Abstract
The study aimed to evaluate the antimicrobial effect of photodynamic therapy (PDT) with the use of Toluidine Blue (TB) on extracted teeth infected with biofilms of Enterococcus faecalis. Fifty-four extracted teeth with single-roots and single canals were mechanically shaped, autoclaved, and contaminated with E. faecalis. They were randomly divided into six groups: two groups were negative and positive control groups, two groups were subjected to mechanical instrumentation and PDT with different pre-irradiation times and irradiation times, and two groups were subjected to chemo-mechanical endodontic treatment and PDT with different pre-irradiation times and irradiation times. In PDT groups, after the application of TB, the canals were irradiated with a diode laser of wavelength 635 nm, with a fiber diameter of 200 μm and 100 mW of power in continuous mode. The bacterial load was evaluated using a BioTimer Assay protocol. The greatest reduction of bacterial load was observed in groups of combined PDT with chemo-mechanical treatment. The reductions of bacterial load in groups of combined PDT with chemo-mechanical treatment, and in the positive control group, were significant (p < 0.01) when compared to that of the negative control group. Photodynamic therapy as an adjunctive modality may improve the disinfection capacity of conventional endodontic treatment against E. faecalis.
1. Introduction
Many complications, such as intra-radicular and extra-radicular infections, cysts containing cholesterol, and foreign body reactions, have been reported to be associated with persistent peri-radicular infections after root canal treatment [1]. Endodontic infections are associated with several bacterial species. Primary root canal infections are usually associated with biofilms in which Gram-negative anaerobic bacteria are usually predominant. Gram-positive bacteria predominate in biofilms that lead to secondary endodontic infections [2].
Enterococcus faecalis is a Gram-positive facultative anaerobe. In 24% to 77% of cases with secondary endodontic infections and peri-radicular lesions, E. faecalis was isolated [3,4]. This may be due to its ability to penetrate deeply in the dentinal tubules, leading to difficulty in its elimination after mechanical instrumentation with common antimicrobial irrigation. Additionally, it has been reported that E. faecalis can resist the antimicrobial effects of intracanal dressings with calcium hydroxide for 10 days [1,5].
Eradication of endodontic infection, elimination of microorganisms, and prevention of further infection are the main goals of endodontic treatment. With the available conventional treatment protocols, it is almost impossible to achieve these goals due to the presence of many factors, such as the complex anatomical structure of the endodontium, the biofilm lifestyle of bacteria, the increase of antibiotic-resistant strains, and the incapability of irrigants to penetrate the dentinal tubules at the apical portion of root canals [6,7,8].
In order to improve the antimicrobial capacities of conventional endodontic techniques, new approaches, instruments, and medications have been proposed. Photodynamic therapy (PDT) in the endodontic field has been proposed as an adjunctive modality to the conventional treatment protocols, especially for the management of refractory endodontic infections [7,8,9,10].
The concept of PDT is the activation of a photosensitizing agent (PS) by light exposure in the presence of oxygen. After light irradiation, the PS transfers from its ground state into an excited state, leading to the production of highly reactive oxygen species (ROS). The resultant ROS may have the ability to target and destroy biomolecules present in the cell walls of bacteria [11,12]. In addition, PDT can stimulate bone formation in the peri-radicular area, resulting in acceleration of the healing process of bone [4].
One of the most remarkable advantages of PDT is its efficiency against multidrug-resistant strains without developing bacterial resistance or modifying bacterial sensitivity to antibiotic therapy with repeated applications of PDT [13,14].
There are many PSs that have been employed in PDT, such as phenothiazines (including Methylene Blue (MB) and Toluidine Blue (TB)), phytotherapic agents, cyanine, hematoporphyrin derivatives, and phthalocyanines [13].
Recent systematic reviews have found that the outcome of PDT in removing endodontic biofilms from infected root canals is still not completely clear. This may be due to the diversity in the studies’ methodology and PDT protocols [13,14,15]. Some authors have demonstrated better results of PDT through improving the evaluation methodology of the antibacterial effect [4]. Others attributed the reduced efficacy of PDT to the reduced penetration ability of PSs; therefore, they have suggested improving the pharmacological characteristics of PSs using semi-synthetic PSs (e.g., chlorophyll derivative [Zn(II)e6Me]) or nanoparticles containing PS (e.g., rose bengal–functionalized chitosan nanoparticle (CSRBnps)) [8,16].
