Superhydrophobic Self-Assembled Silane Monolayers on Hierarchical 6082 Aluminum Alloy for Anti-Corrosion Applications
Abstract
1. Introduction
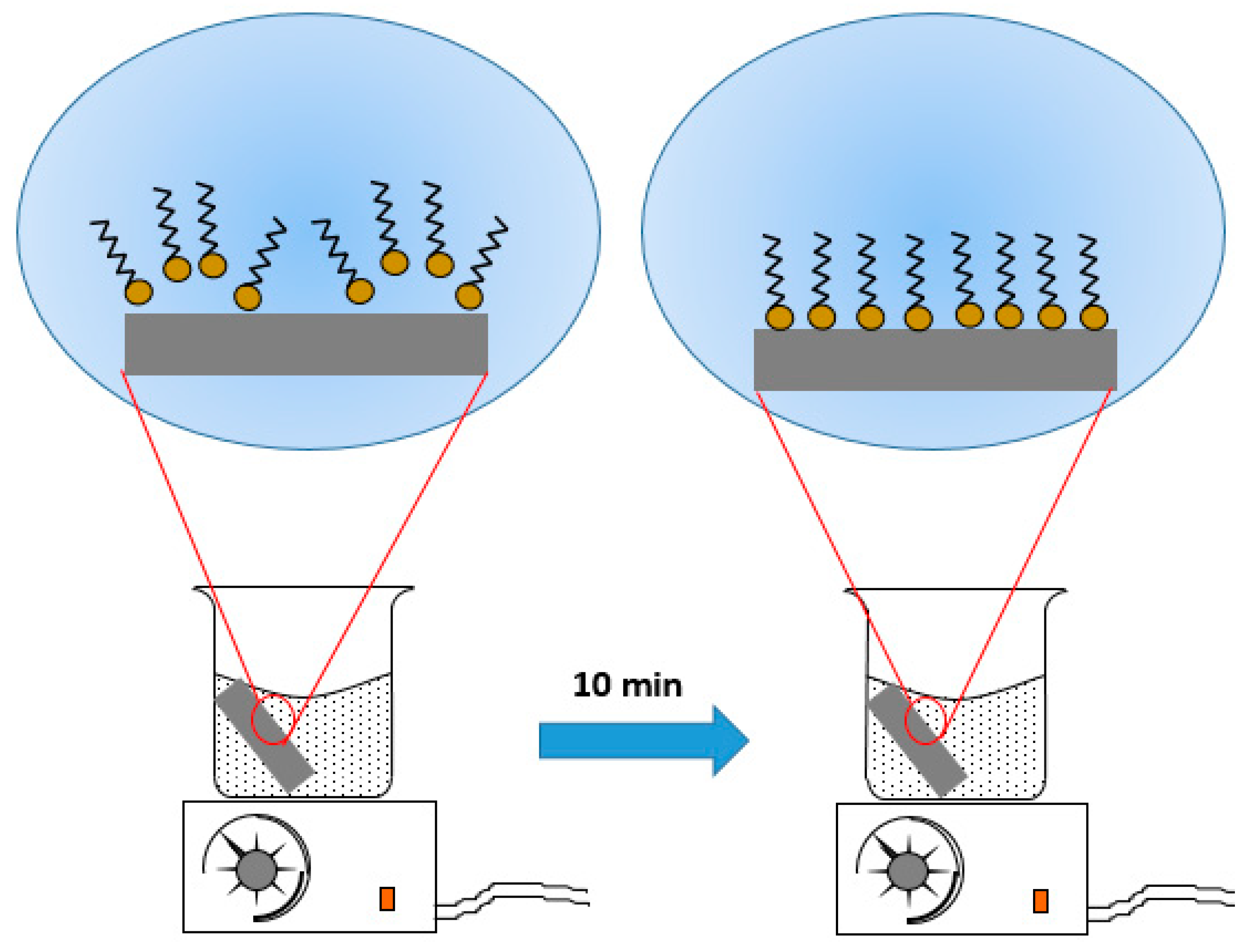
