Rational Design of Spinel Oxide Nanocomposites with Tailored Electrochemical Oxygen Evolution and Reduction Reactions for ZincAir Batteries
Abstract
1. Introduction
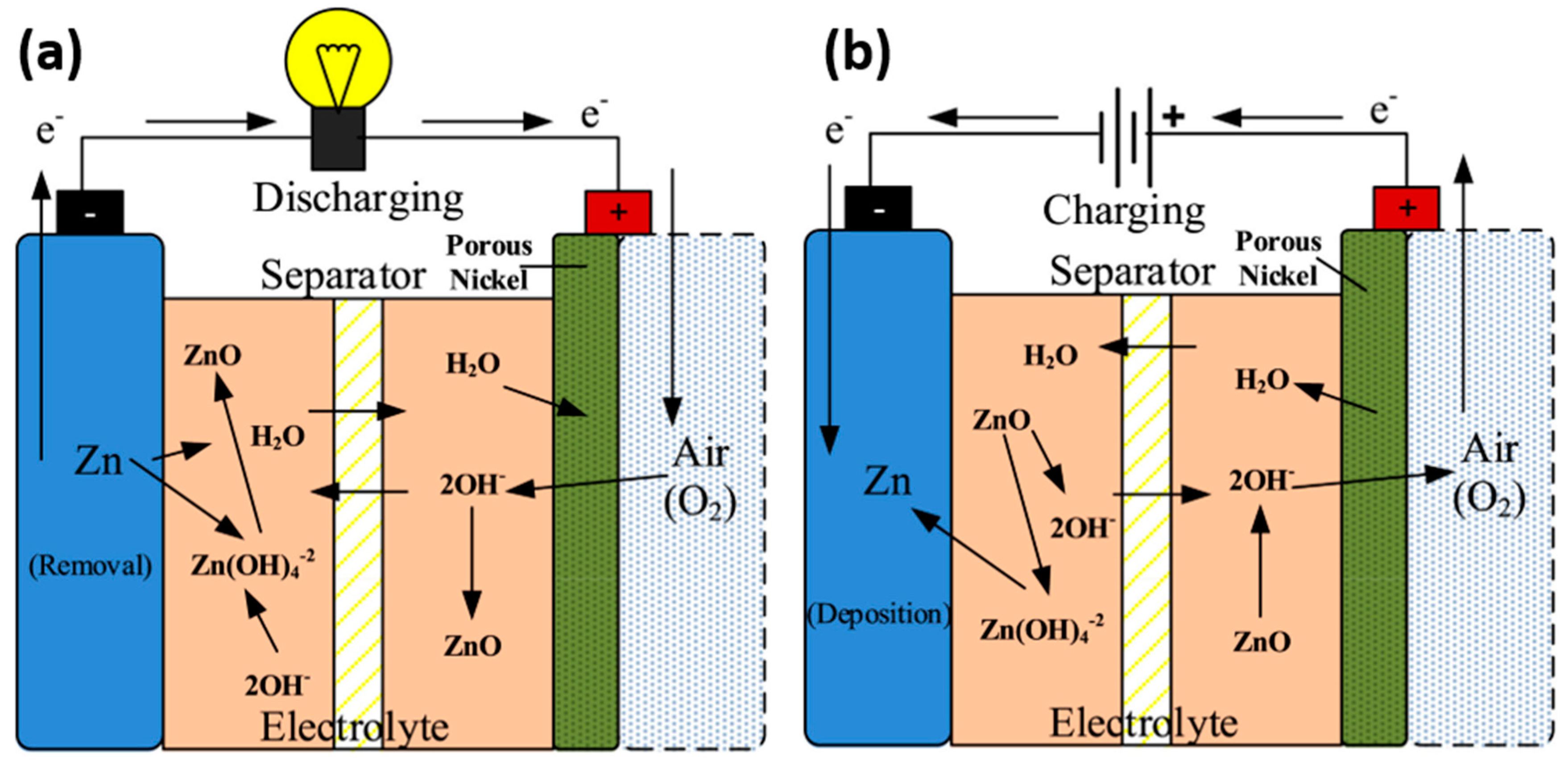
1.1. Zinc–Air Battery
1.2. Cell Configuration of Secondary ZAB (S-ZAB)
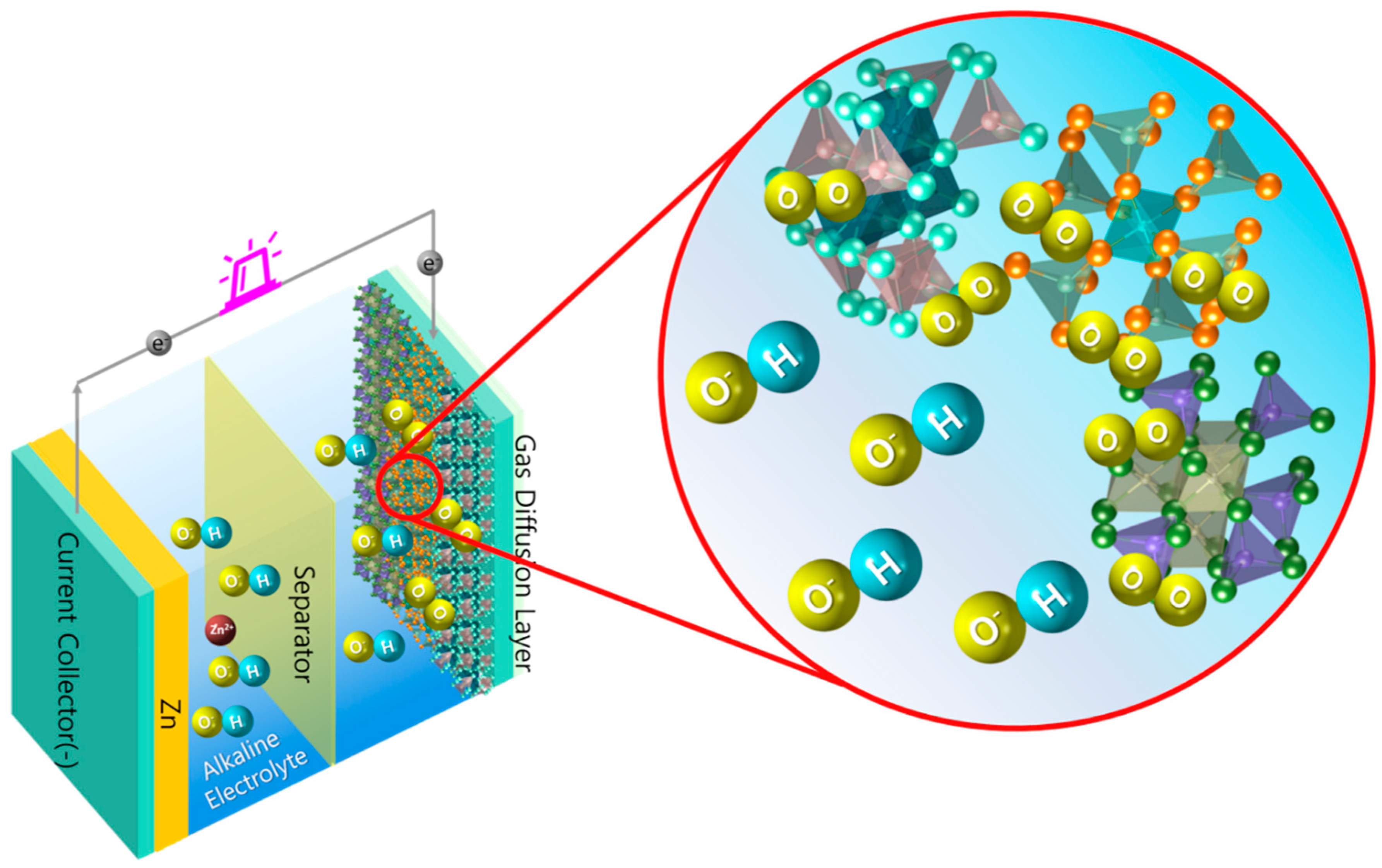
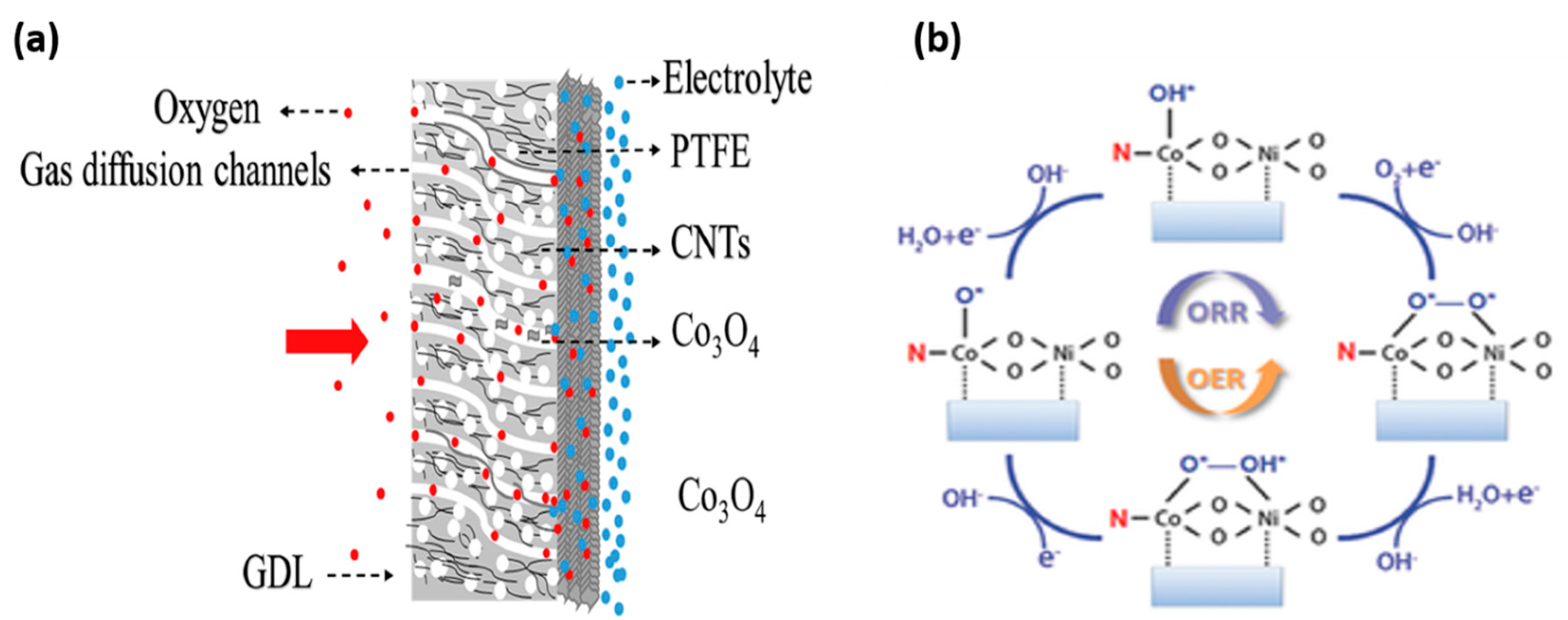
1.3. Cathode Mechanism
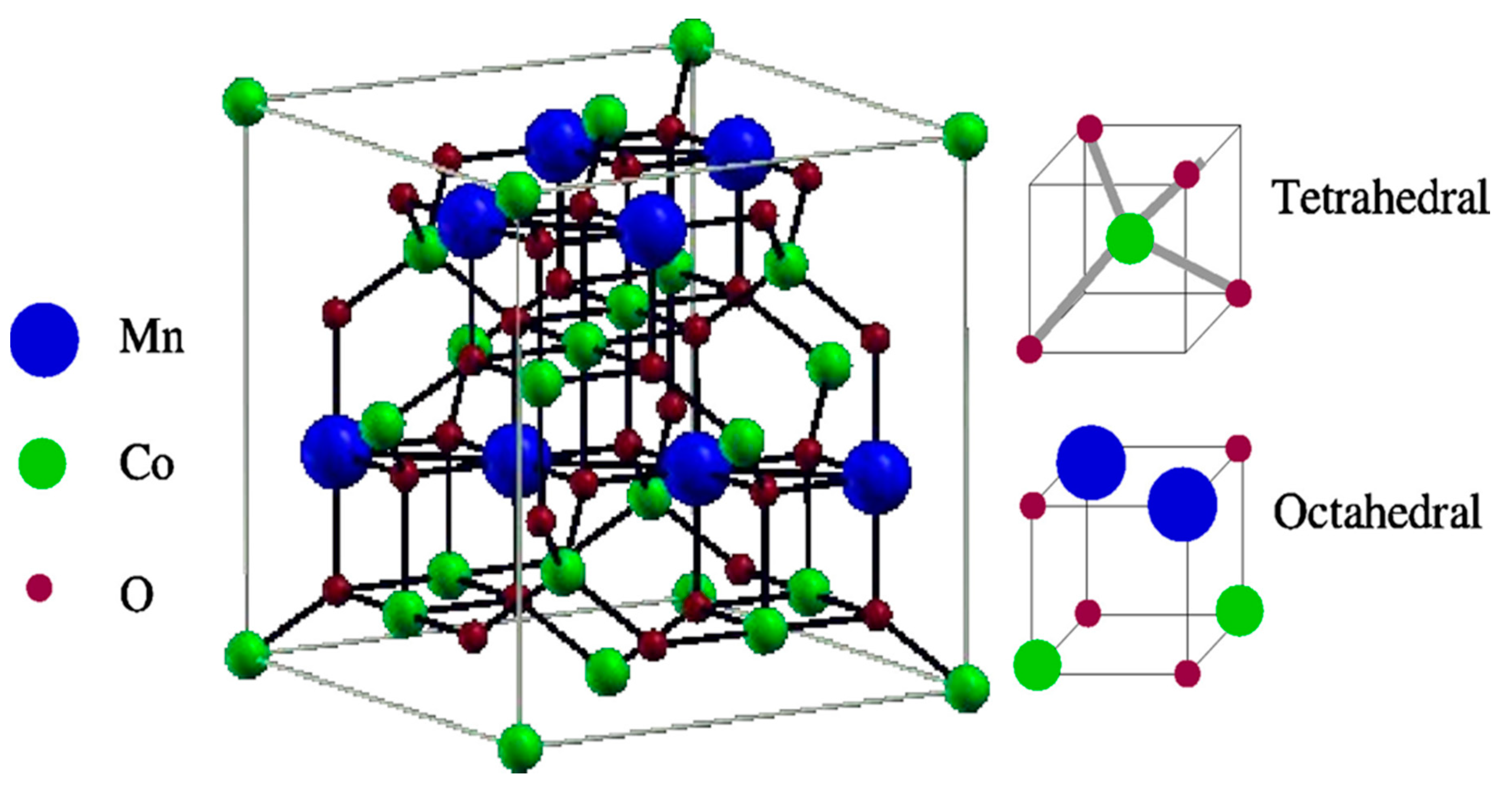
2. Spinels as an Electrocatalyst
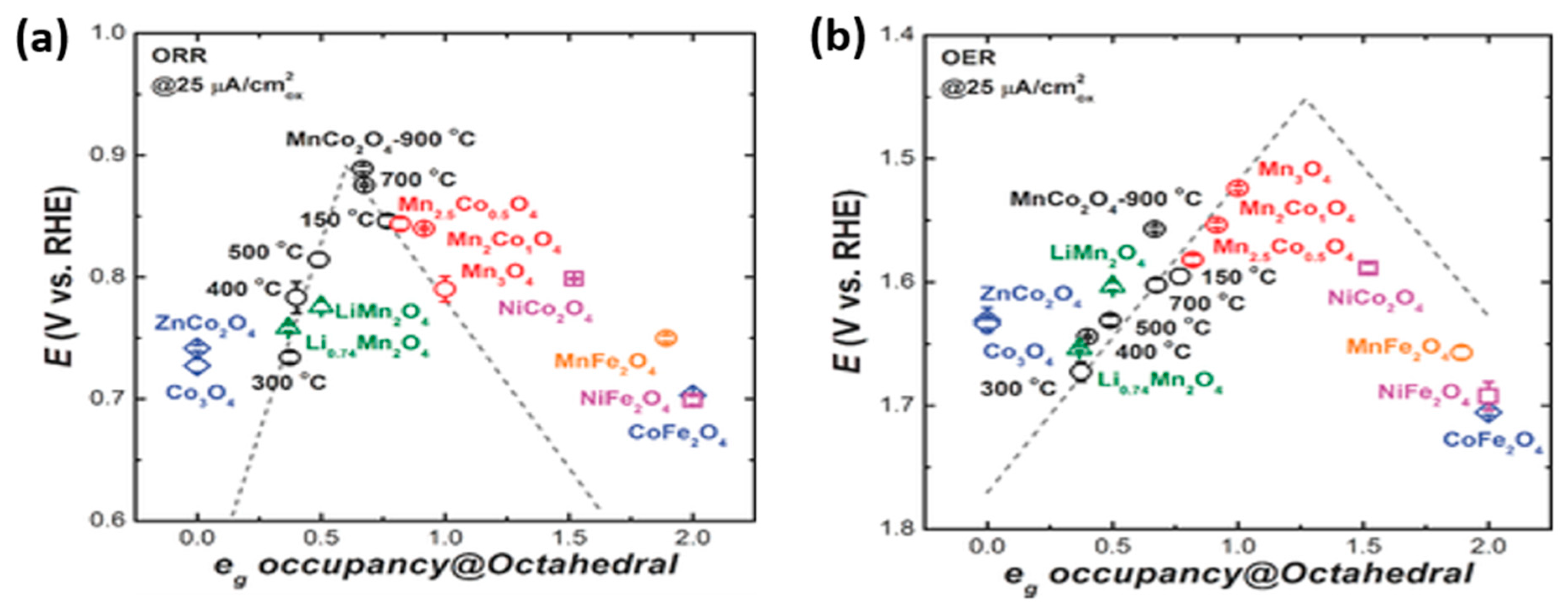
2.1. MnCo2O4 Spinels
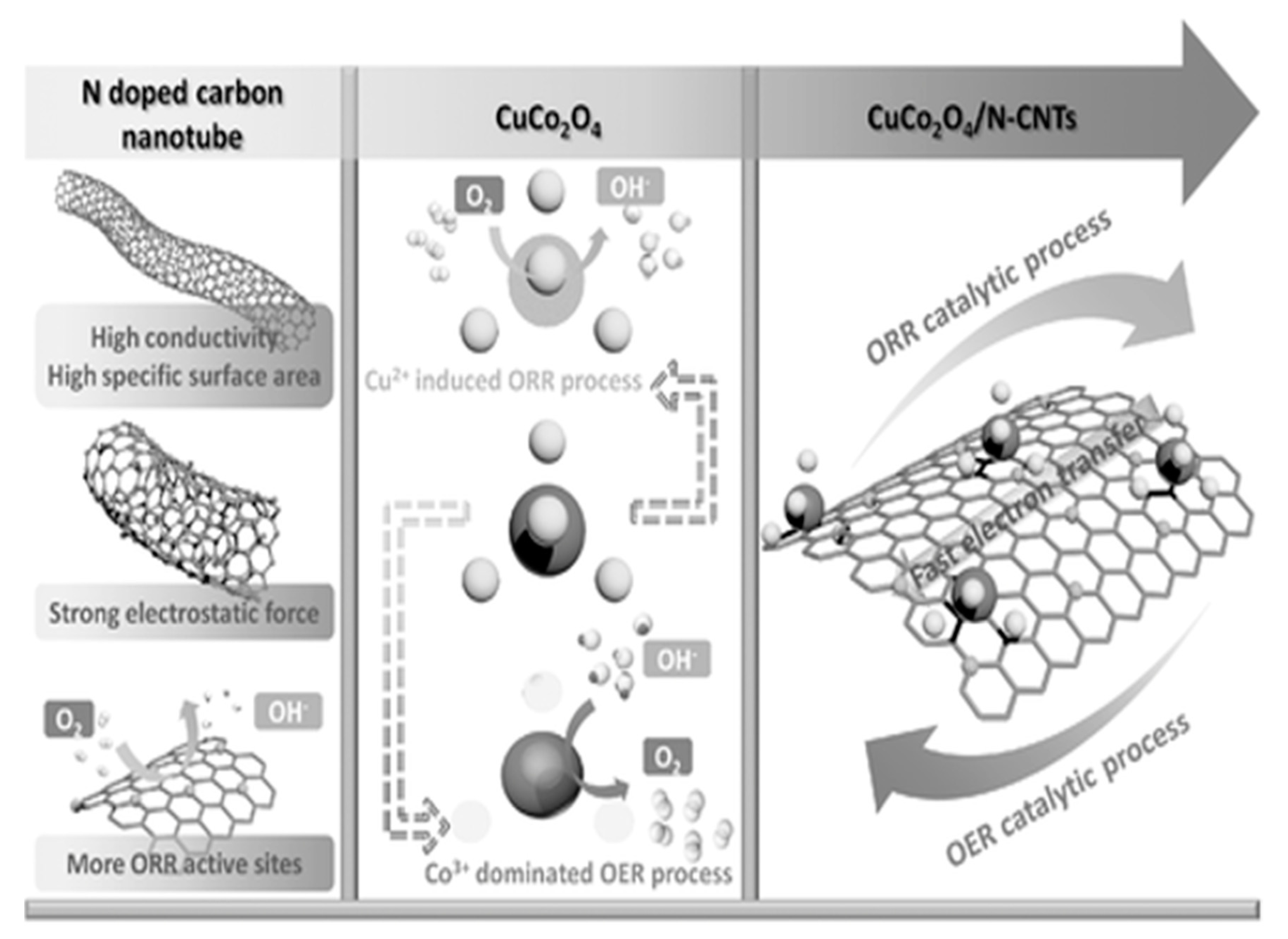
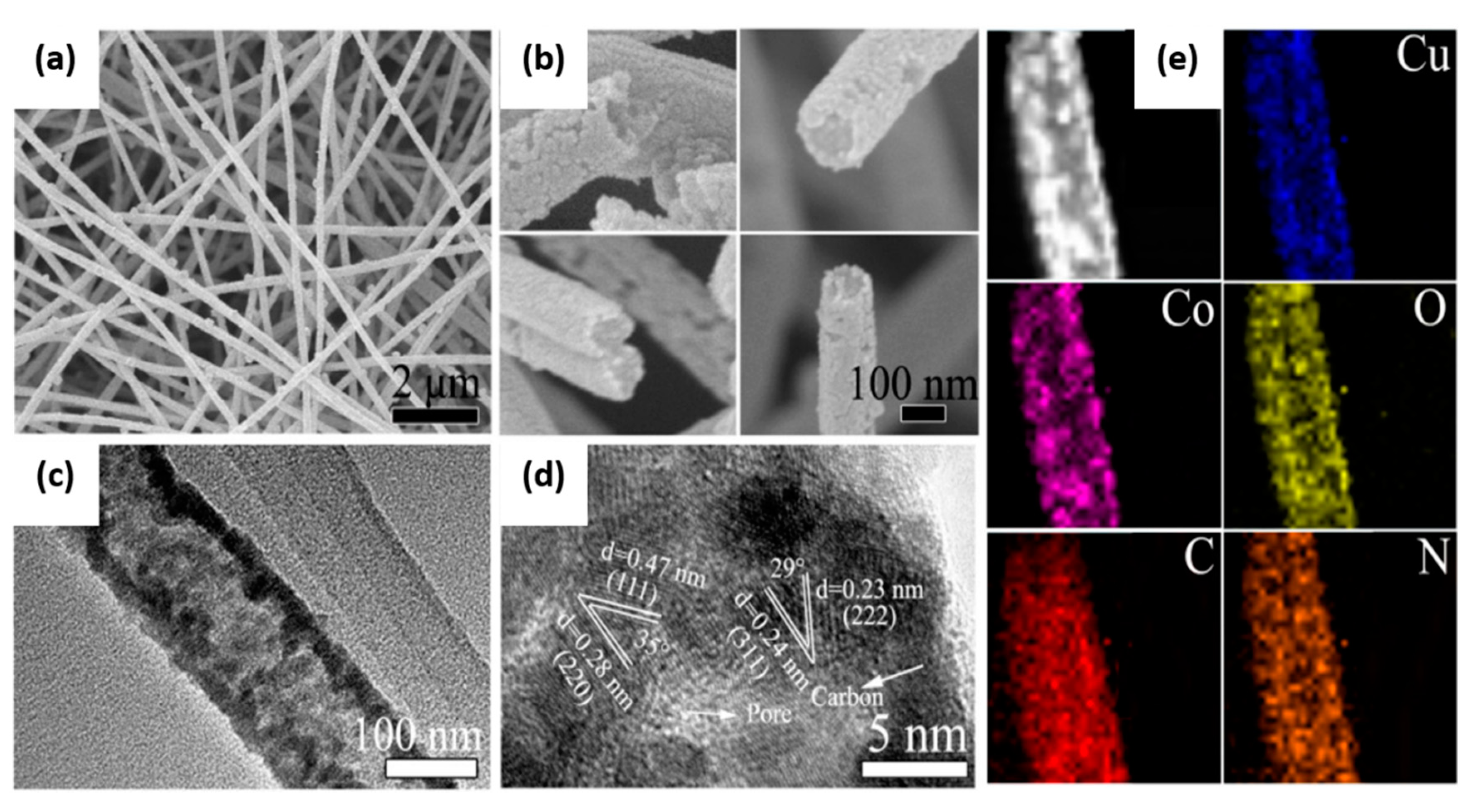
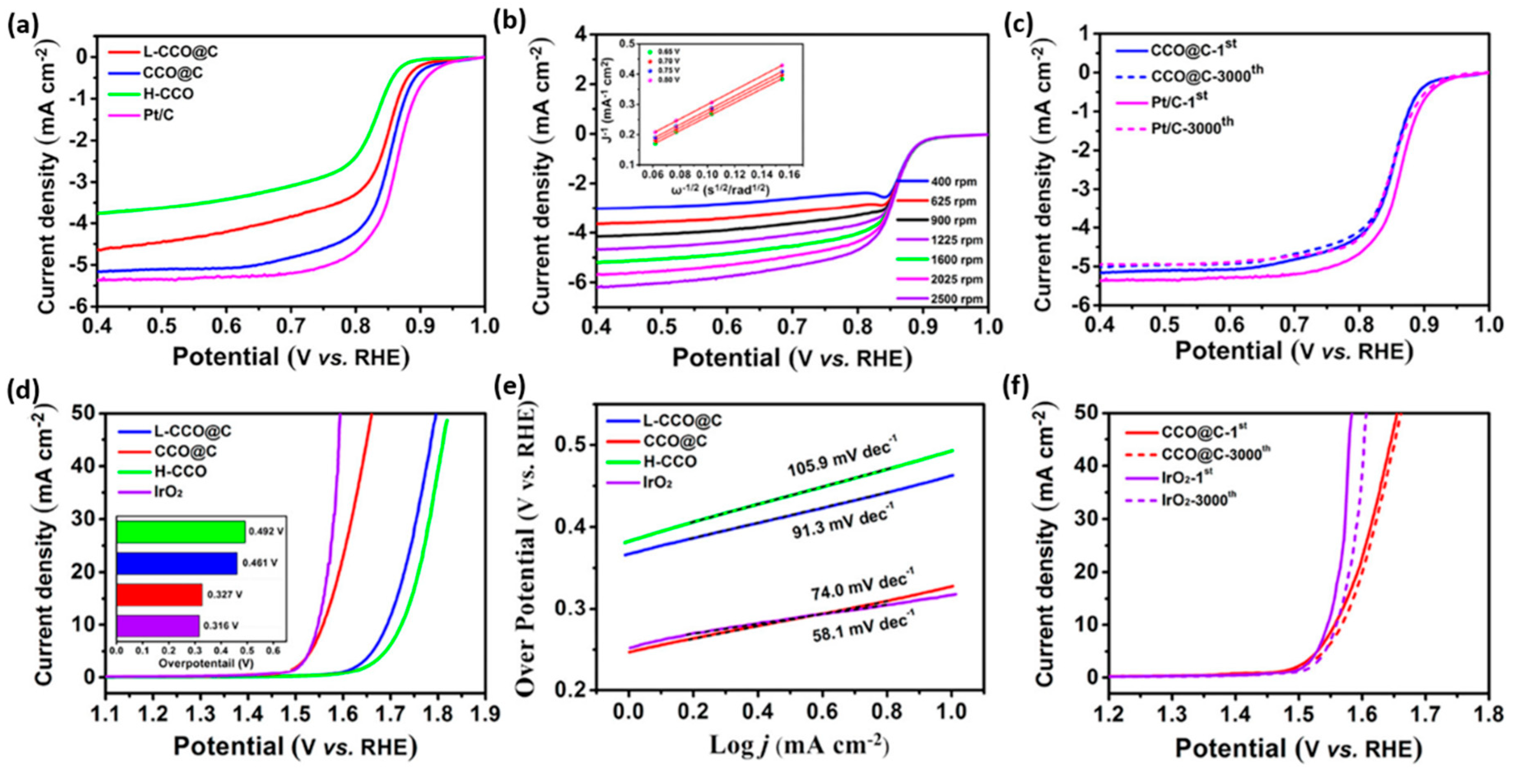
2.2. CuCo2O4 Spinels
2.3. NiCo2O4 Spinels
2.4. Fe-Based Spinels
3. Conclusions and Perspectives
Funding
Acknowledgments
Conflicts of Interest
References
- Pan, J.; Xu, Y.Y.; Yang, H.; Dong, Z.; Liu, H.; Xia, B.Y. Advanced architectures and relatives of air electrodes in zn–air batteries. Adv. Sci. 2018, 5, 1700691. [Google Scholar] [CrossRef]
- Chu, S.; Cui, Y.; Liu, N. The path towards sustainable energy. Nat. Mater. 2016, 16, 16. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, N. Nanostructured electrode materials for high-energy rechargeable li, na and zn batteries. Chem. Mater. 2017, 29, 9589–9604. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, N. Visualizing battery reactions and processes by using in situ and in operando microscopies. Chem 2018, 4, 438–465. [Google Scholar] [CrossRef]
- Qian, Z.; Chen, Y.; Tang, Z.; Liu, Z.; Wang, X.; Tian, Y.; Gao, W. Hollow nanocages of nixco1−xse for efficient zinc–air batteries and overall water splitting. Nano-Micro Lett. 2019, 11, 28. [Google Scholar] [CrossRef]
- Yoo, H.D.; Markevich, E.; Salitra, G.; Sharon, D.; Aurbach, D. On the challenge of developing advanced technologies for electrochemical energy storage and conversion. Mater. Today 2014, 17, 110–121. [Google Scholar] [CrossRef]
- Li, Y.; Dai, H. Recent advances in zinc–air batteries. Chem. Soc. Rev. 2014, 43, 5257–5275. [Google Scholar] [CrossRef]
- Luntz, A.C.; McCloskey, B.D. Nonaqueous li–air batteries: A status report. Chem. Rev. 2014, 114, 11721–11750. [Google Scholar] [CrossRef]
- Wang, Z.-L.; Xu, D.; Xu, J.-J.; Zhang, X.-B. Oxygen electrocatalysts in metal–air batteries: From aqueous to nonaqueous electrolytes. Chem. Soc. Rev. 2014, 43, 7746–7786. [Google Scholar] [CrossRef]
- Harting, K.; Kunz, U.; Turek, T. Zinc-air batteries: Prospects and challenges for future improvement. Z. Phys. Chem. 2012, 226, 151–166. [Google Scholar] [CrossRef]
- Zhang, X.G. Fibrous zinc anodes for high power batteries. J. Power Sources 2006, 163, 591–597. [Google Scholar] [CrossRef]
- Fu, J.; Cano, Z.P.; Park, M.G.; Yu, A.; Fowler, M.; Chen, Z. Electrically rechargeable zinc–air batteries: Progress, challenges, and perspectives. Adv. Mater. 2017, 29, 1604685. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-S.; Tai Kim, S.; Cao, R.; Choi, N.-S.; Liu, M.; Lee, K.T.; Cho, J. Metal–air batteries with high energy density: Li–air versus zn–air. Adv. Energy Mater. 2011, 1, 34–50. [Google Scholar] [CrossRef]
- Kanan, M.W.; Nocera, D.G. In situ formation of an oxygen-evolving catalyst in neutral water containing phosphate and CO2+. Science 2008, 321, 1072. [Google Scholar] [CrossRef] [PubMed]
- Koper, M.T.M. Thermodynamic theory of multi-electron transfer reactions: Implications for electrocatalysis. J. Electroanal. Chem. 2011, 660, 254–260. [Google Scholar] [CrossRef]
