Challenges for the Implantation of Symbiotic Nanostructured Medical Devices
Abstract
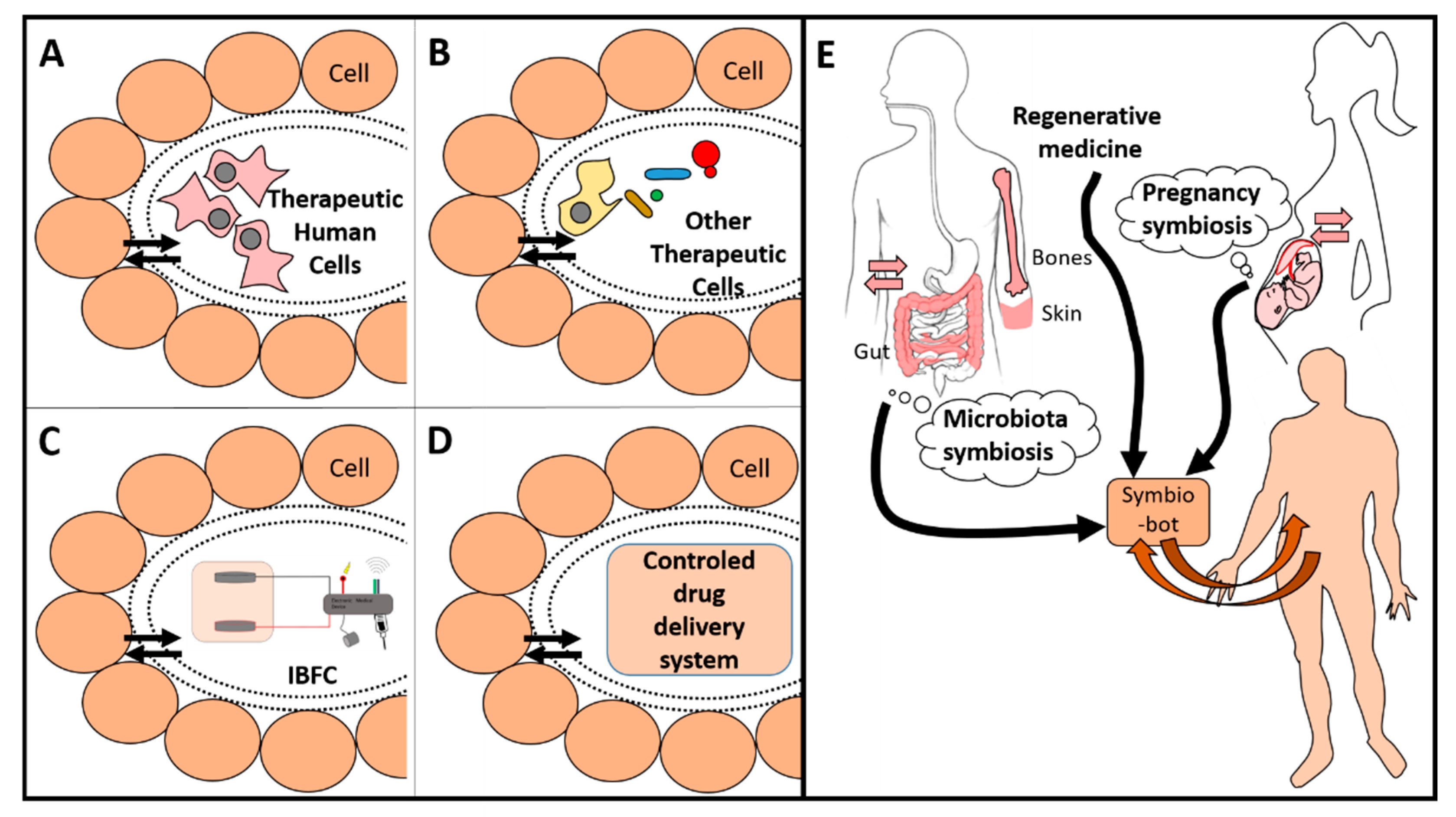
1. Introduction
2. Is it Sufficient to Rely Only on Technological Advances?
3. Biocompatibility is the Major Challenge for Symbiotic Implanted Medical Devices
4. Choices of Membranes for Symbiotic Implanted Medical Devices
5. Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Zsigmondy, R.A. Properties of Colloids. In Nobel Lectures, Chemistry 1922–1941; Elsevier: Amsterdam, The Netherlands, 1966. [Google Scholar]
- Schmitt, O.H. Some interesting and useful biomimetic transforms. In Proceedings of the 3rd Inter Biophysics Congress, Boston, MA, USA, 29 August–3 September 1969. [Google Scholar]
- Feynman, R.P. There’s plenty of room at the bottom. Calif. Inst. Technol. Eng. Sci. 1960, 23, 22–36. [Google Scholar]
- Alcaraz, J.P.; Cinquin, P.; Martin, D.K. Tackling the concept of symbiotic implantable medical devices with nanobiotechnologies. Biotechnol. J. 2018, 13, 1800102. [Google Scholar] [CrossRef]
- Sokic-Lazic, D.; De Andrade, A.R.; Minteer, S.D. Utilization of enzyme cascades for complete oxidation of lactate in an enzymatic biofuel cell. Electrochim. Acta 2011, 56, 10772–10775. [Google Scholar] [CrossRef]
- Xiao, X.; Xia, H.Q.; Wu, R.; Bai, L.; Yan, L.; Magner, E.; Cosnier, S.; Lojou, E.; Zhu, Z.; Liu, A. Tackling the challenges of enzymatic (bio)fuel cells. Chem. Rev. 2019, 119, 9509–9558. [Google Scholar] [CrossRef]
- Zebda, A.; Alcaraz, J.P.; Vadgama, P.; Shleev, S.; Minteer, S.D.; Boucher, F.; Cinquin, P.; Martin, D.K. Challenges for successful implantation of biofuel cells. Bioelectrochemistry 2018, 124, 57–72. [Google Scholar] [CrossRef]
- Langer, R.; Folkman, J. Polymers for the sustained release of proteins and other macromolecules. Nature 1976, 263, 797–800. [Google Scholar] [CrossRef]
- Langer, R.; Brem, H.; Tapper, D. Biocompatibility of polymeric delivery systems for macromolecules. J. Biomed. Mater. Res. 1981, 15, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Ratner, B.D.; Horbett, T.; Hoffman, A.S.; Hauschka, S.D. Cell adhesion to polymeric materials: Implications with respect to biocompatibility. J. Biomed. Mater. Res. 1975, 9, 407–422. [Google Scholar] [CrossRef] [PubMed]
- Ratner, B.D.; Weathersby, P.K.; Hoffman, A.S.; Kelly, M.A.; Scharpen, L.H. Radiation-grafted hydrogels for biomaterial applications as studied by the ESCA technique. J. Appl. Polym. Sci. 1978, 22, 643–664. [Google Scholar] [CrossRef]
- Williams, D.F. Biomaterials and biocompatibility. Med. Prog. Technol. 1976, 4, 31–42. [Google Scholar]
