A Quick, Green and Simple Ultrasound-Assisted Extraction for the Valorization of Antioxidant Phenolic Acids from Moroccan Almond Cold-Pressed Oil Residues
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
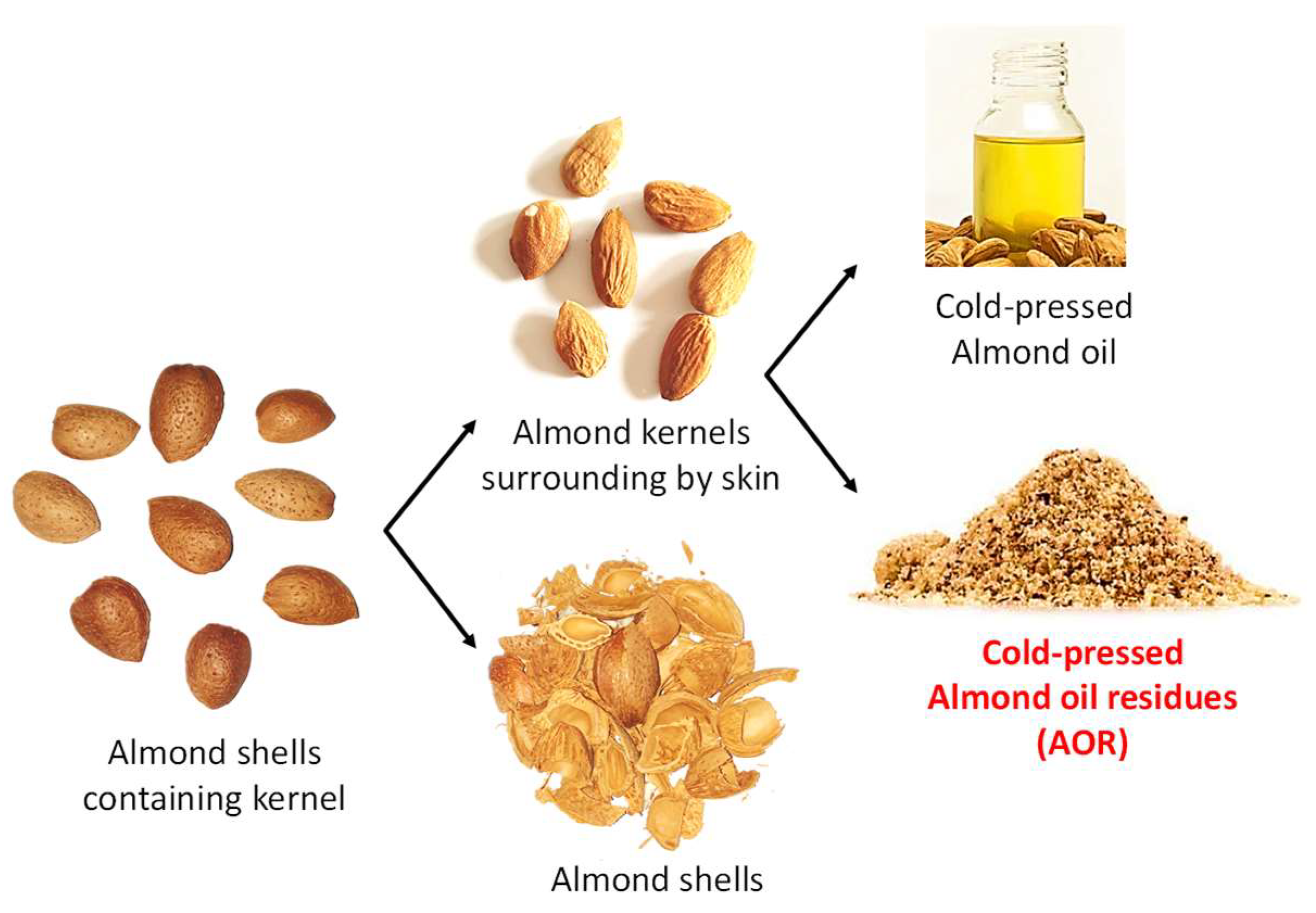
2.2. Plant Materials and Culture Conditions
2.3. Ultrasound-Assisted Extraction Method Development
2.4. Determination of Total Phenolic Content
2.5. Validation Parameters
2.6. HPLC Analysis
2.7. Antioxidant Activities
2.7.1. In Vitro Cell Free DPPH Free Radical Scavenging Assay
2.7.2. In Vitro Cell Free ABTS Antioxidant In Vitro Cell Free Assay
2.7.3. Cupric Ion Reducing Antioxidant Capacity (CUPRAC) In Vitro Cell Free Assay
2.7.4. Determination of Membrane Lipid Peroxidation Using Thiobarbituric Acid-Reactive Substances (TBARS) Assay
2.8. Statistical Analysis
3. Results and Discussion
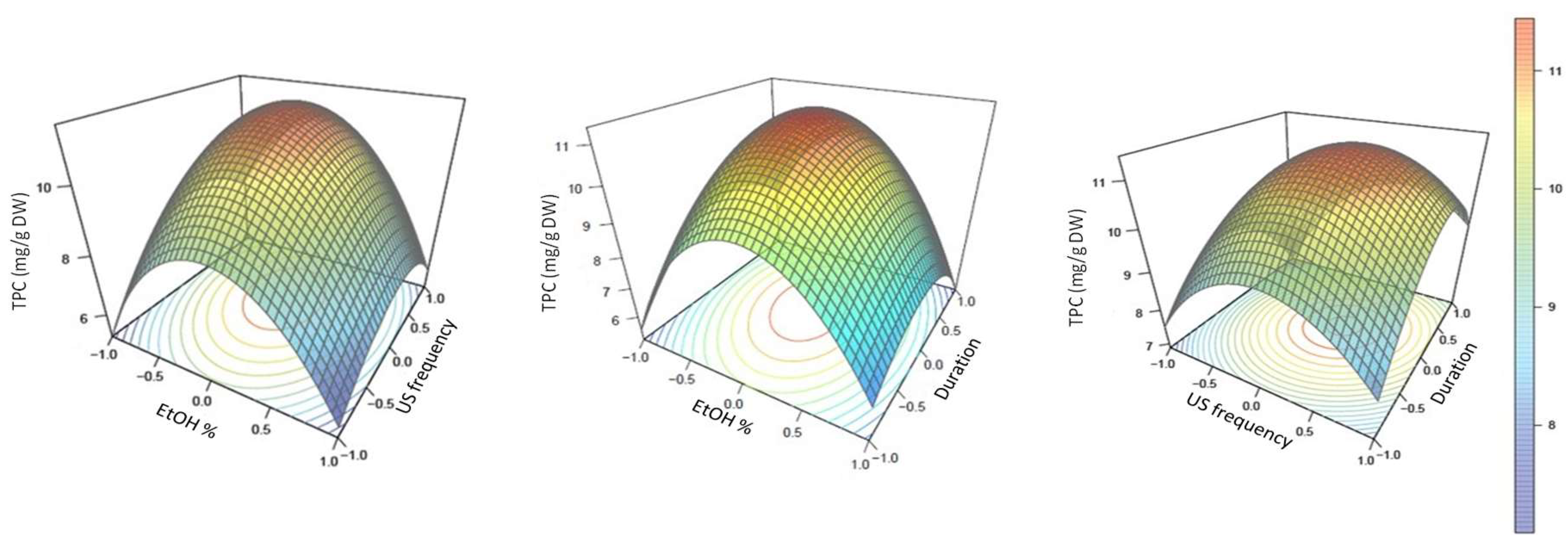
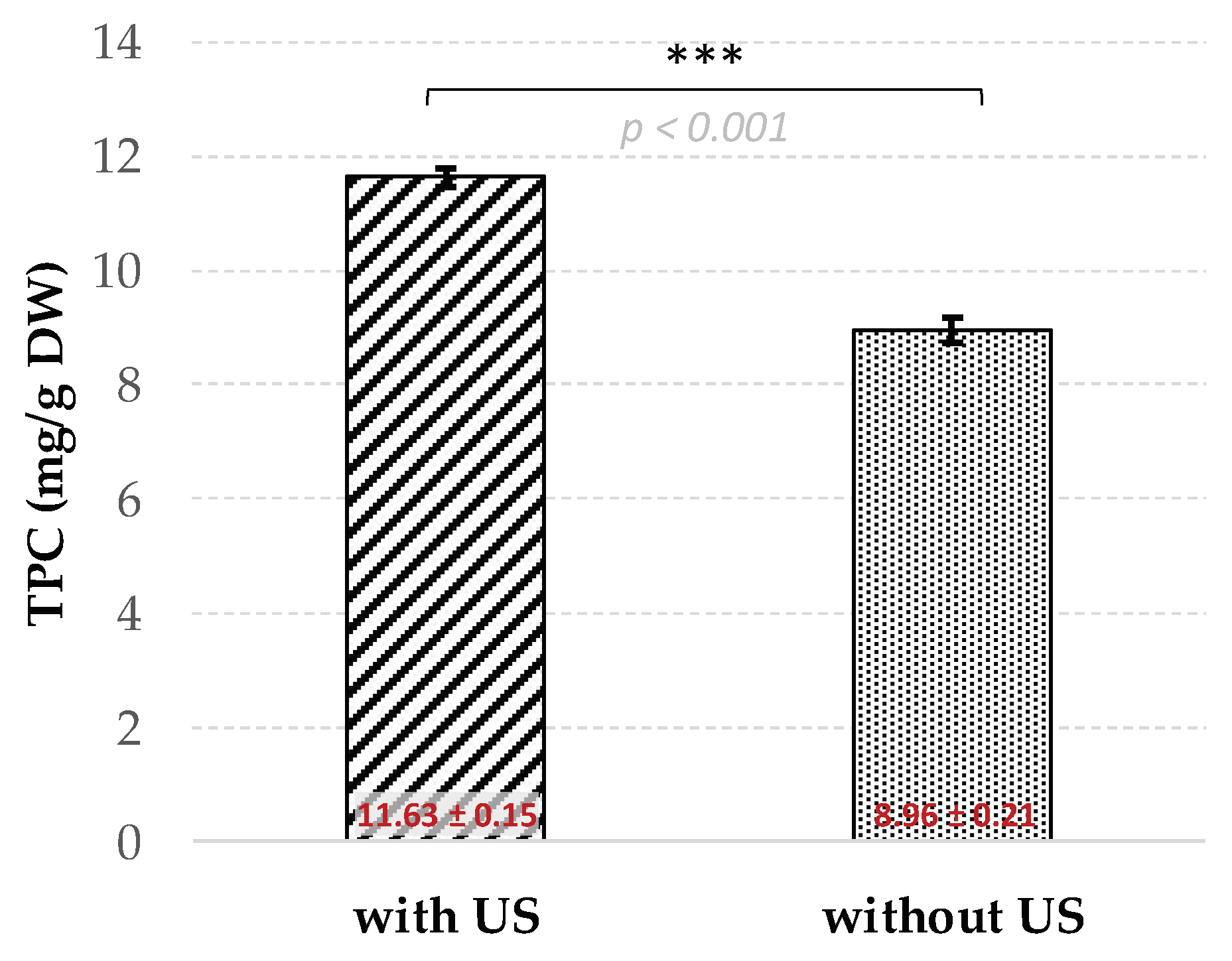
3.1. Development of the Ultrasound-Assisted Extraction Using Box–Behnken Design
3.2. Application to the Analysis of Samples from Different Cultivation Sites
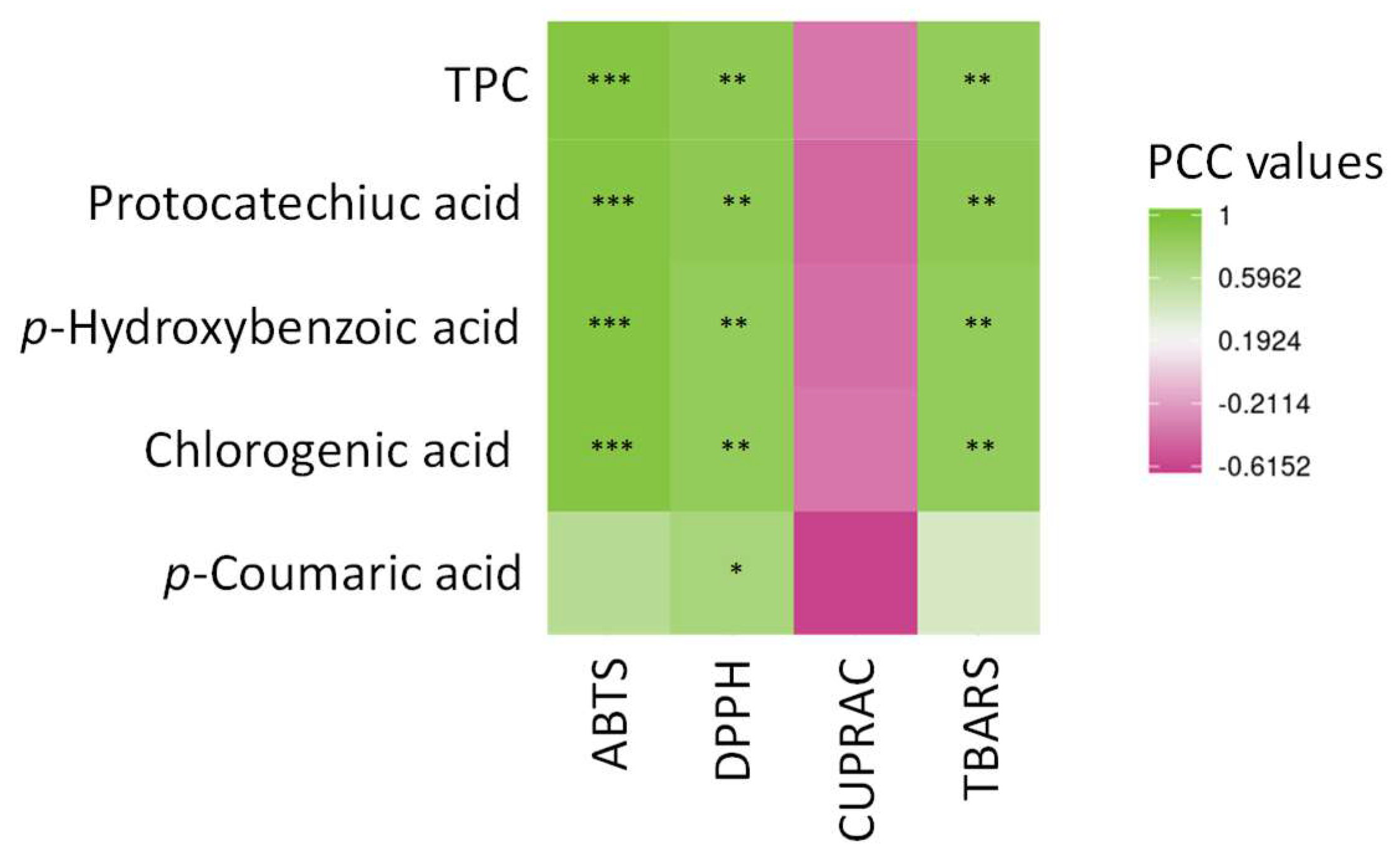
3.3. Determination of the Antioxidant Potential of the Extracts and Correlation Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Oomah, D.B. Flaxseed as a functional food. J. Sci. Food Agric. 2001, 81, 889–894. [Google Scholar] [CrossRef]
- Nayak, B.; Liu, R.H.; Tang, J. Effect of Processing on Phenolic Antioxidants of Fruits, Vegetables, and Grains—A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 887–919. [Google Scholar] [CrossRef] [PubMed]
- Bolling, B.W. Almond polyphenols: Methods of analysis, contribution to food quality, and health promotion. Compr. Rev. Food Sci. Food Saf. 2017, 16, 346–368. [Google Scholar] [CrossRef]
- Barreca, D.; Nabavi, S.M.; Sureda, A.; Rasekhian, M.; Raciti, R.; Silva, A.S.; Annunziata, G.; Arnone, A.; Tenore, G.C.; Süntar, İ. Almonds (Prunus Dulcis Mill. DA Webb): A Source of Nutrients and Health-Promoting Compounds. Nutrients 2020, 12, 672. [Google Scholar] [CrossRef]
- Prgomet, I.; Gonçalves, B.; Domínguez-Perles, R.; Pascual-Seva, N.; Barros, A.I. Valorization challenges to almond residues: Phytochemical composition and functional application. Molecules 2017, 22, 1774. [Google Scholar] [CrossRef]
- Williams, G.M.; Iatropoulos, M.J.; Whysner, J. Safety Assessment of Butylated Hydroxyanisole and Butylated Hydroxytoluene as Antioxidant Food Additives. Food Chem. Toxicol. 1999, 37, 1027–1038. [Google Scholar] [CrossRef]
- Hano, C.; Corbin, C.; Drouet, S.; Quéro, A.; Rombaut, N.; Savoire, R.; Molinié, R.; Thomasset, B.; Mesnard, F.; Lainé, E. The lignan (+)-secoisolariciresinol extracted from flax hulls is an effective protectant of linseed oil and its emulsion against oxidative damage. Eur. J. Lipid Sci. Technol. 2017, 119, 1600219. [Google Scholar] [CrossRef]