- Fang, Z.; Peng, L.; Lv, H.; Zhu, Y.; Yan, C.; Wang, S.; Kalyani, P.; Wu, X.; Yu, G. Metallic transition metal selenide holey nanosheets for efficient oxygen evolution electrocatalysis. ACS Nano 2017, 11, 9550–9557. [Google Scholar] [CrossRef]
- Masa, J.; Xia, W.; Muhler, M.; Schuhmann, W. On the role of metals in nitrogen-doped carbon electrocatalysts for oxygen reduction. Angew. Chem. Int. Ed. 2015, 54, 10102–10120. [Google Scholar] [CrossRef]
- Bera, R.K.; Park, H.; Ryoo, R. Co3o4 nanosheets on zeolite-templated carbon as an efficient oxygen electrocatalyst for a zinc–air battery. J. Mater. Chem. A 2019, 7, 9988–9996. [Google Scholar] [CrossRef]
- Ibraheem, S.; Chen, S.; Li, J.; Wang, Q.; Wei, Z. In situ growth of vertically aligned fecoooh-nanosheets/nanoflowers on fe, n co-doped 3d-porous carbon as efficient bifunctional electrocatalysts for rechargeable zinc–o2 batteries. J. Mater. Chem. A 2019, 7, 9497–9502. [Google Scholar] [CrossRef]
- Zhao, Q.; Yan, Z.; Chen, C.; Chen, J. Spinels: Controlled preparation, oxygen reduction/evolution reaction application, and beyond. Chem. Rev. 2017, 117, 10121–10211. [Google Scholar] [CrossRef]
- Xu, M.; Hou, X.; Yu, X.; Ma, Z.-F.; Yang, J.; Yuan, X. Spinel nico2s4 as excellent bi-functional cathode catalysts for rechargeable li-o2 batteries. J. Electrochem. Soc. 2019, 166, F406–F413. [Google Scholar] [CrossRef]
- Ahuja, B.L.; Dashora, A.; Heda, N.L.; Tiwari, S.; Rajeevan, N.E.; Itou, M.; Sakurai, Y.; Kumar, R. Reversal of orbital magnetic moment on substitution of bi in multiferroic co2mno4: A magnetic compton scattering study. Appl. Phys. Lett. 2010, 97, 212502. [Google Scholar] [CrossRef]
- Grimes, R.W.; Anderson, A.B.; Heuer, A.H. Predictions of cation distributions in ab2o4 spinels from normalized ion energies. J. Am. Chem. Soc. 1989, 111, 1–7. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Loeb, A.L. Theory of ionic ordering, crystal distortion, and magnetic exchange due to covalent forces in spinels. Phys. Rev. 1955, 98, 391–408. [Google Scholar] [CrossRef]
- Kim, S.Y.; Yu, A.; Lee, Y.; Kim, H.Y.; Kim, Y.J.; Lee, N.-S.; Lee, C.; Lee, Y.; Kim, M.H. Single phase of spinel co2rho4 nanotubes with remarkably enhanced catalytic performance for the oxygen evolution reaction. Nanoscale 2019, 11, 9287–9295. [Google Scholar] [CrossRef]
- Liu, J.; Bao, H.; Zhang, B.; Hua, Q.; Shang, M.; Wang, J.; Jiang, L. Geometric occupancy and oxidation state requirements of cations in cobalt oxides for oxygen reduction reaction. ACS Appl. Mater. Interfaces 2019, 11, 12525–12534. [Google Scholar] [CrossRef]
- Davari, E.; Ivey, D.G. Bifunctional electrocatalysts for zn–air batteries. Sustain. Energy Fuels 2018, 2, 39–67. [Google Scholar] [CrossRef]
- Hardwick, L.J.; De León, C.P. Rechargeable multi-valent metal-air batteries. Johns. Matthey Technol. Rev. 2018, 62, 134–149. [Google Scholar] [CrossRef]
- Linden, D.; Reddy, T. Handbook of Batteries; Mcgraw-Hill: New York, NY, USA, 2001. [Google Scholar]
- Fu, G.; Tang, Y.; Lee, J.-M. Recent advances in carbon-based bifunctional oxygen electrocatalysts for zn−air batteries. ChemElectroChem 2018, 5, 1424–1434. [Google Scholar] [CrossRef]
- Ge, J.; Higier, A.; Liu, H. Effect of gas diffusion layer compression on pem fuel cell performance. J. Power Sources 2006, 159, 922–927. [Google Scholar] [CrossRef]
- Su, H.; Xu, Q.; Chong, J.; Li, H.; Sita, C.; Pasupathi, S. Eliminating micro-porous layer from gas diffusion electrode for use in high temperature polymer electrolyte membrane fuel cell. J. Power Sources 2017, 341, 302–308. [Google Scholar] [CrossRef]
