1. Introduction
In recent years, novel magnetic resonance imaging (MRI) techniques using hyperpolarized (HP) gases as imaging agents for in-vivo imaging of human lungs have been developed [
1]. They successfully complement the standard proton imaging and computed tomography (CT) in providing high resolution structural information, on which very sensitive diagnostic methods for lung diseases such as asthma, COPD (chronic obstructive pulmonary disease), and cystic fibrosis are based [
2]. Moreover, dynamic ventilation studies that are impractical using other techniques can be performed [
3].
Contrary to the standard MRI that utilizes the thermal polarization of water protons in high magnetic field, the hyperpolarization of noble gases is obtained by optical methods. The techniques are described in detail both for
3He [
4] and
129Xe [
5]. There are several specific requirements for their application in the MR imaging that take into account the relaxation of unrecoverable polarization during storage and delivery [
6]. A careful choice of construction materials for the gas container with all connecting tubes is necessary to minimize relaxation losses. Moreover, the magnetic field strength and homogeneity along the transferring route from the gas container to the patient has to be optimized. Additionally, due to recent dramatic rise of
3He cost, it is important to collect the exhaled gas for recycling [
7].
For the method to be reliable and reproducible, it is necessary to employ a dedicated system for monitoring and controlling the patient’s breath cycle and dosing hyperpolarized 3He. It should be compatible with the MRI scanner, which utilizes a high field magnet that is located in the Faraday cage to eliminate any RF interference. Therefore, the components of the ventilator that must be inside the Faraday cage have to be made of non-magnetic material and should not be controlled by the electrical AC power. Additionally, the system should provide a triggering circuitry to synchronize its operation with the image acquisition sequence.
The first MRI compatible ventilators were developed for animal studies applications [
8,
9]. Since the cooperation of an animal is impossible, the ventilators were equipped with the gaseous anesthesia unit, as well as the breath and heart rate measuring devices for triggering the MR imaging sequence [
10]. In the case of human lung studies, full cooperation of the patient with the medical scanner operator is usually feasible, so that a fast MRI sequence can be applied during the breath hold [
11]. However, in the case of children, critical care patients, or those requiring permanent mechanical ventilation, the breath control and the sequence triggering may be still necessary to obtain good image quality. For such applications, the standard ventilators were modified by simply extending the gas delivery tubes that connect the main unit located outside the Faraday cage to the patient [
12]. More advanced devices were also built, in which only non-magnetic components were used [
13,
14]. However, all above systems could not handle the hyperpolarized gases.
The first in-vivo MR images of the rat lungs were obtained in 1994 [
15], using hyperpolarized
129Xe as a gaseous imaging agent inhaled by the animal. Subsequently, in-vivo MR images of human lungs were acquired in 1996, with the application of hyperpolarized
3He [
16,
17]. These results opened new possibilities for the application of the technique in medical diagnostics, and stimulated the development of dedicated ventilators with the noble gas administration capability. A detailed description of a device modified for small animal studies was given in [
18,
19,
20]. It was followed by a fully scalable system that could be used for humans [
21], and a sophisticated ventilation system dedicated to MRI of human lungs with the use hyperpolarized noble gases [
22].
This work describes a novel, reliable system for precise human breath monitoring and control, including the noble gas administration and initial lung flushing by nitrogen capabilities. The exhaled gas is collected for recycling. The performance of the ventilator has been assessed by integrating it with the 1.5T Siemens Avanto MRI medical scanner and the high field
3He MEOP (metastability exchange optical pumping) polarizer that operates inside the MRI magnet bore [
23].