- Anderson, J.M.; Hiltner, A.; Schodt, K.; Woods, R. Biopolymers as biomaterials: Mechanical properties of gamma-benzyl-L-glutamate-L-leucine copolymers. J. Biomed. Mater. Res. 1972, 6, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Gibbons, D.F.; Martin, R.L.; Hiltner, A.; Woods, R. The potential for poly-α-amino acids as biomaterials. J. Biomed. Mater. Res. 1974, 8, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.F. Biocompatibility pathways: Biomaterials-induced sterile inflammation, mechanotransduction, and principles of biocompatibility control. ACS Biomater. Sci. Eng. 2017, 3, 2–35. [Google Scholar] [CrossRef]
- Williams, D.F. Phase changes in biomaterials. Biomaterials 2014, 35, 10007–10008. [Google Scholar] [CrossRef]
- Ratner, B.D. Biomaterials: Been there, done that, and evolving into the future. Annu. Rev. Biomed. Eng. 2019, 21, 171–191. [Google Scholar] [CrossRef]
- Romero-Gavilan, F.; Sánchez-Pérez, A.M.; Araújo-Gomes, N.; Azkargorta, M.; Iloro, I.; Elortza, F.; Gurruchaga, M.; Goni, I.; Suay, J. Proteomic analysis of silica hybrid sol-gel coatings: A potential tool for predicting the biocompatibility of implants in vivo. Biofouling 2017, 33, 676–689. [Google Scholar] [CrossRef]
- Sussman, E.M.; Halpin, M.C.; Muster, J.; Moon, R.T.; Ratner, B.D. Porous implants modulate healing and induce shifts in local macrophage polarization in the Foreign Body Reaction. Ann. Biomed. Eng. 2014, 42, 1508–1516. [Google Scholar] [CrossRef]
- Sneddon, J.B.; Tang, Q.; Stock, P.; Bluestone, J.A.; Roy, S.; Desai, T.; Hebrok, M. Stem cell therapies for treating diabetes: Progress and remaining challenges. Cell Stem Cell 2018, 22, 810–823. [Google Scholar] [CrossRef]
- Li, L.; Marchant, R.E.; Dubnisheva An Roy, S.; Fissell, W.H. Anti-biofouling sulfobetaine polymer thin films on silicon and silicon nanopore membranes. J. Biomater. Sci. 2011, 22, 91–106. [Google Scholar] [CrossRef]
- Nowak, T.; Nishida, K.; Shimoda, S.; Konno, Y.; Ichinose, K.; Sakakida, M.; Shichiri, M.; Nakabayashi, N.; Ishihara, K. Biocompatibility of MPC: In vivo evaluation for clinical application. J. Artif. Org. 2000, 3, 39–46. [Google Scholar] [CrossRef]
- Qin, X.H.; Senturk, B.; Valentin, J.; Malheiro, V.; Fortunato, G.; Ren, Q.; Rottmar, M.; Maniuraweber, K. Cell-membrane-inspired silicone interfaces that mitigate proinflammatory macrophage activation and bacterial adhesion. Langmuir 2019, 35, 1882–1894. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.J.; Ma, L.N.; Su, J.; Jing, W.W.; Wei, M.J.; Sha, X.Z. Biocompatibility assessment of porous chitosan-Nafion and chitosan-PTFE composites in vivo. J. Biomed. Mater. Res. A 2014, 102, 2055–2060. [Google Scholar] [CrossRef] [PubMed]
- El Ichi, S.; Zebda, A.; Alcaraz, J.P.; Laaroussi, A.; Boucher, F.; Boutonnat, J.; Reverdy-Bruas, N.; Chaussy, D.; Belgacem, M.N.; Cinquin, P.; et al. Bioelectrodes modified with chitosan for long-term energy supply from the body. Energy Environ. Sci. 2015, 8, 1017–1026. [Google Scholar] [CrossRef]
- El Ichi-Ribault, S.; Alcaraz, J.P.; Boucher, F.; Boutaud, B.; Dalmolin, R.; Boutonnat, J.; Cinquin, P.; Zebda, A.; Martin, D.K. Remote wireless control of an enzymatic biofuel cell implanted in a rabbit for 2 months. Electrochim. Acta 2018, 269, 360–366. [Google Scholar] [CrossRef]
