1. Introduction
Hydrogels are three-dimensional polymer networks that are capable of retaining large amounts of water or biological fluids, and simulating biological tissue [
1,
2]. Hydrogels have been obtained from various polymers of natural origin, for example, polysaccharides, as well as from numerous synthetic polymers, such as poly(ethylene glycol) [
3]. On the basis of the cross-linking mechanism, hydrogels can be classified into two categories, physical and chemical [
1]. Physical cross-links have been formed by ionic interaction, hydrogen bonding, or crystallite formation. In chemically cross-linked hydrogels, covalent bonds have been present between polymer chains, e.g., as a result of reactions of functional groups occurring along polymeric chains with bi- or multifunctional cross-linkers [
4].
Hydrogels, due to their similarity to natural tissue, are suitable for various biomedical applications, such as drug-delivery systems, scaffolds for tissue engineering, wound dressings, or contact lenses [
5,
6]. In addition, an important area of hydrogel application is personal-hygiene products such as nappies, sanitary pads, or adult-incontinence products [
7]. Moreover, due to their unique properties, hydrogels could also find applications in many other fields, e.g., the food industry [
8] or agriculture [
9].
Poly-γ-glutamic acid (γ-PGA), a biopolymer produced by bacteria, is made of D- and L-glutamic acid units connected by amide linkages [
10]. γ-PGA is produced on an industrial scale (mostly from biomass by microbial fermentation), so it is relatively easily available. This naturally occurring polyamide is water-soluble, biodegradable, nontoxic to humans and the environment, and safe for human consumption [
11]. Due to its edibility, γ-PGA has been tested for various food-related applications, e.g., as a texture modifier for baked foods, bitterness-relieving agent, or cryoprotectant for probiotic bacteria [
12,
13]. γ-PGA has also been used for medical applications, e.g., in tissue engineering [
14] or delivery systems [
15]. In addition, γ-PGA could be used to prepare hydrogels with properties suitable for pharmaceutical applications [
16]. γ-PGA-based hydrogels are not limited to only medical applications; they could also be used to purify turbid water [
17] or as electrolytes in organic electrochemical supercapacitors [
18].
Poly(ethylene glycol) (PEG) is a water-soluble and nontoxic polymer, approved by the United States Food and Drug Administration (FDA). PEG has been extensively used in a variety of medical applications, such as drug-delivery systems or tissue engineering [
19]. Moreover, PEG has been used to obtain hydrogels suitable for biomedical applications, e.g., in delivery systems [
20], tissue engineering [
21], or wound dressings [
22].
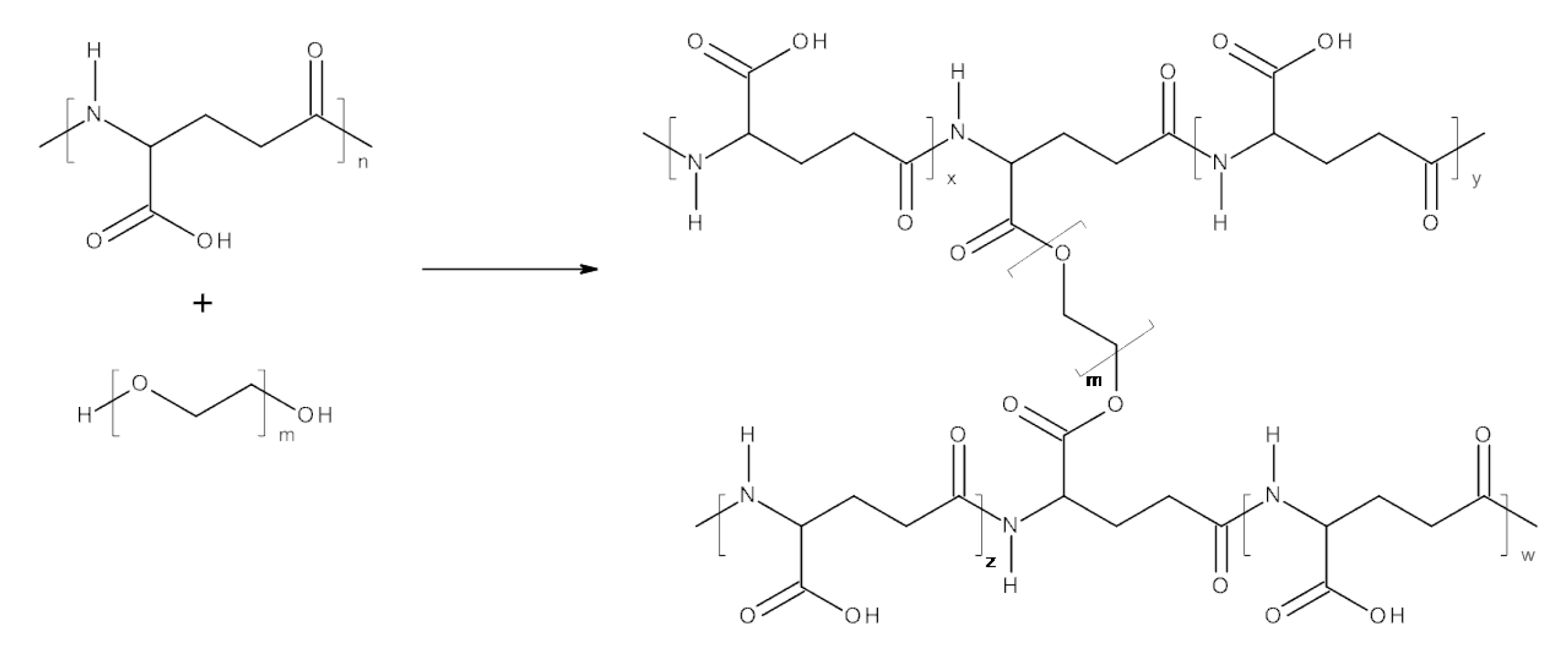
Despite intensive research interest in poly-γ-glutamic acid, there are limited literature data on hydrogels made of poly-γ-glutamic acid and poly(ethylene glycol). One of the few examples is hydrogels obtained via photopolymerisation from methacrylated poly(γ-glutamic acid) and poly(ethylene glycol) diacrylate; on the basis of a cytotoxicity assay of these pH-sensitive hydrogels, they were found suitable for biomedical applications (as a matrix for pH-dependent drug release or scaffolding material in tissue engineering) [
23]. Another example of γ-PGA–PEG hydrogels was prepared in a reaction between γ-PGA-containing maleimide groups and PEG-containing terminal thiol groups. This γ-PGA–PEG hydrogel has the potential to be used as a drug-delivery system [
24]. The synthesis of known γ-PGA–PEG hydrogels was preceded by a modification of γ-PGA biopolymers in which additional functional groups were introduced. The protocol reported in the current study allows to prepare γ-PGA–PEG hydrogels in a one-step method under mild conditions. The hydrogels reported here were synthesised via an esterification reaction between carboxyl groups naturally occurring in γ-PGA, and hydroxyl groups of selected PEG cross-linkers.
