Abstract
Uncaria tomentosa (Willd. ex Schult.) DC. (Family: Rubiaceae), commonly known as cat’s claw, is a tropical medicinal vine originating at the Amazon rainforest and other areas of South and Central America. It has been traditionally used to treat asthma, abscesses, fever, urinary tract infections, viral infections, and wounds and found to be effective as an immune system rejuvenator, antioxidant, antimicrobial, and anti-inflammatory agent. U. tomentosa is rich in many phytoconstituents such as oxindole and indole alkaloids, glycosides, organic acids, proanthocyanidins, sterols, and triterpenes. Biological activities of U. tomentosa have been examined against various microorganisms and parasites, including pathogenic bacteria, viruses, and Plasmodium, Babesia and Theileria parasites. Several formulations of cat’s claw (e.g., tinctures, decoctions, capsules, extracts, and teas) are recently available in the market. The current review covers the chemical constituents, biological activities, pharmacokinetics, and toxic properties of U. tomentosa extracts.
1. Introduction
Medicinal plants have been used for various therapeutic purposes from ancient times and they have also served as an important source for drug discovery [1,2,3]. A large proportion of the population living in developing countries in Asia and Africa depend on plant-based traditional medicines for primary healthcare [4,5]. One of the main reasons for the wide use of plants is due to their easy accessibility and low-cost [6,7,8]. Essentially, herbal therapies contain parts of herbs or unpurified herbal extracts that involve a variety of phytoconstituents that are usually thought to act synergistically together and can be used as a lead compound to discover a huge number of compounds that can be used recently in the treatment of several diseases [7,8].
Uncaria tomentosa (Willd. ex Schult.) DC is commonly known as cat’s claw that is derived from the Spanish word Uña de Gato that identifies the small, curved-back thorns on the stem at the leaf junction. It is a tropical medicinal vine of the Rubiaceae family that is widely distributed in the Amazon rainforest and other areas of South and Central America [9,10]. Thirty-four Uncaria species have been reported including U. guianensis and U. tomentosa that are found in South America. Traditionally, U. tomentosa has been reported to be used to asthma, abscesses, fever, urinary tract infections, viral infections, and wounds [9]. It is also reported to be effective as an immune system rejuvenator, antioxidant, antimicrobial, and anti-inflammatory. U. tomentosa is a potent complimentary herb for treating most parasites [11]. Various chemical constituents are reported from the extracts of U. tomentosa along with their biological activities. The main objective of this review is to review the available scientific literature regarding the chemical constituents, biological activities of U. tomentosa along with the reported side effects and precautions related to drug-drug interactions.
2. Chemical Constituents of U. tomentosa
Previous studies reported the chemical constituents of several Uncaria species and recognized the different molecules present in different parts of the plant. It is worth noting that more than 50 phytochemical molecules have been identified and isolated from U. tomentosa, some of them are considered new to that species [12]. U. tomentosa leaves contain higher oxindole alkaloid content than that present in stem bark and branches. This result is compatible with a study previously described by Laus et al. [13], who documented the accumulation of speciophylline and uncarine F (the main oxindole alkaloids) in leaves that can occur as tetracyclic oxindole alkaloid (TOA) or pentacyclic oxindole alkaloid (POA) derivatives. Both TOA and POA are liable to isomerization that depends mainly on medium polarity, pH, and temperature [13].
A recent study about the chemical variation of a wild population of cat’s claw from Peru reported the existence of three specific chemotypes that producing different alkaloidal constituents [14]. Chemotype I is mainly composed of the POA with the intersection of D/E ring, chemotype II consists primarily of POA with trans D/E ring junction, while chemotype III consists primarily of TOA derivative. Uncarine C and uncarine E are two POA stereoisomers, while mitraphylline, rhynchophylline, and isorhynchophylline are TOAs found in cat’s claw. On the basis of these results, the U.S. Pharmacopeia revealed that dried raw material of cat’s claw included 0.05% (w/w) of the TOA concerning the POA amount, whereas cat’s claw powdered dried extract, tablets, and capsules contained up to 25% (w/w). Cat’s claw contains several active compositions including ajmalicine, campesterol, carboxyl alkyl esters, akuammigine, sitosterols, rutin, chlorogenic acid, speciophylline, catechin, cinchonain [15], corynoxeine, harman, daucosterol, epicatechin, hirsuteine, corynantheine, hirsutine, loganic acid, mitraphylline, iso-pteropodine, oleanolic acid, ursolic acid, lyaloside [16], rhynchophylline, palmitoleic acid, pteropodine quinovic acid glycosides, procyanidins [10], stigmasterol, 3,4-dehydro-5-carboxystrictosidine, vaccenic acid, uncarine A thru F, and strictosidines [10,17]. Moreover, other reports revealed that various compounds other than oxindole alkaloids such as rotundifoline and isorotundifolune, coumarins, flavonoids, quinovic acid glycosides, and triterpenes may be responsible for the cat’s claw medicinal effects [18,19].
3. Biological Activities of U. tomentosa Extracts and Compounds
3.1. Traditional Uses
U. tomentosa bark and root have been traditionally used as a therapy in tropical South America for many conditions, like inflammations, cancer, gastric ulcers, arthritis, and infections. Moreover, it was documented to be used for blood purifications, after child delivery as a wash for wounds to allow skin healing, cleansing the kidneys, asthma, inhibition of several diseases, menstrual irregularity and hemorrhages, fevers, and possess a normalizing activity on body systems [20]. It also was used for the treatment of various ailments including abscesses, urinary tract infections, contraception, rheumatism, and weakness. Additionally, it was used as a treatment option for mental disorders (e.g., anxiety). Some indigenous people in America used the water stored in the stem to quench thirst, and as a restorative drink [21]. Few pharmacological effects of U. tomentosa have shown in Figure 1.