2. Materials and Methods
2.1. Design of the Breath Cycle Control and Hyperpolarized 3He Dosing System
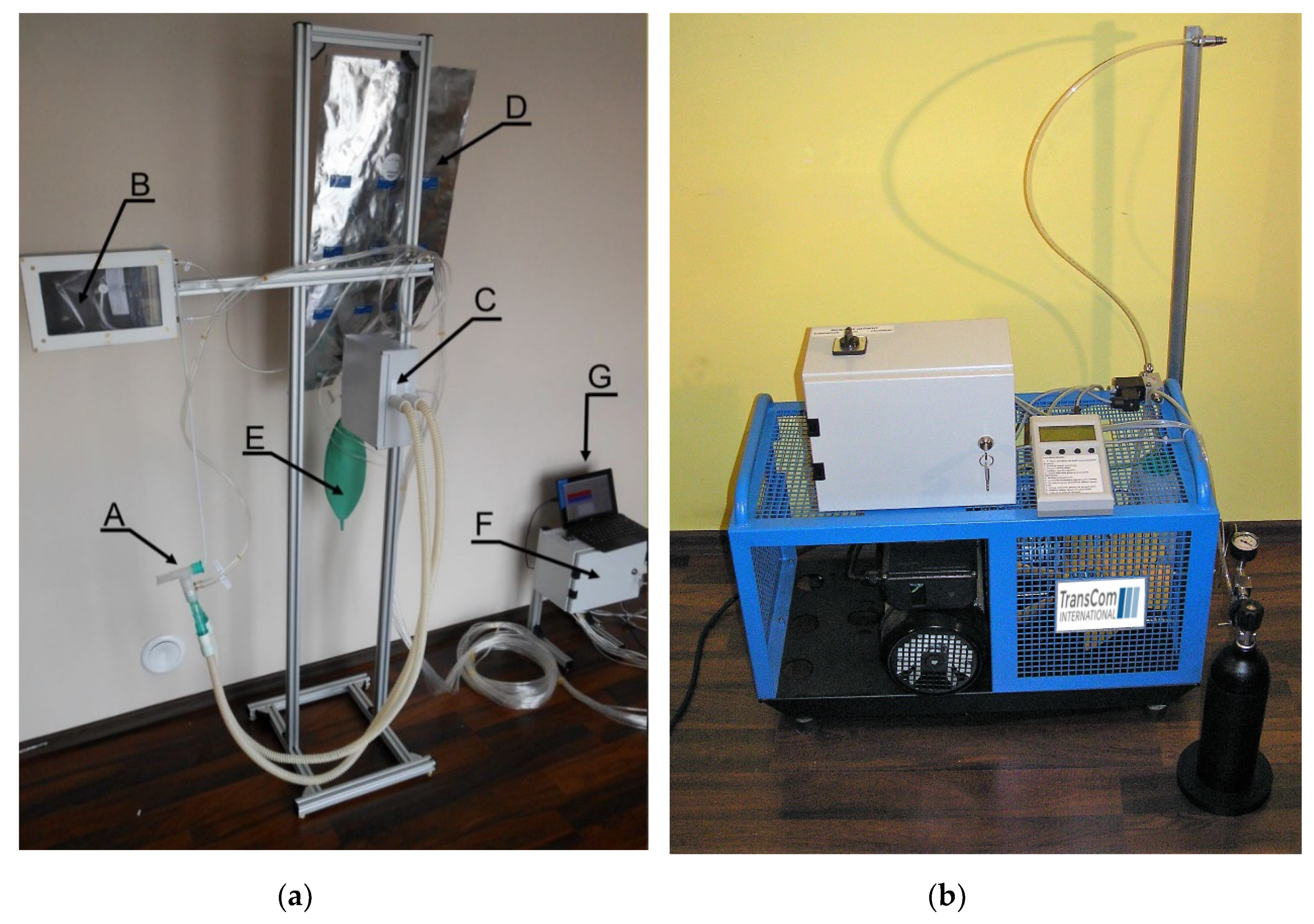
The schematic diagram of the ventilator is presented in
Figure 1 and Figure 3a shows its photograph. The components located both inside and outside the Faraday cage are indicated. Initially, the high field MEOP
3He polarizer is placed in the magnet bore to produce the hyperpolarized gas. After the polarization is complete, the gas is transferred to the plastic Tedlar bag (B), which is inside the rigid, hermetic pressure chamber located in the high and homogeneous field of the magnet. The access to the Tedlar bag is controlled by a nonmetallic pneumatic valve. After the bag is filled with hyperpolarized gas, the polarizer is removed from the magnet to let the patient in. The procedure is described in detail in [
20]. The delivery of hyperpolarized gas is accomplished by applying a small, 10 mbar above the atmospheric pressure to the pressure chamber. The dead volume of the
3He delivery system is less than 1 mL. A set of sterile breathing tubes with the mouthpiece (A) connect the system to the patient, who is able to manipulate them by himself, to achieve the most comfortable position in the scanner. We found it more convenient than the direct application of the Tedlar bag filled with the noble gas. It is required to replace the setup for a new patient to ensure sterility. Inhaling, exhaling and the noble gas delivery processes are controlled by pneumatic one-way valves (C). However, the actual timing is adjusted for each patient by recording his spontaneous breathing. The valves are driven with the 3 bar compressed air delivered by the electronic control module (F), which contains electrical valves and pressure meters. The pneumatic valves are mounted on a lightweight aluminum rack, where the oxygen free nitrogen reservoir for initial lung flushing (E), and the metalized helium-tight bag for exhaled gas collecting (D) are also located.
