Abstract
The unique physical and chemical properties of spinels have made them highly suitable electrocatalysts in oxygen evolution reaction and oxygen reduction reaction (OER & ORR). Zinc–air batteries (ZABs), which are safer and more cost-effective power sources than commercial lithium-ion batteries, hinge on ORR and OER. The slow kinetics of the air electrode reduce its high theoretical energy density and specific capacity, which limits its practical applications. Thus, tuning the performance of the electrocatalyst and cathode architecture is vital for improving the performance of ZABs, which calls for exploring spinel, a material that delivers improved performance. However, the structure–activity relationship of spinel is still unclear because there is a lack of extensive information about it. This study was performed to address the promising potential of spinel as the bifunctional electrocatalyst in ZABs based on an in-depth understanding of spinel structure and active sites at the atomic level.
1. Introduction
The dominance of fossil fuels in energy production has become a massive problem in modern societies because of the resultant global warming and climate change. Because energy obtained from renewable sources faces challenges such as season, climate, and location dependency, the ability to store this energy for uninterrupted use is indispensable if demand is to be met. These circumstances introduce the role of electrochemical energy technology in converting and storing sustainable energy [1,2,3,4,5]. In the quest to store energy from renewable sources, metal–air batteries have been gaining attention because of their safety; they also use abundantly present oxygen as the cathode source, and they have 2–10 times the higher theoretical energy density than commercial lithium-ion batteries [6,7,8,9].
Compared with other metal–air batteries, zinc–air batteries (ZABs) demonstrate superiority owing to their higher safety, their minimal environmental impact, the abundance of zinc, their cost-effectiveness, and their theoretical specific capacity of 820 Ah kg−1 and specific energy of 1084 Wh kg−1 [10,11,12,13].
Generally, the oxygen evolution reaction and oxygen reduction reaction (OER and ORR) at ZAB cathodes are sluggish owing to complex electron transfer [14,15,16,17], and this results in higher overpotential at the cathode than the anode. Because ORR is the surface reaction, O2 is reduced to OH− or H2O2 on the electrode surface after the electron is acquired. The ORR mechanism can happen in two ways: (1) In a two-electron transfer reaction, O2 reduces to OH− in two steps, and (2) in a four-electron transfer reaction, the oxygen reduction happens in one step. The former reaction is more feasible, and the latter is more effective because it avoids the byproduct of H2O2. Practically, the overpotential of OER is higher than that of ORR due to the formation of hydroxide, oxide, and oxy-hydroxide intermediates during the process, and overcoming the overpotential in ORR through four-electron transfer is quite difficult as well.
OER is the tailback of the electrochemical reactions because of the four proton-coupled electron transfers and the generation of an O=O bond. Thus, there is a need for a bifunctional electrocatalyst that catalyzes both the OER and ORR for large-scale applications of these reactions in energy devices [18,19]. Investigators have been using noble metal-based electrocatalysts such as Pt, RuO2, and IrO2 in zinc–air batteries to master the overpotential issues; however, the lower abundance, high cost, and reduced long-term durability of these materials hinder their usage [20]. With a focus on efficient performance, cost-effectiveness, and abundance as the criteria for a better electrocatalyst, transition-metal oxides are gaining more attention for their reactions in energy storage devices, particularly in metal–air batteries [21].
Spinel-structured transition-metal oxides with the formula AB2O4 (A, B = Fe, Co, Ni, Mn, Zn, Cu, and so on), as shown in Figure 1, can be in A3O4 type single-metal oxide, AxB3−xO4 type binary-metal oxide, and AxByC3−x−yO4 type ternary-metal oxide forms. Spinel-based compounds consist of oxygen atoms (32) and one or two transition metals (eight in A site and 16 in B site) with a wide range of valence states. At the A site, one metal is surrounded by the four nearest neighboring oxygen atoms, and the B site has a metal that is surrounded by the six nearest neighboring oxygen atoms. In the spinel frame, A is the divalent cation, B is the trivalent cation, and O is an anion; generally, the spinel structure is classified into three types based on the occupancy of cations, whereas the anions are always accommodated by the polyhedral vertexes. The varieties of spinel are normal, inverse, and mixed spinel.
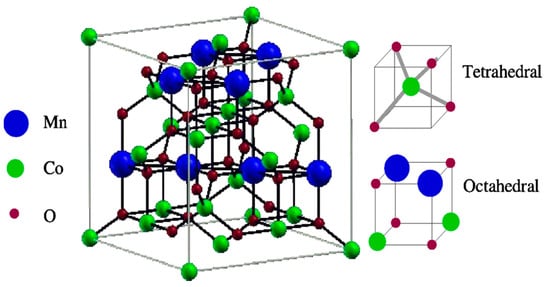
Figure 1.
Normal spinel structure of Co2MnO4. Component structures, namely, tetrahedral and octahedral, are shown on the right-hand side [22]. Copyright 2010, AIP Publishing.
In normal spinel, A2+ has low crystal field stabilization energy (CFSE) and occupies tetrahedral sites. B3+ has high CFSE and occupies octahedral sites. It is represented as (A2+)tet(B3+)octO4. In inverse spinel, A2+ has high CFSE and occupies octahedral sites, and B3+ has low CFSE and occupies some octahedral and some tetrahedral sites. It is represented as (B3+)tet(A2+B3+)octO4. The complex spinel is the intermediate between the normal and inverse spinel structure in which both A2+ and B3+ cations partially occupy the two various sites [23,24]. Among the three types, normal spinel structures are generally cubic close-packed oxides with two tetrahedral and four octahedral sites per formula unit. B3+ ions occupy half the octahedral holes, and A2+ ions occupy one-eighth of the tetrahedral holes. The tetragonal phase presents the different sets of active lattice planes ready for the O2 adsorption and desorption process, which differs from the cubic phase.
Spinels have gained more attention due to their easy synthesis methods, stability, structural flexibility, and a minimum of two orders of magnitude higher electrical conductivity than the equivalent single-metal oxide and helpful contributions from all A and B ions with multiple valence states (A3+/A2+ and B3+/B2+) in the crystal structure [25]. Hence, spinel-based materials possess fair electrochemical activity. Particularly, in the key ZAB reactions such as ORR and OER kinetics (Figure 2), the adsorption and reduction of O2 vastly depend on the d-orbitals of cations placed in octahedral B sites relative to tetrahedral A sites. This phenomenon occurs due to the substantial overlap of the high lying eg-3d orbital in the octahedrally coordinated metal cations with an orbital of O 2p and the weak overlap of the 3d orbital in the tetrahedrally coordinated metal cations with an oxygen atom [26].
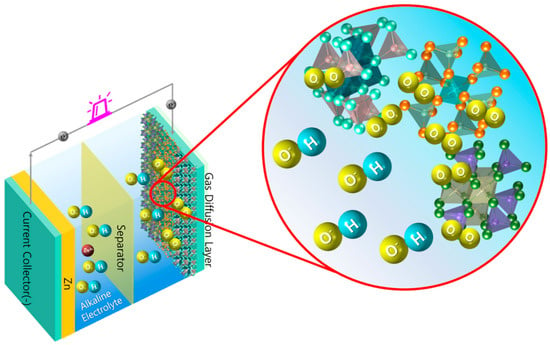
Figure 2.
Schematic representation of the role of spinels in a Zn–air battery (ZAB).
Based on the molecular orbital theory, the presence of octahedral site active cations in, egorbital accounts for spinel activity [27]. Thus, the structural flexibility and variable valence states of the spinel oxides demonstrate the potential to fine-tune their catalytic performance in zinc–air batteries. To date, several researchers have investigated spinel-based bifunctional oxygen electrocatalysts for ZABs. In this review, we summarize the role of spinel-based compounds as the bifunctional oxygen electrocatalysts for ZABs with a brief introduction and discussion of the batteries’ mechanism along with the mechanism in the air cathode.
1.1. Zinc–Air Battery
In 1878, Maiche proposed the primary Zn–air battery, which provided the highest available energy density in the group of primary battery systems. In the beginning, researchers used silver wire as the air electrode and used the gas diffusion electrode with a porous carbon black and nickel current collector in a Walker–Wilkins battery. Primary batteries in the form of button cells bearing specific energy and volumetric energy density up to 442 Wh kg−1 and 1672 Wh L−1, respectively [28]. This century-old technology has been on the market since the 1930s, and its application has expanded to hearing aids, railway signals, and navigation lights; in other words, to diverse medical and telecommunication applications [29]. Later, from 1975 to 2000, ZABs received less attention, and Li-ion batteries gained the research spotlight because of their rechargeability, high energy density, low self-discharge, and low maintenance. ZABs then regained the interest of the scientific community because their theoretical energy density is about four times higher than that of Li-ion batteries and at a fraction of the cost of the Li-ion batteries. Although companies such as EOS Energy Storage, Fluidic Energy, ZincNyx Energy Solutions, Revolt, and EDF France have commercialized rechargeable ZABs, there is still much room for improvement [1,27,30].