The optimization of the antimicrobial effect of PDT might be achieved through the standardization of the PDT protocol. It is believed that studies with similar PS type and light source cannot be compared when the irradiation protocol, irradiation time, laser parameters, and PS concentration are different [13]. Therefore, carrying out further studies to achieve a standardized protocol of PDT is still recommended [13,14,15]. Our study aimed to evaluate the antimicrobial effect of photodynamic therapy using a diode laser 635 nm and TB solution on freshly extracted teeth infected with biofilms of E. faecalis.
2. Materials and Methods
Fifty-four freshly extracted teeth were collected. All of them were extracted due to periodontal disease or orthodontic purposes. All patients gave their informed consent for inclusion before commencing the study. The study was conducted in accordance with the revised Helsinki declaration and the local Ethical Committee.
All collected teeth had a single root and a single canal. The coronal portions of the teeth were cut with a diamond-coated cylindrical bur to obtain standard 12 mm segments. Each root canal was mechanically shaped with a ProTaper® system (Dentsply, Maillefer, Ballaigues, Switzerland) till reaching the F1 file (Ø = 0.20 mm). The canals were irrigated with sterile saline and were dried with sterile paper points. The apex of each tooth was sealed with a flowable light-cured resin (Tetric Flow®, Ivoclar Vivadent, Italy). Each tooth was put into a 1.5 mL Eppendorf tube in an upright position. Then they were sterilized in an autoclave at 121 °C for 15 min.
E. faecalis strain (CCM2541) was grown in brain heart infusion (BHI) (Oxoid Ltd., Hampshire, UK) broth and incubated for 24 h at 37 °C in a humid atmosphere. A dilution (1:50) with a sterile 0.9% NaCl was prepared and incubated for 3 h at 37 °C. After that, the optical density (OD600) was measured to obtain a suspension with OD600 equal to 0.1 (corresponding to about 5 ± 1.3 × 108 colony-forming units/mL (CFUs)/mL) with which teeth were inoculated [6].
A total of 5 μL of this suspension, containing about 2.5 ± 0.7 × 106 CFUs, was carefully injected in each canal using a lab pipette to avoid any contamination of the outer surface of the tooth. Each tooth was placed in an Eppendorf tube and incubated for 72 h in a humid atmosphere at 37 °C. During the experimental procedures, four teeth were damaged and eliminated from the experiment due to the presence of cracks in 3 teeth after the cutting of the coronal portion and due to the occurrence of overcutting in one tooth (<12 mm). The remaining 50 teeth were randomly distributed into six groups (Table 1).

Table 1.
Description of the study groups.
The first group (9 teeth) was considered a negative control group, since the teeth were not subjected to any treatment. Group A (8 teeth) was considered as a positive control group and only chemo-mechanical endodontic treatment was performed. Groups B (8 teeth) and C (8 teeth) were used to evaluate the PDT efficacy; therefore, the teeth were subjected to mechanical instrumentation (with irrigation of 0.9% NaCl) and PDT application. Groups D (8 teeth) and E (9 teeth) were employed to analyze the efficacy of the combination of PDT and chemo-mechanical treatment.
The chemo-mechanical debridement treatment (groups A, D, and E) was as follows: Irrigation with 5% of NaOCl; then mechanical preparation till reaching a file F2 of the Pro Taper® system (Ø = 0.25 mm). Irrigation with 5% of NaOCl for 1 min, washing with 5% sodium thiosulfate in order to neutralize the NaOCl, and washing with sterile saline.
The mechanical instrumentation (groups B and C) was as follows: Irrigation with 0.9% NaCl; then mechanical preparation till reaching a file F2 of the ProTaper® system (Ø = 0.25 mm), and finally, irrigation with sterile saline.
The dental roots of PDT groups (groups B, C, D, and E) were filled with about 15 µL of a 0.05 mM solution of TB (Figure 1). The TB was applied by a Hawe irrigation probe 30 gauge (Kerr). The TB solution was agitated by a K-file #15. Before laser application, the TB solution was left in situ for different pre-irradiation times (T1), and precisely, T1 was 30 s for groups C and E and T1 was 120 s for groups B and D.
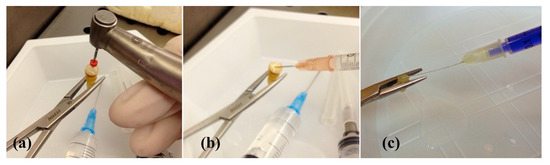
Figure 1.
(a) Mechanical preparation with a file F2 of the ProTaper® system (Ø = 0.25 mm); (b) irrigation of a root canal with 5% of sodium hypochlorite (NaOCl); (c) application of 15 μg/mL of Toluidine Blue (TB).
