The organosolv process is a delignification process that consists of breaking the internal lignin and hemicellulose bonds of the lignocellulose material; in this case, a corncob.
Table 1 shows the chemical composition of corncob before performing the organosolv pretreatment: 45% cellulose, 34% hemicellulose, 16% lignin, and 6% extractives. These values are comparable to the data published by several authors, who indicated that the cellulose, hemicellulose and lignin ratios were 45%, 35% and 15%, respectively [
23,
24]. The high percentage of hemicelluloses in the corncob makes it a suitable material for thermochemical pretreatments such as organosolv. The pretreated corncob had 22% cellulose, 72% hemicellulose, 5% lignin, and 2% extractives, from which it could be verified that the organosolv treatment solubilised and de-polymerised lignin and hemicelluloses, dividing them into separate liquid streams (hydrolysate) and leaving behind solid fractions rich in cellulose for other applications [
25,
26].
The percentages (%; w/w) obtained for C: 46.6 and N: 0.4, as shown in
Table 1, are in agreement with data reported in the literature (C: 41.7% and N: 0.1%) [
22], suggesting that corncob is a material that can be used in an anaerobic process. However, the C:N ratio of corncob is 61:0.5, which is considerably higher than the optimal value reported in the literature for other biomasses used in anaerobic digestion [
3]. The decomposition of materials with high carbon content, greater than 35:1, occurs more slowly, because the multiplication and development of bacteria are low, due to the lack of nitrogen, but the biogas production period is longer. On the other hand, with a C:N ratio less than 8:1, bacterial activity is inhibited due to the formation of an excessive ammonium content, which in large quantities is toxic and inhibits the process [
4].
3.1. Characterisation of Corncob Hydrolysate
To confirm the solubility of the simple sugars in corncob hydrolysate, the hydrolysate obtained after the organosolv pretreatment was analysed.
Table 2 represents the carbohydrates of the hydrolysate corresponding to the available sugars for use by the inoculum. It is important to know that the parameters as proteins constitute a very important substrate in the anaerobic digestion process because, in addition to being a source of carbon and energy, the amino acids derived from their hydrolysis have high nutritional value.
Table 3 shows the results of the sugars present in the organosolv hydrolysate and soda hydrolysate, showing that, with the organosolv pretreatment, the total reducing sugars (TRS) were higher (65.84 g/L) than the TRS with the soda pretreatment (4.64 g/L). This indicates that the hydrolysate is suitable for use in the anaerobic reactor. As with the TRS, the concentration of total sugar in the organosolv pretreatment was 157.7 g/L, which is higher than after the soda pretreatment (20.80 g/L), meaning that a greater amount of total sugars was extracted. Organosolv pretreatment improved the formation of sugars or the ability to subsequently form sugars by enzymatic hydrolysis, thus preventing the loss or degradation of carbohydrates and avoiding the formation of inhibitors, the by-products of subsequent hydrolysis and fermentation processes [
27]. The non-reducing sugars obtained concentrations that exceeded the totals of TRS, demonstrating an incomplete depolymerisation from the organosolv pretreatment.
The chemical oxygen demand (COD) of the corncob hydrolysate was 690 g/L. This result is considered high when compared with soda hydrolysate (195 g/L) and lower results of residues commonly present in the paper industry and its effluents without prior treatment, not exceeding a value of 52 g/L, taking into account that thermochemical processes are also carried out in this industry. The COD of the organosolv hydrolysate exceeded the results characteristic of residues from other types of pretreatment, where values ranging from 179 to 193 g/L for soluble COD and total COD, respectively, have been reported [
28]. The COD increased with the organosolv process because the organic solvents used for the removal of lignin in the corncob leave the hydrolysate rich in depolymerised hemicelluloses (glucose, xylose, galactose, and mannose), thus increasing the organic matter available for the digestion process.
3.2. Anaerobic Digestion Process
The percentage of total solids contained in the mixture with which the digester is loaded is an important factor to consider to ensure that the process is carried out satisfactorily. The accessibility of methanogenic bacteria within the substrate is increasingly limited as the solids content increases, and the gas efficiency and production can therefore be affected.