Flexible tubes are connected to the control module to measure the pressure inside the chamber containing the Tedlar bag, as well as the pressures at the inhaling and exhaling tubes. The flow during inhaling and exhaling is measured by the D6F-PH MEMS Differential Pressure Sensor (OMRON Corporation) mounted on a bypass. The control module also monitors the pressure delivered by the air compressor (not shown), and by the nitrogen bottle (H). A dedicated LabView program with a user-friendly graphical user interface (GUI) is installed on the computer (G) and controls the operation of the system. For convenience the computer is located in the MRI control room.
All parts of the ventilator located inside the Faraday cage are made of non-magnetic materials, and there is not any electronics. Moreover, all components that have contact with hyperpolarized 3He (Masterflex tubes, pyrex glass, sterile breathing tubes and mouthpieces) have been carefully chosen to reduce relaxation effects. The ventilator can be quickly removed from the Faraday cage, when the return to a standard proton MRI operation is required.
After completing the polarization of 300 mL of 3He, it is supplemented by 700 mL of pure nitrogen and transferred to the Tedlar bag (B) by the peristaltic compressor. Typically, it is kept there for about 15 min. In spite of having contact with several components of the polarizer and ventilator, the gas mixture maintains its high chemical and biological purity.
It takes typically about 15 min to remove the polarizer from the magnet, put the patient in, and start the imaging procedure. In the presence of pure nitrogen, which acts as the buffer gas, the T1 relaxation time of 3He is about 3 h. Therefore, the polarization losses are of the order of 8%.
2.2. Operation of the 3He Dosing and Control System
The application of the dedicated ventilator makes it possible to perform successful and reproducible MR imaging of human lungs, providing at the same time maximum possible comfort for the patient. A controlled amount of noble gas of known polarization supplemented with the buffer gas is delivered to fill the lungs entirely, so that the examination takes place in the full inspiration mode. A short training is performed before the actual experiment, to accustom the patient with the apparatus and show him how to quit at any time in case of emergency. For that purpose, the Tedlar bag is filled with ambient air, and the whole procedure is simulated as many times as necessary. During the actual examination, the patient has to focus on the beep signals and verbal instructions signals from the operator, to synchronize his breath hold with the hyperpolarized gas administration and image acquisition.
The operation of the ventilator is software controlled by a dedicated LabView program using the user friendly GUI, which is shown in
Figure 2. It monitors the breathing phases and the status of critical components, and controls the sequence of gas application to the patient. The pressures delivered by the air compressor, in the nitrogen bottle, and in the nitrogen reservoir are shown on the monitors located in the center top of the window. The patient’s breathing cycles are visualized in the graphic display, where the inhaling and exhaling phases are indicated by red and blue colors, respectively. The program records the patient’s breath rate and breathing depth, and sets the timing and amount of the delivered gases (air, nitrogen, polarized
3He) accordingly. The consecutive phases of the automatic gas delivery procedure are monitored by the indicators that are located in the bottom right. Before the actual examination, the program is tested using a calibrated 2 L piston phantom that simulates the patient’s lungs.
Apart from the automatic procedure that is applied during the imaging experiment, the control program provides the capability of manual operation of various valves, using the buttons located in the right top of the GUI window. This is especially important in the preparation of the Tedlar bag, which is flushed with the oxygen-free mixture and evacuated before filling it with hyperpolarized gas from the polarizer. During the initial training of the patient, the Tedlar bag is also manually filled with ambient air.