- Drouet, S.; Doussot, J.; Garros, L.; Mathiron, D.; Bassard, S.; Favre-Réguillon, A.; Molinié, R.; Lainé, É.; Hano, C. Selective Synthesis of 3-O-Palmitoyl-Silybin, a New-to-Nature Flavonolignan with Increased Protective Action against Oxidative Damages in Lipophilic Media. Molecules 2018, 23, 2594. [Google Scholar] [CrossRef]
- Mariod, A.A.; Ibrahim, R.M.; Ismail, M.; Ismail, N. Antioxidant activity and phenolic content of phenolic rich fractions obtained from black cumin (Nigella sativa) seedcake. Food Chem. 2009, 116, 306–312. [Google Scholar] [CrossRef]
- Aditya, N.P.; Hamilton, I.E.; Norton, I.T. Amorphous nano-curcumin stabilized oil in water emulsion: Physico chemical characterization. Food Chem. 2017, 224, 191–200. [Google Scholar] [CrossRef]
- Drouet, S.; Leclerc, E.A.; Garros, L.; Tungmunnithum, D.; Kabra, A.; Abbasi, B.H.; Lainé, É.; Hano, C. A Green Ultrasound-Assisted Extraction Optimization of the Natural Antioxidant and Anti-Aging Flavonolignans from Milk Thistle Silybum marianum (L.) Gaertn. Fruits for Cosmetic Applications. Antioxidants 2019, 8, 304. [Google Scholar] [CrossRef] [PubMed]
- Porter, W.L.; Black, E.D.; Drolet, A.M. Use of polyamide oxidative fluorescence test on lipid emulsions: Contrast in relative effectiveness of antioxidants in bulk versus dispersed systems. J. Agric. Food Chem. 1989, 37, 615–624. [Google Scholar] [CrossRef]
- Prgomet, I.; Gonçalves, B.; Domínguez-Perles, R.; Pascual-Seva, N.; Barros, A.I. A Box-Behnken Design for Optimal Extraction of Phenolics from Almond By-products. Food Anal. Methods 2019, 12, 2009–2024. [Google Scholar] [CrossRef]
- Delplancke, M.; Aumeeruddy-Thomas, Y. Des semis et des clones. Rev. D’ethnoécologie 2017, 27. [Google Scholar] [CrossRef]
- Melhaoui, R.; Fauconnier, M.-L.; Sindic, M.; Addi, M.; Abid, M.; Mihamou, A.; Serghini-Caid, H.; Elamrani, A. Tocopherol Content of Almond Oils. Commun. Appl. Biol. Sci. 2018, 83, 75–77. [Google Scholar]
- Sarkis, J.R.; Michel, I.; Tessaro, I.C.; Marczak, L.D.F. Optimization of phenolics extraction from sesame seed cake. Sep. Purif. Technol. 2014, 122, 506–514. [Google Scholar] [CrossRef]
- Wang, F.; Hu, J.-H.; Guo, C.; Liu, C.-Z. Enhanced laccase production by Trametes versicolor using corn steep liquor as both nitrogen source and inducer. Bioresour. Technol. 2014, 166, 602–605. [Google Scholar] [CrossRef]
- Kahlaoui, M.; Borotto Dalla Vecchia, S.; Giovine, F.; Ben Haj Kbaier, H.; Bouzouita, N.; Barbosa Pereira, L.; Zeppa, G. Characterization of Polyphenolic Compounds Extracted from Different Varieties of Almond Hulls (Prunus dulcis L.). Antioxidants 2019, 8, 647. [Google Scholar] [CrossRef]
