Abstract
Open field burning and tilling the rice straw (RS) back into the fields causes environmental threats by contributing to the increased greenhouse gas emissions. Energy and nutrient recovery from RS through anaerobic digestion (AD) is an effective solution for its utilization. Although RS has good methane potential, its characteristics make it a difficult substrate for AD. This paper reviews the characteristics of RS, mass balance, and distribution of nutrients into liquid and solid digestate in the AD. The present review also discusses the effect of temperature, co-digestion, mixing, inoculum, organic loading rate, recycling liquid digestate, the addition of trace elements, and their bioavailability on the enhancement of biogas/methane yield in the AD of RS. In addition, the digestion of RS at various scales is also covered in the review.
1. Introduction
Rice is ranked as the third-largest crop in the world [1] and it is one of the most important staple foods of humans [2]. Rice straw (RS) is an agricultural residue obtained at the time of harvesting of the rice crop [2]. The rate of RS generated per ton of rice crop varies from 0.4 to 4.0 [3,4,5] due to cultivated rice variance, cultivation technique, and harvesting method [3]. According to the International Rice Research Institute (IRRI), an average of 1.1 ton of RS are generated with each ton of rice produced (range of 0.7–1.4) [6]. There has been an increasing trend in rice production and in the generation of RS in the last decade. A total of 686 million tons of rice and approximately 754 million tons of RS were produced in 2009 [7] while their production was 769 million tons and 846 million tons in 2017 globally, according to the Food and Agriculture Organization of the United Nations [7].
RS is used for feeding animals, heating homes, construction of buildings, and making paper. However, probably the largest share of straw is left unused in the fields [2]. Open-field burning and tilling the RS back into the fields are the common practices for its disposal. Both cause environmental threats by contributing to the increased greenhouse gas emissions [8,9]. Furthermore, the incorporation of RS in the soil increases foliar diseases, which resulted in reduced crop yields [10].
Conversion of RS into more valuable products like methane, ethanol, nutrient-rich soil conditioner, or biofuels are better options compared to its conventional utilization as energy challenges and environmental concerns can be addressed together [2]. The conversion can be done by thermochemical or biological processes [11]. The thermochemical processes like gasification [1], combustion [12], and pyrolysis [13] have been used as energy recovery methods but can cause a threat to the environment. Composting is a biological process that has been used for the conversion of RS into a valuable fertilizer with other substrates [14,15]. Anaerobic digestion (AD) is another biological process which converts biomass into various biofuels or platform chemicals such as methane, ethanol, hydrogen, etc. [1]. It is considered to be one of the most environmentally friendly processes [2]. Moreover, AD technology can be used economically for dry and wet biomasses on a variety of scales, and it requires less input energy as compared to thermochemical processes [16]. Moreover, RS is a bulky material because of its low density (70 kg/m3) which makes it difficult to transport to a storage place for energy recovery by thermochemical processes [17].
During the AD process in biogas technology, organic material is degraded biologically by microorganisms in the absence of molecular oxygen and converted into biogas. The biogas contains primarily methane (CH4) and carbon dioxide (CO2) with small quantities of water vapor (H2O(g)), ammonia (NH3), and hydrogen sulfide (H2S) [11]. Appels et al. [18] explained the AD in four stages: hydrolysis, acidogenesis, acetogenesis, and methanogenesis. Since carbon (C) bound in organic matter is released as CH4 and CO2 during the AD, the C/N ratio of the remaining digestate decreases [19]. Additionally, the plant availability of N is enhanced through the AD process, as organically bound N is mineralized into ammonium (NH4+) which is readily available [20].
The utilization of RS for the recovery of nutrients and energy through AD technology is more desirable than its common disposal practices (open-field burning and tilling in soil). AD technology can be viable for RS because of its high generation rate and potential to reduce greenhouse gas emissions. Energy recovery through AD of food crops is controversial since the demand for food is expected to rise due to an increasing world population [21]. RS as an agricultural residue and does not affect the food security. Furthermore, the calculated and measured biogas and methane yields of RS render it an attractive substrate for AD [22].
According to Baserga [23], biogas and methane potentials of RS can be calculated based on the crude nutrients (ash, fat, fiber, N-free extracts, protein), resulting in 409–415 and 207–211 L/kg VS, respectively [24]. The CH4 yield measured under different experimental conditions were reported from 92 to 280 L/kg VS with various inocula and temperatures [11]. Other authors reported different CH4 yields in their studies such as 226–281 L/kg VS [25] and 100–300 L/kg VS [26].
In addition, many emerging factors advance the utilization of RS for energy recovery through AD, like the usage of appropriate inocula and pretreatment methods. Researchers have become increasingly interested in the AD of RS as the Web of Science ® shows an increasing trend of published papers with “anaerobic digestion” and “rice straw” as topics for the last 10 years as shown in Figure 1.
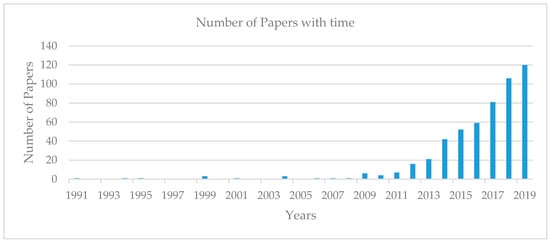
Figure 1.
Web of Science ® bibliometric study with topics “anaerobic digestion” and “rice straw” from 1991 until December 2019. The number of publications from 1991 to 1999 was 6; from 2000 to 2008 was 7; and from 2009 to 2019 was 512.
Apart from its availability and CH4 potential, RS has a complex, lignocellulosic structure and ligno-carbohydrate complexes that create a barrier for the microbial conversion, which make it a difficult substrate to decompose by AD [4,27]. Other challenges associated with mono-digestion of RS are the unbalanced nutritional composition (high C/N ratio) [28], low content of essential trace elements (TEs) [29], and its floatation behavior in wet AD systems [30]. Therefore, RS has not been used as an attractive substrate for the recovery of energy and nutrients through AD.
Significant research and development have taken place in the last 10 years in the field of AD of RS as evidenced by the number of published papers in the scientific literature, as shown in Figure 1. In 2013, Mussoline et al. [11] reviewed the AD of RS. This review provides insights only into certain aspects such as greenhouse gas emissions from rice fields, energy recovery potential of RS, various pretreatment strategies to enhance biogas from RS, optimal nutrient balance, and microbiological considerations. However, new knowledge and technologies are continuously unfolding in the field of AD of RS for energy and nutrients recovery.
The process parameters such as temperature, organic loading rate (OLR), and mixing are fundamental in the AD and here referred to as “factors”. The other process techniques, such as adding co-substrates, usage of appropriate inoculum in a suitable amount, recycling liquid digestate (LD), the addition of TEs, and their bioavailability, are also relevant to the AD of RS and termed as “methods” in this review. The objectives of this paper are to provide an overview of the effect of these factors and methods on the enhancement of energy recovery (biogas/methane yield) from RS through AD. Additionally, the mass balance of nutrients and their distribution into LD and solid digestate (SD) during AD are discussed in this review. Moreover, the digestion of RS at various scales is also part of the current paper. The focus is the detailed highlighting of these aspects in this review and those studies with other similar substrates have also been included for comparison where fewer studies with RS were available.
The effects of various pretreatment techniques on the optimization of AD of RS have been discussed in the review conducted by Mussoline et al. [11]. The enhancement in biogas yield from lignocellulosic substrates including RS by using various pretreatment methods (mechanical, chemical, biological, and physiochemical) has been reviewed recently by Hernández-Beltrán et al. [31]. Moreover, a recent review conducted by Ahmed et al. [32] discusses the optimization of the AD of lignocellulosic substrates by hydrothermal pretreatment. These studies reviewed the effect of various pretreatment methods on the enhancement of energy recovery from RS and other similar substrates and, hence, have not been included in this review. Some aspects of the AD of RS have been reviewed by Mussoline et al. [11] in 2013, and some are discussed in this paper. Therefore, Table 1 indicates the part of the AD discussed by Mussoline et al. [11] and the part which is presented in the current review to show the complementarity.

Table 1.
Aspects of anaerobic digestion (AD) of rice straw (RS) discussed in both studies.
RS is a difficult substrate to degrade under anaerobic conditions due to its complex structure [27]. It is critical to achieve a stable AD process to get efficient biogas production. The project “BIORIST” aimed at designing technically feasible and innovative RS digestion processes. During a 3 year project phase, the process technology was developed and successfully tested in a pilot-scale plant [33].
This paper focuses on factors supporting a stable AD process with RS as an input substrate to produce biogas. This will help to further enhance process technology which mostly exists at a laboratory or pilot-scale to full-scale application. All further post-treatments or optimizations such as biogas purifications can be regarded once a stable biogas technology for RS is established. Further processing of the biogas to biomethane is not considered here, although various post-treatments exist to increase the proportion of valuable biomethane from biogas up to the quality of natural gas. These treatments include water scrubbing, cryogenic separation, physical absorption, chemical absorption, pressure swing adsorption, membrane technology, in-situ methane enrichment, hydrate formation, biological methods, and photoautotrophic methods [34,35]. However, the detailed description of the principles of these treatments is not the scope of this review.
Considering the low number of published data on the experience of pilot- and farm-scale plants for RS digestion resulting from missing implementation of AD of RS, an economic comparison is not reasonable and therefore excluded from this review.
2. Characteristics of RS
RS is produced when harvesting rice crop, and it can be collected from the field once it contains a dry matter of more than 75%.
RS looks like flat fibers having approximate dimensions of 20−60 cm in length and 0.5 cm in width [4].
The general characteristics of RS such as total solids (TS), volatile solids (VS), total carbon (TC), total nitrogen (TN), and C/N ratio are usually reported as a single value or mean values with a variation in the literature. The original data is further processed to generate median, maximum, minimum, and quartiles as shown in Figure 2. Box plots of TS and VS are smaller, and the whiskers do not extend as far, indicating that these properties do not differ much over the various analyzed studies, while the C/N ratio is different for the RS used in these studies with spread-out data points [10,26,28,36,37,38,39,40,41,42].
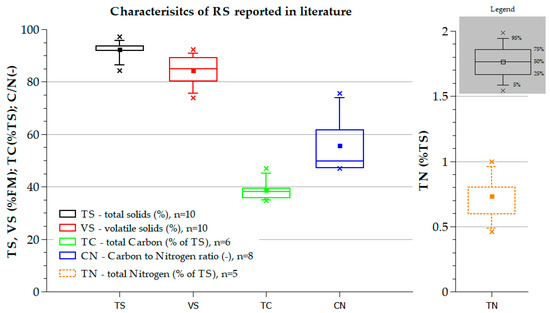
Figure 2.
Statistical evaluation of RS characteristics reported in the literature. “n” is the number of values to calculate the median, maximum, minimum, and quartiles [10,26,28,36,37,38,39,40,41,42].
Other nutrients and TEs, like Phosphorus (P), Calcium (Ca), Magnesium (Mg), Potassium (K), Sodium (Na), Nickel (Ni), Copper (Cu), Zinc (Zn), Iron (Fe), etc., are usually not analyzed in the RS, except in two studies (Table 2).

Table 2.
Nutrients and TEs in RS reported in the literature. (“± “ stands for the standard deviation of the mean as reported by the authors).