The advantage of already known γ-PGA-based hydrogels has been proven in various fields, including medical areas. Taking this into consideration, it seemed reasonable to conduct research on developing γ-PGA-based hydrogels. In this study, a one-step method of obtaining γ-PGA-based hydrogels in a reaction between a γ-PGA biopolymer and selected PEGs as cross-linkers was developed. PEGs were chosen as cross-linkers due to their widespread use in the pharmaceutical and food industries; hence, the human organism has frequent contact with these materials. Well-defined PEGs with desired molecular weights are easily available and relatively cheap. The influence of molecular weight and structure (linear or branched) of the cross-linker on swelling behaviour has been investigated. Moreover, possibilities of using these hydrogels as drug- and probiotic-delivery vehicles were examined. Studies on the cytocompatibility and antimicrobial activity of hydrogels loaded with antibiotics on Gram-positive and -negative bacteria were undertaken. We investigated the protective effect of γ-PGA-based hydrogels on entrapped probiotic cells subjected to low pH. The possibilities of using γ-PGA–PEG hydrogels as probiotic-delivery vehicles had not been investigated until now. Conducting research on γ-PGA–PEG hydrogels in the direction of oral-delivery vehicles seemed reasonable, as both polymers have already been used as food additives.
2. Materials and Methods
2.1. Materials
Poly-γ-glutamic acid (Mw = 200,000–500,000 g/mol) was purchased from Wako Chemicals Europe GmbH (Neuss, Germany). Poly(ethylene glycol) PEG1000 (Mw = 1000 g/mol) and glycerol ethoxylate PEG1000-3-arm (Mn = 1000 g/mol) were purchased from Sigma-Aldrich Chemie GmbH (Steinheim, Germany). Poly(ethylene glycol) PEG400 (Mw = 400 g/mol), poly(ethylene glycol) PEG200 (Mw = 200 g/mol), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC), 4-dimethylaminopyridine (DMAP), and phthalate buffer solution (pH = 3.0) were purchased from Acros Organics (Geel, Belgium). DMSO, acetone, phosphate buffered saline (PBS, pH = 7.4) were purchased from Avantor Performance Materials Poland S.A. (Gliwice, Poland). Dialysis membrane Spectra/Por (MWCO 6000–8000) was purchased from Carl Roth (Karlsruhe, Germany). Chloramphenicol powder was purchased from Fisher Scientific UK (Loughborough, UK). Chloramphenicol discs were purchased from Thermo Scientific (Hampshire, UK). Tryptic soy agar (TSA) was purchased from Lab M Ltd. (Heywood, UK). Dulbecco’s Modified Eagle Medium (DMEM) was purchased from Gibco (UK); foetal bovine serum (FBS), L-glutamine, and antibiotic antimycotic (10,000 units/mL penicillin, 10,000 µg/mL streptomycin, and 25 µg/mL amphotericin B) were purchased from Gibco (UK). Thiazolyl blue tetrazolium bromide (MTT) was purchased from Sigma-Aldrich (UK). NaCl (5.8 g/L) and glycine (7.6 g/L), used for preparing Sorensen’s glycine buffer, were purchased from Sigma-Aldrich (UK). MSTO-211H (human mesothelioma, CRL-2081) and PANC 1 (human pancreatic ductal adenocarcinoma, CRL-1469) were purchased from ATCC (UK). Staphylococcus aureus NCIMB 6571 and Escherichia coli NCIMB 9481 were obtained from the University of Wolverhampton culture collection. A mixture of Lactobacillus strains (L. acidophilus, L. casei, and L. rhamnosus) was obtained from Holland and Barrett, Ltd. (Nuneaton, UK).
2.2. Hydrogel Synthesis
γ-PGA (0.25 g) and DMSO (20 ml) were placed inside a round bottom flask equipped with a magnetic stirring bar. After γ-PGA was dissolved, the remaining reagents were added (mol % toward glutamic acid residue of γ-PGA): PEG (30 mol %), EDC (30 mol %), and DMAP (10 mol %) (quantities in
Table 1). The reaction mixture was stirred at room temperature. After 6 h, the mixture was precipitated in acetone and centrifuged for 5 min at 7000 rpm. During centrifugation, hydrogel samples were formed into discs. For purification, hydrogel samples were put into dialysis membranes and kept in distilled water overnight. The resultant swollen hydrogels were lyophilised.