Figure 1.
Schematic representation of different pharmacological activities of Uncaria tomentosa (cat’s claw).
3.2. Antioxidant Activity
The antioxidant activities of U. tomentosa have been attributed to the existence of alkaloids, flavan-3-ol monomers, and polyphenols. The preclinical assessment revealed that the cat’s claw defends toward various oxidative stresses, involving peroxynitrite that has been included in arthritis and other chronic inflammatory diseases along with inhibiting acute or chronic gastritis caused by high doses of nonsteroidal anti-inflammatory drugs (NSAIDs) [22,23]. U. tomentosa aqueous extract was found to protect against oxidative stress in human erythrocytes and relieve chronic intestinal inflammation in rats caused by indomethacin [24,25]. Another study documented that hydroxybenzoic acids, proanthocyanidins acids hydroxycinnamic were responsible for potent radical scavenging and anti-inflammatory activities of the cat’s claw [26,27]. In an in vitro experiment, U. tomentosa bark showed high antioxidant efficacy manifested by trolox equivalent antioxidant capacity, free radical diphenylpicrylhydrazyl capacity, superoxide radical scavenging activity, and peroxyl radical-trapping capacity. Moreover, it protected membrane lipids from the peroxidation caused by the iron/ascorbate system and was also evaluated by the formation of thiobarbituric acid-reactive substances (TBARs) [26]. Another in vitro study revealed that the cat’s claw prevented the inducible nitric oxide synthase (iNOS) gene expression caused by lipopolysaccharide, nitrite formation, cell death, and the NF-kappaB activation. Cat’s claw possessed a cytoprotective effect due to its ability to interact with the injurious oxidant, therefore, it may act on regulating cell death [22].
3.3. Anti-Neoplastic Activity
Cat’s claw was supposed to have antitumor and immunostimulatory effects because of its oxindole alkaloids content [10,23,28]. U. tomentosa extracts were found to have antiproliferative efficacy against SW620 colon adenocarcinoma, MCF7 breast cancer, and AGS gastric cells [19]. Interestingly, several studies suggested the antiproliferative effect of U. tomentosa against several cancer cell lines, namely cervical carcinoma, osteosarcoma, and breast cancer. For instance, an in vitro study reported that U. tomentosa hot water extract prevents inflammatory responses as well as tumor cell proliferation by inhibiting the transcriptional regulator nuclear factor kappa beta (NF-κB) activation without interfering with interleukin-2 (IL-2) production or IL-2 receptor signaling [29]. Cheng et al. [30] documented the antiproliferative effect of cat’s claw extracts against several cell lines, including glioma, premyelocytic leukemia, MCF7 breast cancer, acute lymphoblastic leukaemia, and neuroblastoma.
3.4. Anti-Inflammatory Activity
Recently, POA isolated from U. tomentosa extract has been documented to enhance the lymphocyte proliferation-regulating factor released from human endothelial cells; however, TOA was found to reduce POA activity on these cells in a dose-related manner [22,31]. Additionally, U. tomentosa stem bark extracts have been revealed to stimulate the in vitro production of IL-6 and IL-1 in rat alveolar and lipopolysaccharide-stimulated macrophages in a dose-related manner and its suppressive activities on cancer cell multiplication appear to be due to apoptosis induction [18,32]. Moreover, Xiao et al. [33] examined the hypotensive efficacy of isorhynchophylline in rats and dogs, whereas Xiang et al. [34] documented the ability of rhynchophylline to suppress rabbit and rat platelet accumulation ex vivo. Additionally, the anti-inflammatory activity of the standardized aqueous extract of U. tomentosa (AC11 of U. tomentosa extract) was attributed to NF-κB inhibition [35]. Recently, several studies reported the antioxidant, anti-neoplastic and immunomodulant activities of the alkaloids isolated from the cat’s claw [36,37,38]. For instance, Lopes et al. [39] revealed that U. tomentosa extract encourages the myeloid precursor’s proliferation by increasing serum colony-stimulating growth factors (CSFs). Moreover, in vivo studies demonstrated the effectiveness of aqueous U. tomentosa extract on leukocyte counts in healthy animals and doxorubicin-induced neutropenia [23,40,41]. Interestingly, Cisneros et al. [42] reported that lung inflammation was reduced in all mice treated with U. tomentosa bark extract. Additionally, Dreifuss et al. [19] examined the in vivo anti-inflammatory efficacy of quinovic acid glycoside separated from the aqueous cat’s claw extracts.
3.5. Antimicrobial, Antiprotozoal and Antiviral Activities
The previous study documented the antimicrobial effect of U. tomentosa bark extracts against several morphological forms of Borrelia burgdorferi and respiratory pathogens namely Enterococcus faecalis, Pseudomonas aeruginosa and Staphylococcus aureus and this activity were attributed to the presence of proanthocyanidins, including dimers and oligomers up to undecamers [43]. U. tomentosa showed remarkable antifungal efficacy against various anidulafungin, terbinafine and fluconazole-resistant non-albicans species [44]. The antiprotozoal activity has been recently documented by Batiha et al. [45] against Babesia and Theileria parasites and this efficacy was attributed to its ability to digest harmful microorganisms. In addition to that, it has been documented to treat many parasites except Giardia. Therefore, U. tomentosa could be a good complementary antiprotozoal herb [11,45]. The antiviral activity of quinovic acid glycosides has been demonstrated in vitro against vesicular stomatitis, ribonucleic acid (RNA), a minus-strand RNA virus, and rhinovirus 1B [25]. Caon et al. [46] assessed the in vitro antiherpetic activity of hydroethanolic U. tomentosa extract, as well as the purified fractions of oxindole alkaloids and quinovic acid glycosides against herpes simplex virus (HSV) infections as well as the protective activity of these preparations on UV-induced DNA damage.