3. Nutrients Balance and Distribution into Liquid and Solid Digestate during Anaerobic Digestion
AD is an appropriate technology to convert agricultural residues into biogas [37] and to transform the nutrients contained in the substrates into plant-available forms so that the final digestate can be used as fertilizers [43,44]. The effective use of nutrients available in the digestate is a way to improve the overall efficiency of AD technology [37]. The mass balance of nutrients in the AD is crucial for the quantification of their recovery potential [45]. It is also essential to evaluate the fate of nutrients like N, P, K, Ca, Mg, and Sulfur (S) and their distribution into LD and SD to assess the agronomic, environmental, and economic benefits of AD technology [46].
The mass balance of nutrients has been investigated in the AD of different substrates [37,46,47,48,49]. The recovery rate (RR) of nutrients is defined as the amount of nutrients available in the digestate after the AD process relative to their initial value (before the AD) and expressed as a percentage (%) [37]. The RR is usually calculated by the authors and provided in the studies.
Various substrates have been used in the studies for the investigation of the mass balance of nutrients. The categories are defined here on the basis of the nature of the substrates. The category “straws” consists of rice, corn, and wheat straw while, “manures” contains swine, cattle, and chicken manure. The RR of N from various studies using different substrates is summarized in Table 3. N showed up to 82% RR for different substrates used in these various experiments at different scales, specifically, 93–100% for manures [37,46], 98.4% for pig slurry (PS) [47], 85–91% for straws [37], 90.8% for organic fraction of municipal waste (OFMSW) [48], and 82–94% for mixed substrates [48,49]. Total N is often reported to be conserved during AD [43]. However, the primary cause of less than 100% RR of N may be gaseous NH3 migration with the biogas flux [48,49]. The RRs of N in these studies are comparable; however, the results of one study are contradictory, as the amount of total N in the digestate after AD was 8–23% higher than before AD [45]. The authors explained that the amount of hydrolyzed microalgae and the quantity of N released to the aqueous phase might have been overestimated since all the parameters were calculated as the difference between the microalgae tests and the control test [45].

Table 3.
RR of nutrients during AD of various substrates.
There is also a wide range (64–100%) of RRs of P when the different substrates are used in AD (Table 3). A RR of P of up to 91% during AD is not considered far from 100% [37], while up to 75% [46] and 64% [47] of P RR have been observed in two studies. The retention of the sludge in psychrophilic anaerobic sequencing batch reactors (PASBR) could be the possible reason for such a low RR of P [46]. In another study, crystal lining was observed on the opening of the digesters, and scanning electron microscopy (SEM-EDS) clearly showed them to be composed of P, Ca, Mg, and Mn (Manganese) [47].
K is conserved in the digestate in all these studies during the AD as shown in Table 3.
There are a few studies found addressing the mass balance of Ca, Mg, and S in AD. For example, on average, 8.7% of Ca, 18.7% of Mg, and 67.7% of S were retained in the bioreactors. However, the authors did not find statistically significant Ca and Mg retention in the digesters except for S [46]. Significant retention of Ca and Mg with 44% and 32.5% has been found in another study as they crystallize as carbonates and phosphates [47].
Li et al. [37] investigated the mass balance of nutrients (N and P) during the AD of RS in batch experiments. The RRs of N and P were 85–91% and 91–97%, respectively. The mass balance of other nutrients (K, Ca, Mg, and S) were not investigated in this study. This is the only study found in which RS was used and mass balance of nutrients (N and P) was performed, thus indicating the scarcity of nutrient mass balance on AD of RS.
The ranges of all nutrients’ RRs for specific substrates are shown in Table 3. The results mentioned in Table 3 are reported as a single value or as a range in the studies. The arithmetic mean is first determined based on the minimum and maximum values if the data points in the study are available as a range. Then these means are further used to calculate the overall average RR of N and P in the AD irrespective of a specific substrate. The results of 12 and 14 experiments are used to calculate the average RR of N and P, respectively, in AD, which is shown in Figure 3. It is assumed that the RR of N is not 100% due to its release over the gas phase, whereas P is suspected to crystalize with other elements and remains on the reactor walls [47]. The overall average RRs of Ca, Mg, and S was not calculated because only two studies were available.
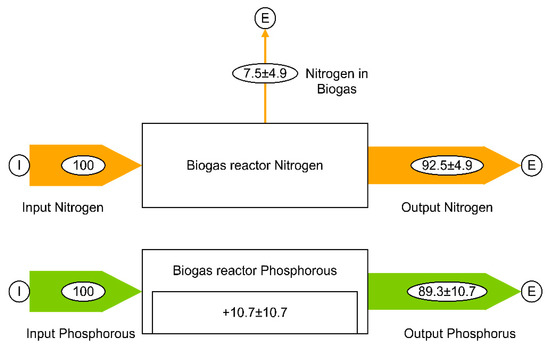
Figure 3.
Calculated mass balance of N and P in percent, relative to the input amount. N is assumed to be released in the the gas phase; P is assumed to remain in the digesters as it forms crystals with other elements. The standard deviation of the mean is displayed as “±”. The results of 12 [37,46,47,48,49] and 14 [37,45,46,47,48,49] experiments are used to calculate the average RR of N and P, respectively.
Concerning the effect of AD on the RR of nutrients, Figure 3 shows that 92.5% of N and 89.3% of P, on average, are recoverd. It can be concluded that N, P, and K are conserved in the digestate, as their RRs are close to 100%.
The separation of digestate into LD and SD with the use of separation techniques like press screws have been considered simple and low-cost practices for the management of the digestate from biogas plants and have been widely used in the European Union [43]. It has been reported that LD has a higher concentration of N in the form of ammonia, and it can be used as a fertilizer to substitute mineral fertilizer to some extent [43]. Moreover, the N/P ratio tends to increase in the LD because of separation, therefore, reducing the accumulation of P in the soil, as it offers a more balanced nutrient content for the crops [50]. The resulting SD generally used in the soil as an organic amendment [51], and it is also exported to other nearby farms to provide the solution of the excess of nutrients problem in that farm [52].
Tambone et al. [52] investigated thirteen full-scale biogas plants fed with a variety of substrates to study the distribution of nutrients into LD and SD after the usage of the screw press as a separation technique. Their results indicated that, on average, 87% of N measured as Total Kjeldahl Nitrogen (TKN) was available in the LD. In another study conducted on a full-scale biogas plant, treating the PS revealed that 85% of output N was also found in the LD [47]. Li et al. [37] carried out AD experiments in batch mode to study the distribution of N and P into LD and SD. They used three kinds of straws (rice, corn, wheat) and three kinds of manures (swine, cattle, and chicken) in their experiments. Their results indicated that on average, 34% of initial N was available in LD and 53% in SD, while the difference between initial and final N in case of straws was 9–15%. Similarly, for the manures, on average, 36% of initial N was available in LD and 59% in SD, while the difference in initial and final N after AD was 3–7% (as presented in Table 4). One possible reason for different proportions of nutrients in LD among these studies could be the usage of varying separation techniques like centrifugation [37], settling [46], and press screw [52].

Table 4.
Distribution of nutrients into LD and SD. “n” is the number of experiments to calculate the standard deviation of the mean (±).
Results obtained by Tambone et al. [52] indicated that the P available in LD accounted for 72% of the output (measured as P2O5). When the straws and PS used as substrates, 82% and 90% of P were available in the LD, respectively [37,47]. For manures, a significant portion of P (86%) was available in the SD [37]. One possible explanation could be that the P in the manures precipitate in solid form. This phenomenon is more likely due to a higher concentration of Mg and Ca which precipitates P [53].
A total of 14 full-scale AD plants, six batch experiments (three for straws and three for manures), and one PASBR were used in these studies to investigate the distribution of nutrients into LD and SD as presented in Table 4. The LD contained about 34–87% of N and 9–90% of P.
There is a wide range of nutrients (N and P) available in LD and SD. The explanation could be the usage of different separation techniques, various substrates, and different modes of AD. More studies need to be conducted to draw better conclusions about the distribution of nutrients into LD and SD. As data on the mass distribution of Ca, K, Mg, and S to LD and SD is scarce. Only N and P have been considered in this study. For RS, only one batch study was available on nutrients mass balance and LD/SD distribution. Future research into the mass balance of other important nutrients (Ca, K, Mg, and S) for RS co-digestion with manure or other suitable substrates, at pilot-scale or farm-scale plants, would help to evaluate the overall efficiency of AD technology and thus support establishing a mechanism to use the digestate.
4. Enhancement of Energy Recovery (Biogas/Methane Yield) from the Anaerobic Digestion of Rice Straw
The effects of the “factors” and “methods” on the improvement of energy recovery from AD of RS are discussed in the following section of this review.
4.1. Temperature
Temperature is one of the most significant parameters affecting activities, survival, and growth of microorganisms in the AD [54]. It also influences the biogas yield and digestate quality [55] in both batch and continuous mode of digestion. Temperature is a crucial parameter to maximize the efficiency of the AD but also in regards to economic input [11]. Generally, there are three different temperature ranges at which anaerobic bacteria can grow. These are: psychrophilic (10−30 °C), mesophilic (30–40 °C), and thermophilic (50–60 °C) [56].
Contreras et al. [25] conducted a study in which RS was digested for 36 days at 37 °C and 55 °C in the presence of mesophilic and thermophilic inocula, respectively. The cumulative CH4 yield was 226 L/kg VS at 37°C (with average 56% CH4 content) and 281 L/k VS at 55 °C (average CH4 content: 67%). At thermophilic conditions, RS produced approximately 25% more CH4 than at mesophilic conditions. Similar results have been obtained by Sathish et al. [54] who co-digested RS with press mud in digesters of 1 m3 capacity. Sathish et al. [54] pointed out that cumulative biogas yield for the duration of 20 days under thermophilic conditions (45–55 °C) was 34% higher than mesophilic conditions (25–40 °C). RS mono-digestion has also been evaluated by Lianhua et al. [57] at mesophilic (35 °C) and thermophilic conditions (55 °C) at different TS (7.5% and 20%) in batch tests. Under wet AD, their results showed the CH4 yield of 120 and 136 L/kg VS at mesophilic and thermophilic conditions, respectively. While CH4 yield was 123 and 76 L/kg VS at mesophilic and thermophilic conditions, respectively, under dry AD. Yu et al. [42] investigated the effect of increasing temperature from 39 to 50 °C on the biogas yield from a 300 m3 digester. The RS was added daily to the digester. The biogas yield increased by approximately 100% (from 200 L/kg TS to 402 L/kg TS) when the temperature of the system increased from 39 to 50°C. Moreover, the CH4 content was improved from 50% to 60%.
The studies mentioned above investigated the effect of mesophilic and thermophilic conditions on the biogas/methane yield. The biogas/methane yields at both temperatures in these studies [25,42,54,57] are shown in Figure 4. About 13–100% more methane is yielded at thermophilic as compared to mesophilic conditions in the four experiments, while in one AD test, 62% more methane was produced at mesophilic conditions.
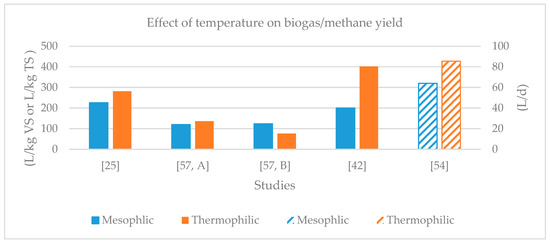
Figure 4.
Effect of temperature on biogas/methane yield [25,42,54,57].