After that, a diode laser with wavelength of 635 nm (LAMBDA SpA, Brendola (Vi), Italy) was applied to all the PDT groups (group B, C, D, and E) with the following parameters: Power of 100 mW in continuous mode (CW) (Figure 2). An optical fiber (diameter of 200 μm) was used to deliver the laser beam. A rubber stopper was used and positioned on the laser fiber at the designed working length (1 mm from the apex). Two irradiation times (T2) were carried out, and more precisely, T2 was 30 s for groups C and E and T2 was 150 s for groups B and D. During the irradiation, a helical movement from the apical to the cervical portion of each canal was performed.
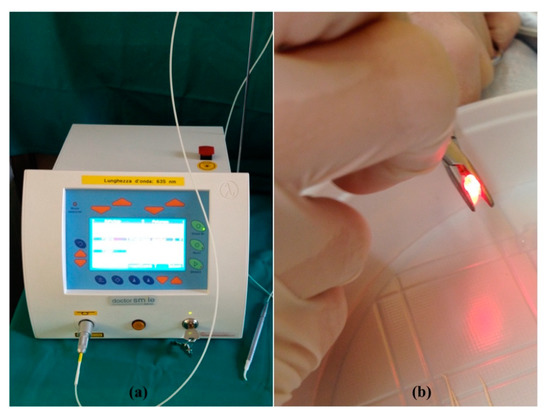
Figure 2.
(a) The diode laser with a wavelength of 635 nm (LAMBDA SpA, Brendola (Vi), Italy); (b) irradiation of a root canal.
To quantitatively evaluate the biofilm on the inner walls of the root canals, BioTimer Assay (BTA) was employed. BTA measures bacterial metabolism and employs Phenol Red-BTA (PR-BTA) reagent, whose color shifts red-to-yellow due to bacterial metabolic acidification [17,18,19,20]. The time (in hours) required for the reagent shift was inversely related to the E. faecalis planktonic concentration through a correlation line whose equation and linear correlation coefficient were y = −0.4767x + 10.022 and r2 0.9977, where “y” corresponds to log10 (CFUs/mL) and “x” corresponds to the hours required for color switch [6]. To evaluate the E. faecalis populations colonizing the inner walls of the roots, infected teeth were placed in 1.5 mL Eppendorf tubes containing 1 mL of BT-PR reagent and incubated at 37 °C. The time for red-to-yellow color switch was recorded and used to evaluate the number of E. faecalis through the correlation line.
To normalize the CFU values obtained with the different teeth, the data were correlated to the areas of the root canals. The area of each root canal was calculated by assuming that the instrumented root canal was a truncated cone, with a surface equal to the canal master. The surface area (neglecting the area of the bases) was given by the following equation: lateral surface area = π (r + R) √ (R − r)2 + H2, where “R” is the upper portion radius, “r” is the lower portion radius, and “H” is the height.
After placing the infected teeth in Eppendorf tubes containing BT-PR medium, the bacterial load for each root canal was calculated through linking the time required for color switch of each root with the correlation line.
Mean survival rates and bacterial load values were calculated from at least three independent experiments. A Student’s t-test for unpaired data was used for statistical analysis. A statistically significant difference was achieved when a p value was ≤0.05.
3. Results
In a previous study, we showed that E. faecalis grew in biofilms on the inner walls of dental roots after 72 h of incubation and that BTA was reliable in evaluating the biofilm load before and after different treatments [6]. Here, the same experimental procedure to colonize the dental roots was used. As expected, dental roots were colonized, and a load equal to 6.00 ± 0.98 log10 CFUs/mm2 was detected (Table 2). The mean bacterial load after treatment, mean survival rate, and p values are reported in Table 2. The greatest reduction of bacterial load was observed in group E with a survival rate of 23.16%. Group D came at the second position with a survival rate of 32.50%, followed by group A (positive control group) with a survival rate of 33.33%. In test groups, the least reduction of bacterial load was reported in group B, as the survival rate was 94.33% (Figure 3).

Table 2.
Enterococcus faecalis CCM2541 counts in control and experimental groups.
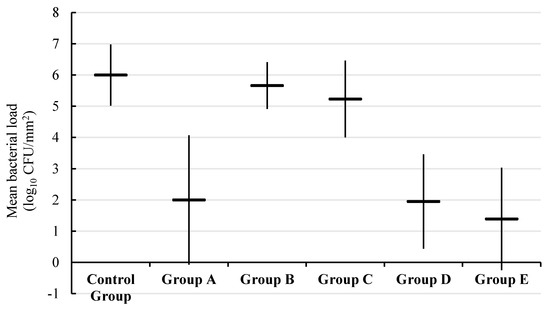
Figure 3.