3.6. Immunomodulatory Activity
Smith et al. [47] reported that the POA isolated from U. tomentosa extracts improved the cellular immune system, while the TOA suppressed this immunostimulating effect of this POA in vitro. Another in vitro study showed the effect of different cat’s claw extracts and mixtures of alkaloids in modulating the immunobiochemical pathways enhanced by interferon-gamma [48]. Notably, in vivo experiments revealed that U. tomentosa extracts exhibited immunomodulatory activity indirectly and promoted a higher provide of myeloid progenitors in the bone marrow as a result of the release of biologically active cytokines (e.g., CSFs, IL-6, and IL-1) [49]. Moreover, Allen-Hall et al. [50] documented that U. tomentosa extracts prevented the mitogen-activated protein kinases (MAPK) signaling pathway and change cytokine expression in the human acute monocytic leukemia cell line THP-1.
3.7. Cardiovascular Activity
Hirsutine isolated from U. rhynchophylla extract was found to decrease intracellular calcium concentrations in rat aortas by inhibiting the calcium channels and effecting calcium stores [51]. Moreover, it showed a vasodilated, negative chronotropic, and antiarrhythmic effect. TOA namely corynoxeine, isocorynoxiene, rhynchophylline, and isorhynchophylline exhibited a Ca2+ channel blocking effect, which resulted in low blood pressure and may affect the central nervous system [52].
3.8. Anti-Alzheimer’s Disease (AD) Activity
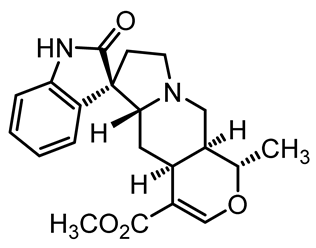
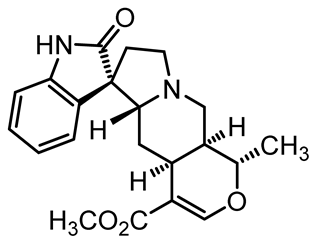
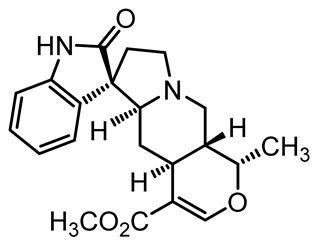
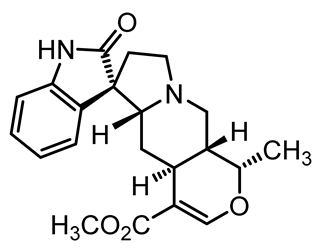
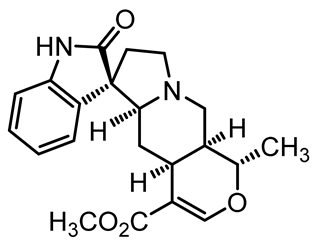
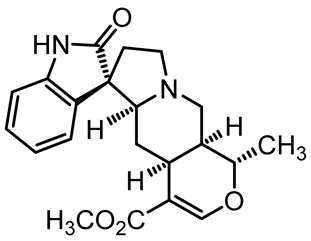
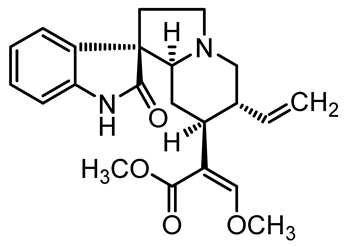
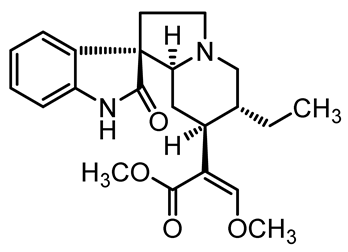
U. tomentosa is reported to act as a strong medicinal extract eliminator of Aβ plaques and it is considered as a potential plant for Alzheimer’s Disease (AD) therapy. This activity was attributed to the fact that U. tomentosa contains newly identified polyphenolic components namely specific proanthocyanidins that possess both “plaque and tangle” reducing and inhibitory effects. Proanthocyanidin B2 (epicatechin-4β-8-epicatechin) is one major cat’s claw-identified specific polyphenol that markedly diminished the brain plaque load and enhanced short-term memory in younger and older Aβ precursor protein (APP) transgenic mice “plaque-producing”. Moreover, proanthocyanidin B2 has been shown to be a strong inhibitor of the brain inflammation as evidenced by a decrease in astrocytosis and gliosis in TASD-41 transgenic mice [53]. List of some of POA and TOA alkaloids along with their structures and biological activities are provided in Table 1.

Table 1.
List of some of bioactive alkaloids isolated from Uncaria tomentosa.
4. Reported Side Effects
The American Herbal Products Association (AHPA) classified cat’s claw as a class-4 safety rating, although, it was known traditionally to be safe and nontoxic, indicating the lack of scientific data for herb safety consideration [31]. Previous reports noted several adverse effects after administration of high doses of cat’s claw including nausea, acute renal failure, slow heart rate, stomach discomfort, hormonal effects, diarrhea, hepatotoxicity, decrease progesterone and estrogen levels, neuropathy [58,59], and increased risk of bleeding when administered with blood thinner agents such as warfarin, therefore, patients may be recommended to stop cat’s claw administration before surgeries [31,60,61]. Signs of allergic reactions including swelling of face, lips, tongue, or throat, difficulty breathing, and hives have been observed [62]. Additionally, acute renal failure was noticed in systemic lupus erythematosus patients after the daily administration of four capsules of the cat’s claw [31,63,64].