The MR lung imaging experiment is carried out in two stages. First the patient is asked to breathe normally with the ambient air, so that his individual breathing parameters are recorded by the program. Then the consecutive gas deliveries that are synchronized with the inhaling phases of the breath cycles are applied. The values of the following automatic procedure parameters are chosen by the operator: The number of initial flushings with nitrogen (from 0 to 1); the number of hyperpolarized gas doses, with or without an additional buffer gas filling; and the number of exhaling cycles before the valve to the metalized bag is closed. The nitrogen flushing can be necessary to decrease the residual oxygen content in the lungs, which effects the relaxation of hyperpolarized gas [
24]. The
3He gas delivery is initiated by the beep signal accompanied by a verbal instruction from the operator for the patient to take a deep breath and hold it for the time needed to acquire the data. It varies from about 5 to 30 s and is determined by the required number of orientations and slices. Depending on the lungs volume and the amount of
3He that is available, an additional portion of nitrogen can be supplied at this moment to fill up the lungs. After the imaging sequence is complete, another beep signal tells the patient to breathe normally, and the exhaled gas is collected for recycling.
2.3. Exhaled Gas Storage System for 3He Recycling
It is of the utmost importance to recover as much of the
3He isotope as possible, due to its extremely high cost. The recycling technology has already been developed for that purpose [
7]. In the presented design the exhaled gas mixture that was collected in the metalized bag is transferred to the high pressure aluminum bottle by a commercial 4-stage compressor that is capable of compressing the gas up to 250 bar. In order to obtain the highest possible gas recovery ratio, the compressor was modified to reduce the compression time and minimize its dead volume. The electronics controlling the compressor operation allows for setting the duration of compression, number of compression cycles, and the output pressure. After the procedure is complete, the compressor volume is flushed with ambient air, so that the residual amount of the gas mixture in the whole installation is negligible. The compressor includes a simple separator to reduce the water vapor content in the compressed air and a manual release valve to remove the water condensate. Due to high acoustic noise generated by the compressor, it is located outside the diagnostic area.
Our software allows to choose the number of exhalation cycles to be stored in the metalized bag (D). Typically it is set to three. Due to some dead space in the tubing, we estimate that about 95% of the exhaled gas is recovered. The compressing process also consists of three cycles, to flush the dead space of the compressor. The resulted gas mixture can be sent to an external company for chemical and biological cleaning and 3He recovery. The efficiency of the entire process is about 80−90%.
The photographs of the ventilator rack that is located in the Faraday cage, and the compression unit are shown in
Figure 3a,b, respectively. In the final step of the
3He recycling procedure, the bottle with the compressed gas mixture is sent to the external company for the noble gas extraction and removal of any chemical and biological contaminants.
The ventilator control unit that is shown on the right side of
Figure 3a is in fact located in the technical room, which is adjacent to the medical scanner room. The MRI console including the control computer, RF and gradient amplifiers are also there. Two additional feedthroughs made of 1 m long, 10 cm diameter metal tubes connect the technical room with the medical scanner room, without affecting the shielding performance of the Faraday cage. As indicated in the schematic diagram shown in
Figure 1, the components A−E and F−H are located inside and outside of the Faraday cage, respectively. The rack with the C to E components is located behind the magnet. The A and B components are put into the magnet, as close to the patient’s head as possible. The ventilator control module G is connected with the gas valves C by 4 m long plastic tubes going through the feedthroughs. After completing the imaging procedure, the helium tight metalized bag D containing the exhaled gas is detached from the ventilator rack and transferred to a distant compressor unit (shown in
Figure 3b), to avoid any electromagnetic interference from the 250 bar compressor.
All components of the ventilator that are located in the medical scanner room are made of nonmagnetic material. Standard proton phantoms provided by the medical scanner producer were used to assess possible artifacts. The EPI imaging sequence, which is very sensitive to magnetic field inhomogeneities, was applied to obtain MR images of the phantoms with and without the presence of the ventilator. No artifacts were observed after subtraction of the images. The fast FLASH sequence that was used to obtain 3He images is characterized by rather poor spatial resolution, and it gives sometimes artifacts in the form of one-pixel-wide horizontal lines. They were not observed in the acquired images.