Box and whisker plot of mean bacterial load (log10 CFUs/mm2) in each experimental group.
The reductions of bacterial load in groups of combined PDT with chemo-mechanical treatment (groups D and E), and group A (positive control group) were significant (p < 0.01) when compared to that of the negative control group. No statistically significant difference was observed between the positive control group (group A) and groups of combined PDT with chemo-mechanical treatment (group D (p = 0.89) and group E (p = 0.42)).
The groups B and C (without NaOCl irrigation) showed slight reductions of bacterial load without statistical significance (p > 0.05) when compared to the negative control group. A significant reduction of bacterial load in group A (positive control group) was observed when compared with groups B and C (p < 0.01).
4. Discussion
The literature seems to be agreeing with the use of PDT as an adjunctive tool to the chemo-mechanical endodontic techniques, particularly in persistent endodontic infection conditions [13,15]. Pourhajibagher et al. showed a significant reduction of diversity and number of bacteria in an in-vivo study on patients with secondary or persistent endodontic infections after PDT with TB [21]. The same results were demonstrated by Jurič et al., as it was found that the chemo-mechanical preparation combined with PDT with TB reduced the number of species of bacteria and no colony forming units were found in 11 out of 21 root canals with secondary endodontic infections [22].
De Oliveira et al. carried out a study to evaluate the effectiveness of PDT and NaOCl irrigation against E. faecalis with the same methodology as our study but a different PS type (MB). The study demonstrated relatively similar results, as the lowest survival rate was observed in the group of combined PDT with NaOCl. However, the survival rates were relatively lower in the 5.25% NaOCl group, PDT group, and combined PDT with NaOCl group when compared to the survival rates of our study groups [23].
Moreover, conflicting results were demonstrated in the literature. In a study on infected extracted teeth with E. faecalis, the greatest culture-negative samples were noted with the 3% NaOCl-irrigation-alone protocol in the secondary infection cases [24]. In fact, the heterogeneity of reported data and the absence of a standardized protocol of PDT were noted in a recent systemic review [15].
Many authors demonstrated the positive effect of PDT on E. faecalis reduction on extracted human teeth when it was accompanied by various protocols [25,26,27,28,29]. Some authors demonstrated that the effect of PDT in combination with various antimicrobial irrigants (NaOCl and EDTA) was more pronounced against E. faecalis, while the effect of irrigants alone was greater against Streptococcus mutans [25]. Others demonstrated that repeated PDT cycles enhanced the positive effect of PDT from 45% of E. faecalis reduction to 95% and emphasized that the most effective antibacterial protocol is still the conventional chemo-mechanical debridement [30]. Others suggested the additional use of other irrigants such as EDTA or citric acid in combination with PDT and common antibacterial irrigants [29].
The heterogeneity of outcomes may be due to the presence of several sensitive factors in the PDT protocol that may affect the outcomes, including the type of PS, its concentration, its pre-irradiation time, the laser’s wavelength, the irradiance time, the type of irrigant, its exposure time, the bacterial biofilms, and the utilized microbiological technique to evaluate the colony counts [14,15,23].
The selection of an appropriate PS should be based on the kind of the targeted bacterial species [13], whereas the antimicrobial capacity of PDT depends on the PS’s abilities to interact with the bacterial membrane, to penetrate, to act inside the cell, and to form ROS after irradiance around the bacterial cell. Some authors found that Gram-negative bacteria, which are predominant in primary root canal infections, showed resistance against PDT because of the different outer membrane structure of Gram-negative bacteria, and because of the hydrophobic and charge effects of PSs [13].
In contrast, PDT showed clear effectiveness against Gram-positive bacteria—which play a role in secondary persistent root infections—due to the presence of relatively porous layer outside the cytoplasmic membrane of the bacteria that permits the diffusion of PS in sensitive sites of bacterial cells [12,31].
The most commonly employed PSs in the endodontic field are TB and MB [15]. The antimicrobial effect of TB against Gram-positive and Gram-negative bacteria has been validated with more effectiveness against Gram-positive bacteria [31]. There is inconsistency of data regarding the recommended concentration of TB [15]. There were reported concerns about the possible occurrence of tooth staining and discoloration after PS application, particularly with MB. However, these concerns would be limited with appropriate PS concentrations and pre-irradiation time [13]. In prior studies, the concentrations of TB were between 15 μg/mL and 15 mg/mL [15].
There is controversy about the pre-irradiation time in the literature [15]. In fact, the pre-radiation time is a key element of PDT, in which the PS takes its time penetrating the dentinal tubules, diffusing inside the bacteria, exerting its antibacterial effect, and consequently resulting in a deeper antimicrobial effect after irradiation [13]. In our study, the increase of pre-irradiation time did not lead to greater reduction of bacterial load.
