites carcinoma. Bioelectromagnetics 2009, 30, 343–351. [Google Scholar] [CrossRef]
- Novikov, V.V.; Ponomarev, V.O.; Novikov, G.V.; Kuvichkin, V.V.; Yablokova, E.V.; Fesenko, E.E. Effects and molecular mechanisms of the biological action of weak and extremely weak magnetic fields. Biophysics 2010, 55, 565–572. [Google Scholar] [CrossRef]
- Novikov, V.V.; Yablokova, E.V.; Novikov, G.V.; Fesenko, E.E. The role of lipid peroxidation and myeloperoxidase in priming a respiratory burst in neutrophils under the action of combined constant and alternating magnetic fields. Biophysics 2017, 62, 759–763. [Google Scholar] [CrossRef]
- Novikov, V.V.; Yablokova, E.V.; Fesenko, E.E. The role of hydroxyl radicals and calcium ions in the priming of a respiratory burst in neutrophils and the increase in luminol-dependent blood chemiluminescence on exposure to combined magnetic fields with a very weak low-frequency alternating component. Biophysics 2017, 62, 440–443. [Google Scholar] [CrossRef]
- Novikov, V.V.; Yablokova, E.V.; Fesenko, E.E. The Role of Oxygen in the Priming of Neutrophils on Exposure to a Weak Magnetic Field. Biophysics 2018, 63, 193–196. [Google Scholar] [CrossRef]
- Karelina, E.A.; Ganina, K.K.; Kosmachev, V.N.; Tarasov, S.A. Results of the blind placebo-controlled trial of the novel anti-stress drug Anoten efficacy against neurotic disoders in dogs. Ross. Vet. (Russ. Vet. J.) 2018, 2, 39–42. [Google Scholar]
- Penkov, N. Peculiarities of the Perturbation of Water Structure by Ions with Various Hydration in Concentrated Solutions of CaCl 2, CsCl, KBr, and KI. Phys. Wave Phenom. 2019, 27, 128–134. [Google Scholar] [CrossRef]
- Epstein, O. The spatial homeostasis hypothesis. Symmetry 2018, 10, 103. [Google Scholar] [CrossRef]
- D’Emilia, E.; Ledda, M.; Foletti, A.; Lisi, A.; Giuliani, L.; Grimaldi, S.; Liboff, A.R. Weak-field H3O(+) ion cyclotron resonance alters water refractive index. Electromagn. Biol. Med. 2017, 36, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Noachtar, S.; Binnie, C.; Ebersole, J.; Mauguiere, F.; Sakamoto, A.; Westmoreland, B. A glossary of terms most commonly used by clinical electroencephalographers and proposal for the report form for the EEG findings. The International Federation of Clinical Neurophysiology. Electroencephalogr. Clin. Neurophysiol. Suppl. 1999, 52, 21–41. [Google Scholar] [PubMed]
- Fesenko, E.E.; Gluvstein, A. Changes in the state of water, induced by radiofrequency electromagnetic fields. FEBS Lett. 1995, 367, 53–55. [Google Scholar] [CrossRef]
- Fesenko, E.E.; Geletyuk, V.I.; Kazachenko, V.N.; Chemeris, N.K. Preliminary microwave irradiation of water solutions changes their channel-modifying activity. FEBS Lett. 1995, 366, 49–52. [Google Scholar] [CrossRef]
- Liboff, A.R. Geomagnetic cyclotron resonance in living cells. J. Biol. Phys. 1985, 13, 99–102. [Google Scholar] [CrossRef]
- Liboff, A.R. ION cyclotron resonance: Geomagnetic strategy for living systems? Electromagn. Biol. Med. 2019, 38, 143–148. [Google Scholar] [CrossRef]
- D’Emilia, E.; Giuliani, L.; Lisi, A.; Ledda, M.; Grimaldi, S.; Montagnier, L.; Liboff, A. Lorentz force in water: Evidence that hydronium cyclotron resonance enhances polymorphism. Electromagn. Biol. Med. 2015, 34, 370–375. [Google Scholar] [CrossRef]
- Novikov, V.V.; Zhadin, M.N. Combined action of weak constant and variable low-frequency magnetic fields on ionic currents in aqueous solutions of amino acid. Biophysics 1994, 39, 41–45. [Google Scholar]
- Novikov, V.V. Initiate action of weak magnetic fields on intermolecular bond formation in aqueous solution of amino acids. Biophysics 1994, 39, 851–856. [Google Scholar]