Table 4 shows the results of the characterisation of the substrate of cow manure and corncob hydrolysate obtained in this project. Experimentally, it has been shown that the load in semicontinuous digesters should not have more than 8% to 12% total solids to ensure the proper functioning of the process, unlike discontinuous digesters, which can handle between 40% and 60% total solids. In this case, the total solids value was 14.82%, which is attributed to the cow manure [
29].
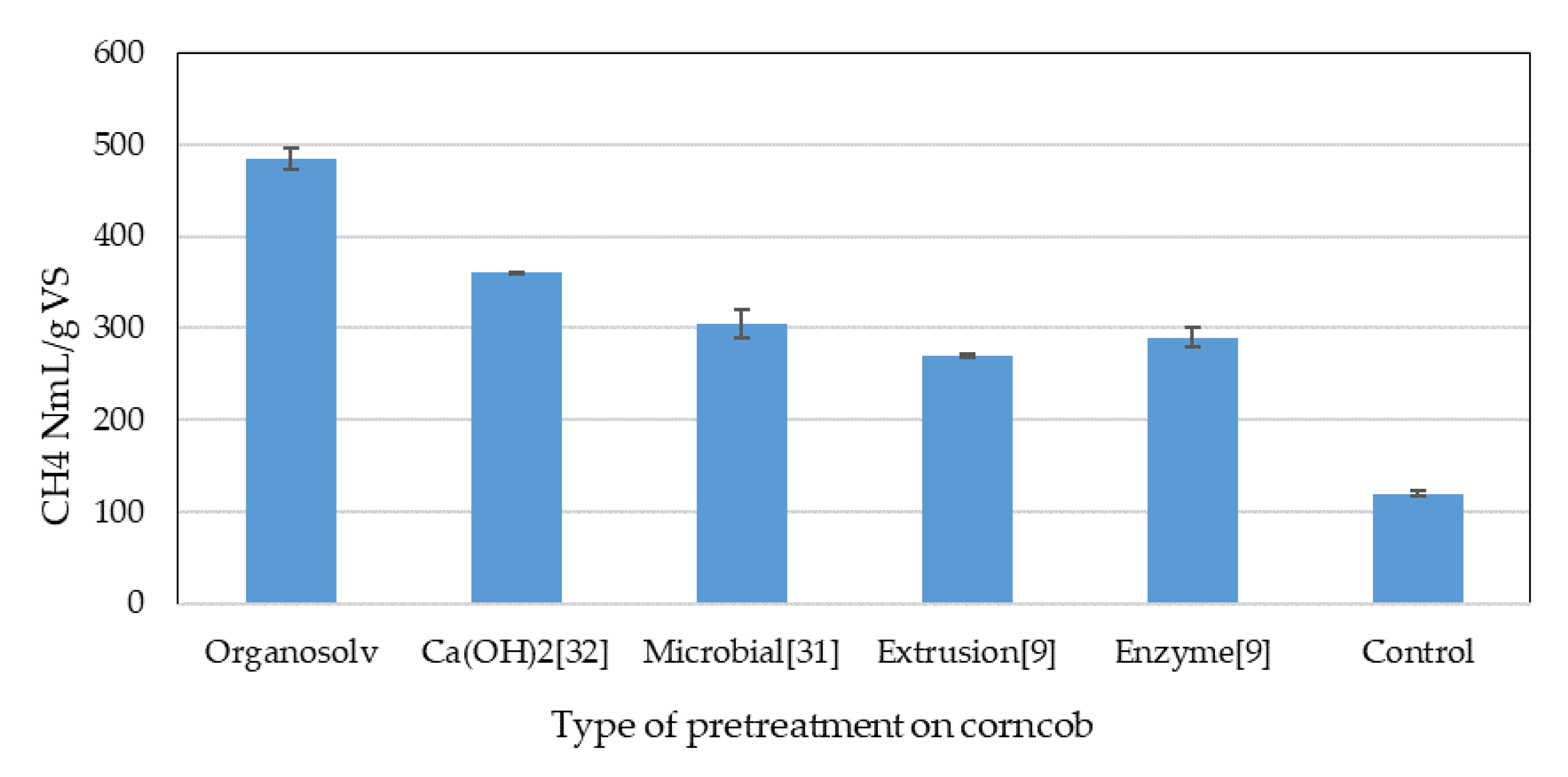
Figure 1 shows the maximum value of biogas production in the bottles reactor obtained with the hydrolysate corncob at 15 days compared with a control corncob without the hydrolysate organosolv pretreatment and with different pretreatments in the bottles reactor reported by other authors [
9,
30,
31]. Hua (2016) reported the effect of microbial pretreatment on the biogas production of non-sterile, rotted silage maize straw, finding 305 NmL/gVS [
30], while Perez-Rodríguez (2017) reported extrusion and enzymatic hydrolysis, finding 279 and 290 NmL/gVS, respectively [
9]. Shah (2018) reported 360 NmL/gVS using lime (Ca(OH)
2) soaking as a pretreatment [
31]. It was clearly observed that, with the use of pretreatment with organosolv, the production of biogas was superior, because 484 NmL/gVS was obtained compared to the other reported treatments. It was also observed that adding the hydrolysate organosolv increased the production because the values of the control without hydrolysate were 120 NmL/gVS.
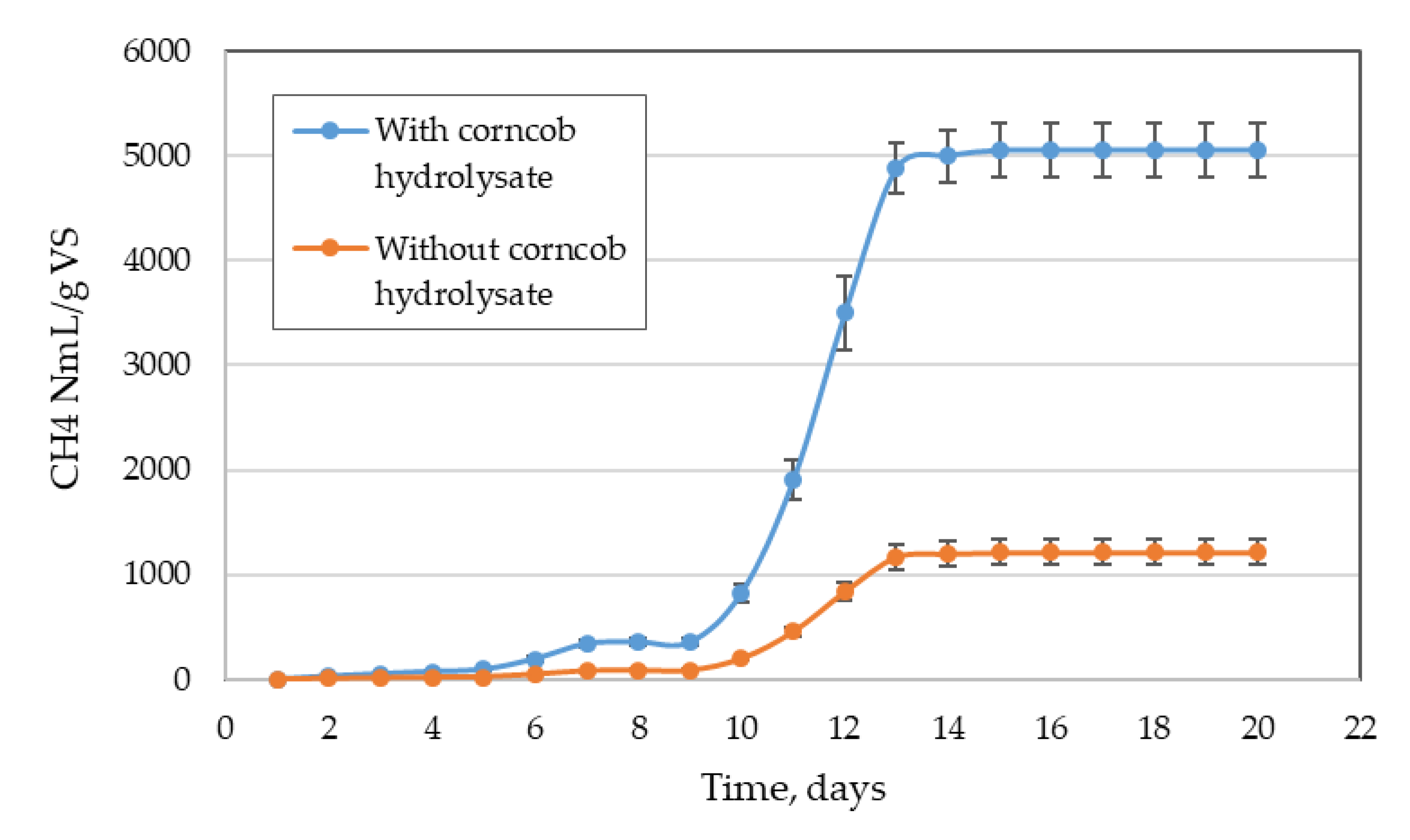
