Probiotic Streptococcus salivarius Reduces Symptoms of Denture Stomatitis and Oral Colonization by Candida albicans
Abstract
:1. Introduction
2. Experimental Section
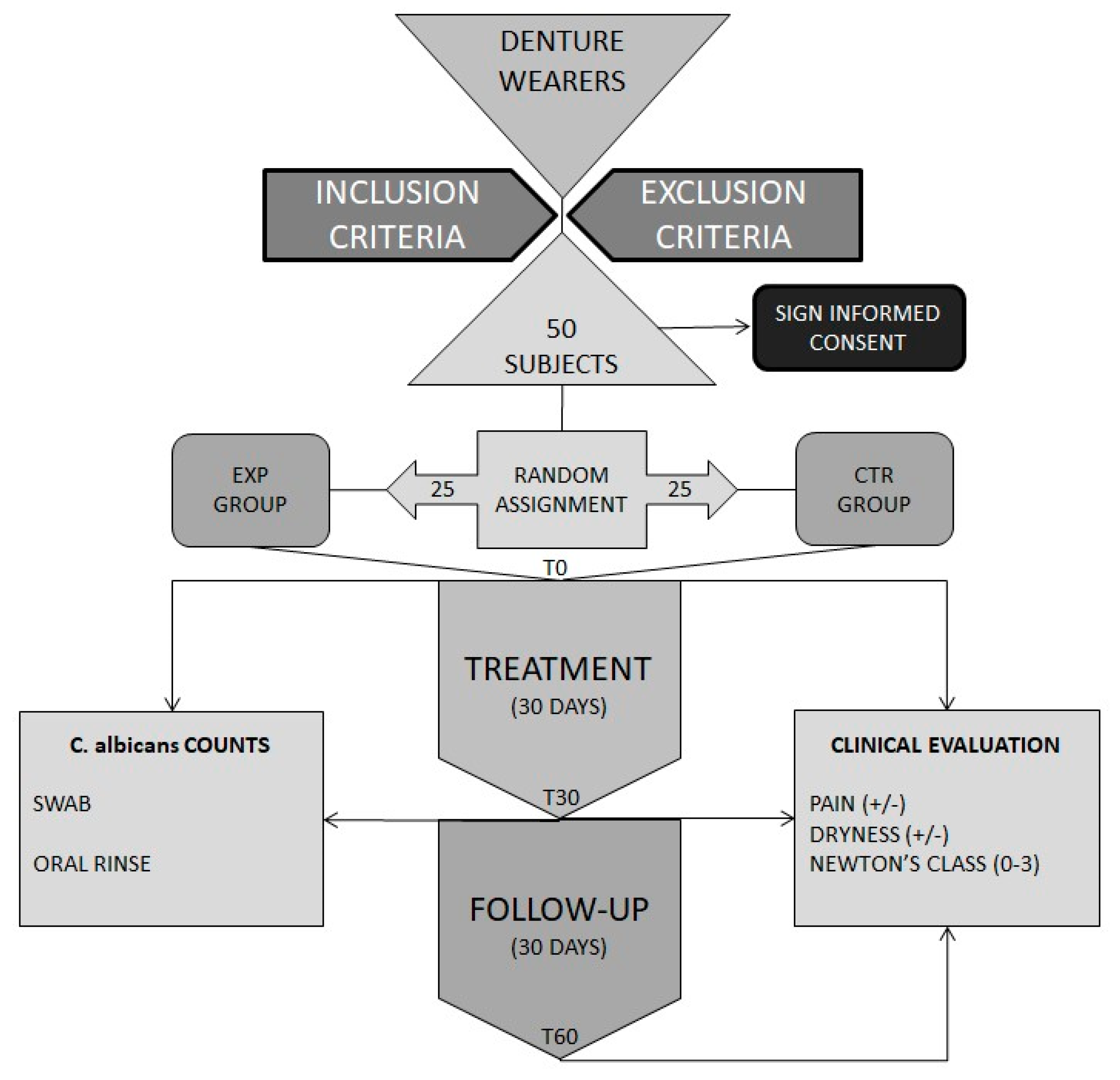
2.1. Studied Subjects and Study Design
2.2. Clinical Evaluation
2.3. Microbiological Samples
2.4. Quantification of Candida Albicans in Samples
2.5. Statistical Analysis
3. Results
3.1. Studied Population
3.2. Probiotic S. salivarius Improved Clinical Conditions
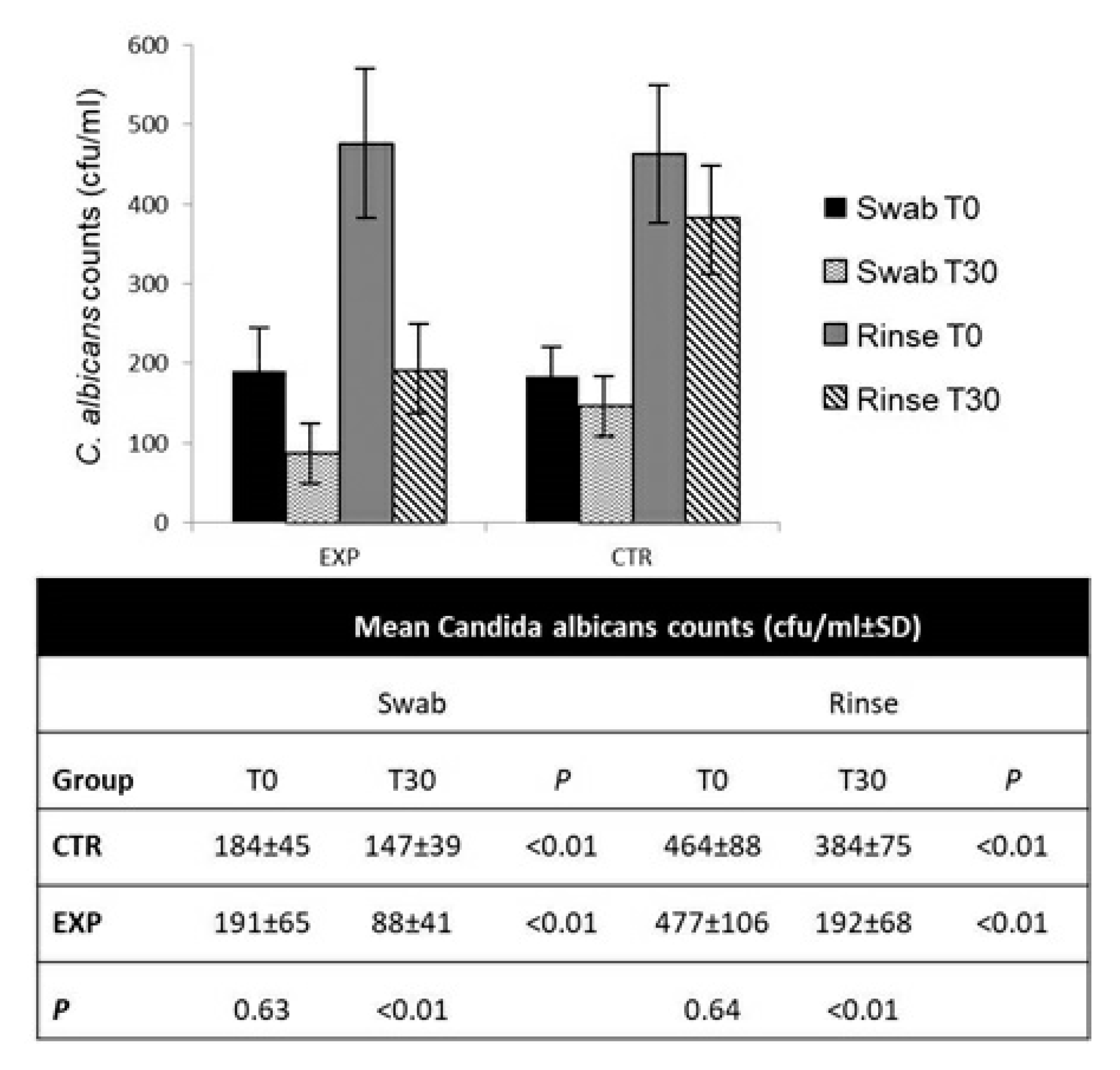
3.3. Probiotic S. salivarius Reduced C. albicans Counts
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bongaarts, J. Human population growth and the demographic transition. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2985–2990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffin, S.O.; Jones, J.A.; Brunson, D.; Griffin, P.M.; Bailey, W.D. Burden of Oral Disease Among Older Adults and Implications for Public Health Priorities. Am. J. Public Health 2012, 102, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Meurman, J.H.; Hämäläinen, P. Oral health and morbidity-implications of oral infections on the elderly. Gerodontology 2006, 23, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Kassebaum, N.; Bernabe, E.; Dahiya, M.; Bhandari, B.; Murray, C.; Marcenes, W. Global Burden of Severe Tooth Loss. J. Dent. Res. 2014, 93, 20S–28S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gendreau, L.; Loewy, Z.G. Epidemiology and Etiology of Denture Stomatitis. J. Prosthodont. 2011, 20, 251–260. [Google Scholar] [CrossRef]
- Gauch, L.M.R.; Pedrosa, S.S.; Silveira-Gomes, F.; Esteves, R.A.; Marques-Da-Silva, S.H. Isolation of Candida spp. from denture-related stomatitis in Pará, Brazil. Braz. J. Microbiol. 2017, 49, 148–151. [Google Scholar] [CrossRef]
- Chevalier, M.; Ranque, S.; Precheur, I. Oral fungal-bacterial biofilm models in vitro: A review. Med. Mycol. 2017, 56, 653–667. [Google Scholar] [CrossRef] [Green Version]
- Morales, D.; Hogan, D.A. Candida albicans Interactions with Bacteria in the Context of Human Health and Disease. PLoS Pathog. 2010, 6, e1000886. [Google Scholar] [CrossRef]