1.2. Cell Configuration of Secondary ZAB (S-ZAB)
In secondary ZABs, the negative anode, positive cathode, electrolyte, and membrane separator are electrochemically coupled, as shown in Figure 3a,b. The anode for alkaline secondary ZABs is a zinc electrode in the form of zinc metal foil, zinc powder, or zinc fiber, and the air electrode has two layers, the gas diffusion, and catalytic layers. There is a three-phase (solid-liquid-gas) reaction at the cathode. Here, the diffusion layer provides the physical support for the catalyst and runway for the passage of electrons to be collected as the current; the hydrophobic section of the gas diffusion layer has to permit the liquid electrolyte as well as protect the electrolyte from leaking out. Thus, an optimal gas–electrolyte–catalyst three-phase interface is required [31,32].
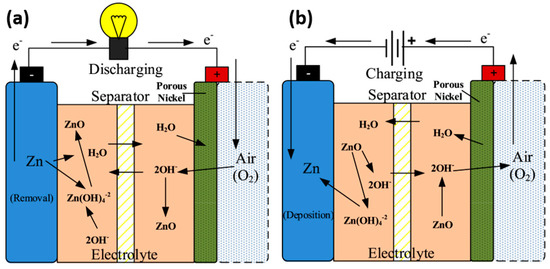
Figure 3.
Schematic of a zinc–air battery under (a) discharging and (b) charging conditions [36]. Copyright 2017, Elsevier.
In general, to carry out the ion transportation between the Zn anode and air cathode, alkaline electrolytes such as KOH or NaOH have been used widely in ZABs because of the better ionic conductivity and high Zn reaction kinetics. The acidic electrolyte reacts with the Zn anode and produces hydrogen; this leads to zinc corrosion, shape change, and production of heat, and results in poor battery efficiency. These reasons restrict the use of acidic electrolytes in ZABs. Neutral electrolytes are not commonly used because of their low conductivity and because they are less friendly toward oxygen electrocatalysis than alkaline electrolytes. [33,34]. The separator, a selectively permeable membrane, plays a vital role in blocking unwanted materials from passing through, such as zinc dendrites between the anode and the cathode [35].
As the battery discharges, in the anode, the zinc reacts with hydroxide and oxidizes to form zincate ion, and this process continues until the zincate ion is supersaturated; then, it decomposes to insoluble white zinc oxide powder and precipitates on the active zinc surface [37]; the insulated ZnO powder produced makes the active area less active. Simultaneously, in the cathode, the pressure gradient induces O2 entry through the diffusion layer and reduction to hydroxyl ions at the catalytic layer by reacting with water and electrons. Hence, oxygen is reduced during battery discharging (ORR) and oxidized (OER) during charging. Along with O2, CO2 enters the cathode and forms carbonates by reacting with KOH. This formation clogs the pore and restricts the entry of air to the cathode. These mechanisms are shown in Equations (1)–(6) [38].
Discharge condition
Zinc electrode:
Zn(s) → Zn2+(aq) + 2e−
Zn2+(aq) + 4OH− → Zn (OH)42− (aq)
Zn (OH)42− (aq) → ZnO(s) + H2O(l) + 2OH− (E⁰ = −1.25 V vs. SHE)
Air electrode:
O2(g) + 2H2O(aq)+ 4e− → 4OH− (aq) (E⁰ = 0.401 V vs. SHE)
Overall reaction:
2Zn (s) + O2 (g) →2ZnO (s) (Cell potential: 1.65 V)
Parasitic carbonate formation:
2KOH (aq) + CO2(g)/K2CO3(s) + H2O (aq)
1.3. Cathode Mechanism
In S-ZABs, two types of electrode arrangements are available, a three-electrode system with a separate electrode for OER and ORR, or a two-electrode system that uses the same electrode for OER and ORR. The second system is more commonly used, in which the electrochemical reactions occur at the bifunctional air cathode [39,40]. The benefits are that using a single electrocatalyst for the two reactions reduces the cost and eliminates the side effects that come with using two catalysts. The redox reaction at the cathode corresponds to the charging and discharging of the battery. In the cathode, oxygen reduction and evolution take place, which involves a series of complex electrochemical reactions, either the Langmuir–Hinshelwood (LH) or the Eley–Rideal (ER) pathway; in the first one, intermediates react on the surface, and in the second, the electrolyte reacts with the surface intermediate. Due to the lower reaction energy barrier, many researchers have theoretically studied OER and ORR based on the ER mechanism, which takes one of two reaction paths, with either two or four electrons depending on the electron transferred during the reduction of O2 on the catalyst [41,42,43]. The four-electron transfer is the direct dissociation of an O–O bond without any byproduct, which requires more energy to dissociate O2.
Two-electron transfer dissociates O2 by generating intermediate species of HO2− or H2O2. Among these two, four-electron transfer is more capable because it does not spend its energy in byproduct formation.
In an alkaline electrolyte,
O2 + 2H2O + 4e− → 4OH− (Four-electron transfer)
O2 + H2O + 2e− → HO2 − + OH− (Two-electron transfer)
H2O + HO2 − + 2e− → 3OH−
During charging, OER occurs in which the formed hydroxyl ions give away electrons and O2 forms. As in ORR, intermediates also form, such as hydroxide, oxide, and oxy-hydride. The four elementary steps of the OER mechanism are described as follows:
OH− + M → MOH ads + e−
MOH ads + OH− → MO ads + H2O(l) + e−
MO ads + OH− → MOOH ads + e−
MOOH ads + OH− → M + O2(g) + H2O(l) + e−
Figure 4a,b depicts a schematic illustration of the gas diffusion layer, the part of the air cathode at which the oxygen electrocatalysis takes place, and the OER and ORR reaction pathways of NiCo/Pyri-NG in multiple steps. The round-trip efficiency of rechargeable ZAB decreases because of the overpotential at the cathode, and the cell potential decreases as well. These complex intermediate reactions must be accelerated to achieve ZABs with commercial value.
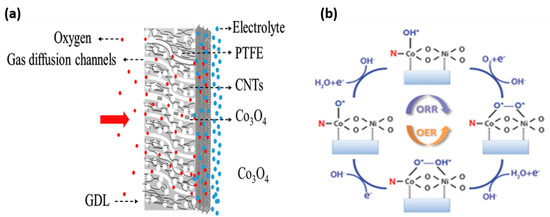
Figure 4.
(a) Schematic illustration of fabricating a self-supported Co3O4/Ni/Gas Diffusion Layer [44]. Copyright 2019, Elsevier (b) oxygen reduction reaction (ORR, clockwise), and oxygen evolution reaction (OER, anticlockwise) mechanisms for NiCo/Pyri-NG, where pyridinic N bonds with surface three-fold coordinated Co [45]. Copyright 2018, John Wiley and Sons.
2. Spinels as an Electrocatalyst
Spinel-based materials exhibit better catalysis because of their controllable composition, structure, valence, and morphology. In particular, transition metal oxides with a spinel structure have received attention as catalysts for ORR and OER because these structures enable good electrical conductivity due to the ready electron hopping between metals at different valence states. The spinel structure also provides surface redox centers for oxygen adsorption and stimulation. The existence of the different transition metals in the interlaced structure of the spinel-based transition-metal oxides is the reason for the high electrocatalytic activity because it is well established that the catalytically energetic element always governs the surface [46]. In addition to spinel, other structural forms can be used as substitute electrocatalysts. However, their usage has been limited because of the high synthesis temperature of perovskite oxides (ABO3) [47], the properties of metal–organic frameworks such as electrical insulating and bad stability in electrolytes [48], the difficulty in raising the concentration of active sites in graphene [49], and the poor OER activity of metal−N-decorated nanocarbon materials [17]. Even the noble metals rarely possess bifunctional electrocatalytic activity for both ORR and OER [50,51].
Therefore, spinel-based compounds with their diverse compositions, bifunctional catalytic activity, and low cost are suitable oxygen electrocatalysts. Synergism between the elements in the structure, spinel frame, compositing, and the supporting material escalates the electrocatalytic activity. Above and beyond, the incorporated cations can form redox couples for the favored catalytic actions. To date, materials with cobalt, manganese, nickel, copper, and iron in spinel structure are widely used as oxygen electrocatalysts in ZABs. Since 1980 Co3O4 has been widely investigated as an electrocatalyst owing to its chemical and physical properties. The efficiency of electron transport between the Co2+ and Co3+ ions promotes catalytic activity in an alkaline medium. The spinel structures with Cobalt in the octahedral sites are more resourceful for the OER and ORR [52,53]. Co3O4, with the advantages of high activity and stability, has been identified as a catalytically active material for air batteries [54,55,56].
To further improve the catalytic performance, the active sites in the single metal oxide should be increased by adding other metals while synthesizing the spinel structure. For example, MnCo2O4, NiCo2O4, CuCo2O4, and MnFe2O4 have been reported as active catalysts for air batteries [57,58,59,60,61]. The above-mentioned spinel structures as the electrocatalyst for Zinc–air batteries are discussed in the following sections.
2.1. MnCo2O4 Spinels
To date, many cationic distributions of MnCo2O4, such as Co2+[Co2+Mn4+]O4 and Co3+[Mn2+ Co3+]O4, have been proposed. Through different synthetic methods, these cations can be tuned to enhance the physical, physicochemical, and interfacial properties. Besides the porosity and surface area, they offer a fast pathway for electrolyte/electron diffusion and more electroactive sites [62]. Tatsumi Ishihara et al. studied mesoporous MnCo2O4 spinel oxide because it consisted of two transition metals in a lattice; thus, they expected high ORR/OER activity because the catalytically active element always dominates on the surface. The authors prepared mesoporous MnCo2O4 by a hard template method with mesoporous silica as an inorganic template. This process formed material with more surface area, an average pore size of 2 nm, and lower overpotential in ORR/OER. Ishihara et al. then evaluated the material’s catalyzing property in a ZAB with 4 M KOH aqueous electrolyte and Hg/HgO as a standard electrode, and it showed a capacity of about 700 mAh/g-Zn, discharge potential of 1.05 V, and could withstand more than 200 charge–discharge cycles [46].