Figure 2 shows a representative evolution in cumulative biogas production in the Labfors batch-type biological reactor. In this section, the values found with and without corncob hydrolysate are compared. The biogas production was observed to be very low for the first nine days in both cases, but began from day 10. In the corncob hydrolysate test, the biogas production was higher than the non-hydrolysate test. Biogas production was monitored until day 20, but there was no production from days 15 to 20, so there was a constant line, and all the added organic substrate was consumed by day 15. The total biogas accumulated in 15 days of production was 5050 ± 253 NmL/gVS and 1212 ± 120 NmL/g VS with and without hydrolysate, respectively. The increase in biogas production was notable when the organosolv hydrolysate of corncob was added.
Table 5 makes clear that the concentration of total reducing sugars is one of the factors that can affect methanogenic production in the anaerobic digestion process, directly and correlationally [
32]. The consumption of TRS and total sugars during the anaerobic digestion was 96.8% and 85.75%, respectively. The COD was reduced to 79% with a COD reduction of 7.9 g, which is proportional to 639.24 NmLbiogas/g COD. Clearly confirming the influence of sugars in biogas production, the sugar content had the maximum reduction on the final day, demonstrating the potential of corncob residues, rich in sugars, as viable substrates in biogas production.
Table 6 shows the results obtained from the daily biogas production and composition. The biogas composition was CH
4 with a concentration of 35.7% to 72.80% (v / v), CO
2 of 63.8% to 26.18%, and 0.5% to 1% (v/v) for other biogas (H
2, O
2, etc.) during anaerobic digestion (15 days). The CH