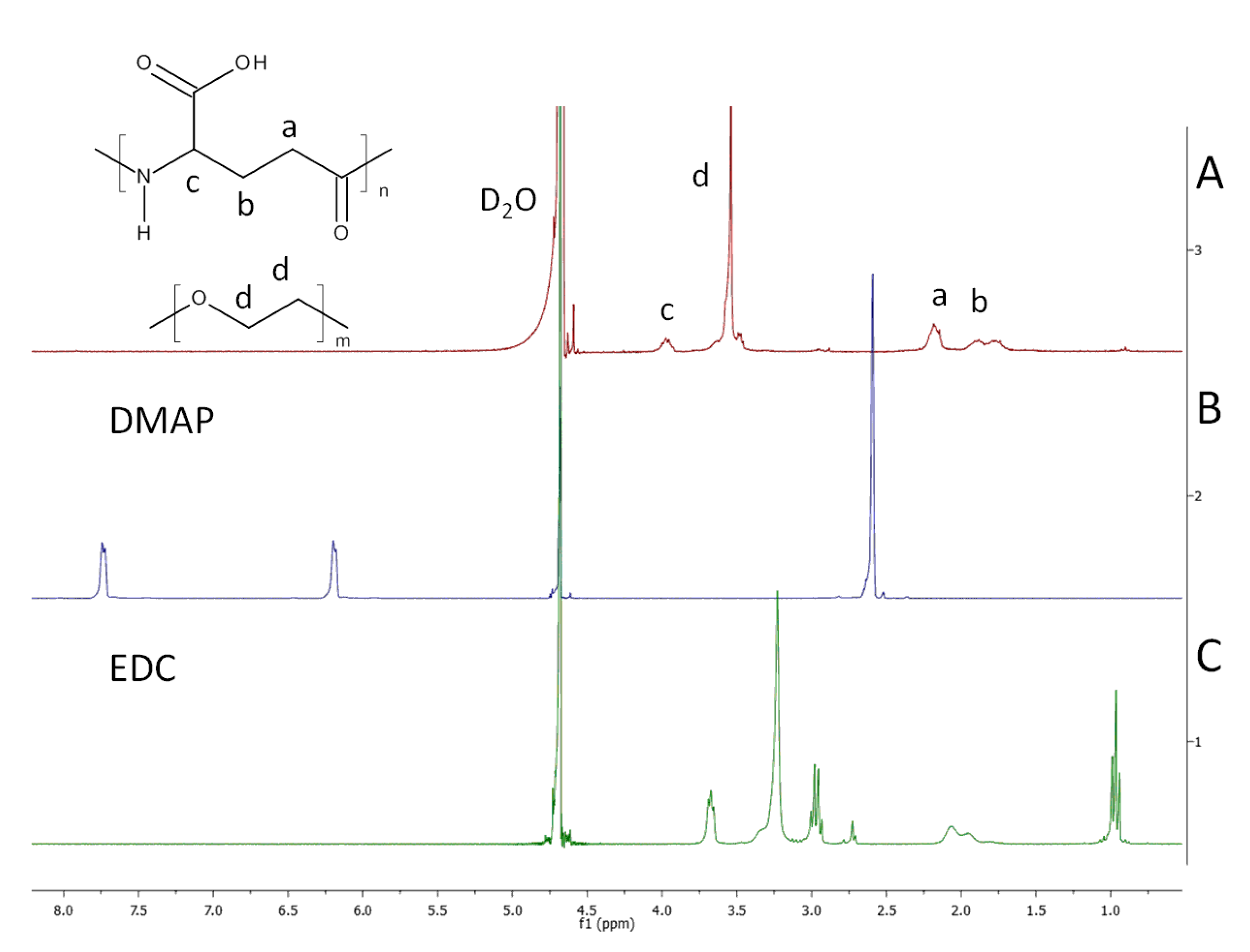
2.3. Nuclear-Magnetic-Resonance (NMR) Characterisation
NMR analyses were performed at room temperature using NMR Varian 300 MHz (Palo Alto, CA, USA). Each spectrum was recorded with 32 scans, 1.71 s acquisition time, and 10 s relaxation delay. Before analyses, samples were hydrolysed in a D
2O solution of NaOH. Samples of dry hydrogels (10 mg) were put into tubes containing 2.0 mL of D
2O solution of NaOH (pH 10.0). The content of the tubes was mixed at 60 °C for 24 h. Trimethylsilylpropanoic acid (TSP) was used as the internal standard. The content of cross-linker X (mol %) of each hydrogel was calculated on the basis of
1H NMR spectra from Equation (1).
where A
d is the peak area of cross-linker protons (signal d in
Figure 1),
n is the number of repeating units in PEG cross-linker, and A
c is the peak area of the γ-proton of PGA (signal c in
Figure 1).
2.4. SEM Analysis
Hydrogel morphology was evaluated using a Phenom ProX scanning electron microscope (SEM) using accelerating voltage of 5 kV. Prior to the scanning process, lyophilised samples were sprayed with a 10 nm gold layer using sputter Quorum Q150R ES.
2.5. Swelling Studies
The dried samples (with mass of 5–9 mg each) of the obtained hydrogels were put into 2.5 mL of deionised water, phthalate buffer, and phosphate-buffered saline, and kept for 24 h at room temperature. After incubation in the solutions, swollen samples were placed on a mesh sheet (Flow-Mesh™) to remove excess of water and weighed. Then samples were lyophilised and weighted. Swelling experiments were carried out in triplicate. Swelling ratio (SR; g/g) was calculated according to the following formula.
where Ws is the weight of the swollen hydrogel (g), and Wd is the weight of the dry hydrogel (g).
2.6. Studies on Antimicrobial Activity of Hydrogel Loaded with Antibiotics
The antimicrobial activity of hydrogels loaded with antibiotics was tested in a disc-diffusion assay. A slightly soaked sheet of pure hydrogel was cut into discs with a diameter of 8 mm. Antibiotics were introduced into the discs, and we applied to the centre of each hydrogel disc 200 μL of suspension of chloramphenicol (30 μg) dropwise in Ringer’s solution with a pipette, and it was left to be absorbed. Paper discs containing chloramphenicol (30 μg in each) and pure hydrogel discs were used as controls. The antimicrobial activity of hydrogel discs containing antibiotics was tested against Staphylococcus aureus and Escherichia coli. Overnight cultures of both pathogens were seeded on the surface of TSA plates. Hydrogel discs containing chloramphenicol, paper disc with chloramphenicol, and pure hydrogel discs were aseptically placed on the TSA plates and incubated at 37 °C for 24 h. Then, the diameter of the zones of inhibition was measured, and the discs of the selected hydrogels were transferred onto freshly seeded TSA plates and further incubated at 37 °C. The diameter of the zones of inhibition was measured again after 24 and 96 h. The tests of antimicrobial activity of each hydrogel were carried out in triplicate.