- Shirtliff, M.; Peters, B.M.; Jabra-Rizk, M.A. Cross-kingdom interactions: Candida albicans and bacteria. FEMS Microbiol. Lett. 2009, 299, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Gendron, R.; Grenier, D.; Maheu-Robert, L.-F. The oral cavity as a reservoir of bacterial pathogens for focal infections. Microbes Infect. 2000, 2, 897–906. [Google Scholar] [CrossRef]
- O’Donnell, L.E.; Smith, K.; Williams, C.; Nile, C.J.; Lappin, D.F.; Bradshaw, D.; Lambert, M.; Robertson, D.; Bagg, J.; Hannah, V.; et al. Dentures are a Reservoir for Respiratory Pathogens. J. Prosthodont. 2015, 25, 99–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coulthwaite, L.; Verran, J. Potential pathogenic aspects of denture plaque. Br. J. Biomed. Sci. 2007, 64, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.; Kuldeep; Shamimul, H. Denture Stomatitis: A Literature Review. J. Orofac. Health Sci. 2015, 6, 65. [Google Scholar] [CrossRef]
- Hilgert, J.B.; Giordani, J.M.D.A.; De Souza, R.F.; Wendland, E.M.; D’Avila, O.P.; Hugo, F.N. Interventions for the Management of Denture Stomatitis: A Systematic Review and Meta-Analysis. J. Am. Geriatr. Soc. 2016, 64, 2539–2545. [Google Scholar] [CrossRef] [PubMed]
- Bueno, M.; Urban, V.; Barbério, G.; Da Silva, W.J.; Porto, V.; Pinto, L.; Neppelenbroek, K. Effect of antimicrobial agents incorporated into resilient denture relines on the Candida albicans biofilm. Oral Dis. 2013, 21, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Ramage, G.; Tomsett, K.; Wickes, B.L.; Lopez-Ribot, J.L.; Redding, S.W. Denture stomatitis: A role for Candida biofilms. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2004, 98, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Perfect, J.R.; Lindsay, M.H.; Perfect, J.R.; Drew, R.H. Adverse Drug Reactions to Systemic Antifungals. Drug Saf. 1992, 7, 323–363. [Google Scholar] [CrossRef]
- White, T.C.; Marr, K.A.; Bowden, R.A. Clinical, Cellular, and Molecular Factors That Contribute to Antifungal Drug Resistance. Clin. Microbiol. Rev. 1998, 11, 382–402. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, K.; Mayer, M.P.A.; Miyazima, T.Y.; Matsubara, V.H.; Silva, E.G.; Paula, C.R.; Campos, T.T.; Nakamae, A. A Multispecies Probiotic Reduces Oral Candida Colonization in Denture Wearers. J. Prosthodont. 2014, 24, 194–199. [Google Scholar] [CrossRef]