- Melhaoui, R.; Fauconnier, M.-L.; Sindic, M.; Addi, M.; Abid, M.; Mihamou, A.; Serghini-Caid, H.; Elamrani, A. Tocopherol content of almond oils produced in eastern Morocco. In Proceedings of the 23rd National Symposium for Applied Biological Sciences (NSABS), Brussels, Belgium, 8 February 2018; pp. 75–77. [Google Scholar]
- Teh, S.-S.; Bekhit, A.E.-D.; Birch, J. Antioxidative polyphenols from defatted oilseed cakes: Effect of solvents. Antioxidants 2014, 3, 67–80. [Google Scholar] [CrossRef]
- Corbin, C.; Fidel, T.; Leclerc, E.A.; Barakzoy, E.; Sagot, N.; Falguiéres, A.; Renouard, S.; Blondeau, J.; Ferroud, C.; Doussot, J.; et al. Development and validation of an efficient ultrasound assisted extraction of phenolic compounds from flax (Linum usitatissimum L.) seeds. Ultrason. Sonochem. 2015, 26, 176–185. [Google Scholar] [CrossRef]
- Fliniaux, O.; Corbin, C.; Ramsay, A.; Renouard, S.; Beejmohun, V.; Doussot, J.; Falguières, A.; Ferroud, C.; Lamblin, F.; Lainé, E.; et al. Microwave-Assisted Extraction of Herbacetin Diglucoside from Flax (Linum usitatissimum L.) Seed Cakes and Its Quantification using an RP-HPLC-UV System. Molecules 2014, 3025–3037. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, C.; Leclerc, É.A.; Corbin, C.; Doussot, J.; Serrano, V.; Vanier, J.R.; Seigneuret, J.M.; Auguin, D.; Pichon, C.; Lainé, É.; et al. Nettle (Urtica dioica L.) as a source of antioxidant and anti-aging phytochemicals for cosmetic applications. C. R. Chim. 2016, 19, 1090–1100. [Google Scholar] [CrossRef]
- Renouard, S.; Hano, C.; Corbin, C.; Fliniaux, O.; Lopez, T.; Montguillon, J.; Barakzoy, E.; Mesnard, F.; Lamblin, F.; Lainé, E. Cellulase-assisted release of secoisolariciresinol from extracts of flax (Linum usitatissimum) hulls and whole seeds. Food Chem. 2010, 122, 679–687. [Google Scholar] [CrossRef]
- Terpinc, P.; Polak, T.; Makuc, D.; Ulrih, N.P.; Abramovič, H. The occurrence and characterisation of phenolic compounds in Camelina sativa seed, cake and oil. Food Chem. 2012, 131, 580–589. [Google Scholar] [CrossRef]
- Wildermuth, S.R.; Young, E.E.; Were, L.M. Chlorogenic acid oxidation and its reaction with sunflower proteins to form green-colored complexes. Compr. Rev. Food Sci. Food Saf. 2016, 15, 829–843. [Google Scholar] [CrossRef]
- Ahmad, B.S.; Talou, T.; Saad, Z.; Hijazi, A.; Cerny, M.; Kanaan, H.; Chokr, A.; Merah, O. Fennel oil and by-products seed characterization and their potential applications. Ind. Crops Prod. 2018, 111, 92–98. [Google Scholar] [CrossRef]
- Lavilla, I.; Bendicho, C. Fundamentals of Ultrasound-Assisted Extraction. Water Extr. Bioact. Compd. From Plants Drug Dev. 2017, 291–316. [Google Scholar] [CrossRef]