2.7. Probiotic Survival in Acid Environments
The protective effect of the selected γ-PGA-based hydrogels was investigated using mixture of three probiotic strains, L. acidophilus, L. casei, and L. rhamnosus, under pH = 1.5 and pH = 3.0. The hydrogel samples (25 or 50 mg each) absorbed 0.5 mL of the suspension of Lactobacillus in Ringer’s solution. Hydrogels with probiotic bacteria, as well as 0.5 mL of suspension of free cells in Ringer’s solution, were subjected to an acidic solution and incubated at 37 °C. Triplicate samples were withdrawn after 2 and 4 h. Hydrogel samples in Ringer’s solution were shaken on a vortex mixer to release the bacteria. A serial dilution of bacteria suspension was prepared up to 10−6; then, 20 μL of each cell suspension was aseptically plated out in duplicate on the TSA plates. Plates were incubated at 37 °C, and cell viability was determined by counting the CFU/mL.
2.8. Cytotoxicity Assays
In vitro cytotoxicity was evaluated after direct contact of the samples of hydrolysed hydrogel with MSTO-211H (human mesothelioma, CRL-2081 from ATCC, UK) and PANC 1 (human pancreatic ductal adenocarcinoma, CRL-1469 from ATCC, UK) cell lines at a ratio of 5 mg of hydrolysate/mL of medium. Before the cytotoxicity assays, two more purification cycles of the hydrogel samples were performed. During one cycle, samples were kept in distilled water overnight and lyophilised. In order to obtain the hydrolysate, hydrogel samples were incubated for 72 h at 37 °C in Dulbecco’s Modified Eagle’s Medium (DMEM) medium. Cell lines were cultured in DMEM containing 4.5 g/L glucose, supplemented with foetal bovine serum (FBS), antibiotic antimycotic, and L-glutamine at 37 °C in a humidity incubator with 5% CO
2. Briefly, 5000 cells were seeded per well (96-well plate) for 24 h at 37 °C in a 5% CO