The effect of increasing temperature on the CH4 yield from co-digestion of dairy manure (DM), chicken manure (CM), and RS has been investigated [26], and the results are shown in Figure 5. RS was co-digested with DM, CM, and also in a mixture with both manures in batch digesters at 20, 30, 40, 50, and 60 °C. CH4 yield continuously increased by increasing the temperature. CH4 yield in three mixtures was on average 2.5 times higher at 40 than at 20 °C, while only about 1.2 times higher at 60 than at 40 °C. No significant difference was observed in CH4 yields between 50 and 60 °C [26].
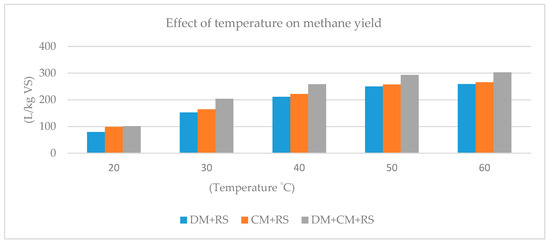
Figure 5.
Effect of temperature on methane yield. Values are presented as the mean of three replicates (n = 3). DM, Dairy manure; CM, Chicken manure; RS, Rice straw [26].
The results of these studies demonstrate that psychrophilic AD of RS has the lowest CH4 yield compared to mesophilic and thermophilic conditions. It illustrates the critical role of temperature in AD digester concerning energy recovery. Compared to mesophilic conditions, the AD of RS under thermophilic conditions yielded up to 100% more biogas yields. Moreover, the quality of biogas in terms of CH4 content is also higher at higher temperatures. Thus, optimal energy recovery from the AD of RS is attained under thermophilic conditions.
Apart from the advantages of higher energy recovery, there are some disadvantages to the thermophilic AD process. For instance, variation in temperature should not exceed 0.6 °C/day to maintain a stable digestion process [18]. The mesophilic process is more robust and less sensitive to environmental changes than the thermophilic process [58].
4.2. Co-digestion of RS
RS is a promising substrate for AD because of its high biogas potential [11]. Untreated RS has a higher amount of total C (e.g., 35–47% TS) and very low content of N (e.g., ≤ 1% TS) with a typical C/N ratio of approximately 47–75 [10,39]. Optimum C/N ratio ranges from 20 to 30 [59,60]. As the optimal C/N ratio is crucial for efficient AD, an external source of N is required for the digestion of RS.
The term co-digestion describes the simultaneous digestion of a mixture of two or more substrates. Co-digestion offers various advantages like improved system stability, adjusted C/N ratio, a better nutrient balance, dilution of toxic compounds, improved buffer capacity, increased biodegradation, adjusted moisture content, a supply of TEs, and increasing OLR. It can therefore, enhance volumetric biogas recovery [61,62,63] and improves the fertilizer value of the digestate [64]. These advantages of co-digestion are presented in Figure 6. Co-digestion of RS with different animal manures is considered a more cost-effective method for nutrition regulation than the addition of N-containing chemical reagents like ammonium bicarbonate or urea [40].
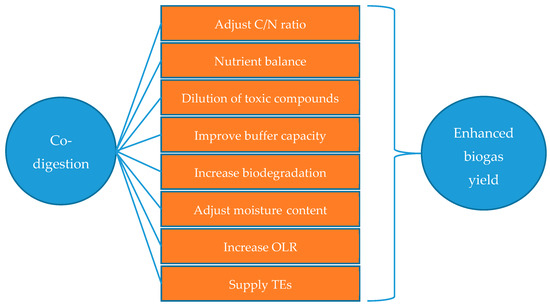
Figure 6.
Advantages of anaerobic co-digestion [61,62,63].
Ye et al. [39] attained about 55%, 72%, and 47% higher biogas yield compared to RS mono-digestion when kitchen waste (KW) and pig manure (PM), were co-digested with RS at a mass ratio of 0:2:1, 0.4:1.6:1, and 0.8:1.2:1, respectively. Similar results were obtained by Zhan-Jiang et al. [65], where 70% more biogas was recovered in co-digestion of RS with food waste (FW) as compared to mono-digestion of RS. RS digested with goat manure (GM) performed best, with a mass mixing ratio of 50:50 (vs. 100:0, 90:10, 70:30, 30:70, 10:90 and 0:100) and yielding a 83% higher biogas yield than RS only [66].
Similarly, co-digestion of RS with various co-substrates such as cow manure (CoM), PM, CM, paper mill sludge (PMS), piggery wastewater (PWW), and municipal sewage sludge (MSS) has been reported by other researchers. The results of these studies (Table 5) show the enhancement in energy recovery from the co-digestion of RS compared to its mono-digestion [22,28,41,67,68].

Table 5.
Effects of co-digestion on the methane yield of RS with other substrates.
Co-digestion of RS can be realized with several different co-substrates as the results of the above-mentioned studies illustrate. It enhances biogas yield from 26% to 83% and also the CH4 yield from 2% to 640% relative to RS mono-digestion when optimized for the appropriate C/N ratio. Co-digestion of RS with proper C/N ratio, which can be achieved by mixing one or even more substrates in a suitable amount, is a good approach for the enhancement of energy recovery. This approach can be applied in pilot-scale or farm-scale plants.
4.3. Inoculum
The starting of the AD system is a vital and delicate stage to achieve a successful operation [69]. Inoculum contains active microorganisms and is added to the digester to initiate the AD [38,70]. Usual inoculum is from digested sludge of active biogas plants or organic animal material [71]. A suitable inoculum can improve the biogas yield by increasing the degradation rate, stabilizing the AD, and also shorten the start-up time [72]. Other positive aspects of inoculum are contained N and micronutrients [73]. The micronutrients can enhance the enzyme activity [74] while the extra N can be provided to meet the needs of the methanogens [38]. Therefore, the sources of inoculum, its treatments and the substrate to inoculum ratio (S/I) are important for the AD of RS. The term S/I ratio determines the amounts of substrate and inoculum that should be added in the AD batch digester to prevent the inhibition by avoiding the overlarge portion of the substrate [75].
Gu et al. [38] found that the sources (digested dairy manure, digested swine manure, digested chicken manure, digested municipal sludge, anaerobic granular sludge, and paper mill sludge) of the inoculum can significantly affect the AD of RS. The digested dairy manure resulted in the highest biogas yield of 325 L/kg VS as it provided suitable nutrients and the highest enzyme activities. RS inoculated with other inocula attained biogas yields of 30–280 L/kg VS. In addition, Gu et al. [38] also suggested that a suitable inoculum can also provide N, macronutrients, and micronutrients for the AD.
Haider et al. [69] compared five S/I ratios, i.e., 0.25, 0.5, 1.0, 1.5, and 2.0 for anaerobic co-digestion of FW and rice husk using the fresh cow dung as inoculum and the biogas yields were 557, 458, 267, 97 and 71 L/kg VS, respectively. The highest biogas yield was obtained at S/I ratio of 0.25. Higher S/I ratios are attributed to the presence of a higher amount of biodegradable substrate and a lower amount of inoculum. Furthermore, the authors reported an accumulation of volatile fatty acids (VFAs) at higher S/I ratios (1.5 and 2.0), indicating insufficient activity of methanogenic bacteria.
In another study, the inoculum was diluted in order to compare the effect of inoculum dilution on the CH4 yield of pretreated RS at various S/I ratios (2, 4, 6, 8, and 10). The original inoculum (I0) was collected from the anaerobic digester of a wastewater treatment plant. Io was diluted twice with the recycled water to achieve the diluted inoculum (I2). When I0 was used as inoculum, the CH4 yields were 238, 190, < 50, < 50, and < 50 L/kg VS at S/I ratios of 2, 4, 6, 8, and 10, respectively. While the yields were 193, 187, 176, 144 and < 50 L/kg VS when I2 was used in the digesters. These results evidenced that I2 had better dilution capacity for acidic compounds and mass transfer performance due to the availability of additional water in the inoculum. These characteristics of I2 improve methanogenic activity [76].
Deng et al. [70] studied the effects of co-inoculating RS with ruminal microbiota (RM) and anaerobic sludge (AS). Five co-inoculum mixtures were prepared with RM: AS as 1:1, 1:2, 2:1, 1:0, and 0:1 based on VS content, while the S/I ratio in all mixtures was approximately 0.25. The results showed that the co-inoculation had almost threefold higher CH4 yield than the digestion of RS in the presence of RM alone.
Kim et al. [77] pointed out that heat treatment (heating the sludge at 100 °C for 15 min and then cooling to room temperature to enrich the spore-forming bacteria of the Clostridium species) of sewage sludge, which was used as inoculum for the AD of RS, caused less biogas yield in comparison to untreated sludge.
It is demonstrated from the results of the studies that the source of inoculum affects the biogas yield. Digested dairy manure proved to be optimal inoculum for RS among the various sources of inocula. Biogas/methane yields tend to decrease with increasing S/I ratios. A high amount of substrate relative to inoculum may cause problems in AD, such as the accumulation of VFAs and lower biogas production rate. Heat treatment may cause the inoculum to be less effective. The use of co-inoculation seems to increase the biogas yield from the AD of RS.
4.4. Organic Loading Rate (OLR)
Process stability and efficiency are proved to be the basic criteria to evaluate the performance of AD [78]. OLR is an important operation parameter that greatly influences the performance of the AD, and it can be described as the amount of substrate added to the digester per day per unit volume. OLR is expressed as Equation (1):
where C is the feeding concentration of substrate in g.VS/L, and tHR is the hydraulic retention time in days (d).
Higher OLR can produce higher biogas because of the higher quantity of substrate for digestion [79]. With a higher OLR, the rate of hydrolysis/acidogenesis could be higher than methanogenesis, and the accumulation of VFAs can eventually cause process failure [80]; therefore, a suitable OLR is always necessary for stable operation.
Thermophilic co-digestion of RS with animal manures ( PM, CoM, and CM) was carried out at different OLRs of 3.0, 3.6, 4.2, 4.8, 6.0, 8.0, and 12.0 g VSL−1 d−1 by Li et al. [40]. Results from co-digestion of RS and PM illustrated that a stable biogas yield, on average, 434 L/kg VS was achieved without inhibition by ammonia or VFAs while, for the co-digestion of RS with CoM, the average yield was 455 L/kg VS at all OLRs. In contrast, the AD process was inhibited by ammonia when the OLR was ≥ 6 g V S L−1 d−1 during the co-digestion of RS with CM and the specific biogas yield was 422 L/kg VS at 4.8 g VSL−1 d−1.
Similarly, the effect of various OLRs on the anaerobic mesophilic co-digestion of RS with the PM [41], CoM [28], and CM [67] have been investigated, and at 6−8, 6, and 4.8 g VS L−1 d−1, there was no inhibition in the digesters due to the accumulation of VFAs or ammonia, respectively. However, co-digestion of RS with PM and CoM was severely inhibited by VFAs at OLR of 12 g VS L−1 d−1. The co-digestion of RS with CM was inhibited at OLR ≥ 6 g VS L−1 d−1 due to the accumulation of ammonia.
The effect of various OLRs on the yield of biogas from the AD of RS, and their recommended values from the literature are summarized in Table 6.

Table 6.
Effects of OLR on the biogas yield in AD of RS.