5. Precautions
5.1. Drug-Drug Interactions
5.1.1. Immunosuppressant Drugs
Theoretically, it was believed that POA isolated from cat’s claw possesses an immunostimulatory effect, therefore, it is contraindicated to be used with immunosuppressant drugs including cyclosporine, azathioprine, daclizumab, basiliximab, mycophenolate, muromonab-CD3, tacrolimus, sirolimus, corticosteroids, prednisone, or other chemotherapeutic drugs recommended for autoimmune disease treatment or after organ transplantation [39,62].
5.1.2. Anticoagulants
Cat’s claw contains TOA that increased risk of bleeding when administered with aspirin, anticoagulant drugs such as warfarin or heparin, NSAIDs such as ibuprofen and naproxen, antiplatelet drugs like clopidogrel due to rhynchophylline inhibitory efficacy on platelet aggregation, therefore, patients may be recommended to stop cat’s claw administration before surgeries [31,61].
5.1.3. Diuretics
Cat’s claw has a diuretic effect, so it is contraindicated to be used with other diuretics, as they act by the same mechanism and thus increases the risk of electrolyte imbalance. Moreover, it may interact with hormonal drugs, cholesterol-lowering drugs, and drugs that affect the kidney [65].
5.1.4. Antihypertensive Drugs
Hirsutine extracted from cat’s claw was reported to have a hypotensive effect, therefore it is not recommended to be used to hypotensive people or those administering antihypertensive drugs (e.g., casein protein, coenzyme Q-10 (ubiquinone), fish oil, L-arginine, Lycium, or stinging nettle) due to rhynchophylline and isorhynchophylline hypotensive effects as it may reduce the blood pressure to be too low [33].
5.1.5. Cytochrome P450 Substrates
Cat’s claw prevents the microsomal CYP 3A4 activity, and thus, increased the serum levels of drugs that are metabolized by CYP 3A4 suchlike nonnucleoside reverse-transcriptase inhibitors, cyclosporine, and some benzodiazepines and increased the serious adverse effects of these drugs [66]. Moreover, the cat’s claw may interact with allergic drugs like fexofenadine, anti-cancer agents as paclitaxel, antifungals like ketoconazole, antiviral drugs, and oral contraceptives [67].
5.2. Drug Safety
Based on the possible safety data, U. tomentosa extracts appears to be safe when administered to several cases of inflammation. Cat’s claw safety has not been documented in breastfeeding and pregnant women, or children under three years of age because of insufficient safety research [31,64].
6. Recommended Doses
The typical and recommended dose of U. tomentosa is one gram given two to three times daily. A standardized extract attributed to specific chemotype of this species consisting of less than 0.5% oxindole alkaloids and 8% to 10% carboxy alkyl esters has been used in doses of 250 to 300 mg in several clinical studies [68,69]. In rats, it was determined that the average lethal dose for a single dose of water extract from U. tomentosa is higher than 8 g/kg. In humans, no toxic symptoms were noticed with frequent administration of 350 mg/day for 6 successive weeks [18,70,71]. Tinctures, decoctions, capsules, extracts, and teas are recently prepared from the cat’s claw. For instance, 250–1000 mg capsule is taken orally in divided doses per day [31], while in a decoction, up to 25 g of raw bark has been used, although this based on traditional management practices. Although U. tomentosa is commercially applicable in skin formulation, its typical dose has not yet been documented [31].
7. Conclusions
The existing review investigates the medicinal activities and all phytochemical molecules extracted from U. tomentosa. U. tomentosa (cat’s claw) is used in the tradition medicine as a treatment option against wide range of health problems, including immune system deficiencies, neurodegenerative disorders, cancer, chronic fatigue syndrome, Crohn’s disease, digestive complaints, parasitic and microbial infections, kidney cleanser, inflammatory problems, irritable and leaky bowel syndrome. Moreover, U. tomentosa has many phytochemical molecules that are attributed to its therapeutic activities and exist in different degrees in the herb. U. tomentosa acts as an effective natural herbal extract eliminator of Aβ protein “plaques”. Thence, U. tomentosa could be a potential herb for AD treatment. Although these medicinal properties, the cat’s claw shows several adverse effects such as nausea, acute renal failure, stomach discomfort, hormonal effects, diarrhea, hepatotoxicity, neuropathy and it is contraindicated to be used with anticoagulants, antihypertensive, and immunosuppressant drugs.