- Cheng, F.; Chen, J. Metal–air batteries: From oxygen reduction electrochemistry to cathode catalysts. Chem. Soc. Rev. 2012, 41, 2172–2192. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Wang, X.; Wen, C. High energy density metal-air batteries: A review. J. Electrochem. Soc. 2013, 160, A1759–A1771. [Google Scholar] [CrossRef]
- Fu, J. Material design and engineering for polymer electrolyte membrane zinc-air batteries. UWSpace, 23 March 2018. [Google Scholar]
- Hannan, M.A.; Hoque, M.M.; Mohamed, A.; Ayob, A. Review of energy storage systems for electric vehicle applications: Issues and challenges. Renew. Sustain. Energy Rev. 2017, 69, 771–789. [Google Scholar] [CrossRef]
- Farmer, E.D.; Webb, A.H. Zinc passivation and the effect of mass transfer in flowing electrolyte. J. Appl. Electrochem. 1972, 2, 123–136. [Google Scholar] [CrossRef]
- Pei, P.; Wang, K.; Ma, Z. Technologies for extending zinc–air battery’s cyclelife: A review. Appl. Energy 2014, 128, 315–324. [Google Scholar] [CrossRef]
- Cheng, H.; Li, M.-L.; Su, C.-Y.; Li, N.; Liu, Z.-Q. Cu—Co bimetallic oxide quantum dot decorated nitrogen-doped carbon nanotubes: A high-efficiency bifunctional oxygen electrode for zn–air batteries. Adv. Funct. Mater. 2017, 27, 1701833. [Google Scholar] [CrossRef]
- Lu, Y.-T.; Chien, Y.-J.; Liu, C.-F.; You, T.-H.; Hu, C.-C. Active site-engineered bifunctional electrocatalysts of ternary spinel oxides, m0.1ni0.9co2o4 (m: Mn, fe, cu, zn) for the air electrode of rechargeable zinc–air batteries. J. Mater. Chem. A 2017, 5, 21016–21026. [Google Scholar] [CrossRef]
- Wu, X.; Niu, Y.; Feng, B.; Yu, Y.; Huang, X.; Zhong, C.; Hu, W.; Li, C.M. Mesoporous hollow nitrogen-doped carbon nanospheres with embedded mnfe2o4/fe hybrid nanoparticles as efficient bifunctional oxygen electrocatalysts in alkaline media. ACS Appl. Mater. Interfaces 2018, 10, 20440–20447. [Google Scholar] [CrossRef]
- Fu, G.; Chen, Y.; Cui, Z.; Li, Y.; Zhou, W.; Xin, S.; Tang, Y.; Goodenough, J.B. Novel hydrogel-derived bifunctional oxygen electrocatalyst for rechargeable air cathodes. Nano Lett. 2016, 16, 6516–6522. [Google Scholar] [CrossRef]
- Yin, J.; Li, Y.; Lv, F.; Fan, Q.; Zhao, Y.-Q.; Zhang, Q.; Wang, W.; Cheng, F.; Xi, P.; Guo, S. Nio/con porous nanowires as efficient bifunctional catalysts for zn–air batteries. ACS Nano 2017, 11, 2275–2283. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wan, L.; Lin, Y.; Wang, B. Construction of mass-transfer channel in air electrode with bifunctional catalyst for rechargeable zinc-air battery. Electrochim. Acta 2019, 320, 134564. [Google Scholar] [CrossRef]
- Wang, X.-R.; Liu, J.-Y.; Liu, Z.-W.; Wang, W.-C.; Luo, J.; Han, X.-P.; Du, X.-W.; Qiao, S.-Z.; Yang, J. Identifying the key role of pyridinic-n–co bonding in synergistic electrocatalysis for reversible orr/oer. Adv. Mater. 2018, 30, 1800005. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, T.; Yokoe, K.; Miyano, T.; Kusaba, H. Mesoporous mnco2o4 spinel oxide for a highly active and stable air electrode for zn-air rechargeable battery. Electrochim. Acta 2019, 300, 455–460. [Google Scholar] [CrossRef]
- Li, B.-Q.; Tang, C.; Wang, H.-F.; Zhu, X.-L.; Zhang, Q. An aqueous preoxidation method for monolithic perovskite electrocatalysts with enhanced water oxidation performance. Sci. Adv. 2016, 2, e1600495. [Google Scholar] [CrossRef]