Cui Shenghai et al. coated carbon on MnCo2O4 nanowire by self-templating from a NiCo2O4-nitrogen triacetic acid (MnCo2-NTA) nanowire precursor. The resulting material, MnCo2O4@C, showed better ORR than MnCo2O4. The authors proposed that the improved ORR was attributable to the conductivity of MnCo2O4@C nanowire, where the carbon layer helped in the electron movement of MnCo2O4 nanoparticles, thereby decreasing the charge transfer resistance. Additionally, the porous texture and large surface promoted the diffusion of mass transfer and enlarged the triple-phase contact area, and the rigid carbon shell provided mechanical strength to gain progressed stability. The evaluated bifunctionality ΔE, which is a small difference, is the reason for excellent reversibility in oxygen electrocatalysis. The authors obtained galvanostatic charge–discharge plots at 10 mA cm−2 and power density status that revealed admirable stability of 70 h in a homemade ZAB (Figure 5) [54].
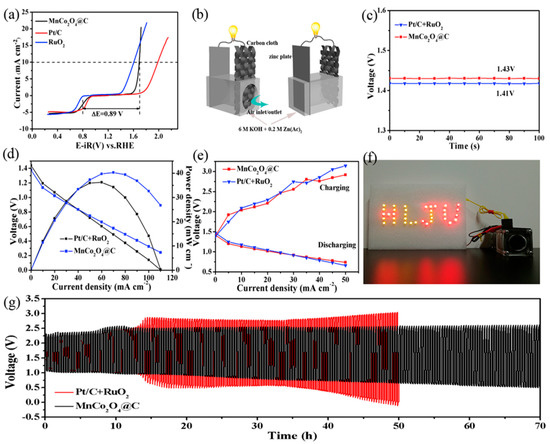
Figure 5.
(a) Linear-sweep curves of MnCo2O4@C, Pt/C, and RuO2 electrodes in O2-saturated 0.1 M KOH solution at a scan rate of 5 mV⋅s−1. (b) Schematic illustration of the homemade Zn–air battery (ZAB). (c) Open-circuit potential plots. (d) Discharge curves of ZAB and the corresponding power density plots. (e) Charge and discharge polarization curves for the ZAB. (f) Photograph of LEDs powered by two serially connected ZABs with MnCo2O4@C cathodes. (g) Long-term galvanostatic charge–discharge plots at 10 mA⋅cm−2 [54]. Copyright 2019, Elsevier.
A combination of spinel compounds and N-doped carbon enriches the electrocatalytic activity of ORR and OER. Duan et al. adopted this strategy and achieved good OER activity of MnCo2O4 because OER and ORR are the core components of ZABs. Therefore, the authors decorated MnCo2O4 on the N-doped macroporous carbon nanofiber arrays (MnCo2O4/NMCNA). The researchers synthesized the macroporous carbon nanofiber array (MCNA) using the surfactant-templating self-assembly of organic resol and a natural crab shell comprising a well-aligned porous macrostructure as a hard template; they attained an interpenetrating ordered array. The pores were larger in the pristine form than in the MnCo2O4/NMCNA; the authors analyzed the porosity by N2 adsorption-desorption and confirmed microporous structure formation.
Chao Wei et al. stated that the eg occupancy of the active cation in the octahedral site is the activity descriptor for the ORR/OER of spinels, consolidating the role of electron orbital filling in metal oxide catalysis. The spinel structure affords multiple sites for transition-metal cations and an extensive range of valence states to give rise to a large number of oxides. In this work, the researchers varied the temperature to tune the electronic structure of MnCo2O4 cubic spinels, which they prepared by solid-state chemistry. Activity on spinel as a function of the Mn valence state of the active element at the octahedral site resulted in a volcano shape, as displayed in Figure 6a,b. Hence, it was demonstrated that eg theory applies to not only the perovskite family but also to the spinel family [63].
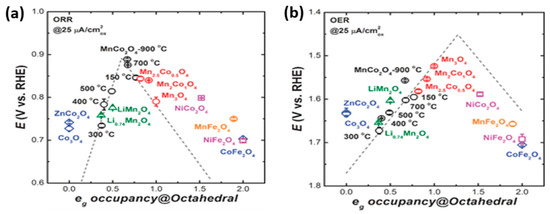
Figure 6.
(a) ORR and (b) OER activity on various spinels as a function of eg occupancy of the active element at the octahedral site. The black circle represents cubic MnCo2O4 spinels with Mn as the active element. The red circle represents the Mn of tetragonal spinels (Mn2CoO4, Mn2.5Co0.5O4, and Mn3O4). The orange circle represents the Mn of ferrite. The green “up” triangle represents the Mn of cubic LixMn2O4 spinels. The blue diamond represents the Co of cubic spinels (Co3O4, ZnCo2O4, and CoFe2O4). The pink square represents the Ni of cubic spinels (NiCo2O4 and NiFe2O4) [63]. Copyright 2017, John Wiley and Sons.
2.2. CuCo2O4 Spinels
The spinel structures with cobalt in the octahedral sites are more resourceful for the OER and ORR, and copper-based materials have exhibited excellent ORR and OER catalytic activity due to the intense interfacial contact of Cu and its surroundings [64,65]. In Cheng et al.’s work, nitrogen-doped carbon nanotubes were ornamented by spinel CuCo2O4 quantum dots (CuCo2O4/N-CNTs) with successful Co3O4 doping without changing its crystalline structure. Carbon nanotubes (CNTs) are used to confer high electrical conductivity, large surface area, and intrinsic flexibility. The CNTs are doped to solve the problem of weak catalytic performance. CuCo2O4/N-CNTs was tested in liquid alkaline and a solid-state electrolyte because of its ORR (onset potential of −0.04 V, the large diffusion-limited current density of −5.53 mA⋅cm−2, and low Tafel slope of 76.53 mV⋅dec−1) and OER (−0.61 V with a potential of 0.69 V to get 10 mA⋅cm−2 and low Tafel slope of 118.80 mV⋅dec−1 compared with Co3O4/N-CNTs, CuO/N-CNTs, and CuCo2O4). The capability of Cu and Co as ORR and OER catalysts gives a ΔE value of 0.90 V. It is evident from the analysis of the other materials that CuO/N-CNTs outperform the Co3O4/N-CNTs in ORR, and the reverse was true for OER.
To learn the role of the N-CNTs, researchers performed electrochemical impedance spectrum (EIS), electrochemical double-layer capacitance (Cdl), and XPS analysis. In the findings, with lower resistance (EIS) and greater capacitance (electrochemically active surface area) along with BET measurement (11.18 m2 g−1), N-CNT provided a large surface area and restricted the aggregation and bonding between N and Cu; additionally, Co offered pyridine nitrogen for good ORR by generating more catalytic sites in CuCo2O4. The catalytic process of CuCo2O4/N-CNT is illustrated in Figure 7. Further, the authors measured the chronoamperometry and chronopotentiometry to identify the stability and used a rotational ring disk electrode to identify the number of electrons transferred.
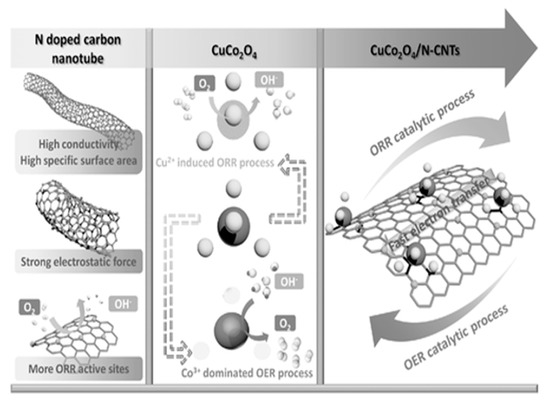
Figure 7.
The catalytic process of CuCo2O4/N-CNTs [39]. Copyright 2017, John Wiley and Sons.
Methanol tolerance results are demonstrated as a control measure to examine the analyzed material as a stable catalyst for the ORR and ve high durability towards the poisoning of any oxidized products [39,41,66,67,68]; CuCo2O4/N-CNTs was found to be a promising oxygen electrocatalyst comparable with Pt/C (ORR) and IrO2 (OER) with a power density of 83.83 mW cm−2 and energy density of 653.9 Wh kg−1. Investigators tested the ZAB stability at 20 mA cm−2, and after 24 h, both the Pt/IrO2 electrode and CuCo2O4/N-CNTs showed some slipping. CuCo2O4/N-CNTs, with their lower overpotential than that of noble metal electrodes, showed very stable charging and discharging potential even after 48 h [39].