- Medina-Torres, N.; Ayora-Talavera, T.; Espinosa-Andrews, H.; Sánchez-Contreras, A.; Pacheco, N. Ultrasound Assisted Extraction for the Recovery of Phenolic Compounds from Vegetable Sources. Agronomy 2017, 7, 47. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Garros, L.; Drouet, S.; Renouard, S.; Lainé, E.; Hano, C. Green Ultrasound Assisted Extraction of trans Rosmarinic Acid from Plectranthus scutellarioides (L.) R.Br. Leaves. Plants 2019, 8, 50. [Google Scholar] [CrossRef]
- Abbasi, B.H.; Siddiquah, A.; Tungmunnithum, D.; Bose, S.; Younas, M.; Garros, L.; Drouet, S.; Giglioli-Guivarc’h, N.; Hano, C. Isodon rugosus (Wall. ex Benth.) codd in vitro cultures: Establishment, phytochemical characterization and in vitro antioxidant and anti-aging activities. Int. J. Mol. Sci. 2019, 20, 452. [Google Scholar] [CrossRef]
- Shah, M.; Ullah, M.A.; Drouet, S.; Younas, M.; Tungmunnithum, D.; Giglioli-Guivarc’h, N.; Hano, C.; Abbasi, B.H. Interactive effects of light and melatonin on biosynthesis of silymarin and anti-inflammatory potential in callus cultures of silybum marianum (L.) gaertn. Molecules 2019, 24, 1207. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.A.; Tungmunnithum, D.; Garros, L.; Drouet, S.; Hano, C.; Abbasi, B.H. Effect of Ultraviolet-C Radiation and Melatonin Stress on Biosynthesis of Antioxidant and Antidiabetic Metabolites Produced in In Vitro Callus Cultures of Lepidium sativum L. Int. J. Mol. Sci. 2019, 20, 1787. [Google Scholar] [CrossRef] [PubMed]
- Garros, L.; Drouet, S.; Corbin, C.; Decourtil, C.; Fidel, T.; De Lacour, J.L.; Leclerc, E.A.; Renouard, S.; Tungmunnithum, D.; Doussot, J.; et al. Insight into the influence of cultivar type, cultivation year, and site on the lignans and related phenolic profiles, and the health-promoting antioxidant potential of flax (linum usitatissimum L.) seeds. Molecules 2018, 23, 2636. [Google Scholar] [CrossRef] [PubMed]
- Babicki, S.; Arndt, D.; Marcu, A.; Liang, Y.; Grant, J.R.; Maciejewski, A.; Wishart, D.S. Heatmapper: Web-enabled heat mapping for all. Nucleic Acids Res. 2016, 44, W147–W153. [Google Scholar] [CrossRef]
- Ferreira, S.L.C.; Silva Junior, M.M.; Felix, C.S.A.; da Silva, D.L.F.; Santos, A.S.; Santos Neto, J.H.; de Souza, C.T.; Cruz Junior, R.A.; Souza, A.S. Multivariate optimization techniques in food analysis—A review. Food Chem. 2019, 273, 3–8. [Google Scholar] [CrossRef]
- Ferreira, S.L.C.; Bruns, R.E.; Ferreira, H.S.; Matos, G.D.; David, J.M.; Brandão, G.C.; da Silva, E.G.P.; Portugal, L.A.; dos Reis, P.S.; Souza, A.S.; et al. Box-Behnken design: An alternative for the optimization of analytical methods. Anal. Chim. Acta 2007, 597, 179–186. [Google Scholar] [CrossRef]
- Chemat, F.; Abert-Vian, M.; Fabiano-Tixier, A.S.; Strube, J.; Uhlenbrock, L.; Gunjevic, V.; Cravotto, G. Green extraction of natural products. Origins, current status, and future challenges. Trends Anal. Chem. 2019, 118, 248–263. [Google Scholar] [CrossRef]