2 incubator. The cells were then exposed to the hydrolysate of the hydrogel sample in DMEM for 24 h to investigate the effect on cell viability. Cells in DMEM were used as a control. Cell viability was evaluated following the standard MTT assay, as previously reported [
6].
2.9. Statistical Analysis
Experiments were performed in at least triplicate, and data are expressed as mean ± standard deviation; p < 0.05 was considered to be statistically significant. Data recorded in MTT assays were analysed statistically by two-way analysis of variance (ANOVA) with Tukey’s multi comparison test using GraphPad Prism.
4. Discussion
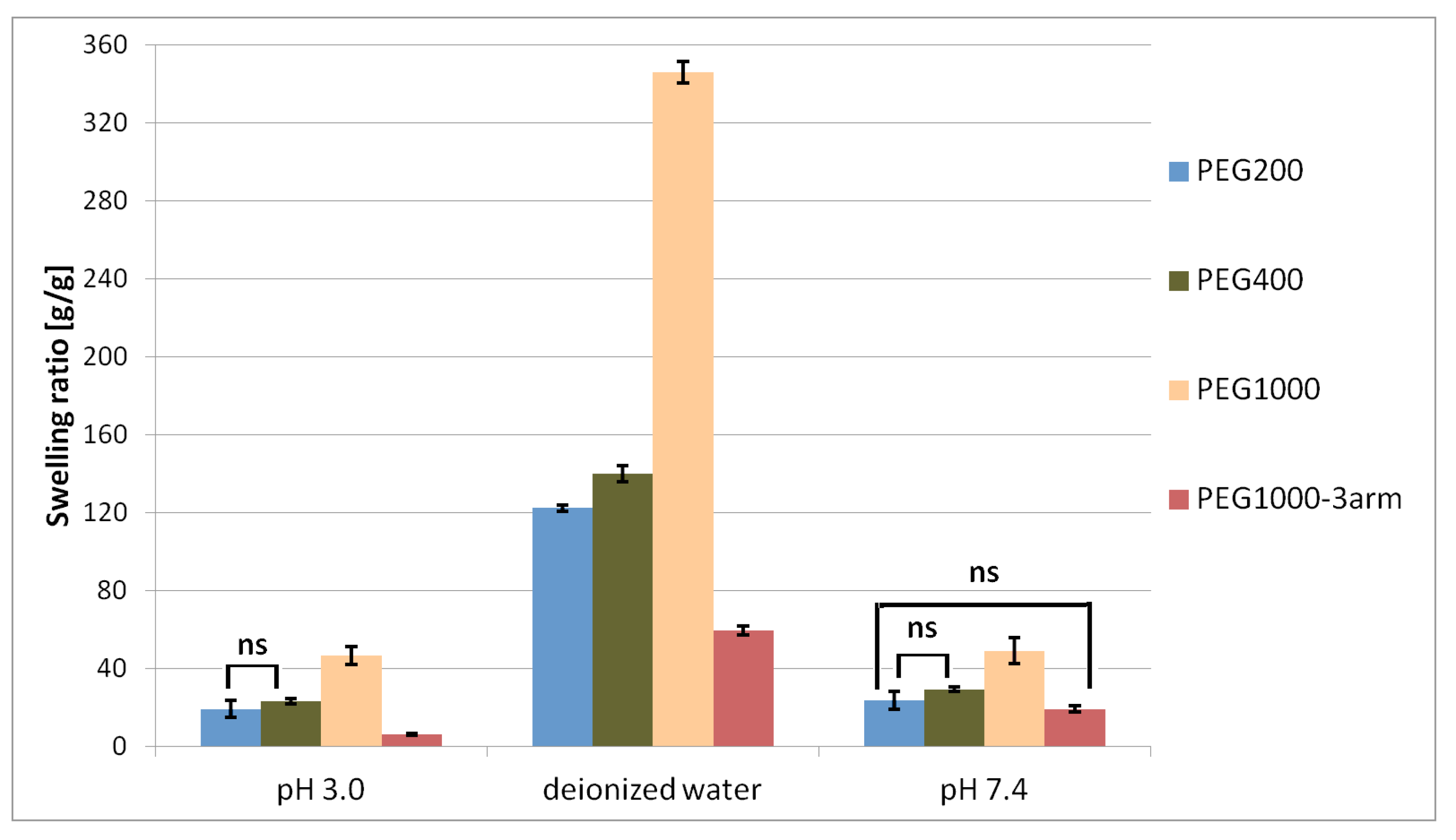
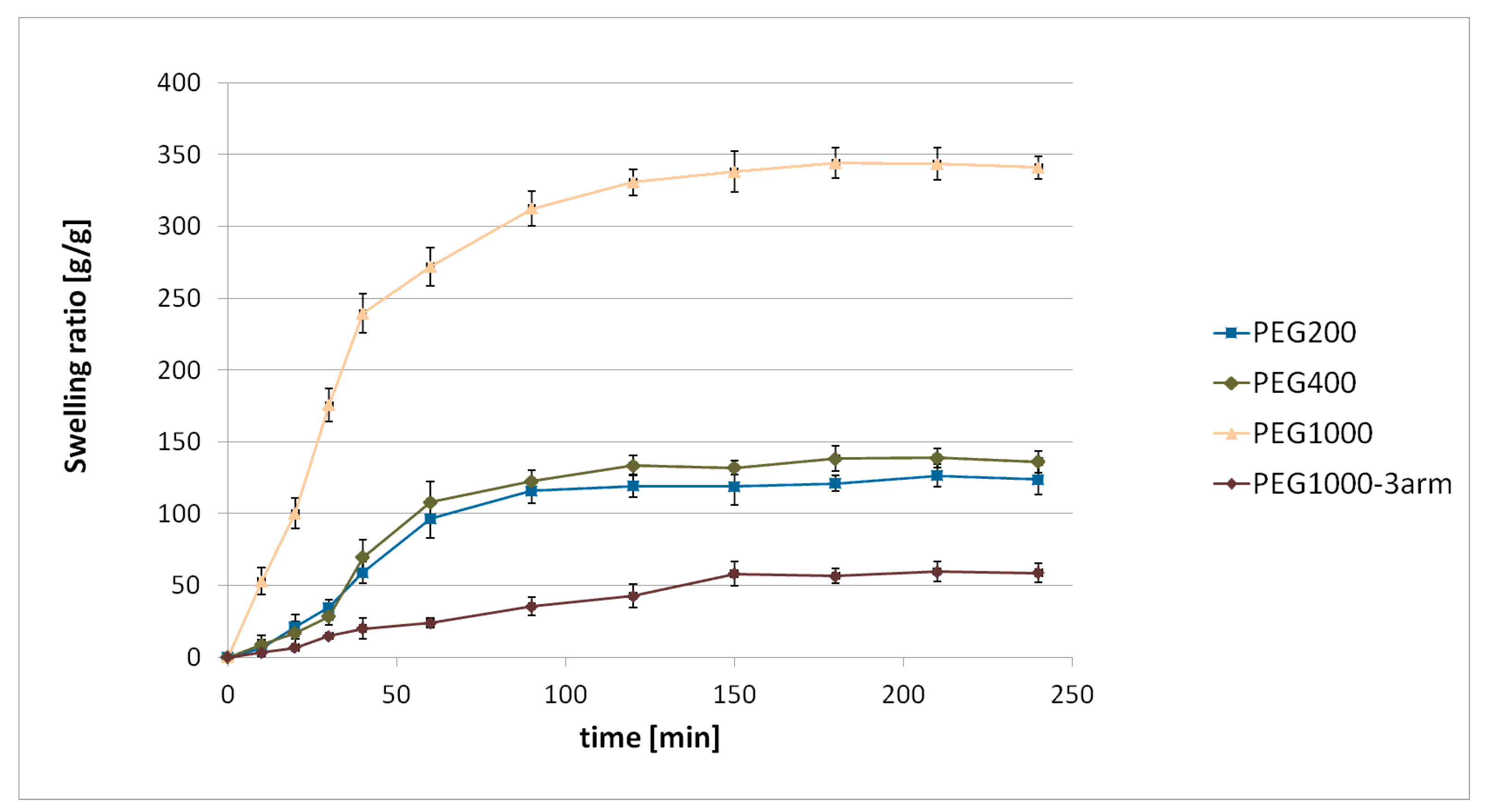
γ-PGA–PEG hydrogels were obtained from an esterification reaction between γ-PGA biopolymers and linear PEG cross-linkers with different chain lengths, as well as with a branched PEG cross-linker. The swelling behaviour of the obtained hydrogels was investigated in deionised water, in phthalate buffer at pH = 3.0, and in phosphate-buffered saline at pH = 7.4. The highest swelling ratio was achieved after soaking the hydrogel samples in deionised water. While the SR of each hydrogel soaked in phthalate buffer and in phosphate-buffered saline was significantly lower than the SR achieved after soaking in deionised water, the achieved SR values at both buffers were quite similar. Differences in swelling in deionised water and in the buffers might be associated with the influence of ionic concentration. According to the literature, the swelling behaviour of γ-PGA hydrogels is associated with repulsive forces between -COO
− groups. The absence of cations in deionised water allows strong electrostatic repulsion between -COO
− groups, which enables high swelling values. In buffers, the number of additional cations caused weakened electrostatic repulsion between –COO
− due to the chelation between –COO
− groups and cations, which presumably lead to a depressed swelling degree [
25].