To rectify the drawback of less surface area between the active material and the electrolyte in the carbon matrix, Xiaojun Wang et al. electrospun the CuCo2O4@C nanotubes via coaxial electrospinning. The researchers studied the morphology and nanostructure of the obtained cubic spinel-type CuCo2O4 by scanning electron microscopy (SEM) and transmission electron microscopy (TEM). SEM images showed that CuCo2O4@C nanotubes formed with an ample number of pores with a rough texture and unremitting length; this could be the reason for the increased diffusion kinetics of hydroxide and the adsorption–desorption of oxygen. The TEM image showed the outer and inner diameters of the nanotube (130 nm and ∼80 nm), wall thickness (25 nm), and double the active surface area, which enhanced the contact between hydroxyl, oxygen, and catalyst. The small CuCo2O4 nanoparticles increased the utilization rate. Figure 8 shows the uniform distribution of Cu, Co, O, C, and N with a hollow carbon substrate confirmed by TEM elemental mapping. The carbonization temperature varied from 700 to 900 °C. Compared with H-CuCo2O4 and L-CuCo2O4@C (higher and lower oxidation temperature), CuCo2O4@C exhibited improved OER (low overpotential of 327 mV at 10 mA cm−2), ORR (positive half-wave potential of 0.850 V), and electrochemically active surface area; CuCo2O4@C (313.8 cm2), L-CuCo2O4@C (80.0 cm2), and H-CuCo2O4 (61.3 cm2) showed better interaction of reactants with the active surface area, as shown in Figure 9. The results showed the excellent catalytic activity of CuCo2O4@C. In the full cell test, the discharge−charge voltage gap of 0.79 V at 10 mA cm−2 cycled up to 160 numbers for 80 h. The methanol tolerance of the material is higher than that of Pt. Additionally, N-doping and interconnected 1D open-ended nanotubes might play roles in oxygen catalysis and conductivity [66].
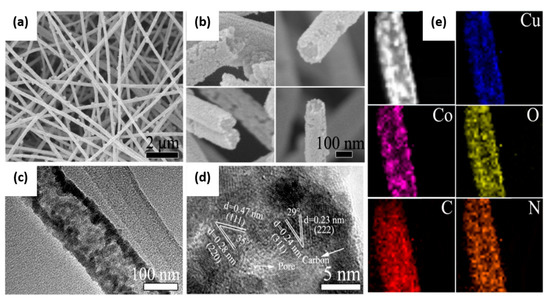
Figure 8.
(a,b) Scanning electron microscopy (SEM) and (c,d) transmission electron microscopy (TEM) images of CuCo2O4@C. (e) TEM elemental mapping images of CuCo2O4@C [66]. Copyright 2017, American Chemical Society.
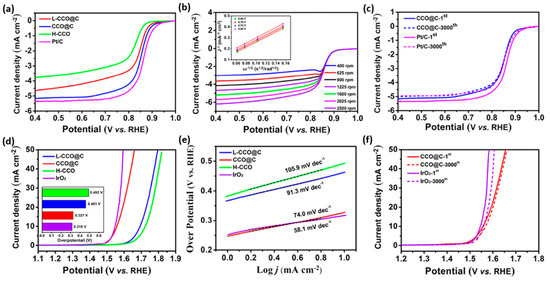
Figure 9.
(a) ORR polarization curves of L-CuCo2O4@C, CuCo2O4@C, H-CuCo2O4, and Pt/C samples. (b) ORR polarization curves of the CuCo2O4@C nanotubes at different rotation speeds and the corresponding K−L plots at different potentials (inset). (c) ORR polarization curves of CuCo2O4@C and Pt/C samples before and after 3000 potential cycles. (d) OER polarization curves of L-CuCo2O4@C, CuCo2O4@C, H-CuCo2O4, IrO2, and the overpotential schematic at 10 mA cm−2 (inset). (e) Tafel plots derived from (d). (f) OER polarization curves of CuCo2O4@C and IrO2 during the cycling durability test [66]. Copyright 2017, American Chemical Society.
2.3. NiCo2O4 Spinels
In the group of transition metals, nickel has emerged as one of the most hopeful electrocatalysts due to its exciting electronic properties and anticipated synergistic effect to alter surface properties of materials to dramatically favor electrocatalysis. The poor conductivity of electrons and noteworthy self-aggregation of Ni particles in the Nickel-based spinels control their application as electrocatalysts [69]. Bohong Chen et al. synthesized NixCo(3-x)O4 (x = 1.25) on aminated carbon nanotubes (NH2-CNTs) in a composition-controlled technique to rectify the above issues. NH2-CNTs make the catalyst more active by providing conductivity and more surface area for fast charge transfer, and the easy diffusion of the catalyst. This took place through a hydrothermal reaction in the presence of nickel ions from Co3O4/NH2-CNTs, in which the cation exchanged. Nickel ions amend the metal oxide nucleation and growth due to the hydrolysis difference between cobalt and nickel ions. This exchange does not change the size, crystal structure, and weight of the metal oxide. Nitrogen in NH2-CNTs fastens the nanoparticles and, in so doing, improved the electronic coupling along with amine. As usual, the researchers verified ORR in N2/O2-saturated 0.1 M KOH electrolyte, and the O2-saturated material performed better. Though its onset and half-wave potential were close to those of Pt/C, its limiting current density was more than in Pt/C. The researchers also confirmed the electron transfer number that resulted from the KL plot slope and rotating ring disk electrode technique. After ORR, investigators verified OER in O2-saturated 0.1 M KOH solution and found that it has a small overpotential and Tafel slope. They analyzed the bifunctionality of NixCo(3−x)O4/NH2-CNTs from the ΔE, the difference in the potentials between ORR and OER (OER (the potential of the current density at 10 mA cm−2 -Ej10) and ORR current density at 3 mA cm−2). ΔE of this material was lower than the best bifunctional electrocatalysts reported. There was a nil effect in the methanol tolerance test, as shown in Figure 10. NixCo(3−x)O4/NH2-CNTs was applied in a ZAB and exhibited a power density of 168 mW⋅cm−2 and 100 charge–discharge cycles without a change in voltage [67].
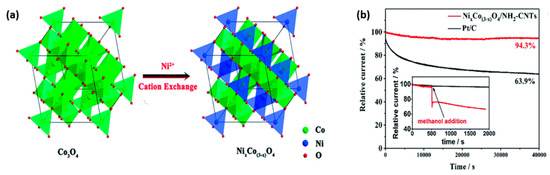
Figure 10.
(a) Illustration of crystal phase preservation during cation exchange. (b) Chronoamperometric curves of the NixCo(3x)O4/NH2-CNTs and Pt/C at 0.7 V. The inset shows the Chronoamperometric curves of the NixCo(3x)O4/NH2-CNTs and Pt/C with the addition of 1.0 M methanol into O2-saturated 0.1 M KOH [67]. Copyright 2013, Royal Society of Chemistry.
Designing efficient bifunctional electrocatalysts for OER/ORR requires a deep understanding of the mechanisms at the atomic level in the applied material. As such, in 2018, Xue-Rui Wang et al. found that pyridinic-N-Co bonding promoted reversible synergistic oxygen electrocatalysis better than pyrrolic-N bonding in the hybrid catalyst, NiCo2O4 nanoparticle/N-doped mesoporous graphene. Using low-intensity pulsed-laser irradiation, the researchers could engineer pyridinic and pyrrolic N contents in NiCo2O4/N-graphene (NiCo/NG) hybrids by tuning the in-plane mesopores onto graphene oxide (GO) sheets.
The authors also tuned the pyridinic and pyrrolic nitrogen content by doping nitrogen atoms into GO and mesoporous graphene oxides with different mesopore densities by a conventional hydrothermal route using ammonia as a nitrogen precursor and the doped samples. The mesopores paved the way for the formation of pyridinic-N-Co by providing the edges as space. Pyridinic-N-Co contributed to the synergistic effect, coordinating with the metal ions at the interface of the hybrid material for the more-activated oxygen reduction and evolution reaction. Thus, NiCo2O4/270 energy pulsed-laser irradiation-treated N-doped graphene oxide (NiCo/NLG-270) displays better charge and discharge performance and long-term durability than the noble metal electrocatalysts in the oxidation and reduction of oxygen (Figure 11), and this was also demonstrated from the DFT calculations.
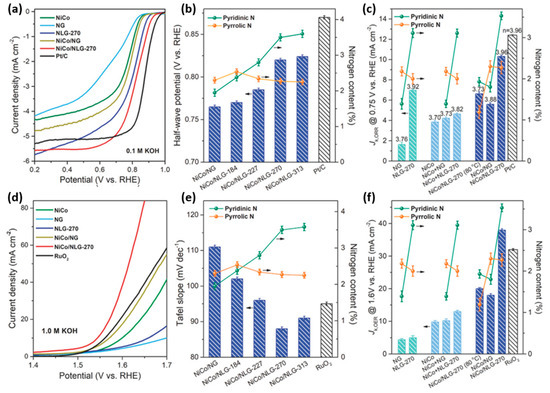
Figure 11.
(a) ORR linear sweep voltammetry (LSV) curves of NG, NLG-270, NiCo2O4 NPs, NiCo/NG, NiCo/NLG-270, and Pt/C, in 0.1 M KOH solution (rotation rate: 1600 rpm). (b) ORR half-wave potential of the hybrid catalysts with different contents of pyridinic and pyrrolic N bonded with Co ions, and Pt/C. (c) Comparison of the kinetic currents and the electron transfer number for the ORR process of the control samples, along with the corresponding contents of pyridinic and pyrrolic N bonded with Co. (d) ORR LSVs of NG, NLG-270, NiCo2O4 NPs, NiCo/NG, NiCo/NLG-270, and Pt/C, in 1 M KOH solution. (e) OER Tafel plots of the hybrid catalysts with different contents of pyridinic and pyrrolic N bonded with Co ions, and RuO2. (f) Comparison of the kinetic currents for the OER process of the control samples, along with the corresponding contents of pyridinic and pyrrolic N bonded with Co [45]. Copyright 2018, John Wiley and Sons.