The recommended OLRs for commercial co-digestion of RS with PM, CoM, and CM at mesophilic conditions are 6.0–8.0, 6.0, and 4.8 g VS L−1 d−1, while recommended values for thermophilic conditions are 12.0, 12.0, and 4.8 g VS L−1 d−1. From the results of these studies, it can be concluded that a choice of a suitable OLR is necessary for attaining a good biogas yield from the AD of the RS. The optimal OLR is influenced by the type of substrate (e.g., manure) and the operating temperature. Higher OLRs with stable biogas yield can be attained at thermophilic conditions compared to mesophilic ones. Moreover, the cause of inhibition is also different in different co-digestion systems. For CM, the process inhibits by free ammonia; VFAs inhibit co-digestion of PM and CoM at higher OLR. The co-digestion system of RS assisted with in situ removal of ammonia can be considered to avoid inhibition by ammonia at higher OLRs [40]. The methods considered for the removal of ammonia are immobilization and adaptation of microorganisms, ultrasonication, microwave, hollow fiber membranes, and microbial fuel cell applications [82].
4.5. Mixing
Mixing is an important operational factor that determines the performance of the AD. It enhances the mass transfer of organic substrates to microbial biomass due to the homogenization of the content in the digester [83]. Moreover, mixing facilitates the removal of trapped gas bubbles in the digester, preventing the sedimentation of heavy particles and establishing uniform temperature [18]. However, intense continuous mixing disrupts the structure of microbial flocks, which disturbs the syntrophic relationship between organisms and leads to a reduction in biogas yield, instability of the digester, and increased vulnerability to shock loadings [83,84]. Therefore, it is crucial to provide optimal mixing for efficient and stable AD [85].
Generally, gas circulation, mechanical pumping, and mechanical stirring are used for mixing [86]. Mechanical stirring is widely used in process industry operations involving various solid–liquid flows because of its higher homogeneity in practice [87]. Moreover, mechanical agitators are the most efficient mixing device in terms of power consumption [88].
The effect of mixing has been mostly studied for municipal solid waste, biosolids [89,90], and manures [83,91]. It is not well understood how mixing affects the AD of agricultural crop residues such as RS [85]. Hence, the optimized mixing parameters for the well-investigated traditionally used substrates could be very different for crop residues, including RS [87].
RS, like other lignocellulosic substrates, has a complex chemical structure. RS also has some specific physical characteristics like low density, high water holding capacity, poor fluidity, and heterogeneity [87,92]. RS floats on the surface in the digester and does not mix properly, especially when biogas is entrapped in the slurry. Due to poor mixing, the substrate does not get good contact with the microorganisms, thus reducing degradation and, ultimately, lowering biogas yield [92].
Kim et al. [85] conducted batch experiments using RS as a substrate to study the effect of periodic mixing (once a day, once a week, twice a week), continuous mixing (50, 150, and 300 revolutions per minute (rpm)), and no mixing on the CH4 yield. Their results showed that the differences in CH4 yield were not statistically significant among the periodic and no mixing conditions, while the total CH4 yield for the 50, 150, and 300 rpm continuous mixing conditions were 13%, 25%, and 38% lower than for no mixing conditions. Kim et al. [85] also investigated the effect of three modes of continuous mixing during the AD of RS in three continuously stirred tank reactors (CSTR). The three applied regimes were: (R1) one digester with continuous mixing at 50 rpm, (R2) second digester with manually mixing of once a day, and (R3) the third digester was operated at 50 rpm continuous mixing with occasional high speed (150 rpm) mixing events. They concluded that the CH4 content in biogas in R2 (53%) was slightly higher than in R1 (51%), while in R3, it dropped to a minimum value of 7%.
Shen et al. [87] carried out a study to improve the mixing of RS during AD to achieve higher biogas yield by computational fluid dynamics (CFD) and experimental tests. The experiments were performed at different stirring rates (40, 80, 120, and 160 rpm) to investigate their effect on biogas yield in CSTR. The highest biogas yield was achieved at a stirring rate of 80 rpm. It was higher than that of other stirring rates by about 19% (40 rpm), 10% (120 rpm), and 12% (160 rpm). Tian et al. [92] utilized the results of Shen et al. [87] for identifying proper agitation intervals to prevent floating layer formation during AD of corn stover (CS) and to improve the biogas yield. Shen et al. [87] studied the effect of various stirring rates (40, 80, 120, and 160 rpm for 5 min each time) on biogas yield of RS, while Tian et al. [92] further investigated the effect of various agitation intervals (continuous; after 2, 4, 8, and 12 h for 5 min for each time) with different OLRs (1.44, 1.78, and 2.11 g TS L−1 d−1) on the biogas yield from AD of CS in CSTRs. The results of the study showed that, at lower OLR of 1.44 g TS L−1 d−1, there was no floating layer observed in the digesters when the time interval for the agitation was continuous for 2 h, 4 h, and 8 h, while the performance of AD was adversely affected at 12 h. Moreover, the biogas yield for the digester agitated after 12 h was 418 L/kg TS, which was 2%, 9%, 3%, and 0% lower than those digesters stirred continuously, for 2 h, 4 h, and 8 h, respectively. The optimal agitation intervals shifted to 6 h and 2 h when the OLRs increased to higher levels of 1.78 g TS L−1 d−1 and 2.11 g TS L−1 d−1, respectively. When the agitation time intervals were more extended than optimal, floating layers were observed in the digesters, especially at higher OLR of 2.11 g TS L−1 d−1. The results of studies investigating the effects of various mixing conditions on biogas yield from the AD of RS and other straw have been summarized in Table 7.

Table 7.
Effects of mixing on the biogas yield in AD of rice and other straw.
The results in Table 7 show that periodic mixing is better as compared to continuous mixing. The stirring rate of 80 rpm lasting for the 5 min provides proper mixing in the digesters. It has also been observed that higher OLR led to the rapid formation of a floating layer. The agitation interval with highest biogas yields has been identified as 2 h at OLRs of 1.44, 1.78, and 2.11 g TS L−1 d−1 during the AD of straw in CSTRs.
The results of these reported studies verified that the proper agitation speed, mixing time, and the mixing intervals can achieve higher biogas yield. In contrast, the very intense mixing can affect the process adversely. These results are useful for operating the digesters treating the RS or other straw in an efficient and cost-effective way.
4.6. Recycling Liquid Digestate (LD)
A separation machine (press screws) is usually used to separate the digestate from the digester into LD and SD. The SD is rather simple to handle as it is generally used as fertilizer after composting or even directly. However, the utilization or treatment of LD is a challenge. LD can be used as liquid fertilizer or can be disposed of in the water streams after treatment; however, the most promising option is its recycling to the digester [94].
The recycling of LD can help to improve the moisture content for some high solid substrates [94] and provides a range of nutrients and vitamins needed by the microorganisms [95]. However, the excessive recycling may result in process inhibition by the accumulation of VFAs, ammonia, and nonbiodegradable intermediates [94,96,97].
Hu et al. [94] investigated the effect of recycling LD during the AD of MS in the CSTR. They compared three scenarios: No recycling of LD serving as a reference, while in the other two digesters, direct recycling and recycling after aeration carried out. Their results showed that there was no significant difference in CH4 yield, CH4 content, substrate reduction, or pH in all three modes. This indicates that recycling did not adversely affect system stability.
Pezzolla et al. [98] evaluated the effect of the frequency of LD recycling on the biogas yield in the solid-state AD of straw with PS. They compared the impact of different recycling frequencies—once per day, twice per day, and four times per day—with a control system (no recycling). The results suggested that recycling LD had a positive effect on biogas yield when it was more than twice per day. The highest cumulative biogas yield was achieved at a recycling frequency of four times per day.
Mussoline et al. [36] aimed to define and optimize the operational parameters of a farm-scale anaerobic batch digester (13,000 m3) using RS and PWW as substrates where recycling of LD was one of the important strategies. Increasing recycling rates of LD (from 0.04 to more than 0.14 m3/m3straw d−1) resulted in increasing biogas yield. Higher recycling rates are recommended, but these are limited by the accumulation of VFAs which can inhibit the methanogenic process.
The effect of LD recycling in various proportions (relative to recovered amount), with different frequencies and with varying post-treatments on the biogas/methane yield in the AD of various substrates is shown in Table 8.

Table 8.
Effects of recycling LD on the biogas/methane yield in AD of various substrates.
The studies presented in Table 8 show different results of the recycling of LD on the biogas or methane yield in the AD of various substrates. The results of the two studies show no increase or decrease in the biogas yield due to LD recycling, whereas the authors explained that there is no adverse impact of recycling on the process stability [94,96]. Some authors obtained higher biogas yield with the recycling of LD. They attributed this to increase microbial growth, enhanced buffer capacity, higher alkalinity, increased methanogenic activities, higher nutrients availability, and improved hydrolysis [36,98,99,100]. In some other studies, the biogas yield improved at a lower rate of LD recycling, and it reduced when more LD was recycled. The accumulation of VFAs and the dilution of essential nutrients were named as causes [100,102,103].
From these studies, it can be concluded that it is technically feasible to recycle part or all of the LD to the AD system. It is also possible that the process cannot be sustained for a longer time due to the dilution of nutrients. Co-digestion of straw with nutrients rich substrates combined with recycling LD is an excellent approach to achieve a stable AD system. In addition, an appropriate amount of LD is required because its excessive recycling may cause process failure due to the accumulation of VFAs. Therefore, LD recycling may enhance system stability and biogas yield.
4.7. Addition of Trace Elements (TEs)
Several specific TEs such as iron (Fe), cobalt (Co), nickel (Ni), copper (Cu), zinc (Zn), molybdenum (Mo), selenium (Se), and tungsten (W) are essential for enzyme cofactors involved in the biochemistry of CH4 formation [104] and needed in a balanced AD [105]. However, the availability of TEs in higher amounts can cause inhibition to anaerobic organisms due to the disruption of an enzyme’s structure and function [106]. Therefore, an appropriate amount of TEs is required to avoid the inhibition by maintaining effective growth and metabolism of microorganisms. Moreover, it has been found from the literature that the degree of inhibition depends upon several factors such as the total concentration, pH, redox potential, and chemical forms of the elements [107,108].
Recently, Mancini et al. [29] added different TEs (Co, Ni, and Se) in various mass fractions to study the enhancement of CH4 yield in the AD of RS. The results showed a 12% improvement in the CH4 yield with the addition of these TEs. In another similar study, Cai et al. [109] observed 18% more CH4 yield when Fe was added to the batch AD of RS. A study conducted by Mancini et al. [110] using RS as a sole substrate showed different results as compared to these similar studies [29,109]. The authors did not observe any enhancement in CH4 yield due to the addition of Fe and Co. They concluded that it is likely due to the presence of the elevated TEs in the inoculum used in the study. The effect of the addition of various TEs on the methane yield in the AD of straws is summarized in Table 9.

Table 9.
Effect of addition of TEs on the methane yield in the AD of RS and other straws.
The results of the abovementioned studies show that research on several TEs has been carried out for single and multiple elements, while Fe, Ni, and Co are the most investigated. The addition of TEs has been successfully used to enhance biogas and CH4 yield in the AD of various straw types. Specifically, the enhancement in CH4 yield of 0–18% has been observed due to the addition of TEs (Fe, Ni, Co, and Se) to the AD of RS. Similarly, up to 62% enhancement in CH4 yield observed with the addition of TEs to other straw (CS) (Table 9).
Literature data on the impact of TEs addition on the enhancement of CH4 yield in the AD of RS is scarse. There were only three studies found investigating the effect of the addition of Fe, Ni, Co, and Se. Two studies show that the addition of TEs improves the CH4 yield while the third study shows there was no improvement. Therefore, there is a need for future work in this area to understand the effect of the addition of other TEs as well. Likely, the addition of suitable elements in an appropriate combination and amount would enhance the CH4 yield in the AD of RS as evidenced by other straws.