4 concentration is in line with values reported by previous studies of the contents of biogas, ranging from 50% to 65% (v/v) for CH
4 and from 35% to 50% (v/v) for CO
2 during sludge anaerobic digestion [
33]. The production of CH
4 indicated the presence of methanogens, and the anaerobic digestion was effective.
Other important parameters during anaerobic digestion are the concentrations of volatile fatty acids (VFA), which increased with respect to the values obtained in the recovery of the substrate material. This is a clear indicator of the microbiological activity of the system during the time of the experimentation with a single feeding and periodic evaluation of the volume of biogas. During anaerobic degradation, complex organic matter is hydrolysed and fermented in low-molecular-weight compounds, including short-chain fatty acids (C
2-C
6). As shown in
Table 7, the VFAs found in both the substrate (initial) and digestate (day 17) were acetic acid in the highest proportion, followed by butyric and propionic acid and lesser quantities of furfural.
During the hydrolytic–acidogenic stage, different organic acids are generated and even biogas rich in H
2, depending on the operating conditions.
Table 7 shows the concentration of the main metabolic intermediaries detected at this stage. These precursors are metabolised to CH
4 during the methanogenesis stage. It should be noted that during the assays, about 70% of the volume of biogas corresponded to CH
4, which implies benefits for its subsequent exploitation. Finally, no accumulation of organic acid was detected throughout the methanogenesis operating period, indicating that the anaerobic digestion process was carried out efficiently with an overall COD balance of 79%.