- Song, Y.-G.; Lee, S.-H. Inhibitory effects of Lactobacillus rhamnosus and Lactobacillus casei on Candida biofilm of denture surface. Arch. Oral Biol. 2017, 76, 1–6. [Google Scholar] [CrossRef]
- Lee, X.; Vergara, C.; Lozano, C.P. Severity of Candida-associated denture stomatitis is improved in institutionalized elders who consume Lactobacillus rhamnosus SP1. Aust. Dent. J. 2019, 64, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Sutula, J.; Coulthwaite, L.; Thomas, L.; Verran, J. The effect of a commercial probiotic drink on oral microbiota in healthy complete denture wearers. Microb. Ecol. Health Dis. 2012, 23, 175. [Google Scholar] [CrossRef]
- Newton, A.V. Denture sore mouth. A possible etiology. Br. Dent. J. 1962, 112, 357–360. [Google Scholar]
- Koch, J.R.L.G. The Measurement of Observer Agreement for Categorical Data. Biometrics 1977, 33, 159. [Google Scholar] [CrossRef] [Green Version]
- Tooyama, H.; Matsumoto, T.; Hayashi, K.; Kurashina, K.; Kurita, H.; Uchida, M.; Kasuga, E.; Honda, T. Candida concentrations determined following concentrated oral rinse culture reflect clinical oral signs. BMC Oral Health 2015, 15, 150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zomorodian, K.; Haghighi, N.N.; Rajaee, N.; Pakshir, K.; Tarazooie, B.; Vojdani, M.; Sedaghat, F.; Vosoghi, M.; Vossoughi, M. Assessment ofCandidaspecies colonization and denture-related stomatitis in complete denture wearers. Med. Mycol. 2011, 49, 208–211. [Google Scholar] [CrossRef] [Green Version]
- Salazar, S.B.; Simões, R.S.; Pedro, N.A.; Pinheiro, M.J.; Carvalho, M.F.N.N.; Mira, N.P. An Overview on Conventional and Non-Conventional Therapeutic Approaches for the Treatment of Candidiasis and Underlying Resistance Mechanisms in Clinical Strains. J. Fungi 2020, 6, 23. [Google Scholar] [CrossRef] [Green Version]