- Miner, E.M.; Fukushima, T.; Sheberla, D.; Sun, L.; Surendranath, Y.; Dincă, M. Electrochemical oxygen reduction catalysed by ni3(hexaiminotriphenylene)2. Nat. Commun. 2016, 7, 10942. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, H.-L.; Guo, S. Towards high-efficiency nanoelectrocatalysts for oxygen reduction through engineering advanced carbon nanomaterials. Chem. Soc. Rev. 2016, 45, 1273–1307. [Google Scholar] [CrossRef]
- Chen, A.; Holt-Hindle, P. Platinum-based nanostructured materials: Synthesis, properties, and applications. Chem. Rev. 2010, 110, 3767–3804. [Google Scholar] [CrossRef]
- Lee, Y.; Suntivich, J.; May, K.J.; Perry, E.E.; Shao-Horn, Y. Synthesis and activities of rutile iro2 and ruo2 nanoparticles for oxygen evolution in acid and alkaline solutions. J. Phys. Chem. Lett. 2012, 3, 399–404. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Choi, B.; Kim, Y.-B. Development of highly active bifunctional electrocatalyst using Co3O4 on carbon nanotubes for oxygen reduction and oxygen evolution. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Iwakura, C.; Honji, A.; Tamura, H. The anodic evolution of oxygen on Co3O4 film electrodes in alkaline solutions. Electrochim. Acta 1981, 26, 1319–1326. [Google Scholar] [CrossRef]
- Shenghai, C.; Liping, S.; Fanhao, K.; Lihua, H.; Hui, Z. Carbon-coated mnco2o4 nanowire as bifunctional oxygen catalysts for rechargeable zn-air batteries. J. Power Sources 2019, 430, 25–31. [Google Scholar] [CrossRef]
- Black, R.; Lee, J.H.; Adams, B.; Mims, C.A.; Nazar, L.F. The role of catalysts and peroxide oxidation in lithium–oxygen batteries. Angew. Chem. Int. Ed. 2013, 52, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Ryu, W.-H.; Yoon, T.-H.; Song, S.H.; Jeon, S.; Park, Y.-J.; Kim, I.-D. Bifunctional composite catalysts using co3o4 nanofibers immobilized on nonoxidized graphene nanoflakes for high-capacity and long-cycle li–o2 batteries. Nano Lett. 2013, 13, 4190–4197. [Google Scholar] [CrossRef]
- Nikolov, I.; Darkaoui, R.; Zhecheva, E.; Stoyanova, R.; Dimitrov, N.; Vitanov, T. Electrocatalytic activity of spinel related cobalties mxco3− xo4 (m = li, ni, cu) in the oxygen evolution reaction. J. Electroanal. Chem. 1997, 429, 157–168. [Google Scholar] [CrossRef]
- Diaz-Morales, O.; Ledezma-Yanez, I.; Koper, M.T.; Calle-Vallejo, F. Guidelines for the rational design of ni-based double hydroxide electrocatalysts for the oxygen evolution reaction. ACS Catal. 2015, 5, 5380–5387. [Google Scholar] [CrossRef]
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.; Nørskov, J.K.; Jaramillo, T.F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, eaad4998. [Google Scholar] [CrossRef]
- Katsounaros, I.; Cherevko, S.; Zeradjanin, A.R.; Mayrhofer, K.J. Oxygen electrochemistry as a cornerstone for sustainable energy conversion. Angew. Chem. Int. Ed. 2014, 53, 102–121. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Y.; Liang, Y.; Zheng, G.; Li, Y.; Cui, Y.; Dai, H. Rechargeable li–O2 batteries with a covalently coupled MnCo2O4–graphene hybrid as an oxygen cathode catalyst. Energy Environ. Sci. 2012, 5, 7931–7935. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, L.; Vellacheri, R.; Lei, Y. Recent advances in designing and fabricating self-supported nanoelectrodes for supercapacitors. Adv. Sci. 2017, 4, 1700188. [Google Scholar] [CrossRef]
- Wei, C.; Feng, Z.; Scherer, G.G.; Barber, J.; Shao-Horn, Y.; Xu, Z.J. Cations in octahedral sites: A descriptor for oxygen electrocatalysis on transition-metal spinels. Adv. Mater. 2017, 29, 1606800. [Google Scholar] [CrossRef] [PubMed]