Appropriate selection of laser wavelength is a crucial factor in PDT technique. The selected wavelength should match the highly absorbed wavelength by the selected PS to produce the photochemical cascade leading to cell death [32]. The wavelength of maximum absorption is 630 nm for TB [13]. In literature, the output power of lasers used for PDT with wavelengths between 600 and 805 nm ranged from 40 mW to 5 W and the irradiation time ranged from 0.5 to 10 min [1].
Nunes et al. found that the use of optical fiber did not play a crucial role in eliminating bacteria in root canals [33]. Some authors believe that utilization of an optical fiber may improve the antimicrobial efficacy of PDT through the uniform distribution of light along the root canals [15]. Concerns were reported regarding this issue because of the possible extrusion of microbial pathogens. Therefore, it is recommended not to apply the fiber to the full length of the root canal [13].
There are several techniques for the evaluation of bacteria in root canal systems [4]. BTA is an easy and sensitive protocol for estimating the bacterial count. The main advantage of this assay is that BTA does not require sample manipulation, thereby overcoming some limitations related to biofilm-counting methods [20].
This study showed a significant reduction of E. faecalis with the chemo-mechanical procedures alone (with 5% NaOCl irrigation) when compared to the negative control group or PDT groups with 0.9% NaCl irrigation (groups B and C).
Irrigation is a complementary procedure of instrumentation in endodontic treatment. It facilitates and contributes in the elimination of pulp tissue, the smear layer, and the remaining biofilm [34]. The most commonly used and studied irrigants are NaOCl and chlorhexidine (CHX) [35]. NaOCl is characterized by having antibacterial property and tissue dissolving ability [36]. The commonly used concentrations of NaOCl are from 0.5% to 6% [35]. The duration of action ranges in the literature between 15 s and 15 min [32]. Its action and toxicity may be directly proportional to its concentration [37]. One of the main disadvantages of NaOCl is its tissue toxicity, and another is its adverse effect on periradicular tissues when extruded from the apex [37].
CHX has a bactericidal effect and substantivity property that may contribute to prolonging the duration of the antibacterial effect [36]. CHX has been proposed as an alternative of NaOCl for open apex cases and patients with NaOCl allergy [37]. The major disadvantage of CHX is its limited ability to dissolve organic tissues [34].
There is a diversity of the outcomes of in-vitro and ex-vivo studies that compare between the antibacterial effects of NaOCl and CHX [10,28,37]. An in-vitro study compared the antibacterial effects of different irrigants against E. faecalis and Candida albicans and demonstrated that 2% CHX had a greater antibacterial effect than 2.5% NaOCl [37]. Other study compared different disinfection methods including PDT, 2.5% NaOCl, and 2% CHX against different microbial pathogens. The most satisfactory results were observed with 2.5% NaOCl, and followed by 2% CHX [28].
In a clinical study, the antibacterial effects of 2% CHX and 2.5% NaOCl were compared and no significant difference was found [36]. A systemic review was carried out to compare between the antibacterial effects of NaOCl and CHX in clinical studies. It was found that the comparative data were few and inconclusive [35].
Interestingly, an ex-vivo study analyzed the effects of PDT in combination with 2% CHX on E. faecalis bacterial counts and on expression patterns of genes associated with biofilm formation. It has been demonstrated that the synergetic effect of PDT with indocyanine green and 2% CHX resulted in modulation of E. faecalis’s virulence through the suppression of the expression patterns of the biofilm-associated genes [10].
A limitation of this ex-vivo experience could be the evaluation of CHX with and without PDT, that should be investigated in future studies.
Several irrigant activation strategies have been proposed to enhance the conventional chemo-mechanical treatment. The ultrasonically activated irrigation (UAI) showed a superior microbial reduction when compared with other irrigant activation techniques [38]. Further studies are needed to evaluate adding activation strategies, such as UAI to the combined PDT with chemo-mechanical treatment protocol.
5. Conclusions
PDT as an adjunctive modality might have the potential to improve the antimicrobial capacity of conventional endodontic treatment against E. faecalis. There is still a need for further studies to establish the proper treatment protocol of PDT.