- Renouard, S.; Lopez, T.; Hendrawati, O.; Dupre, P.; Doussot, J.; Falguieres, A.; Ferroud, C.; Hagege, D.; Lamblin, F.; Laine, E.; et al. Podophyllotoxin and deoxypodophyllotoxin in juniperus bermudiana and 12 other juniperus species: Optimization of extraction, method validation, and quantification. J. Agric. Food Chem. 2011, 59. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green Extraction of Natural Products: Concept and Principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef]
- Ameer, K.; Shahbaz, H.M.; Kwon, J.H. Green Extraction Methods for Polyphenols from Plant Matrices and Their Byproducts: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 295–315. [Google Scholar] [CrossRef]
- Bolling, B.W.; Blumberg, J.B.; Oliver Chen, C.-Y. The influence of roasting, pasteurisation, and storage on the polyphenol content and antioxidant capacity of California almond skins. Food Chem. 2010, 123, 1040–1047. [Google Scholar] [CrossRef] [PubMed]
- Beejmohun, V.; Fliniaux, O.; Grand, É.; Lamblin, F.; Bensaddek, L.; Christen, P.; Kovensky, J.; Fliniaux, M.-A.; Mesnard, F. Microwave-assisted extraction of the main phenolic compounds in flaxseed. Phytochem. Anal. 2007, 18, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Rubilar, M.; Pinelo, M.; Shene, C.; Sineiro, J.; Nuñez, M.J. Separation and HPLC-MS Identification of Phenolic Antioxidants from Agricultural Residues: Almond Hulls and Grape Pomace. J. Agric. Food Chem. 2007, 55, 10101–10109. [Google Scholar] [CrossRef] [PubMed]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: Oxford, UK, 1998; ISBN 0198502346. [Google Scholar]
- Monagas, M.; Garrido, I.; Lebrón-Aguilar, R.; Bartolome, B.; Gómez-Cordovés, C. Almond (Prunus dulcis (Mill.) D.A. Webb) Skins as a Potential Source of Bioactive Polyphenols. J. Agric. Food Chem. 2007, 55, 8498–8507. [Google Scholar] [CrossRef] [PubMed]
- Freeman, B.L.; Eggett, D.L.; Parker, T.L. Synergistic and antagonistic interactions of phenolic compounds found in navel oranges. J. Food Sci. 2010, 75, C570–C576. [Google Scholar] [CrossRef]
- Pathak, N.; Rai, A.K.; Kumari, R.; Bhat, K. V Value addition in sesame: A perspective on bioactive components for enhancing utility and profitability. Pharmacogn. Rev. 2014, 8, 147. [Google Scholar]
- Drouet, S.; Abbasi, B.H.; Falguières, A.; Ahmad, W.; Sumaira; Ferroud, C.; Doussot, J.; Vanier, J.R.; Lainé, E.; Hano, C. Single laboratory validation of a quantitative core shell-based LC separation for the evaluation of silymarin variability and associated antioxidant activity of pakistani ecotypes of milk thistle (silybum marianum L.). Molecules 2018, 23, 904. [Google Scholar] [CrossRef]