- Bin, D.; Guo, Z.; Tamirat, A.G.; Ma, Y.; Wang, Y.; Xia, Y. Crab-shell induced synthesis of ordered macroporous carbon nanofiber arrays coupled with mnco2o4 nanoparticles as bifunctional oxygen catalysts for rechargeable zn–air batteries. Nanoscale 2017, 9, 11148–11157. [Google Scholar] [CrossRef] [PubMed]
- Jahan, M.; Liu, Z.; Loh, K.P. A graphene oxide and copper-centered metal organic framework composite as a tri-functional catalyst for her, oer, and orr. Adv. Funct. Mater. 2013, 23, 5363–5372. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Jin, T.; Meng, J.; Jiao, L.; Zhu, M.; Chen, J. Electrospun thin-walled cuco2o4@c nanotubes as bifunctional oxygen electrocatalysts for rechargeable zn–air batteries. Nano Lett. 2017, 17, 7989–7994. [Google Scholar] [CrossRef]
- Chen, B.; Jiang, Z.; Huang, J.; Deng, B.; Zhou, L.; Jiang, Z.-J.; Liu, M. Cation exchange synthesis of ni x co (3− x) o 4 (x= 1.25) nanoparticles on aminated carbon nanotubes with high catalytic bifunctionality for the oxygen reduction/evolution reaction toward efficient zn–air batteries. J. Mater. Chem. A 2018, 6, 9517–9527. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, Y.; Liu, X.; Chen, L.; Jia, J. In situ formed fe–n doped metal organic framework@ carbon nanotubes/graphene hybrids for a rechargeable zn–air battery. Chem. Commun. 2017, 53, 12934–12937. [Google Scholar] [CrossRef]
- Ma, N.; Jia, Y.A.; Yang, X.; She, X.; Zhang, L.; Peng, Z.; Yao, X.; Yang, D. Seaweed biomass derived (ni, co)/cnt nanoaerogels: Efficient bifunctional electrocatalysts for oxygen evolution and reduction reactions. J. Mater. Chem. A 2016, 4, 6376–6384. [Google Scholar] [CrossRef]
- Zhao, J.; He, Y.; Chen, Z.; Zheng, X.; Han, X.; Rao, D.; Zhong, C.; Hu, W.; Deng, Y. Engineering the surface metal active sites of nickel cobalt oxide nanoplates toward enhanced oxygen electrocatalysis for zn–air battery. ACS Appl. Mater. Interfaces 2019, 11, 4915–4921. [Google Scholar] [CrossRef]
- Singh, N.; Tiwari, S.; Anitha, K.; Singh, R. Electrocatalytic properties of spinel-type mnxfe3–x o4synthesized below 100° c for oxygen evolution in koh solutions. J. Chem. Soc. Faraday Trans. 1996, 92, 2397–2400. [Google Scholar] [CrossRef]
- Liu, W.; Bao, J.; Xu, L.; Guan, M.; Wang, Z.; Qiu, J.; Huang, Y.; Xia, J.; Lei, Y.; Li, H. Nico2o4 ultrathin nanosheets with oxygen vacancies as bifunctional electrocatalysts for zn-air battery. Appl. Surf. Sci. 2019, 478, 552–559. [Google Scholar] [CrossRef]
- An, T.; Ge, X.; Hor, T.S.A.; Goh, F.W.T.; Geng, D.; Du, G.; Zhan, Y.; Liu, Z.; Zong, Y. Co3o4 nanoparticles grown on n-doped vulcan carbon as a scalable bifunctional electrocatalyst for rechargeable zinc–air batteries. RSC Adv. 2015, 5, 75773–75780. [Google Scholar] [CrossRef]
- Qin, Q.; Chen, L.; Wei, T.; Wang, Y.; Liu, X. Ni/nim2o4 (m = mn or fe) supported on n-doped carbon nanotubes as trifunctional electrocatalysts for orr, oer and her. Catal. Sci. Technol. 2019, 9, 1595–1601. [Google Scholar] [CrossRef]
- Tong, X.; Chen, S.; Guo, C.; Xia, X.; Guo, X.-Y. Mesoporous nico2o4 nanoplates on three-dimensional graphene foam as an efficient electrocatalyst for the oxygen reduction reaction. ACS Appl. Mater. Interfaces 2016, 8, 28274–28282. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Wu, X.; Zhong, C.; Deng, Y.; Zhao, N.; Hu, W. Nico2s4 nanocrystals anchored on nitrogen-doped carbon nanotubes as a highly efficient bifunctional electrocatalyst for rechargeable zinc-air batteries. Nano Energy 2017, 31, 541–550. [Google Scholar] [CrossRef]