2.4. Medical Scanner for Magnetic Resonance Imaging of Human Lungs
The ventilator is fully compatible with any commercial medical MRI scanner. In recent studies it has been used with the clinical 1.5 T Avanto medical MRI scanner (Siemens, Erlangen, Germany) in the John Paul II Hospital in Krakow. An upgraded software (Idea, Simens, Erlangen, Germany, 2010) and dedicated birdcage coil purchased from Rapid Biomedical (Rapid Biomedical, Rimpar, Germany) made it possible to operate the system at the
3He resonance frequency (48.5 MHz at 1.5 T). A homemade high field MEOP polarizer was used to produce hyperpolarized
3He gas [
23].
The lung imaging experiments were performed using the fast multi-slice FLASH sequence with the following parameters: resolution 128 × 128, field of view 420 × 420 mm2, slice thickness 15 mm, distance between slices 15 mm, repetition time 8 ms, flip angle 70°, and acquisition time 1 s per slice. Typically four to five coronal slices fully covered the entire lung volume. For better localization (in addition to standard proton localizer imaging sequence), a single slice in the transversal plane was acquired right after the coronal ones. The locations of coronal slices could be observed in this transversal slice as dark stripes of the coronal slice’s width, due to significant suppression of gas polarization in these places caused by preceding experiments.
Possible interference effects caused by the presence and operation of the ventilator in the medical scanner room were assessed in various stages of the lung imaging experiment.
2.5. Opinion of the Bioethics Commission
This study was approved by the Bioethical Commission at the District Medical Chamber in Krakow, Poland (no 14/KBL/OIL/2013 of 12 February 2014). All experiments were performed following an informed, written consent of volunteers.
3. Results
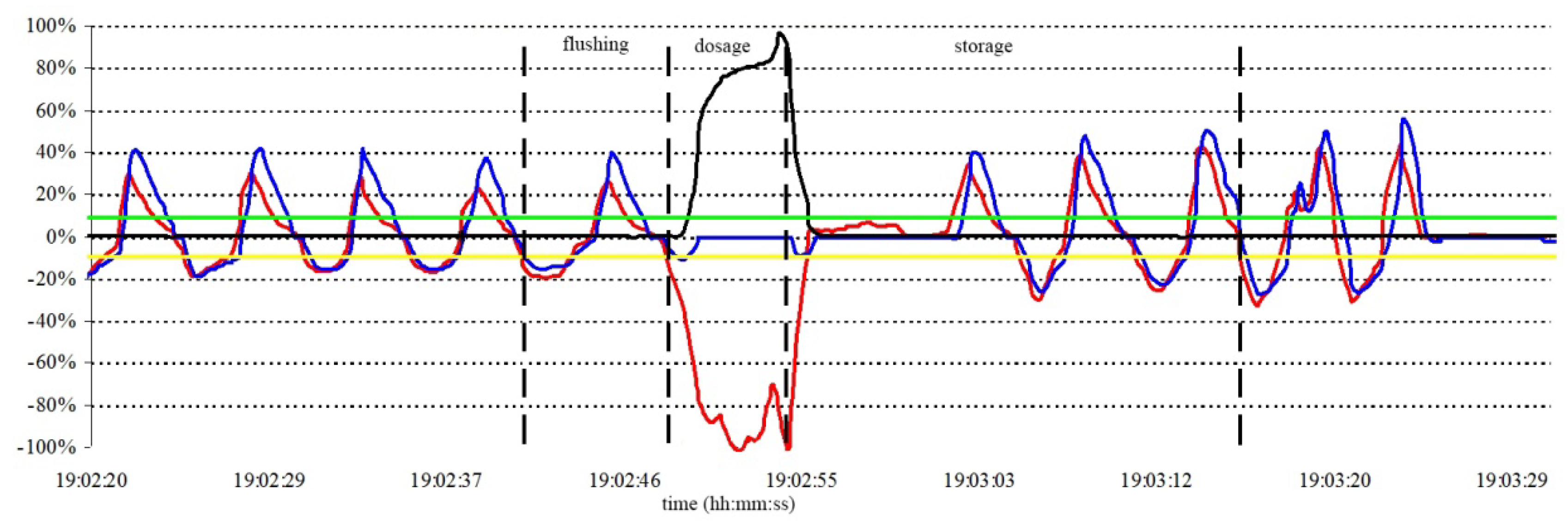
A typical time course of various patient’s breath parameters during the lung imaging experiment is shown in
Figure 4. Directed by a beep signal accompanied with a verbal instruction, a volunteer takes one normal breath of nitrogen to flush his lungs, and right after exhaling it, he inhales 300 mL of the HP gas of about 10% polarization, supplemented by 700 mL of nitrogen to fill the entire lung volume. The breath is held for several seconds to perform imaging experiments. Afterwards three full exhalations are collected to the metalized bag for later helium gas recovery.