On the basis of the conducted research, we found that the swelling ratio of hydrogels increased with the increase of the cross-linker’s chain length. Such a tendency was observed for each tested condition (deionised water, pH = 3.0, and pH = 7.4). Different cross-linker amounts in each hydrogel might have affected the swelling behaviour. Increasing cross-linker concentration could result in a network with more cross-linking points (higher cross-linking density); therefore, there would be less space for water [
26]. However, on the basis of conducted
1H NMR analysis of the hydrolysed hydrogels, the cross-linker amount was similar in all hydrogels. Therefore, it could be assumed that the swelling behaviour of the obtained hydrogels mainly depended on cross-linker chain length. Cross-linker chain length influences swelling behaviour; using a cross-linker with longer chains results in an increase of the hydrogel swelling ratio in comparison to a hydrogel with shorter cross-linker chains [
27,
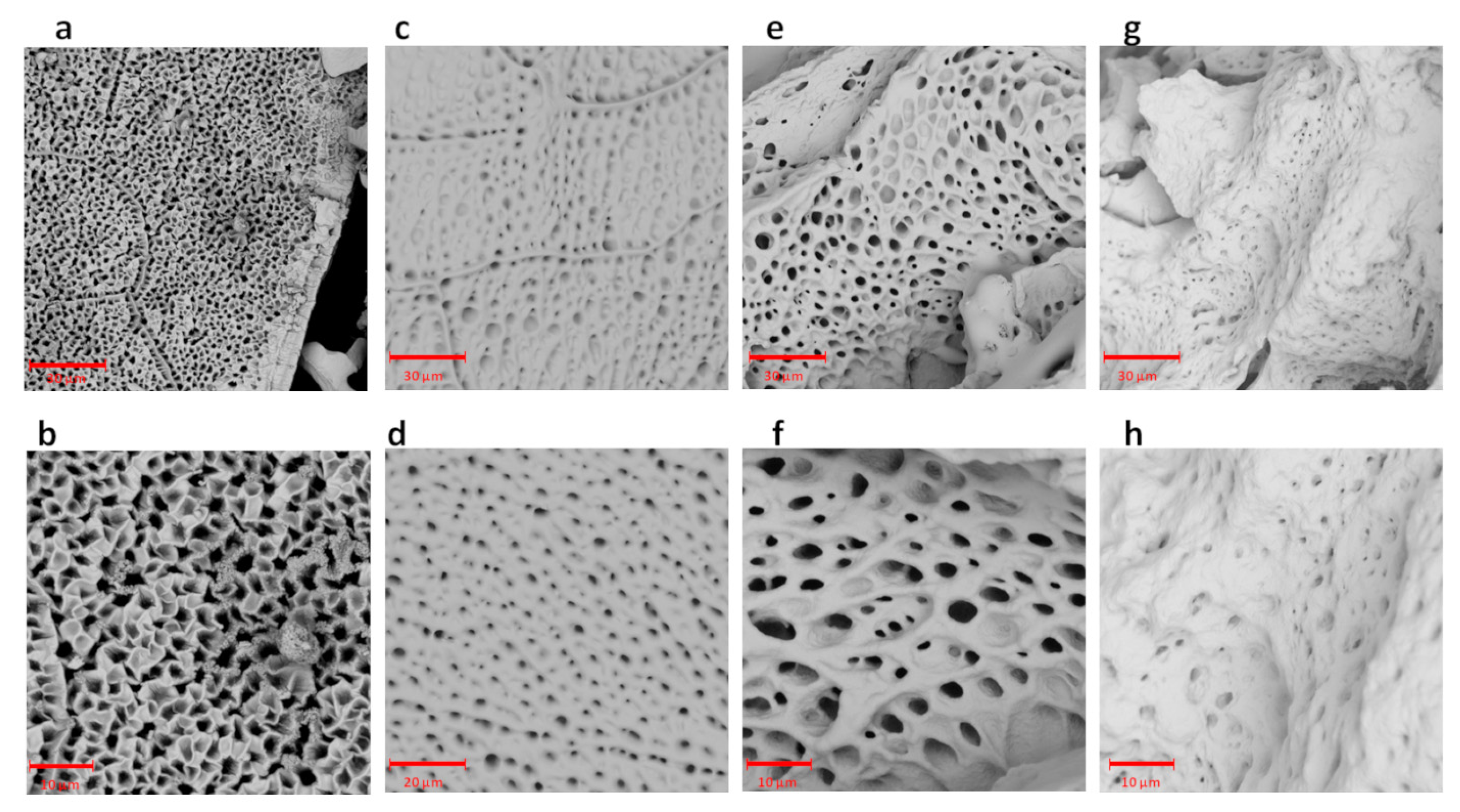
28]. Moreover, it was found that the use of a branched cross-linker resulted in obtaining a hydrogel with a lower swelling ratio in comparison to the application of a linear cross-linker with similar molecular weight. The more water that a hydrogel was able to absorb (the higher SR), the larger the pores that were generated during lyophilisation. Thus, in the SEM image of PEG1000, the biggest pores are visible (
Figure 2e,f), in the SEM image of the PEG1000-3arm, the smallest pores can be observed (
Figure 2g,h), and a rather similar size of pores was observed in PEG200 (
Figure 2a,b) and PEG400 (
Figure 2c,d). On the basis of the obtained results, we established the influence of the molecular weight of the PEG cross-linker, as well as the cross-linker structure (linear or branched), on the swelling ratio of the γ-PGA-based hydrogels. In the current study, the lowest SR = 6.0 ± 0.4 g/g was achieved after soaking the γ-PGA-based hydrogel with 3-arm PEG1000 cross-linker in phthalate buffer at pH = 3.0. The materials that achieved a low swelling ratio under acidic conditions may be useful for intestine-delivery vehicles. This is because such vehicles are first subjected to highly acidic conditions in the stomach, where contact between the contents of those delivery vehicles and the harmful medium should be limited. Then, the release of the active substance from intestine-delivery vehicles should occur after subjecting them to gradually increasing pH, from pH = 5.5 in the small intestine to about pH = 7.5 in the ileocolonic region [
29].
In order to investigate the possibility of using synthesised γ-PGA-based hydrogels as drug-delivery vehicles, studies on the antimicrobial activity of hydrogels loaded with antibiotics were carried out. During a disc-diffusion assay, pure hydrogel discs, hydrogel discs loaded with antibiotics, and a paper disc containing antibiotics were tested against selected pathogens.