Author Contributions
G.E.-S.B., A.M.B., L.W., Y.H.A.E., M.E.A.E.-H., A.E.T., A.A.A.-S., and H.P.D. wrote the paper. G.E.-S.B., A.M.B. and V.T. revised the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research not receive external fund.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| HIV | human immunodeficiency viruses |
| TOA | tetracyclic oxindole alkaloid |
| POA | pentacyclic oxindole alkaloid |
| IUPAC | International Union of Pure and Applied Chemistry |
| NSAIDs | nonsteroidal anti-inflammatory drugs |
| TBARs | thiobarbituric acid-reactive substances |
| iNOS | inducible nitric oxide synthase |
| IL-2 | interleukin-2 |
| AC11 of U. tomentosa extract | standardized aqueous extract of U. tomentosa |
| CSFs | colony-stimulating growth factors |
| RNA | ribonucleic acid |
| HSV | herpes simplex virus |
| MAPK | mitogen-activated protein kinases |
| AD | Alzheimer’s disease |
| Aβ | beta-amyloid |
| AHPA | The American Herbal Products Association |
References
- Batiha, G.E.S.; Beshbishy, A.M.; Tayebwa, D.S.; Adeyemi, O.S.; Shaheen, H.; Yokoyama, N.; Igarashi, I. The effects of trans-chalcone and chalcone 4 hydrate on the growth of Babesia and Theileria. PLoS Negl. Trop. Dis. 2019, 13, e0007030. [Google Scholar] [CrossRef] [PubMed]
- Beshbishy, A.M.; Batiha, G.E.; Yokoyama, N.; Igarashi, I. Ellagic acid microspheres restrict the growth of Babesia and Theileria in vitro and Babesia microti in vivo. Parasit. Vectors 2019, 12, 269. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotech. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed]
- Adeyemi, O.S.; Atolani, O.; Awakan, O.J.; Olaolu, T.D.; Nwonuma, C.O.; Alejolowo, O.; Otohinoyi, D.A.; Rotimi, D.; Owolabi, A.; Batiha, G.E. Focus: Organelles: In vitro screening to identify anti-Toxoplasma compounds and in silico modeling for bioactivities and toxicity. Yale J. Biol. Med. 2019, 92, 369. [Google Scholar]
- Batiha, G.E.S.; Beshbishy, A.A.; Tayebwa, D.S.; Shaheen, M.H.; Yokoyama, N.; Igarashi, I. Inhibitory effects of Syzygium aromaticum and Camellia sinensis methanolic extracts on the growth of Babesia and Theileria parasites. Ticks Tick. Borne Dis. 2019, 10, 949–958. [Google Scholar] [CrossRef]
- Beshbishy, A.M.; Batiha, G.E.S.; Adeyemi, O.S.; Yokoyama, N.; Igarashi, I. Inhibitory effects of methanolic Olea europaea and acetonic Acacia laeta on the growth of Babesia and Theileria. Asian Pac. J. Trop. Med. 2019, 12, 425–434. [Google Scholar]
- Batiha, G.E.S.; Beshbishy, A.A.; Tayebwa, D.S.; Adeyemi, O.S.; Yokoyama, N.; Igarashi, I. Anti-piroplasmic potential of the methanolic Peganum harmala seeds and ethanolic Artemisia absinthium leaf extracts. J. Protoz. Res. 2019, 29, 8–27. [Google Scholar]
- Batiha, G.E.S.; Beshbishy, A.M.; Tayebwa, D.S.; Adeyemi, O.S.; Shaheen, H.; Yokoyama, N.; Igarashi, I. Evaluation of the inhibitory effect of ivermectin on the growth of Babesia and Theileria parasites in vitro and in vivo. Trop. Med. Health. 2019, 47, 42. [Google Scholar] [CrossRef]
- World Health Organization. WHO Monographs on Selected Medicinal Plants; World Health Organization: Geneva, Switzerland, 1999; Volume 2. [Google Scholar]
- Heitzman, M.E.; Neto, C.C.; Winiarz, E.; Vaisberg, A.J.; Hammond, G.B. Ethnobotany, phytochemistry and pharmacology of Uncaria (Rubiaceae). Phytochemistry 2005, 66, 5–29. [Google Scholar] [CrossRef]
- Santos, K.F.; Gutierres, J.M.; Pillat, M.M.; Rissi, V.B.; dos Santos Araújo, M.D.C.; Bertol, G.; Gonçalves, P.B.D.; Schetinger, M.R.C.; Morsch, V.M. Uncaria tomentosa extract alters the catabolism of adenine nucleotides and expression of ecto 5′-nucleotidase/CD73 and P2X7 and A1 receptors in the MDA-MB-231 cell line. J. Ethnopharmacol. 2016, 194, 108–116. [Google Scholar] [CrossRef]
- Navarro Hoyos, M.; Sánchez-Patán, F.; Murillo Masis, R.; Martín-Álvarez, P.J.; Zamora Ramirez, W.; Monagas, M.J.; Bartolomé, B. Phenolic assessment of Uncaria tomentosa L. (cat’s claw): Leaves, stem, bark and wood extracts. Molecules 2015, 20, 22703–22717. [Google Scholar] [CrossRef] [PubMed]
- Laus, G.; Brössner, D.; Kiplinger, K. Alkaloids of Peruvian Uncaria tomentosa. Phytochemistry 1997, 45, 855–860. [Google Scholar] [CrossRef]
- Peñaloza, E.M.C.; Kaiser, S.; Resende, P.E.d.; Pittol, V.; Carvalho, Â.R.; Ortega, G.G. Chemical composition variability in the Uncaria tomentosa (cat’s claw) wild population. Química Nova 2015, 38, 378–386. [Google Scholar]
- Gable, R.S. Risk assessment of ritual use of oral dimethyltryptamine (DMT) and harmala alkaloids. Addiction 2007, 102, 24–34. [Google Scholar] [CrossRef]