4.8. Bioavailability of Trace Elements (TEs)
The presence of a particular TE in the AD does not necessarily imply that it can be taken up by microorganisms and incorporated into the catalytic center of the enzymes [118]. Bioavailability is defined as the degree to which a substance is available for metabolic activities [119]. The bioavailability of TEs can be grouped and approximated by sequential extraction techniques [120]. Water-soluble and exchangeable fractions of TEs are commonly considered as highly bioavailable. TEs bound in carbonates, sulfides, or organic complexes are less bioavailable as their mobility depends on the aqueous solubility of the compounds. The residual fraction is not available to microorganisms because it is non-extractable and non-dissolvable [120]. Ortner et al. [120] examined the applicability of the sequential extraction technique on slurries of AD plants. These slurries were obtained from three biogas plants: an agricultural biogas plant utilizing PM and MSa, a plant using grass silage (GS) and MSa, and a plant processing the slaughterhouse waste (SHW). Their results are presented in Table 10.

Table 10.
Water-soluble and exchangeable fractions (relative to a total concentration of TE) of TEs in the AD of various substrates.
Fe has been added alone and incombination with Co to determine whether their bioavailability could be a limiting factor for CH4 yield using RS as a substrate in the presence of agro-zootechnical digestate as an inoculum in a study carried out by Mancini et al. [110]. The results pointed out that the amount of highly bioavailable Fe was increased from 11% to 23% when it was added alone and from 11% to 20% when it was dosed with Co. Similarly, Co addition resulted in an increase of the highly bioavailable fraction from 8% to 48%. The CH4 yield was the same with and without the addition of TEs. Therefore, the direct relationship between the increased highly bioavailable fractions of TEs and enhanced CH4 yield in the AD of RS was not observed. Cai et al. [109] conducted a similar study to investigate the changes in five fractions of Fe to assess its bioavailability and whether the system was deficient in Fe during the AD of RS. Their results indicated an overall very low (1.7–3.1%) bioavailability of Fe, and most of the Fe was not readily available to microorganisms in the digestion system. Thus, there was a deficiency of bioavailable Fe which may have reduced CH4 yield.
The effect of the bioavailability of Fe, Ni, Co, and Mn on the CH4 yield from the AD of MS in the semi-continuous CSTR was studied by González-Suárez [121]. The results indicated a decreasing trend of highly bioavailable fractions of TEs over time. At the end of the experiment (after 96 days), the highly bioavailable fractions of Fe, Ni, Co, and Mn decreased by 23%, 32%, 33%, and 20%, respectively, compared to day 1. The results confirm that fewer TEs were directly available for microbial uptake at the end of the process leading to an 11% decrease in CH4 yield.
The results of the studies summarized in Table 10 show that the sequential extraction is a useful method to assess the bioavailability of TEs available in various AD systems. The determination of the bioavailability of TEs is a more appropriate approach compared to total available amounts concerning their requirement to monitor the process to achieve a stable and efficient process.
The results of one study show the direct relationship between the reduced amount of highly bioavailable fractions of TEs and reduction in CH4 yield in the AD of MS. While, in another study, the CH4 yield has not enhanced due to the increased amount of highly bioavailable fractions of TEs in the AD of RS. Therefore, the relationship between the higher amount of highly bioavailable fractions of TEs and enhanced CH4 yield is not clear. Moreover, specifically for RS, only Fe and Co have been investigated to assess the effect of bioavailability on the CH4 yield. A large fraction is not available for microbial uptake during the AD of RS and this aspect should be considered for an appropriate supplementation of TEs to achieve enhanced CH4 yield.
5. Pilot-scale and Farm-scale Plants for AD of RS
Energy recovery in the form of biogas/methane from agricultural residues is highly interesting for the agricultural sector, as it is an option to produce local energy, replace fossil fuels, and thus, become self-sufficient and sustainable in terms of energy supply [122]. Introducing AD plants into the farm system, treating the animal manures along with the straws for the recovery of energy has a great potential to reduce the demand for fossil energy and reduction of greenhouse gas emissions [123].
Numerous bench-scale experiments have been carried out on RS digestion to define the optimal temperature [25,26,42], substrates for co-digestion [39,65,66], mixing intensities and duration [87,92], OLR [28,40,41], sources of inoculum [38], S/I ratio [69], recycling LD to the digester [36,98], addition of TEs and their bioavailability [110]. Bench-scale studies are important to understand the principles but it is challenging to design and operate a farm-scale plant based on these microcosms [124]. Besides, the monitoring of a farm-scale plant is necessary to obtain detailed information and a better understanding of technology [125]. It also helps to discover practical operational aspects such as the length of start-up phase and energy and co-substrates input and to check if the system is sustainable on a long-term basis [36,125]. These aspects are often missed in lab tests.
Mussoline et al. [124] conducted a batch pilot-scale study using untreated RS and PWW to define TS, straw-to-wastewater ratio, digestion temperature, and digestion time. RS was co-digested in two pilot-scale batch digesters with different amounts of PWW (Digester A: 50 kg dry straw, 150 L PWW, 20% TS; Digester B: 50 kg dry straw, 60 L PWW, 20% TS) for 189 days. The digestion temperature inside the digester A was between 30 and 40 °C. The digester B was initiated with ambient conditions (20–25 °C) and constant heating was applied later on to maintain mesophilic temperatures. The CH4 yield from digester A was 231 L/kg VS and from the digester, B was 12 L/kg VS. The lower temperature and lesser amount of PWW were the limiting factors for lower CH4 yield from digester B. The amount of PWW was not sufficient to establish a stable microbial community. The results of digester A were compared with the results of another study [57] of the same magnitude where the pretreated RS was digested. The pretreated (cutting into 7–8 cm pieces and treated with white-rot fungi) RS requires only 89 days for digestion while the untreated RS needs 189 days. Moreover, the pretreated RS can be adequately digested at ambient temperature (19–30 °C), while the untreated RS requires the mesophilic conditions in the presence of approximately 67% more PWW [57].
Other than RS, André et al. [126] studied the dry AD of roadside grass (RSG) with manure in a pilot-scale plant at 37 °C with three digesters. The overall average CH4 yield (226 L/kg VS) from three digesters obtained in this study [126] was similar to the yield obtained by Mussoline et al. [124] during the co-digestion of RS with PWW. A pilot-scale plant was successfully operated with a stable AD process treating RS and manure in the BIORIST project [24]. No further studies were found to compare the performance of AD of RS with other substrates at the pilot scale.
Data from a farm-scale system treating RS is limited to one study [36]. During the initial loading event, 727 t of RS and 285 t of PWW was loaded in a batch digester. Approximately 1300 t of water was added to the digester overtime during the first year. The co-digestion of RS with PWW was completed after 422 days, and the cumulative energy production was 295 MWh. The results showed a start-up period of 200 days and the authors attributed this long phase to high straw to wastewater ratio, low ambient temperature (<15 °C), and low recycling rate of LD (< 0.04 m3/m3straw d−1). Similarly, Wandera et al. [127] investigated the performance of a full-scale biogas plant treating the MS. The digester had a capacity of 400 m3 and was operated at a temperature ranging from 55 to 60 °C. They obtained a CH4 yield of 200 L/kg TS.
The abovementioned studies indicate the importance of pilot-scale experimentations to define the operational parameters such as heat input, TS, straw to wastewater ratio, digestion temperature, digestion time, loading frequency, and wastewater volume for the farm-scale plants treating RS. Although the literature reporting data on the AD of RS in farm-scale plants is scared. The implementation of a farm-scale plant using RS co-digested with other suitable substrates (PWW, manures) is possible as evidenced by studies that co-digestion performs better than mono-digestion. The farm-scale biogas plants are good candidates for the utilization of RS to recover energy, to facilitate the development of the agriculture economy and to provide local energy. However, further investigation is needed for a better understanding of the process. Moreover, reseach about the economical viability of farm-scale AD plants for RS should be conducted and published, covering all financial cost of capital and operation, including the logistics of RS and other co-substrates.
6. Conclusions
RS generation will continue to increase as rice production continues to rise to provide food for a still growing global population. The AD of RS is a viable option for the recovery of energy and nutrients. The nutrients (N, P, and K) are mostly conserved in the digestate during the AD, and their significant portion is available in LD. The effective use of nutrients available in the digestate is a way to partially substitute fertilizers for the amendment of agricultural land.
The AD of RS at thermophilic conditions results in higher biogas/methane yield and higher CH4 content in biogas as compared to mesophilic conditions. In contrast, the mesophilic process is more robust and less sensitive to environmental changes. Using a suitable inoculum and co-substrate in an appropriate amount enhances biogas/methane yield in the AD of RS by providing nutrients balance, dilute toxic compounds, adjust moisture content, improve buffer capacity, and stability of the system. Optimal selection of mixing (speed, time, and intervals), OLR, recycling LD, and TEs (with appropriate amount) also enhances the biogas/methane yield.
Pilot-scale plants and experiments are necessary to design and define operational parameters for farm-scale biogas plant treating RS. Technically, the implementation of farm-scale biogas plants utilizing RS together with other suitable substrates such as PWW or other livestock manure is possible. It will provide energy and nutrients recovery to the local farm. However, the economic feasibility of such a plant should be evaluated.
Author Contributions
F.M. reviewed the literature and wrote the first draft of the paper. A.F., V.S.R. reviewed and edited to enhance the scientific quality of the paper. All authors have read and agreed to the published version of the manuscript.
Funding
Furqan Muhayodin is a doctoral student and funded by the Ministry of Higher Education Commission (HEC), Pakistan (SAP 50020935). This work was supported by the German Federal Ministry of Education and Research under the project “BIORIST”- A joint research project for an innovative process technology for biogas production from rice straw (01LY1508A).