S. aureus and
E. coli were chosen to represent Gram-positive and -negative bacteria. Both pathogens are the most common cause of healthcare-associated infections [
30]. Chloramphenicol, an antibacterial agent with a broad spectrum of activity against Gram-positive and -negative bacteria [
31], was used as a model drug. Paper discs containing antibiotics and hydrogel discs loaded with antibiotics exhibited antimicrobial activity against
E. coli and
S. aureus. After 24 h of the experiment, higher antimicrobial activity against both pathogens was observed for antibiotics released from the paper discs. It could be assumed that, during the first period of the experiment, only a small amount of antibiotics was released from the hydrogel discs, and that dose was too low to indicate a similar effect as the antibiotics release from the paper discs. However, after transferring hydrogel discs loaded with antibiotics and paper discs onto freshly seed TSA plates, the hydrogel discs with antibiotics showed noticeably higher antimicrobial activity against the tested pathogens. Along with extending the duration of the experiment, more drugs were released from the hydrogel discs, resulting in observed antibacterial activity up to 120 h. The performed experiment indicated prolonged activity of hydrogels loaded with antibiotics in comparison to paper discs containing antibiotics. Moreover, hydrogel discs dissolved during the experiment, which might be the result of the hydrolysis of ester bonds caused by humidity and/or enzymes produced by bacteria. Such hydrogel behaviour may be desirable for certain applications, such as wound dressing [
32]. The antibacterial effect of pure hydrogel discs observed after 120 h could be explained just by the low pH generated by the hydrolysis of the hydrogel.
The developed γ-PGA–PEG hydrogels were tested as probiotic-delivery systems. The protective effect of γ-PGA-based hydrogels on probiotic cells in low pH was determined. Various hydrogels are known as materials that might improve the survivability of probiotic bacteria under gastrointestinal-track conditions, as well as during storage at various temperatures [
33]. Moreover, the γ-PGA biopolymer was already successfully tested as a delivery vehicle for probiotic bacteria [
12,
34]. Those facts encourage further research, during which the suitability of γ-PGA-based hydrogels for applications as probiotic-delivery systems could be investigated. In the human stomach, probiotic bacteria are subjected to gastric acidic with low pH. Immediately after consuming certain food and drinks, gastric pH could be elevated to a value in the range of 3.0–4.0; however, this decreases over time to a value below 2.0 [
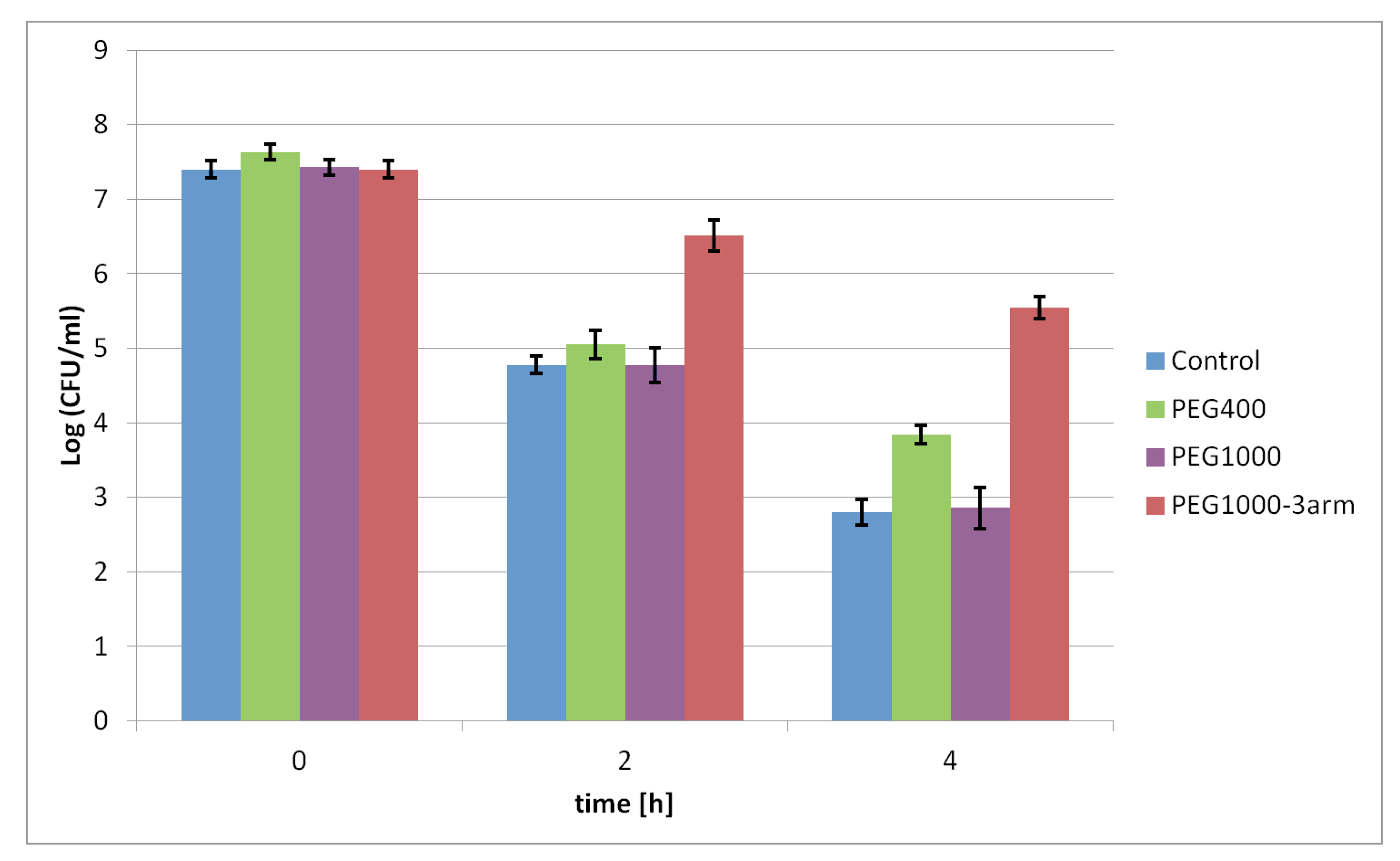
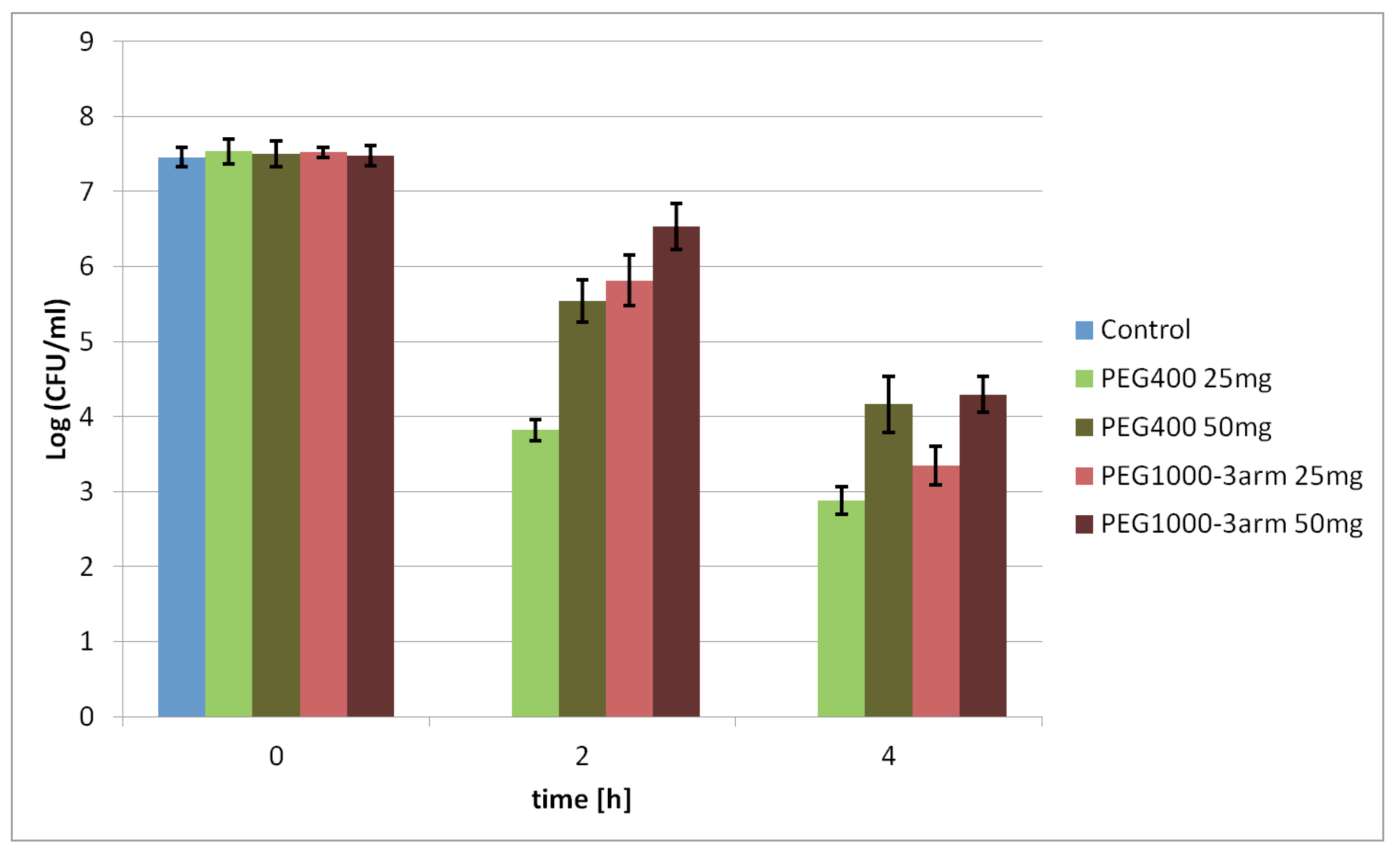