The black vertical lines in
Figure 4 indicate the moments when the ventilator valves are switched. After initial breath stabilization, the nitrogen gas is applied (the first line from the left). Next switching corresponds to the application of HP gas. After emptying the HP storage bag, the system switches the valves again to deliver nitrogen, so that the volunteer may continue the inhalation until his lungs are full. At this moment the system opens the valve to the collector bag, to let the volunteer exhale the gas (the third line). After holding the breath for a few seconds and subsequently completing three full respiration cycles, the collector bag is closed (the fourth line), and the ventilation procedure is complete.
The 3He signal obtained in a single pulse NMR (Nuclear Magnetic Resonance)experiment as well as the proton image of the phantom showed no interference effects that might have been caused by the presence of the ventilator in the scanner room and its operation. The polarization losses of the helium gas during its transfer from the polarizer to the Tedlar bag inside the pressure chamber of the ventilator turned out to be negligible. They are of the same order as the relaxation during storage of the polarized gas in the container made of such material and placed in a homogeneous magnetic field.
Typical results of the lung imaging experiments using the high field
3He polarizer and the ventilator are presented in
Figure 5. The volunteer was a healthy heavy smoker, who used to smoke a pack of cigarettes a day for more than ten years. Five coronal and one transversal slices indicate generally healthy lung parenchyma together with dark areas representing blood vessels. Slight ventilation defects in peripheral regions can be observed.
The experiments with the same healthy heavy smoker were repeated after two weeks, and the images shown in
Figure 5 were reproducible, confirming their diagnostic value.
The dark stripes that are observed in the transversal slice are the interesting demonstration of the unique property of MRI using a hyperpolarized gas: The nuclear magnetization in the areas that were addressed in preceding experiments does not recover. This feature can be used to study the long-range diffusion in the lungs and has some potential diagnostic value [
25].
4. Discussion
The ventilation system for breath monitoring and control of the HP 3He dosing for MR imaging of human lungs has been designed and constructed. It adapts to the patient’s lung volume and his breath cycle, providing maximum achievable comfort during the lung imaging procedure, and making the intubation unnecessary.
The lung imaging procedure was tested in the John Paul II Hospital in Krakow on a group of healthy volunteers of both sexes, different age and body mass. Good quality images were obtained and the usability of the ventilator for the HP 3He lung imaging was confirmed. The presence of the ventilator in the medical scanner room did not interfere with its normal operation, as no artifacts were observed in the MR images.
Although different groups of people were imaged, the total number of successful results was about ten, which is not sufficient for any meaningful statistical analysis. At this stage our work was focused on the optimization of the performance of the polarizer–ventilator system and the MR image was presented as the proof of its successful operation.
In the view of a very high cost of the
3He isotope, the collection and recycling of the exhaled gas mixture is an important factor. The design of a certified recycling unit for the extraction of helium from the gas mixture and removal of any chemical and biological contaminants is planned in the future. The described ventilator can be also used, after small modifications, for the HP
129Xe lung imaging, and this direction is being currently explored in our group [
26].