- Dave Oomah, B.; Mazza, G.; Kenaschuk, E.O. Flavonoid content of flaxseed. Influence of cultivar and environment. Euphytica 1996, 90, 163–167. [Google Scholar] [CrossRef]
- Huang, D.; Zhou, X.; Si, J.; Gong, X.; Wang, S. Studies on cellulase-ultrasonic assisted extraction technology for flavonoids from Illicium verum residues. Chem. Cent. J. 2016, 10, 56. [Google Scholar] [CrossRef]
- Nazir, M.; Tungmunnithum, D.; Bose, S.; Drouet, S.; Garros, L.; Giglioli-Guivarc’h, N.; Abbasi, B.H.; Hano, C. Differential Production of Phenylpropanoid Metabolites in Callus Cultures of Ocimum basilicum L. With Distinct in Vitro Antioxidant Activities and in Vivo Protective Effects against UV stress. J. Agric. Food Chem. 2019, 67, 1847–1859. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Steels, E.L.; Learmonth, R.P.; Watson, K. Stress tolerance and membrane lipid unsaturation in Saccharomyces cerevisiae grown aerobically or anaerobically. Microbiology 1994, 140 Pt 3, 569–576. [Google Scholar] [CrossRef]
- Wolak, N.; Kowalska, E.; Kozik, A.; Rapala-Kozik, M. Thiamine increases the resistance of baker’s yeast Saccharomyces cerevisiae against oxidative, osmotic and thermal stress, through mechanisms partly independent of thiamine diphosphate-bound enzymes. FEMS Yeast Res. 2014, 14, 1249–1262. [Google Scholar] [CrossRef] [PubMed]
- Bisquert, R.; Muñiz-Calvo, S.; Guillamón, J.M. Protective role of intracellular Melatonin against oxidative stress and UV radiation in Saccharomyces cerevisiae. Front. Microbiol. 2018, 9, 318. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Cao, G.; Martin, A.; Sofic, E.; McEwen, J.; O’Brien, C.; Lischner, N.; Ehlenfeldt, M.; Kalt, W.; Krewer, G.; et al. Antioxidant Capacity As Influenced by Total Phenolic and Anthocyanin Content, Maturity, and Variety of Vaccinium Species. J. Agric. Food Chem. 1998, 46, 2686–2693. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D. Antioxidant Property of Coffee Components: Assessment of Methods that Define Mechanisms of Action. Molecules 2014, 19, 19180–19208. [Google Scholar] [CrossRef]


| Variable | Code Unit | Coded Variable Levels | ||
|---|---|---|---|---|
| −1 | 0 | +1 | ||
| Ethanol concentration (% v/v) 1 | X1 | 0 | 50 | 100 |
| US frequency (kHz) | X2 | 0 | 22.5 | 45 |
| Extraction duration (min) | X3 | 20 | 30 | 40 |
| Run ID | Run Order | X1 | X2 | X3 | Experimental TPC (mg/g DW) | Predicted TPC (mg/g DW) |
|---|---|---|---|---|---|---|
| Obs1 | 10 | 0 | +1 | −1 | 7.93 ± 0.14 | 7.93 |
| Obs2 | 6 | +1 | 0 | −1 | 6.16 ± 0.22 | 6.31 |
| Obs3 | 1 | −1 | −1 | 0 | 5.03 ± 0.11 | 5.18 |
| Obs4 | 17 | 0 | 0 | 0 | 11.33 ± 0.10 | 11.37 |
| Obs5 | 15 | 0 | 0 | 0 | 11.37 ± 0.08 | 11.37 |
| Obs6 | 7 | −1 | 0 | +1 | 7.51 ± 0.07 | 7.35 |
| Obs7 | 12 | 0 | +1 | +1 | 9.06 ± 0.13 | 9.23 |