Acknowledgments
The authors extend their thanks and gratitude to Thi Quynh Trang Hoang for providing critical constructive comments to improve the quality of this paper. We acknowledge support by the German Research Foundation and the Open Access Publication Fund of TU Berlin. Our sincere thanks for the two anonymous reviewers who provided critical constructive comments on the manuscript, which resulted in a significantly improved article.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AD | Anaerobic digestion |
| AS | Alfalfa silage |
| AS | Anaerobic sludge |
| C/N | Carbon-to-nitrogen ratio |
| CFD | Computational fluid dynamics |
| CM | Chicken manure |
| CoM | Cow manure |
| CS | Corn stover/straw |
| CSTR | Continuously stirred tank reactor |
| DM | Dairy manure |
| FW | Food waste |
| GM | Goat manure |
| GS | Grass silage |
| IRRI | International Rice Research Institute |
| KW | Kitchen waste |
| LD | Liquid digestate |
| LG | Lawn grass |
| MN | Macronutrients (N and P) |
| MS | Maize stover/straw |
| MSa | Maize silage |
| MSS | Municipal sewage sludge |
| OFMSW | Organic fraction of municipal solid waste |
| OLR | Organic loading rate |
| PASBR | Psychrophilic anaerobic sequencing batch reactor |
| PM | Pig manure |
| PMS | Paper mill sludge |
| PS | Pig slurry |
| PSa | Phragmites straw |
| PWW | Piggery wastewater |
| RM | Ruminal microbiota |
| rpm | Revolutions per minute |
| RR | Recovery rate |
| RS | Rice straw |
| RSG | Roadside grass |
| S/I | Substrate-to-inoculum ratio |
| SD | Solid digestate |
| SEM-EDS | Scanning electron microscopy |
| SHW | Slaughterhouse waste |
| SM | Swine manure |
| SS | Sewage sludge |
| TC | Total carbon |
| TEs | Trace elements |
| tHR | Hydraulic retention time |
| TKN | Total Kjeldahl nitrogen |
| TN | Total nitrogen |
| TS | Total solids |
| VFAs | Volatile fatty acids |
| VS | Volatile solids |
| WS | Wheat straw |
References
- Arvanitoyannis, I.S.; Tserkezou, P. Corn and rice waste: A comparative and critical presentation of methods and current and potential uses of treated waste. Int. J. Food Sci. Technol. 2008, 43, 958–988. [Google Scholar] [CrossRef]
- Sattar, A.; Arslan, C.; Ji, C.; Sattar, S.; Umair, M.; Zia Bakht, M. Quantification of temperature effect on batch production of bio-hydrogen from rice crop wastes in an anaerobic bio reactor. Int. J. Hydrog. Energy 2016, 41, 11050–11061. [Google Scholar] [CrossRef]
- Koopmans, A.; Koppejan, J. Agricultural and forest residues- generation, utilization and availability. Reg. Consult. Mod. Appl. Biomass Energy 1997. [Google Scholar]
- Kadam, K.L.; Forrest, L.H.; Alan Jacobson, W. Rice straw as a lignocellulosic resource: Collection, processing, transportation, and environmental aspects. Biomass Bioenergy 2000, 18, 369–389. [Google Scholar] [CrossRef]
- Purohit, P. Economic potential of biomass gasification projects under clean development mechanism in India. J. Clean. Prod. 2009, 17, 181–193. [Google Scholar] [CrossRef]
- IRRI. Rice Knowledge Bank. Available online: http://www.knowledgebank.irri.org/step-by-step-production/postharvest/rice-by-products (accessed on 11 March 2020).
- FAOSTAT. Global Rice Production by Year. Food and Agriculture of the United Nations. Available online: http://faostat.fao.org (accessed on 19 March 2019).
- Gadde, B.; Bonnet, S.; Menke, C.; Garivait, S. Air pollutant emissions from rice straw open field burning in India, Thailand and the Philippines. Environ. Pollut. 2009, 157, 1554–1558. [Google Scholar] [CrossRef]
- Glissmann, K.; Conrad, R. Fermentation pattern of methanogenic degradation of rice straw in anoxic paddy soil. FEMS Microbiol. Ecol. 2000, 31, 117–126. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Z. Biogasification of rice straw with an anaerobic-phased solids digester system. Bioresour. Technol. 1999, 68, 235–245. [Google Scholar] [CrossRef]
- Mussoline, W.; Esposito, G.; Giordano, A.; Lens, P. The Anaerobic Digestion of Rice Straw: A Review. Crit. Rev. Environ. Sci. Technol. 2013, 43, 895–915. [Google Scholar] [CrossRef]
- Bakker, R.R.; Jenkins, B.M. Feasibility of collecting naturally leached rice straw for thermal conversion. Biomass Bioenergy 2003, 25, 597–614. [Google Scholar] [CrossRef]
- Huang, Y.F.; Kuan, W.H.; Lo, S.L.; Lin, C.F. Hydrogen-rich fuel gas from rice straw via microwave-induced pyrolysis. Bioresour. Technol. 2010, 101, 1968–1973. [Google Scholar] [CrossRef] [PubMed]
- Iranzo, M.; Cañizares, J.V.; Roca-Perez, L.; Sainz-Pardo, I.; Mormeneo, S.; Boluda, R. Characteristics of rice straw and sewage sludge as composting materials in Valencia (Spain). Bioresour. Technol. 2004, 95, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, M.T.; Horiuchi, T.; Oba, S. Composting of rice straw with oilseed rape cake and poultry manure and its effects on faba bean (Vicia faba L.) growth and soil properties. Bioresour. Technol. 2004, 93, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Chynoweth, D.P.; Owens, J.M.; Legrand, R. Renewable methane from anaerobic digstion of biomass. Renew. Energy 2001, 22, 1–8. [Google Scholar] [CrossRef]
- Kargbo, F.R.; Xing, J.; Zhang, Y. Pretreatment for energy use of rice straw. A review. Afr. J. Agric. Res. 2009, 4. [Google Scholar]
- Appels, L.; Baeyens, J.; Degrève, J.; Dewil, R. Principles and potential of the anaerobic digestion of waste-activated sludge. Prog. Energy Combust. Sci. 2008, 34, 755–781. [Google Scholar] [CrossRef]
- Raheman, H.; Mondal, S. Biogas production potential of jatropha seed cake. Biomass Bioenergy 2012, 37, 25–30. [Google Scholar] [CrossRef]
- Grimsby, L.K.; Fjørtoft, K.; Aune, J.B. Nitrogen mineralization and energy from anaerobic digestion of jatropha press cake. Energy Sustain. Dev. 2013, 17, 35–39. [Google Scholar] [CrossRef]
- Dinuccio, E.; Balsari, P.; Gioelli, F.; Menardo, S. Evaluation of the biogas productivity potential of some Italian agro-industrial biomasses. Bioresour. Technol. 2010, 101, 3780–3783. [Google Scholar] [CrossRef]
- Mussoline, W.; Esposito, G.; Lens, P.; Spagni, A.; Giordano, A. Enhanced methane production from rice straw co-digested with anaerobic sludge from pulp and paper mill treatment process. Bioresour. Technol. 2013, 148, 135–143. [Google Scholar] [CrossRef]
- Baserga, U. Landwirtschaftliche CO-Vergarungs-Biogasanlagen. Biogas aus organischen Reststoffen und Energiegras. In FAT-Berichte; Forschungsanstalt für Agrarwirtschaft und Landtechnik: Tänikon, Schweiz, 1998. [Google Scholar]
- Larsen, O.; Rotter, V.S.; Fechter, L.; Spahr, M. BioRist -Entwicklung und Integration eines innovativen Verfahrens zur Biogasherstellung aus Reisstroh in regionale Wertschöpfungsketten im ländlichen Raum in Südostasien unter Berücksichtigung nachhaltiger Entwicklung und Klimaschutz – Beispiel Vietnam 2019.
- Contreras, L.M.; Schelle, H.; Sebrango, C.R.; Pereda, I. Methane potential and biodegradability of rice straw, rice husk and rice residues from the drying process. Water Sci. Technol. 2012, 65, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lu, X.; Li, F.; Yang, G. Effects of temperature and carbon-nitrogen (C/N) ratio on the performance of anaerobic co-digestion of dairy manure, chicken manure and rice straw: Focusing on ammonia inhibition. PLoS ONE 2014, 9, e97265. [Google Scholar] [CrossRef] [PubMed]
- Cann, I.; Kobayashi, Y.; Wakita, M.; Hoshino, H. Effects of 3 chemical treatments on in vitro fermentation of rice straw by mixed rumen microbes in the presence or absence of anaerobic rumen fungi. Reprod. Nutr. Dev. 1994, 34, 47–56. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, D.; Liu, S.; Mi, L.; Li, Z.; Yuan, Y.; Yan, Z.; Liu, X. Effects of feedstock ratio and organic loading rate on the anaerobic mesophilic co-digestion of rice straw and cow manure. Bioresour. Technol. 2015, 189, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Mancini, G.; Papirio, S.; Riccardelli, G.; Lens, P.N.L.; Esposito, G. Trace elements dosing and alkaline pretreatment in the anaerobic digestion of rice straw. Bioresour. Technol. 2018, 247, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Himmel, M.E.; Ding, S.H.; Johnson, D.K.; Adney, W.S.; Nimlos, M.R.; Brady, J.W.; Foust, T.D. Biomass Recalcitrance: Engineering Plants and Enzymes for Biofuels Production. Science 2007, 315, 801–804. [Google Scholar] [CrossRef]
- Hernández-Beltrán, J.U.; Hernández-De Lira, I.O.; Cruz-Santos, M.M.; Saucedo-Luevanos, A.; Hernández-Terán, F.; Balagurusamy, N. Insight into Pretreatment Methods of Lignocellulosic Biomass to Increase Biogas Yield: Current State, Challenges, and Opportunities. Appl. Sci. 2019, 9, 3721. [Google Scholar] [CrossRef]
- Ahmed, B.; Aboudi, K.; Tyagi, V.K.; Álvarez-Gallego, C.J.; Fernández-Güelfo, L.A.; Romero-García, L.I.; Kazmi, A.A. Improvement of Anaerobic Digestion of Lignocellulosic Biomass by Hydrothermal Pretreatment. Appl. Sci. 2019, 9, 3853. [Google Scholar] [CrossRef]
- CERT—Technical University of Berlin, Chair of Circular Economy and Recycling Technology. BioRist Project: Joint Research Project for An Innovative Process Technology for Biogas Production from Rice Straw. Available online: http://www.biorist.tu-berlin.de (accessed on 10 March 2020).