- Hyun, S.; Shanmugam, S. Hierarchical nickel–cobalt dichalcogenide nanostructure as an efficient electrocatalyst for oxygen evolution reaction and a zn–air battery. ACS Omega 2018, 3, 8621–8630. [Google Scholar] [CrossRef]
- Li, C.; Han, X.; Cheng, F.; Hu, Y.; Chen, C.; Chen, J. Phase and composition controllable synthesis of cobalt manganese spinel nanoparticles towards efficient oxygen electrocatalysis. Nat. Commun. 2015, 6, 7345. [Google Scholar] [CrossRef]
- Ma, T.; Li, C.; Chen, X.; Cheng, F.; Chen, J. Spinel cobalt–manganese oxide supported on non-oxidized carbon nanotubes as a highly efficient oxygen reduction/evolution electrocatalyst. Inorg. Chem. Front. 2017, 4, 1628–1633. [Google Scholar] [CrossRef]
- Naik, K.M.; Sampath, S. Two-step oxygen reduction on spinel nife2o4 catalyst: Rechargeable, aqueous solution-and gel-based, zn-air batteries. Electrochim. Acta 2018, 292, 268–275. [Google Scholar] [CrossRef]
- Prabu, M.; Ketpang, K.; Shanmugam, S. Hierarchical nanostructured nico 2 o 4 as an efficient bifunctional non-precious metal catalyst for rechargeable zinc–air batteries. Nanoscale 2014, 6, 3173–3181. [Google Scholar] [CrossRef]
- Prabu, M.; Ramakrishnan, P.; Shanmugam, S. Comn2o4 nanoparticles anchored on nitrogen-doped graphene nanosheets as bifunctional electrocatalyst for rechargeable zinc–air battery. Electrochem. Commun. 2014, 41, 59–63. [Google Scholar] [CrossRef]
- Park, M.G.; Lee, D.U.; Seo, M.H.; Cano, Z.P.; Chen, Z. 3d ordered mesoporous bifunctional oxygen catalyst for electrically rechargeable zinc–air batteries. Small 2016, 12, 2707–2714. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-Q.; Li, M.; Hua, B.; Wang, Y.; Sun, Y.-F.; Luo, J.-L. A strongly cooperative spinel nanohybrid as an efficient bifunctional oxygen electrocatalyst for oxygen reduction reaction and oxygen evolution reaction. Appl. Catal. B Environ. 2018, 236, 413–419. [Google Scholar] [CrossRef]
- Davari, E.; Johnson, A.D.; Mittal, A.; Xiong, M.; Ivey, D.G. Manganese-cobalt mixed oxide film as a bifunctional catalyst for rechargeable zinc-air batteries. Electrochim. Acta 2016, 211, 735–743. [Google Scholar] [CrossRef]
- Wang, Q.; Xue, Y.; Sun, S.; Yan, S.; Miao, H.; Liu, Z. Facile synthesis of ternary spinel co–mn–ni nanorods as efficient bi-functional oxygen catalysts for rechargeable zinc-air batteries. J. Power Sources 2019, 435, 226761. [Google Scholar] [CrossRef]
- Li, G.; Wang, X.; Fu, J.; Li, J.; Park, M.G.; Zhang, Y.; Lui, G.; Chen, Z. Pomegranate-inspired design of highly active and durable bifunctional electrocatalysts for rechargeable metal–air batteries. Angew. Chem. Int. Ed. 2016, 55, 4977–4982. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Liu, Y.; Zhang, X.; Li, X.; Li, A.; Qiao, J.; Zhang, J. Self-assembly formation of bi-functional co3o4/mno2-cnts hybrid catalysts for achieving both high energy/power density and cyclic ability of rechargeable zinc-air battery. Sci. Rep. 2016, 6, 33590. [Google Scholar] [CrossRef]
- Wei, J.; Zhao, S.; Ji, L.; Zhou, T.; Miao, Y.; Scott, K.; Li, D.; Yang, J.; Wu, X. Reuse of ni-co-mn oxides from spent li-ion batteries to prepare bifunctional air electrodes. Resour. Conserv. Recycl. 2018, 129, 135–142. [Google Scholar] [CrossRef]