More detailed mechanisms of the spinel structure and the occupancy of the ions in the octahedral and tetrahedral sites are necessary to engineer better catalysts [45]. Jun Zhao et al. synthesized spinel-shaped nickel cobalt oxide nanoplates (NCO) by coprecipitation, followed by sintering to study the active sites–activity relationship. The authors effectively controlled the valency of the catalyst and engineered the metal active sites of Ni3+/Ni2+ and Co3+/Co2+ in NCO through annealing. The Co(Octahedral)3+ changed to be Co(Tetrahedral)2+ as Ni ions were doped in the structure. Thus, the Co3+/Co2+ ratio was higher in the oxide samples synthesized at higher annealing temperatures. At low temperature, NCO annealed at 250 °C with high Ni3+/Ni2+ sites and low Co3+/Co2+ sites, and porous 2D nanosheets, which increased the surface area and exhibited superior OER and ORR (ΔE = 0.8 V of reduced value). The power density of the battery reached 166 mW cm−2 [70].
2.4. Fe-Based Spinels
Mn–Fe-based spinels are highly electrocatalytically active for the cathode and anode redox reactions on oxygen electrodes. The increment of active sites ensures the further boosted catalytic efficacy [71]. Xiuju Wu et al. prepared spinel MnFe2O4/Fe hybrid nanoparticles in nitrogen-doped mesoporous hollow carbon nanospheres (Fe/Mn-N-C) by pyrolysis to increase the active centers with the addition of Fe. Though the spinel-built oxides showed good catalytic efficacy, the conductivity was inefficient. Here, the porous nature of the catalyst augments the OER and ORR activity of a transition metal spinel oxide.
The ORR and OER evaluation results showed that the material with a 2:1 ratio of Fe3+ to Mn2+ was nearly the same as Pt for OER and better than Pt in terms of methanol tolerance, ORR, and ZAB full cell tests. In ORR, Fe/Mn-N-C exhibited slightly more positive onset potential and half-wave potential and, surprisingly, much lower current density than Pt/C. TEM images suggested that the ordered graphitic carbon was responsible for good conductivity and high corrosion resistance and that the disordered carbon accommodated the number of M-Nx active centers. Although metal agglomeration somewhat increased the current density, the onset value was the same with and without its presence, demonstrating efficient methanol tolerance, durability, and cyclability. The power density of Fe/Mn-N-C (37 mW cm−2) and Pt/C (28 mW cm−2) exhibits its capacity as an excellent oxygen electrocatalyst [41]. Yu-Ju Chien et al. engineered a ternary-spinel oxide based on the octahedral site preference energy (OSPE) model; the OSPE is the difference between crystal field stabilization energies at octahedral and tetrahedral sites (CFSEoct and CFSEtet). The authors synthesized ternary-spinel oxides (i.e., M0.1Ni0.9Co2O4 with M: Mn, Fe, Cu, and Zn) by hydrothermal and annealing processes. The inverse spinel structure of NiCo2O4, in which Ni atoms were in the octahedral sites and Co atoms occupied both octahedral and tetrahedral sites, was confirmed by XRD. Further, the same spinel structures were found in Mn0.1Ni0.9Co2O4, Fe0.1Ni0.9Co2O4, Cu0.1Ni0.9Co2O4, and Zn0.1Ni0.9Co2O4 (MNCO-01, FNCO-01, CNCO-01, and ZNCO-01), respectively. Due to the cation substitution in Ni and Co, the lattice expanded and contracted. The oxidation state of these doped metal ions can alter the mean oxidation states of Ni and Co; therefore, the site preferences of all metallic ions can vary. The activity of the catalyst is based on the metal atoms’ occupancy sites in the spinel structure. The authors calculated the OSPE following the crystal field theory, and there were more cations at the octahedral sites than at the tetrahedral sites at higher OSPE. The inverse spinel structure had Co2+ and Co3+ at the tetrahedral sites and Co3+, Ni2+, and Ni3+ at the octahedral sites. The abovementioned metals were doped by substituting for Co2+, Co3+, Ni2+, or Ni3+ based on their OSPE. The OSPE of the doped metals is given in Figure 12a [11].
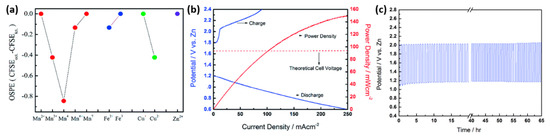
Figure 12.
(a) The Octahedral site preference energy (OSPE) diagram of Mn, Fe, Cu, and Zn ions at various oxidation states. (b) The discharge–charge profiles under galvanodynamic (1 mA⋅s−1) mode and the corresponding power density profile for the ZAB using the FNCO-01-coated air electrode under ambient air and (c) the steady-state discharge–charge profile under galvanostatic (10 mA⋅cm−2, 40 min per cycle) mode for a ZAB using the FNCO-01-coated air electrode in 6 M KOH and 0.2 M Zn(CH3COO)2 in ambient air [40]. Copyright 2013, Royal Society of Chemistry.
Surprisingly, Fe3+ in FNCO-01, with a small OSPE, substituted smoothly for Co3+, with an increased Co2+/Co3+ ratio and decreased Ni2+/Ni3+ (Fe2+ is replaced by Ni2+ because of large OSPE) FNCO-01 with better oxygen-evolving and oxygen-reducing activity because of its high voltammetric charge, onset potential of 1.503 V (vs. RHE), a relatively high order of ORR onset potential, and electron transfer number of 3.65. In the ZAB test, a rare phenomenon occurs because of the redox transitions of Ni and Co species prior to the OER (Figure 12b). In the current density vs. potential vs. power density graph, the cell voltage increases suddenly due to the high overpotential, and a much higher power density of 150 mW cm−2 occurs at a current density of 250 mA cm−2. The wettability of FNCO-01 with an anhydrous surface reduced the discharge cell voltages in the initial five cycles, and after 5–10 charge–discharge cycles, OER improved the wettability of catalysts at nearly 100 cycles without any change (Figure 12c) [40].
In the above reports, researchers designed various spinel structured cathode materials with different morphologies and compositions that greatly improved their OER and ORR performance. Table 1 summarizes the recent research on the bifunctional electrocatalytic activity of the spinel-structured compounds in ZABs, and Figure 13 presents the ZAB power densities for the varied spinels as the electrocatalysts. In summary, spinel-based compounds that consist of Ni, Mn, Cu, and Fe at the A site and Co, Mn, Fe, Ni at the B site with oxygen at the X site have been widely used in ZABs as electrocatalysts. Other halogens can replace the X site, for instance, selenide, sulfide, and nitride. With the better bifunctional activity of spinels, strategies such as improving porosity and surface area, nanostructuring, carbon coating, doping with nitrogen, phase change, etc., could greatly improve the catalytic activity.

Table 1.
Summary of OER/ORR performance of recently reported bifunctional spinel electrocatalysts.
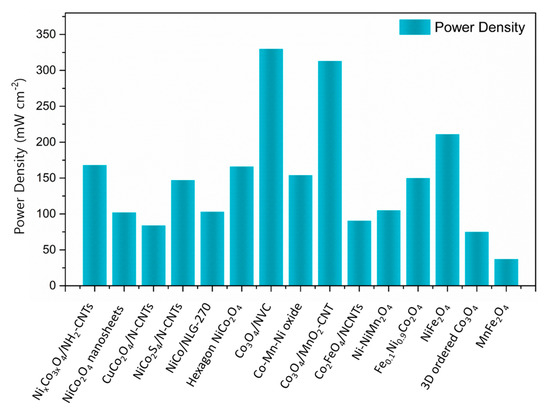
Figure 13.
Power density of ZABs with various electrocatalysts in the spinel structure [39,40,41,45,71,72,74,75,76,78,82,85,88,90,92].
3. Conclusions and Perspectives
In this review, we have summarized some strategies from the literature on applying spinel-based compounds as the bifunctional oxygen electrocatalysts in the air cathodes of ZABs. The authors of all the studies we reviewed had aimed at improving battery performance. Among the post-Li-ion battery energy storage devices, ZABs, which operate in alkaline electrolytes, have the most advantages required for addressing the energy, safety, and environmental problems. The spinel-structured materials catalyze the lethargic ORR and OER activity in ZABs through their better intrinsic properties. ORR and OER can be improved with the engineering of structures, active sites, morphologies, interfaces, surface areas, porosities, etc. by compositing, using conductive substrates, creating defects, modifying the synthesis procedure, and hybridizing with other materials. Thus, the spinel-based materials contribute to electrocatalysts that surpass the noble electrocatalysts. However, the lack of analysis of intrinsic specific activity, synergistic effects between the compounds in spinel and their hybrids, and atomic-level data calls for more detailed investigation of spinels with the help of theoretical inquiries and in operando analysis. In addition, spinels are used to increase the discharge capacity and decrease the discharge−charge gap in both aqueous and aprotic electrolytes. The most promising spinels are transition-metal oxides used as bifunctional electrocatalysts because of their low price and high catalytic efficiency [43,46]. Therefore, the analysis needs to be extended to spinel-sulfides, selenides, and nitrides in acidic and nonaqueous electrolytes. The distribution of cations in spinels also warrants exploration, using factors such as cation radius, Coulombic interactions between the cations, and crystal field effects of the octahedral site preference energy of cations.