- Sun, Q.; Li, H.; Yan, J.; Liu, L.; Yu, Z.; Yu, X. Selection of appropriate biogas upgrading technology-a review of biogas cleaning, upgrading and utilisation. Renew. Sustain. Energy Rev. 2015, 51, 521–532. [Google Scholar] [CrossRef]
- Angelidaki, I.; Treu, L.; Tsapekos, P.; Luo, G.; Campanaro, S.; Wenzel, H.; Kougias, P.G. Biogas upgrading and utilization: Current status and perspectives. Biotechnol. Adv. 2018, 36, 452–466. [Google Scholar] [CrossRef]
- Mussoline, W.; Esposito, G.; Lens, P.; Garuti, G.; Giordano, A. Electrical energy production and operational strategies from a farm-scale anaerobic batch reactor loaded with rice straw and piggery wastewater. Renew. Energy 2014, 62, 399–406. [Google Scholar] [CrossRef]
- Li, H.; Tan, F.; Ke, L.; Xia, D.; Wang, Y.; He, N.; Zheng, Y.; Li, Q. Mass balances and distributions of C, N, and P in the anaerobic digestion of different substrates and relationships between products and substrates. Chem. Eng. J. 2016, 287, 329–336. [Google Scholar] [CrossRef]
- Gu, Y.; Chen, X.; Liu, Z.; Zhou, X.; Zhang, Y. Effect of inoculum sources on the anaerobic digestion of rice straw. Bioresour. Technol. 2014, 158, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Li, D.; Sun, Y.; Wang, G.; Yuan, Z.; Zhen, F.; Wang, Y. Improved biogas production from rice straw by co-digestion with kitchen waste and pig manure. Waste Manag. 2013, 33, 2653–2658. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Mei, Z.; He, W.; Yuan, Y.; Yan, Z.; Li, J.; Liu, X. Biogas production from thermophilic codigestion of air-dried rice straw and animal manure. Int. J. Energy Res. 2016, 40, 1245–1254. [Google Scholar] [CrossRef]
- Li, D.; Liu, S.; Mi, L.; Li, Z.; Yuan, Y.; Yan, Z.; Liu, X. Effects of feedstock ratio and organic loading rate on the anaerobic mesophilic co-digestion of rice straw and pig manure. Bioresour. Technol. 2015, 187, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Tian, Z.; Liu, J.; Zhou, J.; Yan, Z.; Yong, X.; Jia, H.; Wu, X.; Wei, P. Biogas Production and Microbial Community Dynamics during the Anaerobic Digestion of Rice Straw at 39–50 °C: A Pilot Study. Energy Fuels 2018, 32, 5157–5163. [Google Scholar] [CrossRef]
- Möller, K.; Müller, T. Effects of anaerobic digestion on digestate nutrient availability and crop growth: A review. Eng. Life Sci. 2012, 12, 242–257. [Google Scholar] [CrossRef]
- Šimon, T.; Kunzová, E.; Friedlová, M. The effect of digestate, cattle slurry and mineral fertilization on the winter wheat yield and soil quality parameters. Plant Soil Environ. 2016, 61, 522–527. [Google Scholar] [CrossRef]
- Alcántara, C.; García-Encina, P.A.; Muñoz, R. Evaluation of mass and energy balances in the integrated microalgae growth-anaerobic digestion process. Chem. Eng. J. 2013, 221, 238–246. [Google Scholar] [CrossRef]
- Massé, D.I.; Croteau, F.; Masse, L. The fate of crop nutrients during digestion of swine manure in psychrophilic anaerobic sequencing batch reactors. Bioresour. Technol. 2007, 98, 2819–2823. [Google Scholar] [CrossRef]
- Marcato, C.E.; Pinelli, E.; Pouech, P.; Winterton, P.; Guiresse, M. Particle size and metal distributions in anaerobically digested pig slurry. Bioresour. Technol. 2008, 99, 2340–2348. [Google Scholar] [CrossRef]
- Schievano, A.; D’Imporzano, G.; Salati, S.; Adani, F. On-field study of anaerobic digestion full-scale plants (part I): An on-field methodology to determine mass, carbon and nutrients balance. Bioresour. Technol. 2011, 102, 7737–7744. [Google Scholar] [CrossRef]
- Ma, G.; Neiberg, J.S.; Harrison, J.H.; Whitefield, E.M. Nutrient contributions and biogas potential of co-digestion of feedstocks and dairy manure. Waste Manag. 2017, 64, 88–95. [Google Scholar] [CrossRef]
- Lukehurst, C.T.; Frost, P.; Al Seadi, T. Utilisation of Digestate from Biogas Plants as Biofertiliser; IEA Bioenergy Task 37- Energy from Biogas; IEA Bioenergy: Paris, France, 2010; pp. 1–36. [Google Scholar]
- Tambone, F.; Terruzzi, L.; Scaglia, B.; Adani, F. Composting of the solid fraction of digestate derived from pig slurry: Biological processes and compost properties. Waste Manag. 2015, 35, 55–61. [Google Scholar] [CrossRef]
- Tambone, F.; Orzi, V.; D’Imporzano, G.; Adani, F. Solid and liquid fractionation of digestate: Mass balance, chemical characterization, and agronomic and environmental value. Bioresour. Technol. 2017, 243, 1251–1256. [Google Scholar]
- Wild, D.; Kisliakova, A.; Siegrist, H. Prediction of recycle phosphorus loads from anaerobic digestion. Water Resour. 1997, 31, 2300–2308. [Google Scholar] [CrossRef]
- Sathish, S.; Chandrasekaran, M.; Parthiban, A. Effect of co-digestion agricultural-industrial residues: Various slurry temperatures. Int. J. Ambient Energy 2018, 39, 694–697. [Google Scholar] [CrossRef]
- Sanchez, E.; Borja, R.; Weiland, P.; Travieso, L.; Martin, A. Effect of substrate concentration and temperature on the anaerobic digestion of piggery waste in a tropical climate. Process Biochem. 2001, 37, 483–489. [Google Scholar] [CrossRef]
- Zhang, C.; Su, H.; Baeyens, J.; Tan, T. Reviewing the anaerobic digestion of food waste for biogas production. Renew. Sustain. Energy Rev. 2014, 38, 383–392. [Google Scholar] [CrossRef]
- Lianhua, L.; Dong, L.; Yongming, S.; Longlong, M.; Zhenhong, Y.; Xiaoying, K. Effect of temperature and solid concentration on anaerobic digestion of rice straw in South China. Int. J. Hydrog. Energy 2010, 35, 7261–7266. [Google Scholar] [CrossRef]
- Kim, M.; Ahn, Y.H.; Speece, R.E. Comparative process stability and efficiency of anaerobic digestion; mesophilic vs. thermophilic. Water Res. 2002, 36, 4369–4385. [Google Scholar] [CrossRef]
- Li, Y.; Park, S.Y.; Zhu, J. Solid-state anaerobic digestion for methane production from organic waste. Renew. Sustain. Energy Rev. 2011, 15, 821–826. [Google Scholar] [CrossRef]
- Puyuelo, B.; Ponsá, S.; Gea, T.; Sánchez, A. Determining C/N ratios for typical organic wastes using biodegradable fractions. Chemosphere 2011, 85, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zheng, Y.; Xu, F.; Li, Y. Solid-state anaerobic co-digestion of hay and soybean processing waste for biogas production. Bioresour. Technol. 2014, 154, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Zhang, K.; Liu, P.; Khan, A.; Xiong, J.; Tian, F.; Li, X. A critical review on the interaction of substrate nutrient balance and microbial community structure and function in anaerobic co-digestion. Bioresour. Technol. 2018, 247, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Rabii, A.; Aldin, S.; Dahman, Y.; Elbeshbishy, E. A Review on Anaerobic Co-Digestion with a Focus on the Microbial Populations and the Effect of Multi-Stage Digester Configuration. Energies 2019, 12, 1106. [Google Scholar] [CrossRef]
- Shi, X.S.; Yuan, X.Z.; Wang, Y.P.; Zeng, S.J.; Qiu, Y.L.; Guo, R.B.; Wang, L.S. Modeling of the methane production and pH value during the anaerobic co-digestion of dairy manure and spent mushroom substrate. Chem. Eng. J. 2014, 244, 258–263. [Google Scholar] [CrossRef]
- Zhan-jiang, P.; Jie, L.; Feng-mei, S.; Su, W.; Ya-bing, G.; Da-lei, Z. High-solid Anaerobic Co-digestion of Food Waste and Rice Straw for Biogas Production. J. Northeast Agric. Univ. (Engl. Ed.) 2014, 21, 61–66. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, L.; Song, Z.; Ren, G.; Feng, Y.; Han, X.; Yang, G. Biogas production by co-digestion of goat manure with three crop residues. PLoS ONE 2013, 8, e66845. [Google Scholar] [CrossRef]
- Mei, Z.; Liu, X.; Huang, X.; Li, D.; Yan, Z.; Yuan, Y.; Huang, Y. Anaerobic Mesophilic Codigestion of Rice Straw and Chicken Manure: Effects of Organic Loading Rate on Process Stability and Performance. Appl. Biochem. Biotechnol. 2016, 179, 846–862. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, Y.; Zhang, C.; Li, S.; Huang, Z.; Ruan, W. Synergistic and pretreatment effect on anaerobic co-digestion from rice straw and municipal sewage sludge. Bioresources 2014, 9, 5871–5882. [Google Scholar] [CrossRef]
- Haider, M.R.; Zeshan; Yousaf, S.; Malik, R.N.; Visvanathan, C. Effect of mixing ratio of food waste and rice husk co-digestion and substrate to inoculum ratio on biogas production. Bioresour. Technol. 2015, 190, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Huang, Z.; Zhao, M.; Ruan, W.; Miao, H.; Ren, H. Effects of co-inoculating rice straw with ruminal microbiota and anaerobic sludge: Digestion performance and spatial distribution of microbial communities. Appl. Microbiol. Biotechnol. 2017, 101, 5937–5948. [Google Scholar] [CrossRef] [PubMed]
- Croce, S.; Wei, Q.; D’Imporzano, G.; Dong, R.; Adani, F. Anaerobic digestion of straw and corn stover: The effect of biological process optimization and pre-treatment on total bio-methane yield and energy performance. Biotechnol. Adv. 2016, 34, 1289–1304. [Google Scholar] [CrossRef] [PubMed]
- Quintero, M.; Castro, L.; Ortiz, C.; Guzmán, C.; Escalante, H. Enhancement of starting up anaerobic digestion of lignocellulosic substrate: Fique’s bagasse as an example. Bioresour. Technol. 2012, 108, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Wang, F.; Long, L.; Yebo, L. Comparison of digestate from solid anaerobic digesters and dewatered effluent from liquid anaerobic digesters as inocula for solid state anaerobic digestion of yard trimmings. Bioresour. Technol. 2016, 200, 750–760. [Google Scholar] [CrossRef]
- Zhang, L.; Lee, Y.W.; Jahng, D. Anaerobic co-digestion of food waste and piggery wastewater: Focusing on the role of trace elements. Bioresour. Technol. 2011, 102, 5048–5059. [Google Scholar] [CrossRef]
- VDI-Gesellschaft Energietechhnik. Vergärung organischer Stoffe (Fermentation of organic materials) VDI-Richtlinie 4630; VDI-Handbuch Energietechnik: Düsseldorf, Germany, 2006. [Google Scholar]
- Zhou, Y.; Li, C.; Nges, I.A.; Liu, J. The effects of pre-aeration and inoculation on solid-state anaerobic digestion of rice straw. Bioresour. Technol. 2017, 224, 78–86. [Google Scholar] [CrossRef]
- Kim, M.; Yang, Y.; Morikawa-Sakura, M.S.; Wang, Q.; Lee, M.V.; Lee, D.-Y.; Feng, C.; Zhou, Y.; Zhang, Z. Hydrogen production by anaerobic co-digestion of rice straw and sewage sludge. Int. J. Hydrog. Energy 2012, 37, 3142–3149. [Google Scholar] [CrossRef]
- Lv, W.; Schanbacher, F.L.; Yu, Z. Putting microbes to work in sequence: Recent advances in temperature-phased anaerobic digestion processes. Bioresour. Technol. 2010, 101, 9409–9414. [Google Scholar] [CrossRef]
- Liu, X.; Wang, W.; Shi, Y.; Zheng, L.; Gao, X.; Qiao, W.; Zhou, Y. Pilot-scale anaerobic co-digestion of municipal biomass waste and waste activated sludge in China: Effect of organic loading rate. Waste Manag. 2012, 32, 2056–2060. [Google Scholar] [CrossRef] [PubMed]