| Obs8 | 4 | +1 | +1 | 0 | 6.01 ± 0.11 | 5.86 |
| Obs9 | 18 | 0 | 0 | 0 | 11.41 ± 0.12 | 11.37 |
| Obs10 | 5 | −1 | 0 | −1 | 5.52 ± 0.05 | 5.36 |
| Obs11 | 13 | 0 | 0 | 0 | 11.32 ± 0.17 | 11.37 |
| Obs12 | 2 | +1 | −1 | 0 | 5.54 ± 0.18 | 5.56 |
| Obs13 | 8 | +1 | 0 | +1 | 5.18 ± 0.14 | 5.16 |
| Obs14 | 9 | 0 | −1 | −1 | 7.69 ± 0.19 | 7.52 |
| Obs15 | 11 | 0 | −1 | +1 | 6.88 ± 0.13 | 6.88 |
| Obs16 | 16 | 0 | 0 | 0 | 11.44 ± 0.17 | 11.37 |
| Obs17 | 3 | −1 | +1 | 0 | 7.66 ± 0.16 | 7.64 |
| Obs18 | 14 | 0 | 0 | 0 | 11.35 ± 0.15 | 11.37 |
| Source | Value | SD | t | P > |t| |
|---|---|---|---|---|
| Constant | 11.370 | 0.059 | 193.27 | <0.0001 *** |
| X1 | −0.354 | 0.051 | −6.943 | 0.00012 *** |
| X2 | 0.690 | 0.051 | 13.543 | <0.0001 *** |
| X3 | 0.166 | 0.051 | 3.253 | 0.011 ** |
| X12 | −3.554 | 0.069 | −51.516 | <0.0001 *** |
| X22 | −1.756 | 0.069 | −25.459 | <0.0001 *** |
| X32 | −1.724 | 0.069 | −24.988 | <0.0001 *** |
| X1X2 | −0.540 | 0.072 | −7.495 | <0.0001 *** |
| X1X3 | −0.743 | 0.072 | −10.305 | <0.0001 *** |
| X2X3 | 0.485 | 0.072 | 6.731 | 0.00015 *** |
| Source | Sum of Square | df | Mean of Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 106.071 | 9 | 11.786 | 567.558 | <0.0001 *** |
| Lack of fit | 0.166 | 8 | 0.021 | - | - |
| Residual | 0.166 | 8 | 0.021 | - | - |
| Pure Error | 0.000 | 0 | - | - | - |
| Cor. Total | 106.237 | 17 | - | - | - |
| R2 | 0.997 | ||||
| R2 adj | 0.998 | ||||
| CV% | 0.976 |
| Precision (% RSD) | Repeatability (% RSD) | Recovery 1 (%) | |||
|---|---|---|---|---|---|
| Intraday | Interday | 0.5 | 1.0 | 2.0 | |
| 0.05 | 0.28 | 1.30 | 100.26 ± 0.07 | 100.90 ± 0.80 | 101.13 ± 0.50 |
| Compound | RT (min) | λmax (nm) | Linear Range (mg/L) | Equation | R2 | LOD (mg/L) | LOQ (mg/L) |
|---|---|---|---|---|---|---|---|
| Protocatechuic acid | 23.69 | 295 | 0.5–200 | y = 3.429 x + 0.814 | 0.9991 | 0.12 | 0.38 |
| p-Hydroxybenzoic acid | 28.46 | 295 | 0.5–200 | y = 3.018 + 0.732 | 0.9993 | 0.21 | 0.68 |
| Chlorogenic acid | 31.02 | 325 | 0.5–200 | y = 5.041x + 0.324 | 0.9997 | 0.22 | 0.73 |
| p-Coumaric acid | 33.07 | 325 | 0.5–200 | y = 7.561x + 0.623 | 0.9992 | 0.14 | 0.47 |
| Sample ID | TPC (mg/g DW) | Protocatechuic Acid (mg/g DW) | p-Hydroxybenzoic Acid (mg/g DW) | Chlorogenic Acid (mg/g DW) | p-Coumaric Acid (mg/g DW) |
|---|---|---|---|---|---|
| SID#1 | 9.35 ± 0.63 bcd | 1.33 ± 0.10 de | 0.78 ± 0.02 d | 5.53 ± 0.13 e | 0.26 ± 0.05 ab |
| SID#2 | 11.78 ± 1.58 ab | 1.84 ± 0.07 ab | 1.02 ± 0.06 b | 7.02 ± 0.19 bc | 0.29 ± 0.07 ab |
| SID#3 | 11.79 ± 1.29 ab | 1.75 ± 0.09 b | 0.98 ± 0.02 b | 6.97 ± 0.04 b | 0.29 ± 0.06 ab |
| AIN#1 | 8.87 ± 0.31 d | 1.29 ± 0.06 e | 0.75 ± 0.03 d | 5.29 ± 0.12 e | 0.21 ± 0.04 b |