Our ventilator compares favorably with similar systems that were reported in the literature. De Alejo et al. describe the ventilator for animal applications, which impose different requirements [
21]. In contrast to humans, the animals to be imaged do not cooperate and have to be anesthetized. In the case of small animals, like mice or rats, the intubation is not used, and the breathing and heart rate need to be monitored continuously. Stable breathing is achieved by supplying a controlled mixture of air, oxygen, and anesthetic. Moreover, the animal is kept in constant temperature, because the temperature self-control is not active in the sleep phase. The above procedure has to be carried out by a trained personnel, which is not necessary in imaging humans, who can control breathing by themselves. Nevertheless, it is of utmost importance for both the patient and the personnel to get familiar with the ventilator operation before the actual experiment. During initial training, the software automatically records the breathing parameters of the patient, using air in the proper sequence. Therefore, the patient feels comfortable during the actual imaging procedure, when hyperpolarized helium mixed with nitrogen is applied. When compared to self-controlled breathing of the prepared in advance gas mixture, our automatic system is more reliable and reproducible.
Güldner et al. for the first time presented the operation of the ventilator for humans and the resulted MR images of human lungs [
22]. The authors used the medical scanner and the RF coil that was produced by the same company, and we analyzed and utilized their experiences to a large extent. Compared to their design, our system is of significantly smaller size. It cooperates with the high-field optical polarizer, which was designed and built in our lab [
23] and uses the magnet of the medical scanner as a source of high magnetic field. The critical components of the ventilator are also located in the imaging room and the imaging procedure includes the preparation of the hyperpolarized helium gas. Therefore, there is no need for an external production, transport, and delivery unit, as it is in the Mainz design. In the first stage, the polarizer is placed in the magnet and the sufficient amount of the polarized helium is produced in about 20 min. A unique, nonmagnetic peristaltic pump is used both to accumulate the polarized gas and to transfer it to the one-liter administration box, which is visible on the left side of
Figure 3a. In the second stage, the polarizer is removed from the magnet, making place for the patient, who was already trained. The only component that is left in the magnet is the administration box and some plastic tubes which are connected to the patient’s mouthpiece when he is inside. Our design is significantly different from the Mainz system, which cannot cooperate with the high-field polarizer.
The described ventilator is only the first step in making the imaging with HP 3He a routine clinical procedure. The main goal was to design a system that would produce images of diagnostic value with high reproducibility and reliability, independent of the sex, age, and lung volume of the patient. Due to low density of water vapor in the lungs, the standard 1H MR imaging does not provide any structural information, therefore a quantitative comparison of both methods is impossible. We present the best images obtained with the use of our ventilator up to now, and their quality is comparable with the ones reported in literature. According to medical doctors, they contain some diagnostic details that are not visible in the high-resolution computed tomography images, for example some dark areas in the peripheral region of the lung of the healthy heavy smoker.
The problem of the initial flushing of the lungs with pure nitrogen gas is still controversial, especially in the case of a sick patient. Therefore, our software enables the omission of this step. Healthy volunteers did not report any discomfort when a single flushing was applied. However, it is known that several inspirations of pure nitrogen lead to a fast decrease of blood saturation and can be carried out only in the presence of a medical doctor. The blood oxygenation level was monitored by the pulse oximeter during the entire imaging procedure. At this stage, the comparison of images taken with and without nitrogen flushing was not made, but it is planned in the future. An additional limiting factor is the cost of
3He gas, which in recent years has increased by two orders of magnitude. Some quantitative information about the oxygen-related relaxation of the HP helium gas is given in [
24].
Computed tomography is the most widely used technique for lung imaging. However, it is sensitive to soft tissue only and cannot image the air spaces, which are dominant in the lungs. The only comparable method is MRI using hyperpolarized xenon, which is much less expensive. Its main disadvantage when compared to HP
3He MRI is the long time necessary to produce a sufficient amount of hyperpolarized gas. Nevertheless, this direction is actively pursued in our lab [
25]. Apart from standard imaging, the high solubility of xenon in blood makes it possible to apply the localized spectroscopy of
129Xe to study perfusion in the lungs and metabolic processes in the brain.
Bearing in mind the cost and time of examination, the application of our device will be probably limited to special cases, such as lung transplantology. It can also successfully replace CT when frequent examinations are necessary, in order to avoid ionizing radiation damages. The device is still a prototype and needs further improvements to be ready for wider distribution.