Overall, several research efforts have been devoted to investigating ZABs as well as designing bifunctional ZAB electrocatalysts. On considering the practical usage of ZABs, excellent performance and long-term stability are the key properties that promote their adoption. Consequently, the catalyst needs to be designed with the maximum exposure of active sites. Critical anode behavior, cathode architecture, three-phase interfacial reactions at the gas diffusion layer, ZAB fabrication procedure, and electrolyte effects in ZABs should also be considered during catalyst design. With the development of theory, techniques of characterization, innovative synthesis methods, catalyst design, and in operando analysis, the mechanistic features of spinel-based electrocatalysts in ZABs can be unleashed, and practical applications of ZABs will become a reality. In short, the challenge remains to design well-performing spinel-based electrocatalysts along with the commercialization of sustainable clean energy techniques, such as metal–air and metal–ion batteries, water splitting, supercapacitors, and CO2, CO, and NOx reduction.
Funding
This research received no external funding.
Acknowledgments
The authors acknowledge this work supported by the National Research Foundation of Korea grant funded by the Ministry of Science, ICT & Future Planning, Republic of Korea (2018R1A5A1025224) and the Human Resources Development program of the Korea Institute of Energy Technology Evaluation and Planning (KETEP) grant funded by the Korea government Ministry of Trade migrant number 20194030202470.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pan, J.; Xu, Y.Y.; Yang, H.; Dong, Z.; Liu, H.; Xia, B.Y. Advanced architectures and relatives of air electrodes in zn–air batteries. Adv. Sci. 2018, 5, 1700691. [Google Scholar] [CrossRef]
- Chu, S.; Cui, Y.; Liu, N. The path towards sustainable energy. Nat. Mater. 2016, 16, 16. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, N. Nanostructured electrode materials for high-energy rechargeable li, na and zn batteries. Chem. Mater. 2017, 29, 9589–9604. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, N. Visualizing battery reactions and processes by using in situ and in operando microscopies. Chem 2018, 4, 438–465. [Google Scholar] [CrossRef]
- Qian, Z.; Chen, Y.; Tang, Z.; Liu, Z.; Wang, X.; Tian, Y.; Gao, W. Hollow nanocages of nixco1−xse for efficient zinc–air batteries and overall water splitting. Nano-Micro Lett. 2019, 11, 28. [Google Scholar] [CrossRef]
- Yoo, H.D.; Markevich, E.; Salitra, G.; Sharon, D.; Aurbach, D. On the challenge of developing advanced technologies for electrochemical energy storage and conversion. Mater. Today 2014, 17, 110–121. [Google Scholar] [CrossRef]
- Li, Y.; Dai, H. Recent advances in zinc–air batteries. Chem. Soc. Rev. 2014, 43, 5257–5275. [Google Scholar] [CrossRef]
- Luntz, A.C.; McCloskey, B.D. Nonaqueous li–air batteries: A status report. Chem. Rev. 2014, 114, 11721–11750. [Google Scholar] [CrossRef]
- Wang, Z.-L.; Xu, D.; Xu, J.-J.; Zhang, X.-B. Oxygen electrocatalysts in metal–air batteries: From aqueous to nonaqueous electrolytes. Chem. Soc. Rev. 2014, 43, 7746–7786. [Google Scholar] [CrossRef]
- Harting, K.; Kunz, U.; Turek, T. Zinc-air batteries: Prospects and challenges for future improvement. Z. Phys. Chem. 2012, 226, 151–166. [Google Scholar] [CrossRef]
- Zhang, X.G. Fibrous zinc anodes for high power batteries. J. Power Sources 2006, 163, 591–597. [Google Scholar] [CrossRef]
- Fu, J.; Cano, Z.P.; Park, M.G.; Yu, A.; Fowler, M.; Chen, Z. Electrically rechargeable zinc–air batteries: Progress, challenges, and perspectives. Adv. Mater. 2017, 29, 1604685. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-S.; Tai Kim, S.; Cao, R.; Choi, N.-S.; Liu, M.; Lee, K.T.; Cho, J. Metal–air batteries with high energy density: Li–air versus zn–air. Adv. Energy Mater. 2011, 1, 34–50. [Google Scholar] [CrossRef]
- Kanan, M.W.; Nocera, D.G. In situ formation of an oxygen-evolving catalyst in neutral water containing phosphate and CO2+. Science 2008, 321, 1072. [Google Scholar] [CrossRef] [PubMed]
- Koper, M.T.M. Thermodynamic theory of multi-electron transfer reactions: Implications for electrocatalysis. J. Electroanal. Chem. 2011, 660, 254–260. [Google Scholar] [CrossRef]
- Fang, Z.; Peng, L.; Lv, H.; Zhu, Y.; Yan, C.; Wang, S.; Kalyani, P.; Wu, X.; Yu, G. Metallic transition metal selenide holey nanosheets for efficient oxygen evolution electrocatalysis. ACS Nano 2017, 11, 9550–9557. [Google Scholar] [CrossRef]
- Masa, J.; Xia, W.; Muhler, M.; Schuhmann, W. On the role of metals in nitrogen-doped carbon electrocatalysts for oxygen reduction. Angew. Chem. Int. Ed. 2015, 54, 10102–10120. [Google Scholar] [CrossRef]
- Bera, R.K.; Park, H.; Ryoo, R. Co3o4 nanosheets on zeolite-templated carbon as an efficient oxygen electrocatalyst for a zinc–air battery. J. Mater. Chem. A 2019, 7, 9988–9996. [Google Scholar] [CrossRef]
- Ibraheem, S.; Chen, S.; Li, J.; Wang, Q.; Wei, Z. In situ growth of vertically aligned fecoooh-nanosheets/nanoflowers on fe, n co-doped 3d-porous carbon as efficient bifunctional electrocatalysts for rechargeable zinc–o2 batteries. J. Mater. Chem. A 2019, 7, 9497–9502. [Google Scholar] [CrossRef]
- Zhao, Q.; Yan, Z.; Chen, C.; Chen, J. Spinels: Controlled preparation, oxygen reduction/evolution reaction application, and beyond. Chem. Rev. 2017, 117, 10121–10211. [Google Scholar] [CrossRef]
- Xu, M.; Hou, X.; Yu, X.; Ma, Z.-F.; Yang, J.; Yuan, X. Spinel nico2s4 as excellent bi-functional cathode catalysts for rechargeable li-o2 batteries. J. Electrochem. Soc. 2019, 166, F406–F413. [Google Scholar] [CrossRef]
- Ahuja, B.L.; Dashora, A.; Heda, N.L.; Tiwari, S.; Rajeevan, N.E.; Itou, M.; Sakurai, Y.; Kumar, R. Reversal of orbital magnetic moment on substitution of bi in multiferroic co2mno4: A magnetic compton scattering study. Appl. Phys. Lett. 2010, 97, 212502. [Google Scholar] [CrossRef]
- Grimes, R.W.; Anderson, A.B.; Heuer, A.H. Predictions of cation distributions in ab2o4 spinels from normalized ion energies. J. Am. Chem. Soc. 1989, 111, 1–7. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Loeb, A.L. Theory of ionic ordering, crystal distortion, and magnetic exchange due to covalent forces in spinels. Phys. Rev. 1955, 98, 391–408. [Google Scholar] [CrossRef]
- Kim, S.Y.; Yu, A.; Lee, Y.; Kim, H.Y.; Kim, Y.J.; Lee, N.-S.; Lee, C.; Lee, Y.; Kim, M.H. Single phase of spinel co2rho4 nanotubes with remarkably enhanced catalytic performance for the oxygen evolution reaction. Nanoscale 2019, 11, 9287–9295. [Google Scholar] [CrossRef]
- Liu, J.; Bao, H.; Zhang, B.; Hua, Q.; Shang, M.; Wang, J.; Jiang, L. Geometric occupancy and oxidation state requirements of cations in cobalt oxides for oxygen reduction reaction. ACS Appl. Mater. Interfaces 2019, 11, 12525–12534. [Google Scholar] [CrossRef]
- Davari, E.; Ivey, D.G. Bifunctional electrocatalysts for zn–air batteries. Sustain. Energy Fuels 2018, 2, 39–67. [Google Scholar] [CrossRef]
- Hardwick, L.J.; De León, C.P. Rechargeable multi-valent metal-air batteries. Johns. Matthey Technol. Rev. 2018, 62, 134–149. [Google Scholar] [CrossRef]
- Linden, D.; Reddy, T. Handbook of Batteries; Mcgraw-Hill: New York, NY, USA, 2001. [Google Scholar]
- Fu, G.; Tang, Y.; Lee, J.-M. Recent advances in carbon-based bifunctional oxygen electrocatalysts for zn−air batteries. ChemElectroChem 2018, 5, 1424–1434. [Google Scholar] [CrossRef]