- Sheng, Y.; Akesson, C.; Holmgren, K.; Bryngelsson, C.; Giamapa, V.; Pero, R.W. An active ingredient of cat’s claw water extracts identification and efficacy of quinic acid. J. Ethnopharmacol. 2005, 96, 577–584. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, J.; Kim, B.Y.; Lee, H.S.; Ahn, J.S.; Chang, Y.S. Inhibition of phospholipase cgamma1 and cancer cell proliferation by triterpene esters from Uncaria rhynchophylla. J. Nat. Prod. 2000, 63, 753–756. [Google Scholar] [CrossRef]
- Sheng, Y.; Bryngelsson, C.; Pero, R.W. Enhanced DNA repair, immune function and reduced toxicity of C-MED-100, a novel aqueous extract from Uncaria tomentosa. J. Ethnopharmacol. 2000, 69, 115–126. [Google Scholar] [CrossRef]
- Dreifuss, A.A.; Bastos-Pereira, A.L.; Fabossi, I.A.; Lívero, F.A.; Stolf, A.M.; Alves de Souza, C.E.; Gomes Lde, O.; Constantin, R.P.; Furman, A.E.; Strapasson, R.L.; et al. Uncaria tomentosa exerts extensive anti-neoplastic effects against the Walker-256 tumor by modulating oxidative stress and not by alkaloid activity. PLoS ONE 2013, 8, e54618. [Google Scholar] [CrossRef]
- Laus, G. Advances in chemistry and bioactivity of the genus Uncaria. Phytother. Res. 2004, 18, 259–274. [Google Scholar] [CrossRef]
- Azevedo, B.C.; Morel, L.J.F.; Carmona, F.; Cunha, T.M.; Contini, S.H.T.; Delprete, P.G.; Ramalho, F.S.; Crevelin, E.; Bertoni, B.W.; França, S.C.; et al. Aqueous extracts from Uncaria tomentosa (Willd. ex Schult.) DC. reduce bronchial hyper responsiveness and inflammation in a murine model of asthma. J. Ethnopharmacol. 2018, 218, 76–89. [Google Scholar] [CrossRef]
- Sandoval, M.; Charbonnet, R.M.; Okuhama, N.N.; Roberts, J.; Krenova, Z.; Trentacosti, A.M.; Miller, M.J. Cat’s claw inhibits TNF-α production and scavenges free radicals: Role in cytoprotection. Free Radic. Biol. Med. 2000, 29, 71–78. [Google Scholar] [CrossRef]
- Piscoya, J.; Rodriguez, Z.; Bustamante, S.A.; Okuhama, N.N.; Miller, M.J.; Sandoval, M. Efficacy and safety of freeze-dried cat’s claw in osteoarthritis of the knee: Mechanisms of action of the species Uncaria guianensis. Inflamm. Res. 2001, 50, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, M.; Okuhama, N.N.; Zhang, X.J.; Condezo, L.A.; Lao, J.; Angeles, F.M.; Musah, R.A.; Bobrowski, P.; Miller, M.J. Anti-inflammatory and antioxidant activities of cat’s claw (Uncaria tomentosa and Uncaria guianensis) are independent of their alkaloid content. Phytomedicine 2002, 9, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Bors, M.; Bukowska, B.; Pilarski, R.; Gulewicz, K.; Oszmiański, J.; Michałowicz, J.; Koter-Michalak, M. Protective activity of the Uncaria tomentosa extracts on human erythrocytes in oxidative stress induced by 2,4-dichlorophenol (2,4-DCP) and catechol. Food Chem. Toxicol. 2011, 49, 2202–2211. [Google Scholar] [CrossRef]
- Navarro, M.; Arnaez, E.; Moreira, I.; Hurtado, A.; Monge, D.; Monagas, M. Polyphenolic composition and antioxidant activity of Uncaria tomentosa commercial bark products. Antioxidants 2019, 8, 339. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Hoyos, M.; Lebrón-Aguilar, R.; Quintanilla-López, J.E.; Cueva, C.; Hevia, D.; Quesada, S.; Azofeifa, G.; Moreno-Arribas, M.V.; Monagas, M.; Bartolomé, B. Proanthocyanidin characterization and bioactivity of extracts from different parts of Uncaria tomentosa L. (Cat’s Claw). Antioxidants 2017, 6, 12. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, J.J.; Xu, J.; Feng, F.; Qu, W. Medicinal uses, phytochemistry and pharmacology of the genus Uncaria. J. Ethnopharmacol. 2015, 173, 48–80. [Google Scholar] [CrossRef]
- Akesson, C.; Lindgren, H.; Pero, R.W.; Leanderson, T.; Ivars, F. An extract of Uncaria tomentosa inhibiting cell division and NF-kappa B activity without inducing cell death. Int. Immunopharmacol. 2003, 3, 1889–1900. [Google Scholar] [CrossRef]
- Cheng, A.C.; Jian, C.B.; Huang, Y.T.; Lai, C.S.; Hsu, P.C.; Pan, M.H. Induction of apoptosis by Uncaria tomentosa through reactive oxygen species production, cytochrome c release, and caspases activation in human leukemia cells. Food Chem. Toxicol. 2007, 45, 2206–2218. [Google Scholar] [CrossRef]
- Erowele, G.I.; Kalejaiye, A.O. Pharmacology and therapeutic uses of cat’s claw. Am. J. Health Syst. Pharm. 2009, 66, 992–995. [Google Scholar] [CrossRef]
- Rinner, B.; Li, Z.X.; Haas, H.; Siegl, V.; Sturm, S.; Stuppner, H.; Pfragner, R. Antiproliferative and pro-apoptotic effects of Uncaria tomentosa in human medullary thyroid carcinoma cells. Anticancer Res. 2009, 29, 4519–4528. [Google Scholar] [PubMed]
- Xiao, D.; Gu, Z.L.; Qian, Z.N. Effects of quercetin on platelet and reperfusion-induced arrhythmias in rats. Zhongguo Yao Li Xue Bao 1993, 14, 505–508. [Google Scholar] [PubMed]