Author Contributions
Conceptualization, G.T., G.P., G.M., A.M., F.R., G.G., A.I., F.B., and U.R.; methodology, G.T., G.P., G.M., A.M., F.R., G.G., A.I., and F.B.; software, G.T. and A.M.; validation G.T., G.P., G.M., A.M., F.R., G.G., A.I., and F.B.; formal analysis, G.T., G.P., G.M., A.M., F.R., G.G., A.I., Y.S., and F.B.; investigation, G.T., G.P., G.M., A.M., F.R., G.G., A.I., Y.S., and F.B.; resources, A.P. and U.R.; data curation, G.T., G.P., G.M., A.M., F.R., G.G., A.I., Y.S., and F.B.; writing—original draft preparation, G.T., G.P., G.M., A.M., F.R., G.G., A.I., and F.B.; writing—review and editing, G.T., G.P., G.M., A.M., F.R., G.G., A.I., F.B., A.P., and U.R.; visualization, G.T., G.P., G.M., A.M., F.R., G.G., A.I., and F.B.; supervision, G.T., F.B., A.P. and U.R.; project administration, F.B., A.P., and U.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Siddiqui, S.H.; Awan, K.; Javed, F. Bactericidal efficacy of photodynamic therapy against Enterococcus faecalis in infected root canals: A systematic literature review. Photodiagnosis Photodyn. Ther. 2013, 10, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.F. Aetiology of root canal treatment failure: Why well-treated teeth can fail. Int. Endod. J. 2001, 34, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Stuart, C.H.; Schwartz, S.A.; Beeson, T.J.; Owatz, C.B. Enterococcus faecalis: Its Role in Root Canal Treatment Failure and Current Concepts in Retreatment. J. Endod. 2006, 32, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Janani, M.; Jafari, F.; Samiei, M.; Lotfipour, F.; Nakhlband, A.; Ghasemi, N.; Salari, T. Evaluation of Antibacterial Efficacy of Photodynamic Therapy vs. 2.5% NaOCl against E. faecalis-infected Root Canals Using Real-time PCR Technique. J. Clin. Exp. Dent. 2017, 9, e539–e544. [Google Scholar] [CrossRef]
- Ørstavik, D.; Haapasalo, M. Disinfection by endodontic irrigants and dressings of experimentally infected dentinal tubules. Dent. Traumatol. 1990, 6, 142–149. [Google Scholar] [CrossRef]
- Romeo, U.; Palaia, G.; Nardo, A.; Tenore, G.; Telesca, V.; Kornblit, R.; Del Vecchio, A.; Frioni, A.; Valenti, P.; Berlutti, F. Effectiveness of KTP laser versus 980 nm diode laser to killEnterococcus faecalisin biofilms developed in experimentally infected root canals. Aust. Endod. J. 2014, 41, 17–23. [Google Scholar] [CrossRef]
- Vaziri, S.; Kangarlou, A.; Shahbazi, R.; Nazarinasab, A.; Naseri, M. Comparison of the bactericidal efficacy of photodynamic therapy, 2.5% sodium hypochlorite, and 2% chlorhexidine against Enterococcous faecalis in root canals; an in vitro study. Dent. Res. J. 2012, 9, 613–618. [Google Scholar] [CrossRef]
- Diogo, P.; Mota, M.S.; Fernandes, C.; Sequeira, D.B.; Palma, P.J.; Caramelo, F.; Neves, M.G.P.; Faustino, M.; Gonçalves, T.; Santos, J.M. Is the chlorophyll derivative Zn(II)e 6 Me a good photosensitizer to be used in root canal disinfection? Photodiagnosis Photodyn. Ther. 2018, 22, 205–211. [Google Scholar] [CrossRef]
- Soares, J.A.; Soares, S.M.C.S.; Tavarez, R.D.J.R.; Rizzi, C.D.C.; Rodrigues, S.C.G.V.; Filho, E.M.M.; Brito-Junior, M.; Pereira, R.D.; Magalhães, P.P.; Farias, L.D.M. Exploring different photodynamic therapy parameters to optimize elimination of Enterococcus faecalis in planktonic form. Photodiagnosis Photodyn. Ther. 2018, 22, 127–131. [Google Scholar] [CrossRef]
- Bolhari, B.; Pourhajibagher, M.; Bazarjani, F.; Chiniforush, N.; Rad, M.R.; Pirmoazen, S.; Bahador, A. Ex vivo assessment of synergic effect of chlorhexidine for enhancing antimicrobial photodynamic therapy efficiency on expression patterns of biofilm-associated genes of Enterococcus faecalis. Photodiagnosis Photodyn. Ther. 2018, 22, 227–232. [Google Scholar] [CrossRef]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part two—Cellular signaling, cell metabolism and modes of cell death. Photodiagnosis Photodyn. Ther. 2005, 2, 1–23. [Google Scholar] [CrossRef]