- Wang, X.T.; Ouyang, T.; Wang, L.; Zhong, J.H.; Ma, T.; Liu, Z.Q. Redox-inert fe3+ ions in octahedral sites of co-fe spinel oxides with enhanced oxygen catalytic activity for rechargeable zinc–air batteries. Angew. Chem. 2019, 131, 13425–13430. [Google Scholar] [CrossRef]
- Wang, W.; Kuai, L.; Cao, W.; Huttula, M.; Ollikkala, S.; Ahopelto, T.; Honkanen, A.P.; Huotari, S.; Yu, M.; Geng, B. Mass-production of mesoporous mnco2o4 spinels with manganese (iv)-and cobalt (ii)-rich surfaces for superior bifunctional oxygen electrocatalysis. Angew. Chem. Int. Ed. 2017, 56, 14977–14981. [Google Scholar] [CrossRef]
- Xu, N.; Qiao, J.; Nie, Q.; Wang, M.; Xu, H.; Wang, Y.; Zhou, X.-D. Cofe2o4 nanoparticles decorated carbon nanotubes: Air-cathode bifunctional catalysts for rechargeable zinc-air batteries. Catal. Today 2018, 318, 144–149. [Google Scholar] [CrossRef]

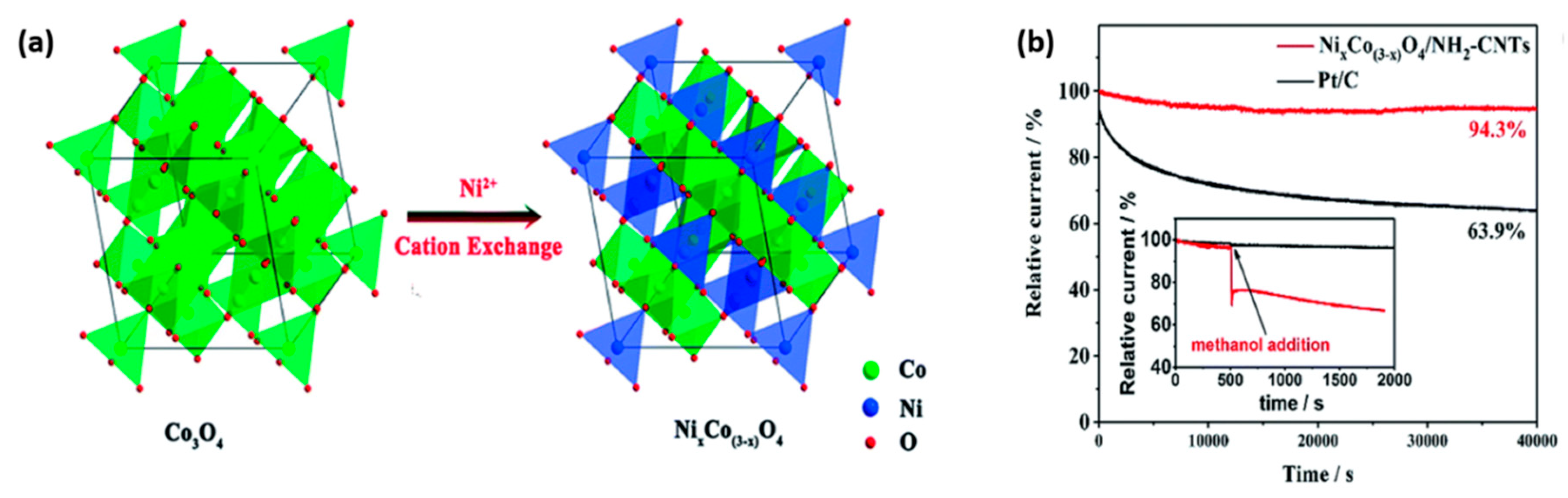
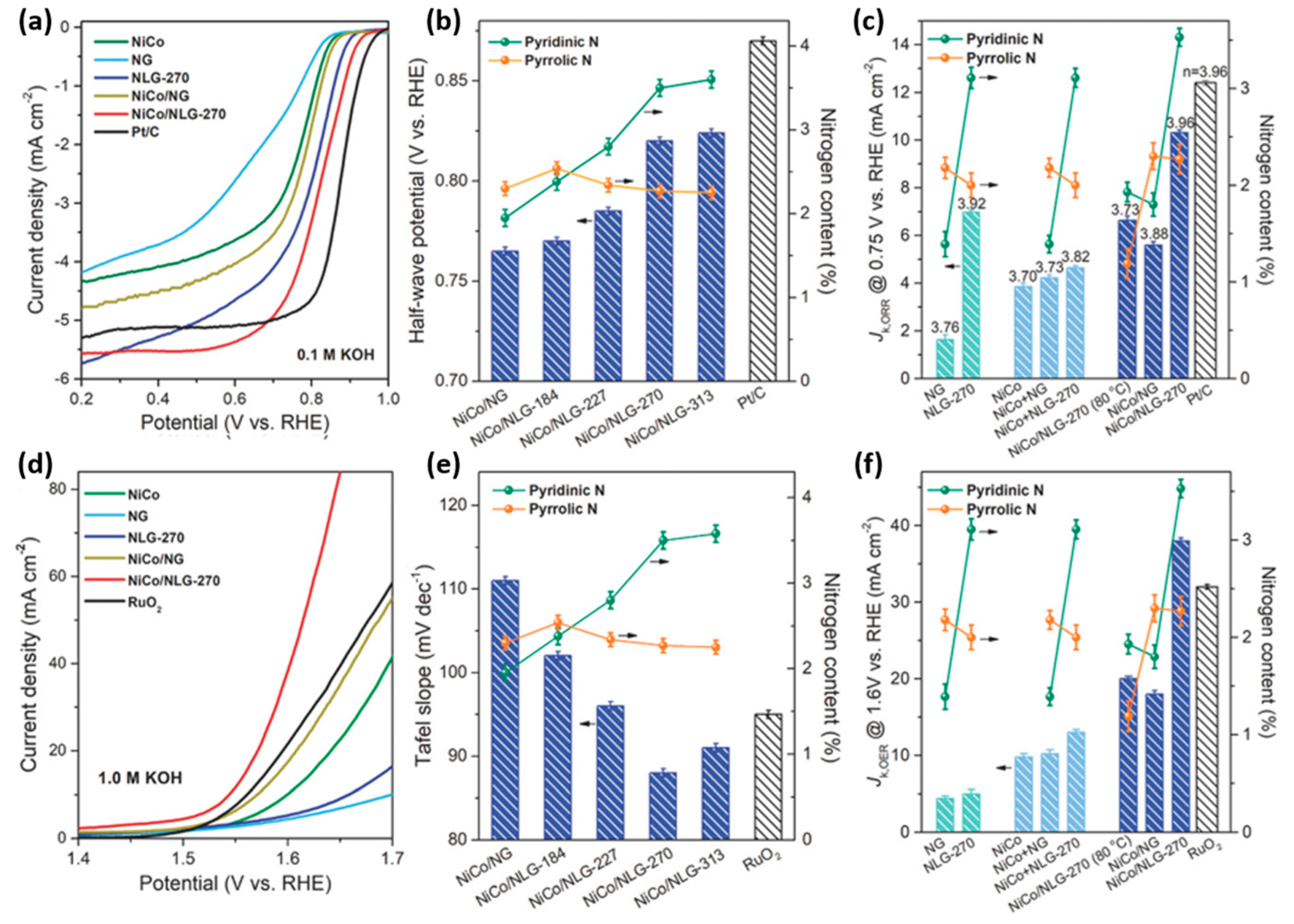
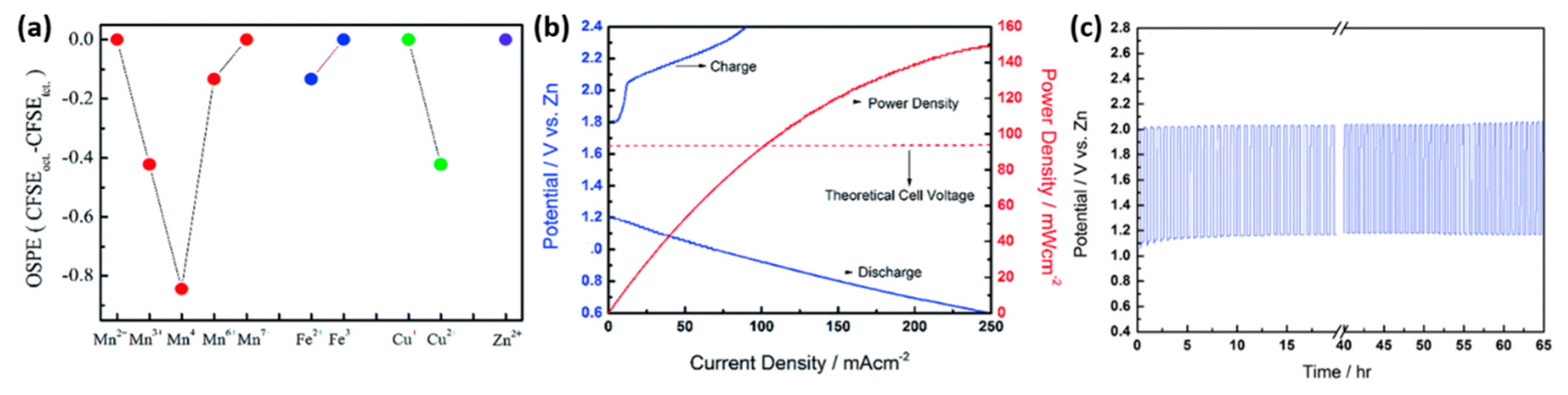

| Spinel | Feature | ORR | OER | ZAB | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Electrolyte (OER &ORR) | E onset [V vs. RHE] | E half [V vs. RHE] | JL [mA cm−2] | Electron Number | ᶯOER (mV) | OER Tafel [mV dec−1] | Electrolyte (Battery) | ΔE (V) | Power Density [mW cm−2] | Cycles | Ref no | ||
| NixCo(3−x)O4 | NixCo(3−x)O4/NH2-CNTs | 0.1 M KOH | 0.954 | 0.863 | 5.5 | >3.9 | 386 | 105.2 | 6 M KOH | 0.764 | 168 | over 100 | [67] |
| NiCo2O4 | NiCo2O4 nanosheets | 0.1 M KOH | 0.85 | 0.74 | 5.2 | 3.94 | 340 | 75 | Mixture of 6 M KOH and 0.2 M Zn(OAc)2 | 0.93 | 102.08 | 1000 | [72] |
| CO3O4 | Co3O4/N-doped Vulcan carbon (NVC) | 0.1 M KOH | 0.92 * | 0.82 * | 5.2 | 4 | - | 47 | 6 M KOH + 20.0 g L−1 ZnCl2 | 0.1 | 330 | - | [73] |
| MnCo2O4 | Mesoporous MnCo2O4 | 4 M KOH | - | - | - | - | 625 | - | 4 M KOH + Zn plate | - | - | - | [46] |
| MnCo2O4 | Carbon-coated MnCo2O4 nanowire | 0.1 M KOH | 0.92 | 0.80 | 5.45 | 3.61 | 430 | - | 6 M KOH containing 0.2 M Zn(OAc)2 | 0.89 | - | - | [54] |
| NiM2O4 (M = Mn or Fe) | N-Doped Carbon Nanotube (NCNT)/Ni- NiMn2O4 | 1 M KOH | - | 0.71 | - | - | 300 | 89 | 6 M KOH | - | 105 | - | [74] |
| CuCo2O4 | CuCo2O4/Nitrogen-Doped Carbon Nanotubes (N-CNTs) | 0.1 M KOH | 0.61 | 0.84 * | 5.05 | >3.7 | 690 | 118.80 | 6 M KOH with 0.2 M Zn(OAc)2 | 0.90 | 83.83 | - | [39] |
| MnCo2O4 | MnCo2O4/Nitrogen-Doped Microporous Carbon Nanofiber Arrays (NMCNA) | 1 M KOH | 0.90 | 0.76 | 4.62 | 3.9 | - | - | 6 M KOH + 0.2 M ZnCl2 | 0.55 | - | - | [64] |
| NiCo2O4 | NiCo2O4 nanoplate arrays/3D-graphene foam | 0.1 M KOH | - | 0.86 | 6.25 | 4 | - | - | - | - | - | 1000 | [75] |
| NiCo2S4 | NiCo2S4/Nitrogen-Doped Carbon Nanotubes (N-CNTs) | 0.1 M KOH | 0.93 | 0.80 | 3.8 | 3.82 | - | - | 6 M KOH with 0.2 M ZnCl2 | 0.80 | 147 | 150 | [76] |
| NiCo2S4 | NiCo2S4 Nanosheet arrays grown on Carbon Cloth (NiCo2S4 NS/CC) | 1 M KOH | - | - | - | - | 260 | 72 | 6 M KOH with 0.2 M Zn(OAc)2 | - | - | - | [77] |
| CoxMn3−xO4 | Both cubic (c)-CoMn2/C | 0.1 M KOH | 0.91 | 0.73 | 4.4 | 3.83 | - | - | 6 M KOH | - | - | - | [78] |
| M0.1Ni0.9Co2O4 | Fe0.1Ni0.9Co2O4 | 0.1 M KOH | - | - | 2.25 | 3.65 | - | 72 | 6 M KOH | - | 150 | 100 | [40] |
| Co-Mn-O | CMO/nonoxidized Carbon Nanotubes (CNTs) | 0.1 M KOH | 0.97 | 0.85 | 0.8 | 3.7 | 1500 | 81.1 | 6 M KOH with 0.2M ZnO | - | - | - | [79] |
| NiFe2O4 | NiFe2O4 | 0.1 M KOH | - | - | - | 3.9 | 620 | 60 | 6 M KOH with 0.2 M ZnCl2 | - | 211 | 100 | [80] |
| NiCo2O4 | 1D- NiCo2O4 | 0.1 M KOH | 0.93 | 0.78 | 4.4 | 4 | 1620 | 87 | 6 M KOH | 0.92 | - | - | [81] |