- El Ichi, S.; Zebda, A.; Laaroussi, A.; Reverdy-Bruas, N.; Chaussy, D.; Belgacem, M.N.; Cinquin, P.; Martin, D.K. Chitosan improves stability of carbon nanotube biocathodes for glucose biofuel cells. Chem. Commun. 2014, 50, 14535–14538. [Google Scholar] [CrossRef]
- Cinquin, P.; Gondran, C.; Giroud, F.; Mazabrard, S.; Pellissier, A.; Boucher, F.; Alcaraz, J.; Gorgy, K.; Lenouvel, F.; Mathe, S.; et al. A glucose biofuel cell implanted in rats. PLoS ONE 2010, 5, e10476. [Google Scholar] [CrossRef]
- Zebda, A.; Cosnier, S.; Alcaraz, J.P.; Holzinger, M.; Le Goff, A.; Gondran, C.; Boucher, F.; Giroud, F.; Gorgy, K.; Lamraoui, H.; et al. Single glucose biofuel cells implanted in rats power electronic devices. Sci. Rep. 2013, 3, 1516. [Google Scholar] [CrossRef]
- Wang, M.; Cheng, X.; Zhu, W.; Holmes, B.; Keidar, M.; Zhang, I.G. Design of biomimetic and bioactive cold plasma-modified nanostructured scaffolds for enhanced osteogenic differentiation of bone marrow-derived mesenchymal stem cells. Tissue Eng. A 2014, 20, 1060–1071. [Google Scholar] [CrossRef]
- Guitian Oliveira, N.; Sirgado, T.; Reis, L.; Pinto, L.F.V.; da Silva, C.L.; Ferreira, F.C.; Rodrigues, A. In vitro assessment of three dimensional dense chitosan-based structures to be used as bioabsorbable implants. J. Mech. Behav. Biomed. Mater. 2014, 40, 413–425. [Google Scholar] [CrossRef]
- Alcaraz, J.P. Conception Guidée par la Physiologie de Biopiles Bioinspirées Implantables. Ph.D. Thesis, Université Grenoble Alpes, Grenoble, France, 2016. [Google Scholar]
- Zhou, J.; Ma, Z.; Hong, X.; Wu, H.M.; Ma, S.Y.; Li, Y.; Chen, D.J.; Yu, H.Y.; Huang, X.J. Top-down strategy of implantable biosensor using adaptable, porous hollow fibrous membrane. ACS Sens. 2019, 4, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Sumayya, A.S.; Muraleedhara Kurup, G. Biocompatibility of subcutaneously implanted marine macromolecules cross-linked bio-composite scaffold for cartilage tissue engineering applications. J. Biomater. Sci. Polym. Ed. 2018, 29, 257–276. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Cao, X.; Shen, X.; Yu, S.; Su, F.; Liu, S.; Liu, F.; Xie, C. Alkyl chitosan film-high strength, functional biomaterials. J. Biomed. Mater. Res. A 2017, 105, 3034–3041. [Google Scholar]
- Chaouat, M.; Le Visage, C.; Baille, W.E.; Escoubet, B.; Chaubet, F.; Mateescu, M.A.; Letourneur, D. A novel cross-linked poly(vinyl alcohol) (PVA) for vascular grafts. Adv. Funct. Mater. 2008, 18, 2855–2861. [Google Scholar] [CrossRef]
- Ino, J.M.; Sju, E.; Ollivier, V.; Yim, E.K.F.; Letourneur, D.; Le Visage, C. Evaluation of hemocompatibility and endothelialization of hybrid poly(vinyl alcohol) (PVA)/gelatin polymer films. J. Biomed. Mater. Res. B 2013, 101, 1549–1559. [Google Scholar] [CrossRef]