- Konopka, K.; Goslinski, T. Photodynamic therapy in dentistry. J. Dent. Res. 2007, 86, 694–707. [Google Scholar] [CrossRef] [PubMed]
- Plotino, G.; Grande, N.M.; Mercade, M. Photodynamic therapy in endodontics. Int. Endod. J. 2018, 52, 760–774. [Google Scholar] [CrossRef] [PubMed]
- Diogo, P.; Faustino, M.; Neves, M.; Palma, P.J.; Baptista, I.P.; Gonçalves, T.; Santos, J.M. An Insight into Advanced Approaches for Photosensitizer Optimization in Endodontics—A Critical Review. J. Funct. Biomater. 2019, 10, 44. [Google Scholar] [CrossRef] [PubMed]
- Bordea, I.R.; Hanna, R.; Chiniforush, N.; Grădinaru, E.; Câmpian, R.S.; Sîrbu, A.; Amaroli, A.; Benedicenti, S. Evaluation of the outcome of various laser therapy applications in root canal disinfection: A systematic review. Photodiagnosis Photodyn. Ther. 2020, 29, 101611. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.; Hamblin, M.R.; Kishen, A. Photoactivated rose bengal functionalized chitosan nanoparticles produce antibacterial/biofilm activity and stabilize dentin-collagen. Nanomed. Nanotechnol. Boil. Med. 2013, 10, 491–501. [Google Scholar] [CrossRef]
- Pantanella, F.; Valenti, P.; Frioni, A.; Natalizi, T.; Coltella, L.; Berlutti, F. BioTimer Assay, a new method for counting Staphylococcus spp. in biofilm without sample manipulation applied to evaluate antibiotic susceptibility of biofilm. J. Microbiol. Methods 2008, 75, 478–484. [Google Scholar] [CrossRef]
- Rosa, L.; Lepanto, M.S.; Cutone, A.; Berlutti, F.; De Angelis, M.; Vullo, V.; Mastroianni, C.M.; Valenti, P.; Oliva, A. BioTimer assay as complementary method to vortex-sonication-vortex technique for the microbiological diagnosis of implant associated infections. Sci. Rep. 2019, 9, 7534. [Google Scholar] [CrossRef]
- Rosa, L.; Cutone, A.; Coletti, M.; Lepanto, M.S.; Scotti, M.; Valenti, P.; Raponi, G.; Ghezzi, M.C.; Berlutti, F. Biotimer assay: A reliable and rapid method for the evaluation of central venous catheter microbial colonization. J. Microbiol. Methods 2017, 143, 20–25. [Google Scholar] [CrossRef]
- Pantanella, F.; Valenti, P.; Natalizi, T.; Passeri, D.; Berlutti, F. Analytical techniques to study microbial biofilm on abiotic surfaces: Pros and cons of the main techniques currently in use. Ann. Ig. 2013, 25, 31–42. [Google Scholar]
- Pourhajibagher, M.; Ghorbanzadeh, R.; Parker, S.; Chiniforush, N.; Bahador, A. The evaluation of cultivable microbiota profile in patients with secondary endodontic infection before and after photo-activated disinfection. Photodiagnosis Photodyn. Ther. 2017, 18, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Juric, I.B.; Plečko, V.; Pandurić, D.G.; Anić, I. The antimicrobial effectiveness of photodynamic therapy used as an addition to the conventional endodontic re-treatment: A clinical study. Photodiagnosis Photodyn. Ther. 2014, 11, 549–555. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, B.P.; Aguiar, C.M.; Câmara, A.C.; De Albuquerque, M.M.; Correia, A.C.R.D.B.; Soares, M.F.D.L.R. The efficacy of photodynamic therapy and sodium hypochlorite in root canal disinfection by a single-file instrumentation technique. Photodiagnosis Photodyn. Ther. 2015, 12, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Tennert, C.; Feldmann, K.; Haamann, E.; Al-Ahmad, A.; Follo, M.; Wrbas, K.-T.; Hellwig, E.; Altenburger, M.J. Effect of photodynamic therapy (PDT) on Enterococcus faecalis biofilm in experimental primary and secondary endodontic infections. BMC Oral Health 2014, 14, 132. [Google Scholar] [CrossRef] [PubMed]
- Susila, A.V.; Sugumar, R.; Chandana, C.S.; Subbarao, C.V. Combined effects of photodynamic therapy and irrigants in disinfection of root canals. J. Biophotonics 2015, 9, 603–609. [Google Scholar] [CrossRef]