- Xiang, L.; Li, Y.; Deng, X.; Kosanovic, D.; Schermuly, R.T.; Li, X. Natural plant products in treatment of pulmonary arterial hypertension. Pulm. Circ. 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Allen-Hall, L.; Arnason, J.T.; Cano, P.; Lafrenie, R.M. Uncaria tomentosa acts as a potent TNF-alpha inhibitor through NF-kappaB. J. Ethnopharmacol. 2010, 127, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Paniagua-Pérez, R.; Madrigal-Bujaidar, E.; Molina-Jasso, D.; Reyes-Cadena, S.; Alvarez-González, I.; Sánchez-Chapul, L.; Pérez-Gallaga, J. Antigenotoxic, antioxidant and lymphocyte induction effects produced by pteropodine. Basic Clin. Pharmacol. Toxicol. 2009, 104, 222–227. [Google Scholar] [CrossRef]
- Bacher, N.; Tiefenthaler, M.; Sturm, S.; Stuppner, H.; Ausserlechner, M.J.; Kofler, R.; Konwalinka, G. Oxindole alkaloids from Uncaria tomentosa induce apoptosis in proliferating, G0/G1-arrested and bcl-2-expressing acute lymphoblastic leukemia cells. Br. J. Haematol. 2006, 132, 615–622. [Google Scholar] [CrossRef]
- Garcia Prado, E.; Garcia Gimenez, M.D.; De la Puerta Vazquez, R.; Espartero Sánchez, J.L.; Sáenz Rodríguez, M.T. Antiproliferative effects of mitraphylline, a pentacyclic oxindole alkaloid of Uncaria tomentosa on human glioma and neuroblastoma cell lines. Phytomedicine 2007, 14, 280–284. [Google Scholar] [CrossRef]
- Lopes, C.M.; Dourado, A.; Oliveira, R. Phytotherapy and nutritional supplements on breast cancer. Biomed. Res. Int. 2017, 2017, 42. [Google Scholar] [CrossRef]
- Sheng, Y.; Pero, R.W.; Wagner, H. Treatment of chemotherapy-induced leukopenia in a rat model with aqueous extract from Uncaria tomentosa. Phytomedicine 2000, 7, 137–143. [Google Scholar] [CrossRef]
- Farias, I.L.G.; Araújo, M.C.S.; Farias, J.G.; Rossato, L.V.; Elsenbach, L.I.; Dalmora, S.L.; Flores, N.M.; Durigon, M.; Cruz, I.B.; Morsch, V.M.; et al. Uncaria tomentosa for reducing side effects caused by chemotherapy in CRC Patients: Clinical trial. Evid. Based Complement. Alternat. Med. 2012, 2012, 892182. [Google Scholar] [CrossRef]
- Cisneros, F.J.; Jayo, M.; Niedziela, L. An Uncaria tomentosa (cat’s claw) extract protects mice against ozone-induced lung inflammation. J. Ethnopharmacol. 2005, 96, 355–364. [Google Scholar] [CrossRef]
- Ccahuana-Vasquez, R.A.; Santos, S.S.; Koga-Ito, C.Y.; Jorge, A.O. Antimicrobial activity of Uncaria tomentosa against oral human pathogens. Braz. Oral Res. 2007, 21, 46–50. [Google Scholar] [CrossRef]
- Moraes, R.C.; Dalla Lana, A.J.; Kaiser, S.; Carvalho, A.R.; de Oliveira, L.F.S.; Fuentefria, A.M.; Ortega, G.G. Antifungal activity of Uncaria tomentosa (Willd.) DC against resistant non-albicans Candida isolates. Ind. Crops Prod. 2015, 69, 7–14. [Google Scholar] [CrossRef]
- Batiha, G.E.S.; Beshbishy, A.A.; Tayebwa, D.S.; Shaheen, M.H.; Yokoyama, N.; Igarashi, I. Inhibitory effects of Uncaria tomentosa bark, Myrtus communis roots, Origanum vulgare leaves and Cuminum cyminum seeds extracts against the growth of Babesia and Theileria in vitro. Jpn. J. Vet. Parasitol. 2018, 17, 1–13. [Google Scholar]
- Caon, T.; Kaiser, S.; Feltrin, C.; de Carvalho, A.; Sincero, T.C.; Ortega, G.G.; Simões, C.M. Antimutagenic and antiherpetic activities of different preparations from Uncaria tomentosa (cat’s claw). Food Chem. Toxicol. 2014, 66, 30–35. [Google Scholar] [CrossRef]
- Smith, V.J.; Brown, J.H.; Hauton, C. Immunostimulation in crustaceans: Does it really protect against infection? Fish Shellfish Immunol. 2003, 15, 71–90. [Google Scholar] [CrossRef]
- Kośmider, A.; Czepielewska, E.; Kuraś, M.; Gulewicz, K.; Pietrzak, W.; Nowak, R.; Nowicka, G. Uncaria tomentosa Leaves Decoction Modulates Differently ROS Production in Cancer and Normal Cells, and Effects Cisplatin Cytotoxicity. Molecules 2017, 22, 620. [Google Scholar] [CrossRef]
- Eberlin, S.; dos Santos, L.M.; Queiroz, M.L. Uncaria tomentosa extract increases the number of myeloid progenitor cells in the bone marrow of mice infected with Listeria monocytogenes. Int. Immunopharmacol. 2005, 5, 1235–1246. [Google Scholar] [CrossRef] [PubMed]
- Allen-Hall, L.; Cano, P.; Arnason, J.T.; Rojas, R.; Lock, O.; Lafrenie, R.M. Treatment of THP-1 cells with Uncaria tomentosa extracts differentially regulates the expression if IL-1beta and TNF-alpha. J. Ethnopharmacol. 2007, 109, 312–317. [Google Scholar] [CrossRef]
- Zhu, K.; Yang, S.N.; Ma, F.F.; Gu, X.F.; Zhu, Y.C.; Zhu, Y.Z. The novel analogue of hirsutine as an anti-hypertension and vasodilatary agent both in vitro and in vivo. PLoS ONE 2015, 10, e0119477. [Google Scholar] [CrossRef]