- Nagao, N.; Tajima, N.; Kawai, M.; Niwa, C.; Kurosawa, N.; Matsuyama, T.; Yusoff, F.M.; Toda, T. Maximum organic loading rate for the single-stage wet anaerobic digestion of food waste. Bioresour. Technol. 2012, 118, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Li, H.; Wu, X.; Wang, Y.; Zhang, Q. Effect of organic loading rate on anaerobic co-digestion of rice straw and pig manure with or without biological pretreatment. Bioresour. Technol. 2018, 250, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Krakat, N.; Demirel, B.; Anjum, R.; Dietz, D. Methods of ammonia removal in anaerobic digestion: A review. Water Sci. Technol. 2017, 76, 1925–1938. [Google Scholar] [CrossRef]
- Kaparaju, P.; Buendia, I.; Ellegaard, L.; Angelidakia, I. Effects of mixing on methane production during thermophilic anaerobic digestion of manure: Lab-scale and pilot-scale studies. Bioresour. Technol. 2008, 99, 4919–4928. [Google Scholar] [CrossRef]
- Lindmark, J.; Thorin, E.; Bel Fdhila, R.; Dahlquist, E. Effects of mixing on the result of anaerobic digestion: Review. Renew. Sustain. Energy Rev. 2014, 40, 1030–1047. [Google Scholar] [CrossRef]
- Kim, M.; Kim, B.-C.; Choi, Y.; Nam, K. Minimizing mixing intensity to improve the performance of rice straw anaerobic digestion via enhanced development of microbe-substrate aggregates. Bioresour. Technol. 2017, 245, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Wu, B. CFD analysis of mechanical mixing in anaerobic digesters. Am. Socity Agric. Biol. Eng. 2009, 52, 1371–1382. [Google Scholar]
- Shen, F.; Tian, L.; Yuan, H.; Pang, Y.; Chen, S.; Zou, D.; Zhu, B.; Liu, Y.; Li, X. Improving the mixing performances of rice straw anaerobic digestion for higher biogas production by computational fluid dynamics (CFD) simulation. Appl. Biochem. Biotechnol. 2013, 171, 626–642. [Google Scholar] [CrossRef]
- Wu, B. CFD simulation of mixing in egg-shaped anaerobic digesters. Water Res. 2010, 44, 1507–1519. [Google Scholar] [CrossRef]
- Stroot, P.G.; McMahon; Mackie, R.I.; Raskin, L. Anaerobic codigestion of municipal solid waste and biosolids under various mixing conditionds-I. Digester performance. Water Resour. 2001, 35, 1804–1816. [Google Scholar]
- McMahon, K.; Stroot, P.G.; Mackie, R.I.; Raskin, L. Anaerobic codigestion of municipal solid waste and biosolids under various mixing conditions-II: Microbial population dynamics. Water Resour. 2001, 35, 1817–1827. [Google Scholar] [CrossRef]
- Ong, H.K.; Greenfield, P.F.; Pullammanappallil, P.C. Effect of mixing on biomethanation of cattle-manure slurry. Environ. Technol. 2002, 23, 1081–1090. [Google Scholar] [CrossRef]
- Tian, L.; Zou, D.; Yuan, H.; Wang, L.; Zhang, X.; Li, X. Identifying proper agitation interval to prevent floating layers formation of corn stover and improve biogas production in anaerobic digestion. Bioresour. Technol. 2015, 186, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kozłowski, K.; Mazurkiewicz, J.; Chełkowski, D.; Jeżowska, A.; Cieślik, M.; Brzoski, M.; Smurzyńska, A.; Dongmin, Y.; Wei, Q. The Effect of Mixing During Laboratory Fermentation of Maize Straw with Thermofilic Technology. J. Ecol. Eng. 2018, 19, 93–98. [Google Scholar] [CrossRef]
- Hu, Y.; Shen, F.; Yuan, H.; Zou, D.; Pang, Y.; Liu, Y.; Zhu, B.; Chufo, W.A.; Jaffar, M.; Li, X. Influence of recirculation of liquid fraction of the digestate (LFD) on maize stover anaerobic digestion. Biosyst. Eng. 2014, 127, 189–196. [Google Scholar] [CrossRef]
- Gerin, P.A.; Vliegen, F.; Jossart, J.-M. Energy and CO2 balance of maize and grass as energy crops for anaerobic digestion. Bioresour. Technol. 2008, 99, 2620–2627. [Google Scholar] [CrossRef]
- Zeb, I.; Ma, J.; Frear, C.; Zhao, Q.; Ndegwa, P.; Yao, Y.; Kafle, G.K. Recycling separated liquid-effluent to dilute feedstock in anaerobic digestion of dairy manure. Energy 2017, 119, 1144–1151. [Google Scholar] [CrossRef]
- Shahriari, H.; Warith, M.; Hamoda, M.; Kennedy, K.J. Effect of leachate recirculation on mesophilic anaerobic digestion of food waste. Waste Manag. 2012, 32, 400–403. [Google Scholar] [CrossRef] [PubMed]
- Pezzolla, D.; Di Maria, F.; Zadra, C.; Massaccesi, L.; Sordi, A.; Gigliotti, G. Optimization of solid-state anaerobic digestion through the percolate recirculation. Biomass Bioenergy 2017, 96, 112–118. [Google Scholar] [CrossRef]
- Nges, I.A.; Wang, B.; Cui, C.; Liu, J. Digestate liquor recycle in minimal nutrients-supplemented anaerobic digestion of wheat straw. Biochem. Eng. J. 2015, 94, 106–114. [Google Scholar] [CrossRef]
- Peng, X.; Nges, I.A.; Liu, J. Improving methane production from wheat straw by digestate liquor recirculation in continuous stirred tank processes. Renew. Energy 2016, 85, 12–18. [Google Scholar] [CrossRef]
- Sibiya, N.T.; Tesfagiorgis, H.B.; Muzenda, E. Influence of digestate recirculation and recirculation percentage on biogas production from lawn grass via anaerobic digestion. Proc. World Congr. Eng. Comput. Sci. 2015, II. [Google Scholar]
- Nordberg, A.; Jarvis, A.; Stenberg, B.; Mathisen, B.; Svensson, B.H. Anaerobic digestion of alfalfa silage with recirculation of process liquid. Bioresour. Technol. 2007, 98, 104–111. [Google Scholar] [CrossRef]
- Qian, M.; Zhang, Y.; Li, R.; Nelles, M.; Stinner, W.; Li, Y. Effects of Percolate Recirculation on Dry Anaerobic Co-digestion of Organic Fraction of Municipal Solid Waste and Corn Straw. Energy Fuels 2017, 31, 12183–12191. [Google Scholar] [CrossRef]
- Demirel, B.; Scherer, P. Trace element requirements of agricultural biogas digesters during biological conversion of renewable biomass to methane. Biomass Bioenergy 2011, 35, 992–998. [Google Scholar] [CrossRef]
- Zandvoort, M.H.; van Hullebusch, E.D.; Gieteling, J.; Lens, P.N.L. Granular sludge in full-scale anaerobic bioreactors: Trace element content and deficiencies. Enzym. Microb. Technol. 2006, 39, 337–346. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef]
- Lin, C.Y.; Chen, C.C. Effect of heavy metals on the methanogenic UASB granule. Water Res. 1998, 33, 409–416. [Google Scholar] [CrossRef]
- Zayed, G.; Winter, J. Inhibition of methane production from whey by heavy metals - protective effect of sulfide. Appl. Microbiol Biotechnol. 2000, 53, 726–731. [Google Scholar] [CrossRef]
- Cai, Y.; Zhao, X.; Zhao, Y.; Wang, H.; Yuan, X.; Zhu, W.; Cui, Z.; Wang, X. Optimization of Fe2+ supplement in anaerobic digestion accounting for the Fe-bioavailability. Bioresour. Technol. 2018, 250, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Mancini, G.; Papirio, S.; Lens, P.; Esposito, G. A Preliminary Study of the Effect of Bioavailable Fe and Co on the Anaerobic Digestion of Rice Straw. Energies 2019, 12, 577. [Google Scholar] [CrossRef]
- Hinken, L.; Urban, I.; Haun, E.; Weichgrebe, D.; Rosenwinkel, K.-H. The valuation of malnutrition in the mono-digestion of maize silage by anaerobic batch tests. Water Sci. Technol. 2008, 58, 1453–1459. [Google Scholar] [CrossRef]
- Evranos, B.; Demirel, B. The impact of Ni, Co and Mo supplementation on methane yield from anaerobic mono-digestion of maize silage. Environ. Technol. 2015, 36, 1556–1562. [Google Scholar] [CrossRef]
- Liu, C.; Yuan, H.; Zou, D.; Liu, Y.; Zhu, B.; Li, X. Improving Biomethane Production and Mass Bioconversion of Corn Stover Anaerobic Digestion by Adding NaOH Pretreatment and Trace Elements. Biomed Res. Int. 2015, 2015, 125241. [Google Scholar] [CrossRef][Green Version]
- Khatri, S.; Wu, S.; Kizito, S.; Zhang, W.; Li, J.; Dong, R. Synergistic effect of alkaline pretreatment and Fe dosing on batch anaerobic digestion of maize straw. Appl. Energy 2015, 158, 55–64. [Google Scholar] [CrossRef]
- Zhang, H.; Tian, Y.; Wang, L.; Mi, X.; Chai, Y. Effect of ferrous chloride on biogas production and enzymatic activities during anaerobic fermentation of cow dung and Phragmites straw. Biodegradation 2016, 27, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zhang, H.; Chai, Y.; Wang, L.; Mi, X.; Zhang, L.; Ware, M.A. Biogas properties and enzymatic analysis during anaerobic fermentation of Phragmites australis straw and cow dung: Influence of nickel chloride supplement. Biodegradation 2017, 28, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.; Tian, Y.; Zhang, H.; Chai, Y. Copper stressed anaerobic fermentation: Biogas properties, process stability, biodegradation and enzyme responses. Biodegradation 2017, 28, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Ortner, M.; Rameder, M.; Rachbauer, L.; Bochmann, G.; Fuchs, W. Bioavailability of essential trace elements and their impact on anaerobic digestion of slaughterhouse waste. Biochem. Eng. J. 2015, 99, 107–113. [Google Scholar] [CrossRef]
- Marcato, C.-E.; Pinelli, E.; Cecchi, M.; Winterton, P.; Guiresse, M. Bioavailability of Cu and Zn in raw and anaerobically digested pig slurry. Ecotoxicol. Environ. Saf. 2009, 72, 1538–1544. [Google Scholar] [CrossRef] [PubMed]
- Ortner, M.; Rachbauer, L.; Somitsch, W.; Fuchs, W. Can bioavailability of trace nutrients be measured in anaerobic digestion? Appl. Energy 2014, 126, 190–198. [Google Scholar] [CrossRef]
- González-Suárez, A.; Pereda-Reyes, I.; Oliva-Merencio, D.; Suárez-Quiñones, T.; José da Silva, A.; Zaiat, M. Bioavailability and dosing strategies of mineral in anaerobic mono-digestion of maize straw. Eng. Life Sci. 2018, 18, 562–569. [Google Scholar] [CrossRef]
- Ahlberg-Eliasson, K.; Nadeau, E.; Levén, L.; Schnürer, A. Production efficiency of Swedish farm-scale biogas plants. Biomass Bioenergy 2017, 97, 27–37. [Google Scholar] [CrossRef]
- Kaparaju, P.; Rintala, J. Mitigation of greenhouse gas emissions by adopting anaerobic digestion technology on dairy, sow and pig farms in Finland. Renew. Energy 2011, 36, 31–41. [Google Scholar] [CrossRef]
- Mussoline, W.; Esposito, G.; Lens, P.; Garuti, G.; Giordano, A. Design considerations for a farm-scale biogas plant based on pilot-scale anaerobic digesters loaded with rice straw and piggery wastewater. Biomass Bioenergy 2012, 46, 469–478. [Google Scholar] [CrossRef]
- Chiumenti, A.; da Borso, F.; Limina, S. Dry anaerobic digestion of cow manure and agricultural products in a full-scale plant: Efficiency and comparison with wet fermentation. Waste Manag. 2018, 71, 704–710. [Google Scholar] [CrossRef]
- André, L.; Zdanevitch, I.; Pineau, C.; Lencauchez, J.; Damiano, A.; Pauss, A.; Ribeiro, T. Dry anaerobic co-digestion of roadside grass and cattle manure at a 60 L batch pilot scale. Bioresour. Technol. 2019, 289, 121737. [Google Scholar] [CrossRef]
- Wandera, S.M.; Qiao, W.; Algapani, D.E.; Bi, S.; Yin, D.; Qi, X.; Liu, Y.; Dach, J.; Dong, R. Searching for possibilities to improve the performance of full scale agricultural biogas plants. Renew. Energy 2018, 116, 720–727. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).