- Ge, J.; Higier, A.; Liu, H. Effect of gas diffusion layer compression on pem fuel cell performance. J. Power Sources 2006, 159, 922–927. [Google Scholar] [CrossRef]
- Su, H.; Xu, Q.; Chong, J.; Li, H.; Sita, C.; Pasupathi, S. Eliminating micro-porous layer from gas diffusion electrode for use in high temperature polymer electrolyte membrane fuel cell. J. Power Sources 2017, 341, 302–308. [Google Scholar] [CrossRef]
- Cheng, F.; Chen, J. Metal–air batteries: From oxygen reduction electrochemistry to cathode catalysts. Chem. Soc. Rev. 2012, 41, 2172–2192. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Wang, X.; Wen, C. High energy density metal-air batteries: A review. J. Electrochem. Soc. 2013, 160, A1759–A1771. [Google Scholar] [CrossRef]
- Fu, J. Material design and engineering for polymer electrolyte membrane zinc-air batteries. UWSpace, 23 March 2018. [Google Scholar]
- Hannan, M.A.; Hoque, M.M.; Mohamed, A.; Ayob, A. Review of energy storage systems for electric vehicle applications: Issues and challenges. Renew. Sustain. Energy Rev. 2017, 69, 771–789. [Google Scholar] [CrossRef]
- Farmer, E.D.; Webb, A.H. Zinc passivation and the effect of mass transfer in flowing electrolyte. J. Appl. Electrochem. 1972, 2, 123–136. [Google Scholar] [CrossRef]
- Pei, P.; Wang, K.; Ma, Z. Technologies for extending zinc–air battery’s cyclelife: A review. Appl. Energy 2014, 128, 315–324. [Google Scholar] [CrossRef]
- Cheng, H.; Li, M.-L.; Su, C.-Y.; Li, N.; Liu, Z.-Q. Cu—Co bimetallic oxide quantum dot decorated nitrogen-doped carbon nanotubes: A high-efficiency bifunctional oxygen electrode for zn–air batteries. Adv. Funct. Mater. 2017, 27, 1701833. [Google Scholar] [CrossRef]
- Lu, Y.-T.; Chien, Y.-J.; Liu, C.-F.; You, T.-H.; Hu, C.-C. Active site-engineered bifunctional electrocatalysts of ternary spinel oxides, m0.1ni0.9co2o4 (m: Mn, fe, cu, zn) for the air electrode of rechargeable zinc–air batteries. J. Mater. Chem. A 2017, 5, 21016–21026. [Google Scholar] [CrossRef]
- Wu, X.; Niu, Y.; Feng, B.; Yu, Y.; Huang, X.; Zhong, C.; Hu, W.; Li, C.M. Mesoporous hollow nitrogen-doped carbon nanospheres with embedded mnfe2o4/fe hybrid nanoparticles as efficient bifunctional oxygen electrocatalysts in alkaline media. ACS Appl. Mater. Interfaces 2018, 10, 20440–20447. [Google Scholar] [CrossRef]
- Fu, G.; Chen, Y.; Cui, Z.; Li, Y.; Zhou, W.; Xin, S.; Tang, Y.; Goodenough, J.B. Novel hydrogel-derived bifunctional oxygen electrocatalyst for rechargeable air cathodes. Nano Lett. 2016, 16, 6516–6522. [Google Scholar] [CrossRef]
- Yin, J.; Li, Y.; Lv, F.; Fan, Q.; Zhao, Y.-Q.; Zhang, Q.; Wang, W.; Cheng, F.; Xi, P.; Guo, S. Nio/con porous nanowires as efficient bifunctional catalysts for zn–air batteries. ACS Nano 2017, 11, 2275–2283. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wan, L.; Lin, Y.; Wang, B. Construction of mass-transfer channel in air electrode with bifunctional catalyst for rechargeable zinc-air battery. Electrochim. Acta 2019, 320, 134564. [Google Scholar] [CrossRef]
- Wang, X.-R.; Liu, J.-Y.; Liu, Z.-W.; Wang, W.-C.; Luo, J.; Han, X.-P.; Du, X.-W.; Qiao, S.-Z.; Yang, J. Identifying the key role of pyridinic-n–co bonding in synergistic electrocatalysis for reversible orr/oer. Adv. Mater. 2018, 30, 1800005. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, T.; Yokoe, K.; Miyano, T.; Kusaba, H. Mesoporous mnco2o4 spinel oxide for a highly active and stable air electrode for zn-air rechargeable battery. Electrochim. Acta 2019, 300, 455–460. [Google Scholar] [CrossRef]
- Li, B.-Q.; Tang, C.; Wang, H.-F.; Zhu, X.-L.; Zhang, Q. An aqueous preoxidation method for monolithic perovskite electrocatalysts with enhanced water oxidation performance. Sci. Adv. 2016, 2, e1600495. [Google Scholar] [CrossRef]
- Miner, E.M.; Fukushima, T.; Sheberla, D.; Sun, L.; Surendranath, Y.; Dincă, M. Electrochemical oxygen reduction catalysed by ni3(hexaiminotriphenylene)2. Nat. Commun. 2016, 7, 10942. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, H.-L.; Guo, S. Towards high-efficiency nanoelectrocatalysts for oxygen reduction through engineering advanced carbon nanomaterials. Chem. Soc. Rev. 2016, 45, 1273–1307. [Google Scholar] [CrossRef]
- Chen, A.; Holt-Hindle, P. Platinum-based nanostructured materials: Synthesis, properties, and applications. Chem. Rev. 2010, 110, 3767–3804. [Google Scholar] [CrossRef]
- Lee, Y.; Suntivich, J.; May, K.J.; Perry, E.E.; Shao-Horn, Y. Synthesis and activities of rutile iro2 and ruo2 nanoparticles for oxygen evolution in acid and alkaline solutions. J. Phys. Chem. Lett. 2012, 3, 399–404. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Choi, B.; Kim, Y.-B. Development of highly active bifunctional electrocatalyst using Co3O4 on carbon nanotubes for oxygen reduction and oxygen evolution. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Iwakura, C.; Honji, A.; Tamura, H. The anodic evolution of oxygen on Co3O4 film electrodes in alkaline solutions. Electrochim. Acta 1981, 26, 1319–1326. [Google Scholar] [CrossRef]
- Shenghai, C.; Liping, S.; Fanhao, K.; Lihua, H.; Hui, Z. Carbon-coated mnco2o4 nanowire as bifunctional oxygen catalysts for rechargeable zn-air batteries. J. Power Sources 2019, 430, 25–31. [Google Scholar] [CrossRef]
- Black, R.; Lee, J.H.; Adams, B.; Mims, C.A.; Nazar, L.F. The role of catalysts and peroxide oxidation in lithium–oxygen batteries. Angew. Chem. Int. Ed. 2013, 52, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Ryu, W.-H.; Yoon, T.-H.; Song, S.H.; Jeon, S.; Park, Y.-J.; Kim, I.-D. Bifunctional composite catalysts using co3o4 nanofibers immobilized on nonoxidized graphene nanoflakes for high-capacity and long-cycle li–o2 batteries. Nano Lett. 2013, 13, 4190–4197. [Google Scholar] [CrossRef]
- Nikolov, I.; Darkaoui, R.; Zhecheva, E.; Stoyanova, R.; Dimitrov, N.; Vitanov, T. Electrocatalytic activity of spinel related cobalties mxco3− xo4 (m = li, ni, cu) in the oxygen evolution reaction. J. Electroanal. Chem. 1997, 429, 157–168. [Google Scholar] [CrossRef]
- Diaz-Morales, O.; Ledezma-Yanez, I.; Koper, M.T.; Calle-Vallejo, F. Guidelines for the rational design of ni-based double hydroxide electrocatalysts for the oxygen evolution reaction. ACS Catal. 2015, 5, 5380–5387. [Google Scholar] [CrossRef]
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.; Nørskov, J.K.; Jaramillo, T.F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, eaad4998. [Google Scholar] [CrossRef]
- Katsounaros, I.; Cherevko, S.; Zeradjanin, A.R.; Mayrhofer, K.J. Oxygen electrochemistry as a cornerstone for sustainable energy conversion. Angew. Chem. Int. Ed. 2014, 53, 102–121. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Y.; Liang, Y.; Zheng, G.; Li, Y.; Cui, Y.; Dai, H. Rechargeable li–O2 batteries with a covalently coupled MnCo2O4–graphene hybrid as an oxygen cathode catalyst. Energy Environ. Sci. 2012, 5, 7931–7935. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, L.; Vellacheri, R.; Lei, Y. Recent advances in designing and fabricating self-supported nanoelectrodes for supercapacitors. Adv. Sci. 2017, 4, 1700188. [Google Scholar] [CrossRef]
- Wei, C.; Feng, Z.; Scherer, G.G.; Barber, J.; Shao-Horn, Y.; Xu, Z.J. Cations in octahedral sites: A descriptor for oxygen electrocatalysis on transition-metal spinels. Adv. Mater. 2017, 29, 1606800. [Google Scholar] [CrossRef] [PubMed]
- Bin, D.; Guo, Z.; Tamirat, A.G.; Ma, Y.; Wang, Y.; Xia, Y. Crab-shell induced synthesis of ordered macroporous carbon nanofiber arrays coupled with mnco2o4 nanoparticles as bifunctional oxygen catalysts for rechargeable zn–air batteries. Nanoscale 2017, 9, 11148–11157. [Google Scholar] [CrossRef] [PubMed]