- Leenen, F.H.; Ruzicka, M.; Huang, B.S. Central sympathoinhibitory effects of calcium channel blockers. Curr. Hypertens. Rep. 2001, 3, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Snow, A.D.; Castillo, G.M.; Nguyen, B.P.; Choi, P.Y.; Cummings, J.A.; Cam, J.; Hu, Q.; Lake, T.; Pan, W.; Kastin, A.J.; et al. The Amazon rain forest plant Uncaria tomentosa (cat’s claw) and its specific proanthocyanidin constituents are potent inhibitors and reducers of both brain plaques and tangles. Sci. Rep. 2019, 9, 561. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Duran, R.; González-Aspajo, G.; Ruiz-Martel, C.; Bourdy, G.; Doroteo-Ortega, V.H.; Alban-Castillo, J.; Robert, G.; Auberger, P.; Deharo, E. Anti-inflammatory activity of Mitraphylline isolated from Uncaria tomentosa bark. J. Ethnopharmacol. 2012, 143, 801–804. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, B.C.; Roxo, M.; Borges, M.C.; Peixoto, H.; Crevelin, E.J.; Bertoni, B.W.; Contini, S.H.T.; Lopes, A.A.; França, S.C.; Pereira, A.M.S.; et al. Antioxidant activity of an aqueous leaf extract from Uncaria tomentosa and its major alkaloids mitraphylline and isomitraphylline in Caenorhabditis elegans. Molecules 2019, 24, 3299. [Google Scholar] [CrossRef]
- Kim, T.J.; Lee, J.H.; Lee, J.J.; Yu, J.Y.; Hwang, B.Y.; Ye, S.K.; Shujuan, L.; Gao, L.; Pyo, M.Y.; Yun, Y.P. Corynoxeine isolated from the hook of Uncaria rhynchophylla inhibits rat aortic vascular smooth muscle cell proliferation through the blocking of extracellular signal regulated kinase 1/2 phosphorylation. Biol. Pharm. Bull. 2008, 31, 2073–2078. [Google Scholar] [CrossRef]
- Chen, C.X.; Jin, R.M.; Li, Y.K.; Zhong, J.; Yue, L.; Chen, S.C.; Zhou, J.Y. Inhibitory effect of rhynchophylline on platelet aggregation and thrombosis. Zhongguo Yao Li Xue Bao 1999, 13, 126–130. [Google Scholar]
- Cosentino, C.; Torres, L. Reversible worsening of Parkinson’s disease motor symptoms after oral intake of Uncaria tomentosa (cat’s claw). Clin. Neuropharmacol. 2008, 31, 293–294. [Google Scholar] [CrossRef]
- Navarro, V.J.; Barnhart, H.; Bonkovsky, H.L.; Davern, T.; Fontana, R.J.; Grant, L.; Reddy, K.R.; Seeff, L.B.; Serrano, J.; Sherker, A.H.; et al. Liver injury from herbals and dietary supplements in the U.S. Drug-Induced Liver Injury Network. Hepatology 2014, 60, 1399–1408. [Google Scholar] [CrossRef] [PubMed]
- Ernst, E. Cardiovascular adverse effects of herbal medicines: A systematic review of the recent literature. Can. J. Cardiol. 2003, 19, 818–827. [Google Scholar]
- Vogel, J.H.; Bolling, S.F.; Costello, R.B.; Guarneri, E.M.; Krucoff, M.W.; Longhurst, J.C.; Olshansky, B.; Pelletier, K.R.; Tracy, C.M.; Vogel, R.A.; et al. Integrating complementary medicine into cardiovascular medicine: A report of the American college of cardiology foundation task force on clinical expert consensus documents (writing committee to develop an expert consensus document on complementary and integrative medicine). J. Am. Coll. Cardiol. 2005, 46, 184–221. [Google Scholar]
- Standard, N. Natural Standard Herb & Supplement Guide-E-Book: An Evidence-Based Reference; Elsevier Health Sciences: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Gabardi, S.; Munz, K.; Ulbricht, C. A review of dietary supplement–induced renal dysfunction. Clin. J. Amer. Soc. Nephr. 2007, 2, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Kemper, K.J. Shark cartilage, cat’s claw, and other complementary cancer therapies. Contemp. Ped. 1999, 16, 101. [Google Scholar]
- Yano, S.; Horiuchi, H.; Horie, S.; Aimi, N.; Sakai, S.; Watanabe, K. Ca2+, channel-blocking effects of hirsutine, an indole alkaloid from Uncaria genus, in the isolated rat aorta. Planta Medica. 1991, 57, 403–405. [Google Scholar] [CrossRef] [PubMed]
- Budzinski, J.W.; Foster, B.C.; Vandenhoek, S.; Arnason, J.T. An in vitro evaluation of human cytochrome P450 3A4 inhibition by selected commercial herbal extracts and tinctures. Phytomedicine 2000, 7, 273–282. [Google Scholar] [CrossRef]
- Scott, G.N.; Elmer, G.W. Update on natural product—Drug interactions. Am. J. Health Syst. Pharm. 2002, 59, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Li, L.; Holmgren, K.; Pero, R.W. DNA repair enhancement of aqueous extracts of Uncaria tomentosa in a human volunteer study. Phytomedicine 2010, 8, 275–282. [Google Scholar] [CrossRef]
- Lehmann-Willenbrock, E.; Riedel, H.H. Clinical and endocrinologic studies of the treatment of ovarian insufficiency manifestations following hysterectomy with intact adnexa. Zentralbl. Gynakol. 1988, 110, 611–618. [Google Scholar]
- Valerio, L.G., Jr.; Gonzales, G.F. Toxicological aspects of the South American herbs cat’s claw (Uncaria tomentosa) and Maca (Lepidium meyenii): A critical synopsis. Toxicol. Rev. 2005, 24, 11–35. [Google Scholar] [CrossRef]
- Williams, J.E. Review of antiviral and immunomodulating properties of plants of the Peruvian rainforest with a particular emphasis on Una de Gato and Sangre de Grado. Altern. Med. Rev. 2001, 6, 567–579. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).