| AIN#2 | 11.29 ± 1.24 ab | 1.66 ± 0.10 bc | 0.95 ± 0.05 bc | 6.69 ± 0.06 c | 0.28 ± 0.05 ab |
| AIN#3 | 13.86 ± 0.91 a | 2.03 ± 0.07 a | 1.13 ± 0.02 a | 8.14 ± 0.10 a | 0.26 ± 0.20 a |
| RIS#1 | 9.34 ± 0.27 cd | 1.36 ± 0.09 de | 0.75 ± 0.04 d | 5.34 ± 0.14 e | 0.22 ± 0.03 b |
| RIS#2 | 10.69 ± 0.73 bc | 1.51 ± 0.05 cd | 0.87 ± 0.06 c | 6.34 ± 0.05 d | 0.29 ± 0.02 a |
| RIS#3 | 11.97 ± 1.51 ab | 1.76 ± 0.02 b | 1.00 ± 0.10 bc | 7.12 ± 0.08 b | 0.30 ± 0.02 a |
| Sample ID | ABTS (µM TEAC/g DW 1) | DPPH (µM TEAC/g DW 1) | CUPRAC (µM TEAC/g DW 1) | TBARS (% inhibition) |
|---|---|---|---|---|
| SID#1 | 233.10 ± 12.52 d | 323.51 ± 19.12 a | 198.07 ± 22.97 ab | 51.81 ± 1.13 c |
| SID#2 | 361.81 ± 14.48 b | 347.40 ± 7.73 a | 141.04 ± 2.16 c | 66.95 ± 1.74 a |
| SID#3 | 366.49 ± 12.97 b | 341.17 ± 5.49 ab | 129.69 ± 0.32 d | 58.73 ± 1.18 b |
| AIN#1 | 216.94 ± 12.32 d | 275.84 ± 34.88 b | 205.92 ± 17.11 a | 50.62 ± 2.46 c |
| AIN#2 | 276.37 ± 13.12 c | 326.88 ± 30.16 ab | 164.79 ± 14.02 b | 51.81 ± 1.45 c |
| AIN#3 | 401.52 ± 11.44 a | 357.33 ± 24.24 a | 178.73 ± 19.10 ab | 69.12 ± 0.34 a |
| RIS#1 | 238.07 ± 15.86 cd | 319.73 ± 14.74 b | 160.93 ± 13.74 bc | 53.13 ± 1.01 c |
| RIS#2 | 244.91 ± 12.02 cd | 315.60 ± 7.43 b | 143.76 ± 5.24 bc | 52.63 ± 1.65 c |
| RIS#3 | 391.29 ± 9.64 ab | 351.07 ± 2.89 a | 173.47 ± 26.29 abc | 66.85 ± 2.57 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tungmunnithum, D.; Elamrani, A.; Abid, M.; Drouet, S.; Kiani, R.; Garros, L.; Kabra, A.; Addi, M.; Hano, C. A Quick, Green and Simple Ultrasound-Assisted Extraction for the Valorization of Antioxidant Phenolic Acids from Moroccan Almond Cold-Pressed Oil Residues. Appl. Sci. 2020, 10, 3313. https://doi.org/10.3390/app10093313
Tungmunnithum D, Elamrani A, Abid M, Drouet S, Kiani R, Garros L, Kabra A, Addi M, Hano C. A Quick, Green and Simple Ultrasound-Assisted Extraction for the Valorization of Antioxidant Phenolic Acids from Moroccan Almond Cold-Pressed Oil Residues. Applied Sciences. 2020; 10(9):3313. https://doi.org/10.3390/app10093313
Chicago/Turabian StyleTungmunnithum, Duangjai, Ahmed Elamrani, Malika Abid, Samantha Drouet, Reza Kiani, Laurine Garros, Atul Kabra, Mohamed Addi, and Christophe Hano. 2020. "A Quick, Green and Simple Ultrasound-Assisted Extraction for the Valorization of Antioxidant Phenolic Acids from Moroccan Almond Cold-Pressed Oil Residues" Applied Sciences 10, no. 9: 3313. https://doi.org/10.3390/app10093313
APA StyleTungmunnithum, D., Elamrani, A., Abid, M., Drouet, S., Kiani, R., Garros, L., Kabra, A., Addi, M., & Hano, C. (2020). A Quick, Green and Simple Ultrasound-Assisted Extraction for the Valorization of Antioxidant Phenolic Acids from Moroccan Almond Cold-Pressed Oil Residues. Applied Sciences, 10(9), 3313. https://doi.org/10.3390/app10093313