- Jahan, M.; Liu, Z.; Loh, K.P. A graphene oxide and copper-centered metal organic framework composite as a tri-functional catalyst for her, oer, and orr. Adv. Funct. Mater. 2013, 23, 5363–5372. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Jin, T.; Meng, J.; Jiao, L.; Zhu, M.; Chen, J. Electrospun thin-walled cuco2o4@c nanotubes as bifunctional oxygen electrocatalysts for rechargeable zn–air batteries. Nano Lett. 2017, 17, 7989–7994. [Google Scholar] [CrossRef]
- Chen, B.; Jiang, Z.; Huang, J.; Deng, B.; Zhou, L.; Jiang, Z.-J.; Liu, M. Cation exchange synthesis of ni x co (3− x) o 4 (x= 1.25) nanoparticles on aminated carbon nanotubes with high catalytic bifunctionality for the oxygen reduction/evolution reaction toward efficient zn–air batteries. J. Mater. Chem. A 2018, 6, 9517–9527. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, Y.; Liu, X.; Chen, L.; Jia, J. In situ formed fe–n doped metal organic framework@ carbon nanotubes/graphene hybrids for a rechargeable zn–air battery. Chem. Commun. 2017, 53, 12934–12937. [Google Scholar] [CrossRef]
- Ma, N.; Jia, Y.A.; Yang, X.; She, X.; Zhang, L.; Peng, Z.; Yao, X.; Yang, D. Seaweed biomass derived (ni, co)/cnt nanoaerogels: Efficient bifunctional electrocatalysts for oxygen evolution and reduction reactions. J. Mater. Chem. A 2016, 4, 6376–6384. [Google Scholar] [CrossRef]
- Zhao, J.; He, Y.; Chen, Z.; Zheng, X.; Han, X.; Rao, D.; Zhong, C.; Hu, W.; Deng, Y. Engineering the surface metal active sites of nickel cobalt oxide nanoplates toward enhanced oxygen electrocatalysis for zn–air battery. ACS Appl. Mater. Interfaces 2019, 11, 4915–4921. [Google Scholar] [CrossRef]
- Singh, N.; Tiwari, S.; Anitha, K.; Singh, R. Electrocatalytic properties of spinel-type mnxfe3–x o4synthesized below 100° c for oxygen evolution in koh solutions. J. Chem. Soc. Faraday Trans. 1996, 92, 2397–2400. [Google Scholar] [CrossRef]
- Liu, W.; Bao, J.; Xu, L.; Guan, M.; Wang, Z.; Qiu, J.; Huang, Y.; Xia, J.; Lei, Y.; Li, H. Nico2o4 ultrathin nanosheets with oxygen vacancies as bifunctional electrocatalysts for zn-air battery. Appl. Surf. Sci. 2019, 478, 552–559. [Google Scholar] [CrossRef]
- An, T.; Ge, X.; Hor, T.S.A.; Goh, F.W.T.; Geng, D.; Du, G.; Zhan, Y.; Liu, Z.; Zong, Y. Co3o4 nanoparticles grown on n-doped vulcan carbon as a scalable bifunctional electrocatalyst for rechargeable zinc–air batteries. RSC Adv. 2015, 5, 75773–75780. [Google Scholar] [CrossRef]
- Qin, Q.; Chen, L.; Wei, T.; Wang, Y.; Liu, X. Ni/nim2o4 (m = mn or fe) supported on n-doped carbon nanotubes as trifunctional electrocatalysts for orr, oer and her. Catal. Sci. Technol. 2019, 9, 1595–1601. [Google Scholar] [CrossRef]
- Tong, X.; Chen, S.; Guo, C.; Xia, X.; Guo, X.-Y. Mesoporous nico2o4 nanoplates on three-dimensional graphene foam as an efficient electrocatalyst for the oxygen reduction reaction. ACS Appl. Mater. Interfaces 2016, 8, 28274–28282. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Wu, X.; Zhong, C.; Deng, Y.; Zhao, N.; Hu, W. Nico2s4 nanocrystals anchored on nitrogen-doped carbon nanotubes as a highly efficient bifunctional electrocatalyst for rechargeable zinc-air batteries. Nano Energy 2017, 31, 541–550. [Google Scholar] [CrossRef]
- Hyun, S.; Shanmugam, S. Hierarchical nickel–cobalt dichalcogenide nanostructure as an efficient electrocatalyst for oxygen evolution reaction and a zn–air battery. ACS Omega 2018, 3, 8621–8630. [Google Scholar] [CrossRef]
- Li, C.; Han, X.; Cheng, F.; Hu, Y.; Chen, C.; Chen, J. Phase and composition controllable synthesis of cobalt manganese spinel nanoparticles towards efficient oxygen electrocatalysis. Nat. Commun. 2015, 6, 7345. [Google Scholar] [CrossRef]
- Ma, T.; Li, C.; Chen, X.; Cheng, F.; Chen, J. Spinel cobalt–manganese oxide supported on non-oxidized carbon nanotubes as a highly efficient oxygen reduction/evolution electrocatalyst. Inorg. Chem. Front. 2017, 4, 1628–1633. [Google Scholar] [CrossRef]
- Naik, K.M.; Sampath, S. Two-step oxygen reduction on spinel nife2o4 catalyst: Rechargeable, aqueous solution-and gel-based, zn-air batteries. Electrochim. Acta 2018, 292, 268–275. [Google Scholar] [CrossRef]
- Prabu, M.; Ketpang, K.; Shanmugam, S. Hierarchical nanostructured nico 2 o 4 as an efficient bifunctional non-precious metal catalyst for rechargeable zinc–air batteries. Nanoscale 2014, 6, 3173–3181. [Google Scholar] [CrossRef]
- Prabu, M.; Ramakrishnan, P.; Shanmugam, S. Comn2o4 nanoparticles anchored on nitrogen-doped graphene nanosheets as bifunctional electrocatalyst for rechargeable zinc–air battery. Electrochem. Commun. 2014, 41, 59–63. [Google Scholar] [CrossRef]
- Park, M.G.; Lee, D.U.; Seo, M.H.; Cano, Z.P.; Chen, Z. 3d ordered mesoporous bifunctional oxygen catalyst for electrically rechargeable zinc–air batteries. Small 2016, 12, 2707–2714. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-Q.; Li, M.; Hua, B.; Wang, Y.; Sun, Y.-F.; Luo, J.-L. A strongly cooperative spinel nanohybrid as an efficient bifunctional oxygen electrocatalyst for oxygen reduction reaction and oxygen evolution reaction. Appl. Catal. B Environ. 2018, 236, 413–419. [Google Scholar] [CrossRef]
- Davari, E.; Johnson, A.D.; Mittal, A.; Xiong, M.; Ivey, D.G. Manganese-cobalt mixed oxide film as a bifunctional catalyst for rechargeable zinc-air batteries. Electrochim. Acta 2016, 211, 735–743. [Google Scholar] [CrossRef]
- Wang, Q.; Xue, Y.; Sun, S.; Yan, S.; Miao, H.; Liu, Z. Facile synthesis of ternary spinel co–mn–ni nanorods as efficient bi-functional oxygen catalysts for rechargeable zinc-air batteries. J. Power Sources 2019, 435, 226761. [Google Scholar] [CrossRef]
- Li, G.; Wang, X.; Fu, J.; Li, J.; Park, M.G.; Zhang, Y.; Lui, G.; Chen, Z. Pomegranate-inspired design of highly active and durable bifunctional electrocatalysts for rechargeable metal–air batteries. Angew. Chem. Int. Ed. 2016, 55, 4977–4982. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Liu, Y.; Zhang, X.; Li, X.; Li, A.; Qiao, J.; Zhang, J. Self-assembly formation of bi-functional co3o4/mno2-cnts hybrid catalysts for achieving both high energy/power density and cyclic ability of rechargeable zinc-air battery. Sci. Rep. 2016, 6, 33590. [Google Scholar] [CrossRef]
- Wei, J.; Zhao, S.; Ji, L.; Zhou, T.; Miao, Y.; Scott, K.; Li, D.; Yang, J.; Wu, X. Reuse of ni-co-mn oxides from spent li-ion batteries to prepare bifunctional air electrodes. Resour. Conserv. Recycl. 2018, 129, 135–142. [Google Scholar] [CrossRef]
- Wang, X.T.; Ouyang, T.; Wang, L.; Zhong, J.H.; Ma, T.; Liu, Z.Q. Redox-inert fe3+ ions in octahedral sites of co-fe spinel oxides with enhanced oxygen catalytic activity for rechargeable zinc–air batteries. Angew. Chem. 2019, 131, 13425–13430. [Google Scholar] [CrossRef]
- Wang, W.; Kuai, L.; Cao, W.; Huttula, M.; Ollikkala, S.; Ahopelto, T.; Honkanen, A.P.; Huotari, S.; Yu, M.; Geng, B. Mass-production of mesoporous mnco2o4 spinels with manganese (iv)-and cobalt (ii)-rich surfaces for superior bifunctional oxygen electrocatalysis. Angew. Chem. Int. Ed. 2017, 56, 14977–14981. [Google Scholar] [CrossRef]
- Xu, N.; Qiao, J.; Nie, Q.; Wang, M.; Xu, H.; Wang, Y.; Zhou, X.-D. Cofe2o4 nanoparticles decorated carbon nanotubes: Air-cathode bifunctional catalysts for rechargeable zinc-air batteries. Catal. Today 2018, 318, 144–149. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).