| CoMn2O4 | CoMn2O4/Nitrogen-reduced Graphene Oxide (N-rGO) | 0.1 M KOH | 0.90 | 0.80 | 9.2 | 4 | 1660 | - | 6 M KOH | 0.86 | - | 100 | [82] |
| Co3O4 | 3D Ordered mesoporous Co3O4 (3DOM Co3O4) | 0.1 M KOH | 0.85 * | 0.69 * | 4.71 | 3.9 | - | 58 | 6 M KOH | 0.76 | 75 | 200 | [83] |
| CuCo2O4 | Mesoporous CuCo2O4@C with abundant nitrogen-doped nanotube | 0.1 M KOH | 0.951 | 0.85 | 5.17 | 3.9 | 327 | 74.0 | 6 M KOH with 0.2 M ZnO | 0.707 | - | - | [66] |
| NiCo2O4 | NiCo2O4/N-graphene hybrids relative concentration of pyridinic and pyrrolic N (NiCo/NLG-270) | 0.1 M KOH | 0.96 | 0.82 | 5.55 | 3.96 | 1570 | 88 | 6 M KOH with 0.2 M ZnCl2 | 0.75 | 103 | 40 | [45] |
| MnFe2O4 | MnFe2O4/metallic Fe hybrid nanoparticles | 0.1 M KOH | - | - | 6.0 | 3.9 | 360 | 60 | 6 M KOH and 0.2 M Zn(OAc)2 | - | 37 | - | [41] |
| MnFe2O4 | MnFe2O4/NiCo2O4 hybrid | 1 M KOH | 0.881 | 0.767 | 5.01 | 4.0 | 344 | 46.7 | 6 M KOH + 0.2 M Zn(OAc)2 | 0.807 | - | 100 | [84] |
| NiCo2O4 | Shape-control of hexagon NiCo2O4 nanosheets | 1 M KOH | 0.95 | 0.75 | 5.31 | 3.98 | 320 | 84 | 6 M KOH with 0.2 M ZnCl2 | 0.8 | 166 | [70] | |
| Mn-Co oxide | Mn/Co:2.5-Carbon Black (CB) | 0.1 M KOH | 1.02 * | - | - | 3.34 | - | 43.7 | 6 M KOH with 2% ZnO | - | - | - | [85] |
| Co-Mn-Ni ternary oxide | CMN-231 | 0.1 M KOH | 0.975 | 0.749 | 6.1 | 3.9 | 410 | 120 | 6 M KOH + 0.2 M Zn(OAc)2 | 0.88 | 154 | 100 | [86] |
| Co3O4 | Co3O4-coated N and B doped graphene hollow spheres | 0.1 M KOH | - | 0.862 | - | 4 | 450 | 63 | - | - | - | 330 | [87] |
| Co3O4 | Co3O4/MnO2-Carbon Nanotube (CNT) | 0.1 M KOH | 0.95 | 0.94 | 3.8 | - | - | 61.5 | 6 M KOH | 0.7 | 313 | - | [88] |
| Ni-Co-Mn oxide | NCM heat-treated at 600 °C for 300 min | 1 M KOH | - | - | 1.23 | 3.6 | 250 | - | 6 M KOH + 0.2 M Zn(OAc)2 | - | - | - | [89] |
| Co2FeO4 | Co2FeO4/N-Doped Carbon Nanotube (NCNTs) | 0.1 M KOH | 0.92 | 0.8 | 5.23 | 3.7 | 1660 | 113.6 | 6 M KOH + 0.2 M Zn(OAc)2 | 0.86 | 90.68 | - | [90] |
| MnCo2O4 | Mesoporous MnCo2O4 | 0.1 M KOH | 0.95 | - | 5.4 | 3.94 | 1630 | 90 | 6 M KOH + 0.2M Zn(OAc)2 | 0.83 | - | 180 | [91] |
| Pt/C | Pt/C (20 wt%) | 0.1 M KOH | 0.96 | 0.858 | 4.8 | - | - | - | 6 M KOH | - | 135 | - | [67] |
| IrO2 | IrO2 | 0.1 M KOH | 0.74 | 0.58 | 3 | 3.07 | 500 | 67 | 6 M KOH | 1.56 | 89 | - | [92] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janani, G.; Chae, Y.; Surendran, S.; Sim, Y.; Park, W.; Kim, J.K.; Sim, U. Rational Design of Spinel Oxide Nanocomposites with Tailored Electrochemical Oxygen Evolution and Reduction Reactions for ZincAir Batteries. Appl. Sci. 2020, 10, 3165. https://doi.org/10.3390/app10093165
Janani G, Chae Y, Surendran S, Sim Y, Park W, Kim JK, Sim U. Rational Design of Spinel Oxide Nanocomposites with Tailored Electrochemical Oxygen Evolution and Reduction Reactions for ZincAir Batteries. Applied Sciences. 2020; 10(9):3165. https://doi.org/10.3390/app10093165
Chicago/Turabian StyleJanani, Gnanaprakasam, Yujin Chae, Subramani Surendran, Yelyn Sim, Woosung Park, Jung Kyu Kim, and Uk Sim. 2020. "Rational Design of Spinel Oxide Nanocomposites with Tailored Electrochemical Oxygen Evolution and Reduction Reactions for ZincAir Batteries" Applied Sciences 10, no. 9: 3165. https://doi.org/10.3390/app10093165
APA StyleJanani, G., Chae, Y., Surendran, S., Sim, Y., Park, W., Kim, J. K., & Sim, U. (2020). Rational Design of Spinel Oxide Nanocomposites with Tailored Electrochemical Oxygen Evolution and Reduction Reactions for ZincAir Batteries. Applied Sciences, 10(9), 3165. https://doi.org/10.3390/app10093165