- Novikov, V.V. Cooperative effect of the resonance amplification of ionic current in aqueous solutions of amino acids under the action of weak electromagnetic fields. Approaches to experimental and theoretical analysis. Biophysics 1996, 41, 983–988. [Google Scholar]
- Zhadin, M.N.; Novikov, V.V.; Barnes, F.S.; Pergola, N.F. Combined action of static and alternating magnetic fields on ionic current in aqueous glutamic acid solution. Bioelectromagnetics 1998, 19, 41–45. [Google Scholar] [CrossRef]
- Novikov, V.V.; Yablokova, E.V.; Fesenko, E.E. Respiratory burst reduction in neutrophils after exposure to certain modes of weak combined magnetic fields. Biophysics 2020, 65, 82–87. [Google Scholar] [CrossRef]
- Fesenko, E.E.; Novikov, V.V.; Kuvichkin, V.V.; Iablokova, E.V. Effect of treated with weak magnetic field aqueous salt solutions on the intrinsic fluorescence of bovine serum albumin. Isolation from solutions and partial characterization of the biologically active fluorescing fraction. Biophysics 2000, 45, 232–239. [Google Scholar]
- Fesenko, E.E.; Popov, V.I.; Novikov, V.V.; Khutsian, S.S. Water structure formation by weak magnetic fields and xenon. Electron microscopic analysis. Biophysics 2002, 47, 389–394. [Google Scholar]
- Novikov, V.V.; Kuvichkin, V.V.; Fesenko, E.E. Effect of weak combined low frequency static and low-frequency alternative magnetic fields on the intrinsic fluorescence of some proteins in aqueous solutions. Biophysics 1999, 44, 224–230. [Google Scholar]
- Novikov, V.V.; Fesenko, E.E. Hydrolysis of various peptides and proteins in weak permanent and low frequency fluctuating magnetic fields. Biofizika 2001, 46, 235–241. [Google Scholar]
- Novikov, V.V.; Sheiman, I.M.; Lisitsyn, A.S.; Kliubin, A.V.; Fesenko, E.E. Dependence of effects of weak combined low-frequency variable and constant magnetic fields on the intensity of asexual reproduction of planarians Dugesia tigrina on the magnitude of the variable field. Biofizika 2002, 47, 564–567. [Google Scholar]
- Fukushima, M.; Mohri, K.; Kataoka, T.; Matsumoto, M. Milli Gauss pursed Magnetic Field Applied Phosphate Buffeted Saline Solution Elevates Intracellular Ca2+ Level and Stimulates Phagocytic Activity of Human Neutrophils. Trans. Magn. Soc. Jpn. 2002, 2, 15–18. [Google Scholar] [CrossRef]
- Ayrapetyan, S.N.; Grigorian, K.V.; Avanesian, A.S.; Stamboltsian, K.V. Magnetic fields alter electrical properties of solutions and their physiological effects. Bioelectromagnetics 1994, 15, 133–142. [Google Scholar] [CrossRef]
- Ayrapetyan, S.; Hunanyan, A.S.; Hakobyan, S. 4 Hz EMF treated physiological solution depresses Ach-induced neuromembrane current. Bioelectromagnetics 2004, 25, 397–399. [Google Scholar] [CrossRef] [PubMed]
- Foletti, A.; Ledda, M.; Lolli, M.G.; Grimaldi, S.; Lisi, A. Electromagnetic information transfer through aqueous system. Electromagn. Biol. Med. 2017, 36, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Yinnon, T.; Kalia, K.; Kikar, D. Very Dilute Aqueous Solutions—Structural and Electromagnetic Phenomena. Water 2017, 9, 28–66. [Google Scholar]
- Konovalov, A.; Ryzhkina, I.; Maltzeva, E.; Murtazina, L.; Kiseleva, Y.; Kasparov, V.; Palmina, N. Nanoassociate formation in highly diluted water solutions of potassium phenosan with and without permalloy shielding. Electromagn. Biol. Med. 2015, 34, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Góra, U.; Podeszwa, R.; Cencek, W.; Szalewicz, K. Interaction energies of large clusters from many-body expansion. J. Chem. Phys. 2011, 135, 224102. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).