- Penven, G. Encapsulation de Dispositifs Symbiotiques Implantables: Évaluation de la Biocompatibilité et des Performances. Ph.D. Thesis, Université Grenoble Alpes, Grenoble, France, 2016. [Google Scholar]
- Burczak, K.; Gamian, E.; Kochman, A. Long-term in vivo performance and biocompatibility of poly(vinyl alcohol) hydrogel macrocapsules for hybrid-type artificial pancreas. Biomaterials 1996, 17, 2351–2356. [Google Scholar] [CrossRef]
- Shah, R.; Stodulka, P.; Skopalova, K.; Saha, P. Dual crosslinked collagen/chitosan film for potential biomedical applications. Polymers 2019, 11, 2094. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.E.; Truong, K.P.; Hagen, M.W.; Yim, E.K.; Hinds, M.T. Biomimetic modification of poly (vinyl alcohol): Encouraging endothelialization and preventing thrombosis with antiplatelet monotherapy. Acta Biomater. 2019, 86, 291–299. [Google Scholar] [CrossRef]
- Dolega, M.E.; Wagh, J.; Gerbaud, S.; Kermarrec, F.; Alcaraz, J.P.; Martin, D.K.; Gidrol, X.; Picollet-d’hahan, N. Facile bench-top fabrication of enclosed circular microchannels provides 3D confined structure for growth of prostate epithelial cells. PLoS ONE 2014, 9, e99416. [Google Scholar] [CrossRef]
- Picollet-d’hahan, N.; Gerbaud, S.; Kermarrec, F.; Alcaraz, J.P.; Obeid, P.; Bhajun, R.; Guyon, L.; Sulpice, E.; Cinquin, P.; Dolega, M.E.; et al. The modulation of attachment, growth and morphology of cancerous prostate cells by polyelectrolyte nanofilms. Biomaterials 2013, 34, 10099–10108. [Google Scholar] [CrossRef]
- Baraket, A.; Alcaraz, J.P.; Gondran, C.; Costa, G.; Nonglaton, G.; Gaillard, F.; Cinquin, P.; Cosnier, M.; Martin, D.K. Long duration stabilization of porous silicon membranes in physiological media: Application for implantable reactors. Mater. Sci. Eng. C 2020, 108, 110359. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alcaraz, J.-P.; Menassol, G.; Penven, G.; Thélu, J.; El Ichi, S.; Zebda, A.; Cinquin, P.; Martin, D.K. Challenges for the Implantation of Symbiotic Nanostructured Medical Devices. Appl. Sci. 2020, 10, 2923. https://doi.org/10.3390/app10082923
Alcaraz J-P, Menassol G, Penven G, Thélu J, El Ichi S, Zebda A, Cinquin P, Martin DK. Challenges for the Implantation of Symbiotic Nanostructured Medical Devices. Applied Sciences. 2020; 10(8):2923. https://doi.org/10.3390/app10082923
Chicago/Turabian StyleAlcaraz, Jean-Pierre, Gauthier Menassol, Géraldine Penven, Jacques Thélu, Sarra El Ichi, Abdelkader Zebda, Philippe Cinquin, and Donald K. Martin. 2020. "Challenges for the Implantation of Symbiotic Nanostructured Medical Devices" Applied Sciences 10, no. 8: 2923. https://doi.org/10.3390/app10082923
APA StyleAlcaraz, J.-P., Menassol, G., Penven, G., Thélu, J., El Ichi, S., Zebda, A., Cinquin, P., & Martin, D. K. (2020). Challenges for the Implantation of Symbiotic Nanostructured Medical Devices. Applied Sciences, 10(8), 2923. https://doi.org/10.3390/app10082923