- Pinheiro, S.L.; Da Silva, J.N.; Gonçalves, R.O.; Villalpando, K.T. Manual and Rotary Instrumentation Ability to Reduce Enterococcus faecalis Associated with Photodynamic Therapy in Deciduous Molars. Braz. Dent. J. 2014, 25, 502–507. [Google Scholar] [CrossRef][Green Version]
- Soares, J.A.; Soares, S.M.C.S.; César, C.A.S.; De Carvalho, M.A.R.; Brito-Júnior, M.; De Sousa, G.R.; Soares, B.M.; Farias, L.D.M. Monitoring the effectiveness of photodynamic therapy with periodic renewal of the photosensitizer on intracanal Enterococcus faecalis biofilms. Photodiagnosis Photodyn. Ther. 2016, 13, 123–127. [Google Scholar] [CrossRef]
- Gergova, R.T.; Gueorgieva, T.; Kalchinov, V.; Mitov, I.; Kamenoff, J.; Dencheva-Garova, M.S.; Krasteva, A. Antimicrobial activity of different disinfection methods against biofilms in root canals. J. Investig. Clin. Dent. 2015, 7, 254–262. [Google Scholar] [CrossRef]
- Tennert, C.; Drews, A.; Walther, V.; Altenburger, M.J.; Karygianni, L.; Wrbas, K.-T.; Hellwig, E.; Al-Ahmad, A. Ultrasonic activation and chemical modification of photosensitizers enhances the effects of photodynamic therapy against Enterococcus faecalis root-canal isolates. Photodiagnosis Photodyn. Ther. 2015, 12, 244–251. [Google Scholar] [CrossRef]
- Prażmo, E.J.; Godlewska, R.; Mielczarek, A. Effectiveness of repeated photodynamic therapy in the elimination of intracanal Enterococcus faecalis biofilm: An in vitro study. Lasers Med. Sci. 2017, 32, 655–661. [Google Scholar] [CrossRef]
- Usacheva, M.N.; Teichert, M.C.; Biel, M.A. Comparison of the methylene blue and toluidine blue photobactericidal efficacy against gram-positive and gram-negative microorganisms. Lasers Surg. Med. 2001, 29, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Arneiro, R.A.S.; Nakano, R.D.; Antunes, L.S.; Ferreira, G.B.; Fontes, K.B.F.C.; Antunes, L.S. Efficacy of antimicrobial photodynamic therapy for root canals infected with Enterococcus faecalis. J. Oral Sci. 2014, 56, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Nunes, M.R.; Mello, I.; Franco, G.C.N.; De Medeiros, J.M.F.; Dos Santos, S.S.F.; Habitante, S.M.; Lage-Marques, J.L.; Raldi, D.P. Effectiveness of Photodynamic Therapy Against Enterococcus faecalis, With and Without the Use of an Intracanal Optical Fiber: An In Vitro Study. Photomed. Laser Surg. 2011, 29, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Teves, A.; Blanco, D.; Casaretto, M.; Torres, J.; Alvarado, D.; E Jaramillo, D. Effectiveness of different disinfection techniques of the root canal in the elimination of a multi-species biofilm. J. Clin. Exp. Dent. 2019, 11, e978–e983. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, L.; Rodrigues, R.C.V.; Junior, C.V.A.; Soares, R.G.; Vettore, M.V. The Effect of Sodium Hypochlorite and Chlorhexidine as Irrigant Solutions for Root Canal Disinfection: A Systematic Review of Clinical Trials. J. Endod. 2016, 42, 527–532. [Google Scholar] [CrossRef]
- Rôças, I.N.; Provenzano, J.C.; Neves, M.A.; Siqueira, J.F. Disinfecting Effects of Rotary Instrumentation with Either 2.5% Sodium Hypochlorite or 2% Chlorhexidine as the Main Irrigant: A Randomized Clinical Study. J. Endod. 2016, 42, 943–947. [Google Scholar] [CrossRef]
- Jose, J.; Krishnamma, S.; Peedikayil, F.; Aman, S.; Tomy, N.; Mariodan, J.P. Comparative Evaluation of Antimicrobial Activity of QMiX, 2.5% Sodium Hypochlorite, 2% Chlorhexidine, Guava Leaf Extract and Aloevera Extract Against Enterococcus faecalis and Candida albicans—An in-vitro Study. J. Clin. Diagn. Res. 2016, 10, ZC20–ZC23. [Google Scholar] [CrossRef]
- Nagendrababu, V.; Jayaraman, J.; Suresh, A.; Kalyanasundaram, S.; Neelakantan, P. Effectiveness of ultrasonically activated irrigation on root canal disinfection: A systematic review of in vitro studies. Clin. Oral Investig. 2018, 22, 655–670. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).