- Rossoni, R.D.; De Barros, P.P.; De Alvarenga, J.A.; Ribeiro, F.D.C.; Velloso, M.D.S.; Fuchs, B.B.; Mylonakis, E.; Jorge, A.O.C.; Junqueira, J.C. Antifungal activity of clinical Lactobacillus strains against Candida albicans biofilms: Identification of potential probiotic candidates to prevent oral candidiasis. Biofouling 2018, 34, 212–225. [Google Scholar] [CrossRef] [Green Version]
- Wescombe, P.A.; Hale, J.D.; Heng, N.C.; Tagg, J.R. Developing oral probiotics fromStreptococcus salivarius. Futur. Microbiol. 2012, 7, 1355–1371. [Google Scholar] [CrossRef]
- Hu, L.; Mao, Q.; Zhou, P.; Lv, X.; Hua, H.; Yan, Z. Effects of Streptococcus salivarius K12 with nystatin on oral candidiasis—RCT. Oral Dis. 2019, 25, 1573–1580. [Google Scholar] [CrossRef]
- Aarti, C.; Ameer, K.; Varghese, R.; Arasu, M.V.; Agastian, P.; Al-Dhabi, N.A.; Ilavenil, S.; Choi, K.C. In vitro investigation on probiotic, anti-Candida, and antibiofilm properties of Lactobacillus pentosus strain LAP1. Arch. Oral Biol. 2018, 89, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kang, S.-S. Antifungal activities against Candida albicans, of cell-free supernatants obtained from probiotic Pediococcus acidilactici HW01. Arch. Oral Biol. 2019, 99, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, V.; Dos Santos, S.S.F.; Silva, C.; Jorge, A.; Leão, M. Lactobacillus is able to alter the virulence and the sensitivity profile of Candida albicans. J. Appl. Microbiol. 2016, 121, 1737–1744. [Google Scholar] [CrossRef] [PubMed]
- Reid, P.; Heng, N.C.; Hale, J.D.; Krishnan, D.; Crane, J.; Tagg, J.R.; Milne, T.J. A TaqMan™-based quantitative PCR screening assay for the probiotic Streptococcus salivarius K12 based on the specific detection of its megaplasmid-associated salivaricin B locus. J. Microbiol. Methods 2020, 170, 105837. [Google Scholar] [CrossRef]
| Group | ||
|---|---|---|
| Experimental | Control | |
| Age (mean ± SD) | 74.3 ± 3.8 | 73.1 ± 3.6 |
| Age range | 68–82 | 67–83 |
| Gender (Males/Females) | 13/12 | 11/14 |
| Smokers a (%) | 24 | 20 |
| Diabetes b (%) | 36 | 28 |
| Arterial hypertension c (%) | 64 | 52 |
| Group | |||||
|---|---|---|---|---|---|
| Control | Experimental | ||||
| Count | Column N % | Count | Column N % | ||
| Newton class at T0 | 0 | 0 | 0.0% | 0 | 0.0% |