29]. Therefore, the protective effect of the selected γ-PGA-based hydrogels on probiotic bacteria was tested under various pH values, 3.0 and 1.5, respectively. The viability of the tested probiotic bacteria entrapped in the hydrogels was mostly higher than that of free cells subjected to pH 3.0 and pH 1.5. Certain differences in the viability of bacteria protected by PEG400, PEG1000, and PEG1000-3arm were observed. Those differences might be related to the different swelling behaviours of those hydrogels; the PEG1000-3arm hydrogel had a lower SR under acidic conditions than that of the PEG400 and PEG1000 hydrogels. Less of the harmful medium could penetrate inside a less swollen PEG1000-3arm hydrogel; thus, contact between entrapped probiotic bacteria and acid medium is more limited. This is in contrast to the hydrogel with the linear PEG1000 cross-linker, which had the highest swelling ratio between the tested hydrogels. Presumably, the high SR value is the reason why the viability rate of the bacteria entrapped in this hydrogel was similar to the viability of the free cells. The main differences in the survival level of probiotic bacteria was visible after incubation in pH 1.5; under these conditions, free cells were not able to survive for 2 h, while protected cells could maintain a certain level of viability after 4 h. Moreover, it was found that increasing the hydrogel amount may further improve bacterial survival under acidic conditions. A larger gel amount might provide a thicker barrier between probiotic bacteria and harmful environments, especially for bacteria in the core of hydrogel samples. The obtained results proved the bacterial protective ability of the synthesised γ-PGA-based hydrogels in a solution with low pH. It was established that the hydrogel swelling ratio and amount had noticeable influence on the survival rate of bacteria protected by hydrogels.
Products that claim to contribute to the improvement of intestinal microflora should contain a minimum 10
6 CFU/mL of probiotic bacteria [
35]. Depending on the consumed food and drinks, gastric-residence time could vary between 15 and 194 min [
29]. Probiotic bacteria entrapped in 50 mg of the γ-PGA–PEG1000-3arm hydrogel maintained a cell population of 6.53 ± 0.31 Log CFU/mL after incubation for 2 h in pH = 1.5. Despite visible loss in cell viability for bacteria protected by the hydrogels after 4 h of incubation in pH 1.5, the γ-PGA-based hydrogels could be further investigated to develop probiotic-delivery systems. Synthesised hydrogels demonstrated improved viability of the entrapped probiotic bacteria under acidic conditions in comparison to free cells. If a sufficiently high starting dose of bacteria were introduced into the appropriate γ-PGA-based hydrogel amount, and subjected to low pH, such a delivery system could contain the required amount of viable bacteria.
In order to evaluate in vitro cytotoxicity, two cell lines, MSTO and PANC 1, were treated with hydrolysed γ-PGA–PEG400 hydrogel. On the basis of the obtained results, it was observed that the presence of hydrolysis products negatively affected the cellular viability of both tested lines. However, percentages of cell viability in the range of 80%–60% were still recognised as weak cytotoxicity [
36].