| 1 | 2 | 8.0% | 4 | 16.0% | |
| 2 | 17 | 68.0% | 15 | 60.0% | |
| 3 | 6 | 24.0% | 6 | 24.0% | |
| Newton class at T30 | 0 | 6 | 24.0% | 15 | 60.0% |
| 1 | 16 | 64.0% | 10 | 40.0% | |
| 2 | 3 | 12.0% | 0 | 0.0% | |
| 3 | 0 | 0.0% | 0 | 0.0% | |
| Newton class at T60 | 0 | 4 | 16.0% | 18 | 72.0% |
| 1 | 13 | 52.0% | 7 | 28.0% | |
| 2 | 8 | 32.0% | 0 | 0.0% | |
| 3 | 0 | 0.0% | 0 | 0.0% | |
| Group | |||||
|---|---|---|---|---|---|
| Control | Experimental | ||||
| Count | Column N % | Count | Column N % | ||
| Pain T0 | No | 0 | 0.0% | 0 | 0.0% |
| Yes | 25 | 100.0% | 25 | 100.0% | |
| Pain T30 | No | 11 | 44.0% | 18 | 72.0% |
| Yes | 14 | 56.0% | 7 | 28.0% | |
| Pain T60 | No | 8 | 32.0% | 21 | 84.0% |
| Yes | 17 | 68.0% | 4 | 16.0% | |
| Dryness T0 | No | 14 | 56.0% | 16 | 64.0% |
| Yes | 11 | 44.0% | 9 | 36.0% | |
| Dryness T30 | No | 20 | 80.0% | 23 | 92.0% |
| Yes | 5 | 20.0% | 2 | 8.0% | |
| Dryness T60 | No | 18 | 72.0% | 23 | 92.0% |
| Yes | 7 | 28.0% | 2 | 8.0% | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Passariello, C.; Di Nardo, F.; Polimeni, A.; Di Nardo, D.; Testarelli, L. Probiotic Streptococcus salivarius Reduces Symptoms of Denture Stomatitis and Oral Colonization by Candida albicans. Appl. Sci. 2020, 10, 3002. https://doi.org/10.3390/app10093002
Passariello C, Di Nardo F, Polimeni A, Di Nardo D, Testarelli L. Probiotic Streptococcus salivarius Reduces Symptoms of Denture Stomatitis and Oral Colonization by Candida albicans. Applied Sciences. 2020; 10(9):3002. https://doi.org/10.3390/app10093002
Chicago/Turabian StylePassariello, Claudio, Francesco Di Nardo, Antonella Polimeni, Dario Di Nardo, and Luca Testarelli. 2020. "Probiotic Streptococcus salivarius Reduces Symptoms of Denture Stomatitis and Oral Colonization by Candida albicans" Applied Sciences 10, no. 9: 3002. https://doi.org/10.3390/app10093002
APA StylePassariello, C., Di Nardo, F., Polimeni, A., Di Nardo, D., & Testarelli, L. (2020). Probiotic Streptococcus salivarius Reduces Symptoms of Denture Stomatitis and Oral Colonization by Candida albicans. Applied Sciences, 10(9), 3002. https://doi.org/10.3390/app10093002