2. Materials and Methods
3. Results and Discussions
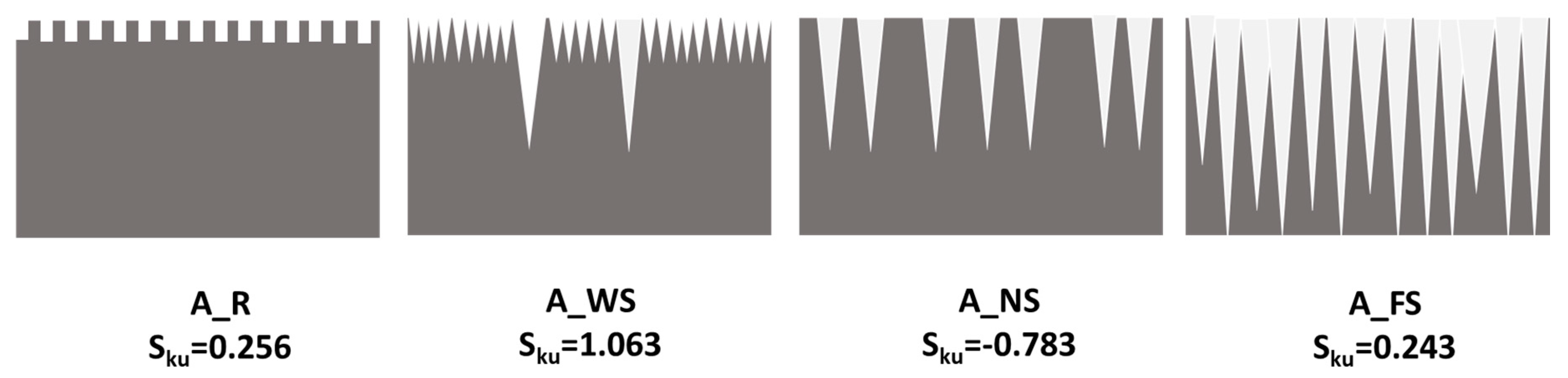
3.1. Surface Morphology and Roughness
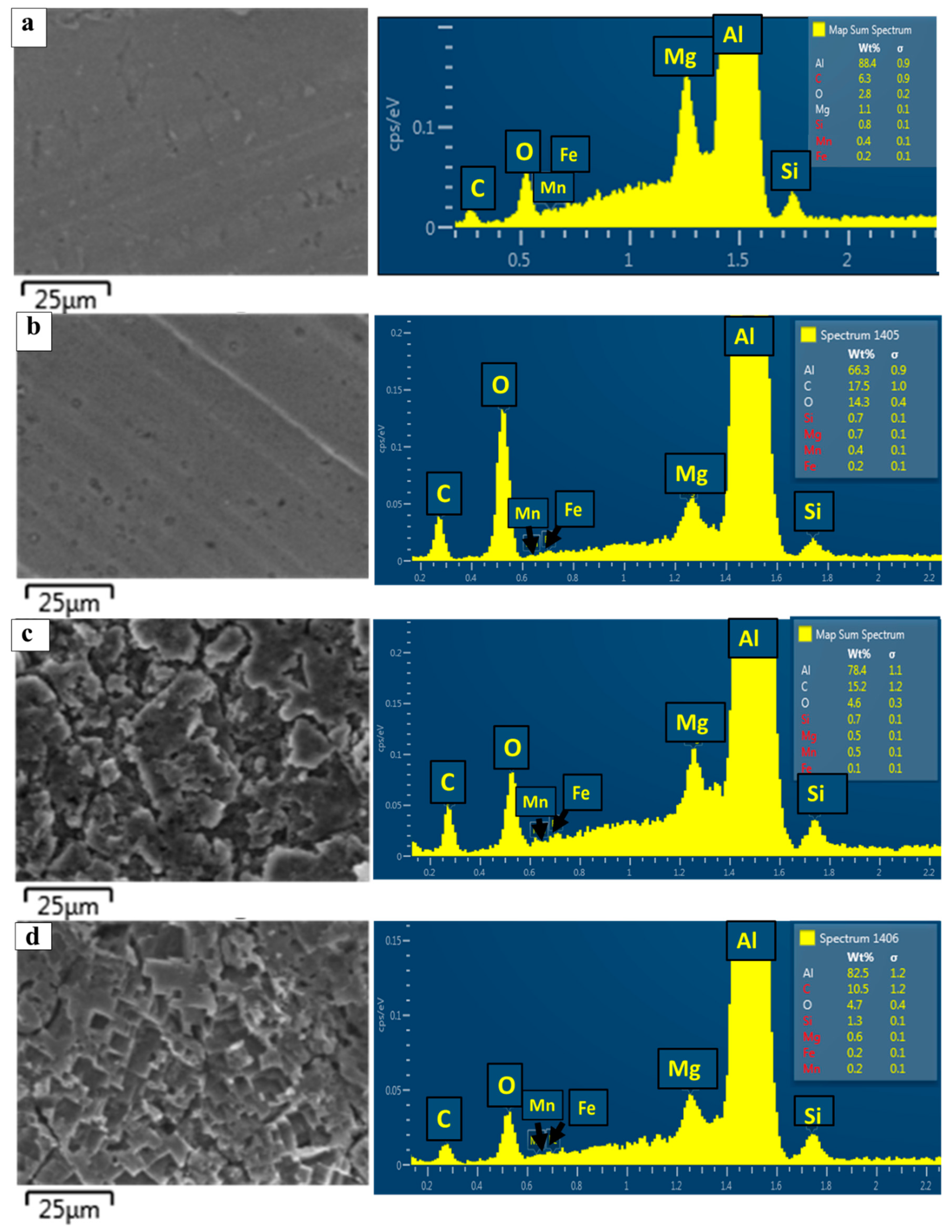
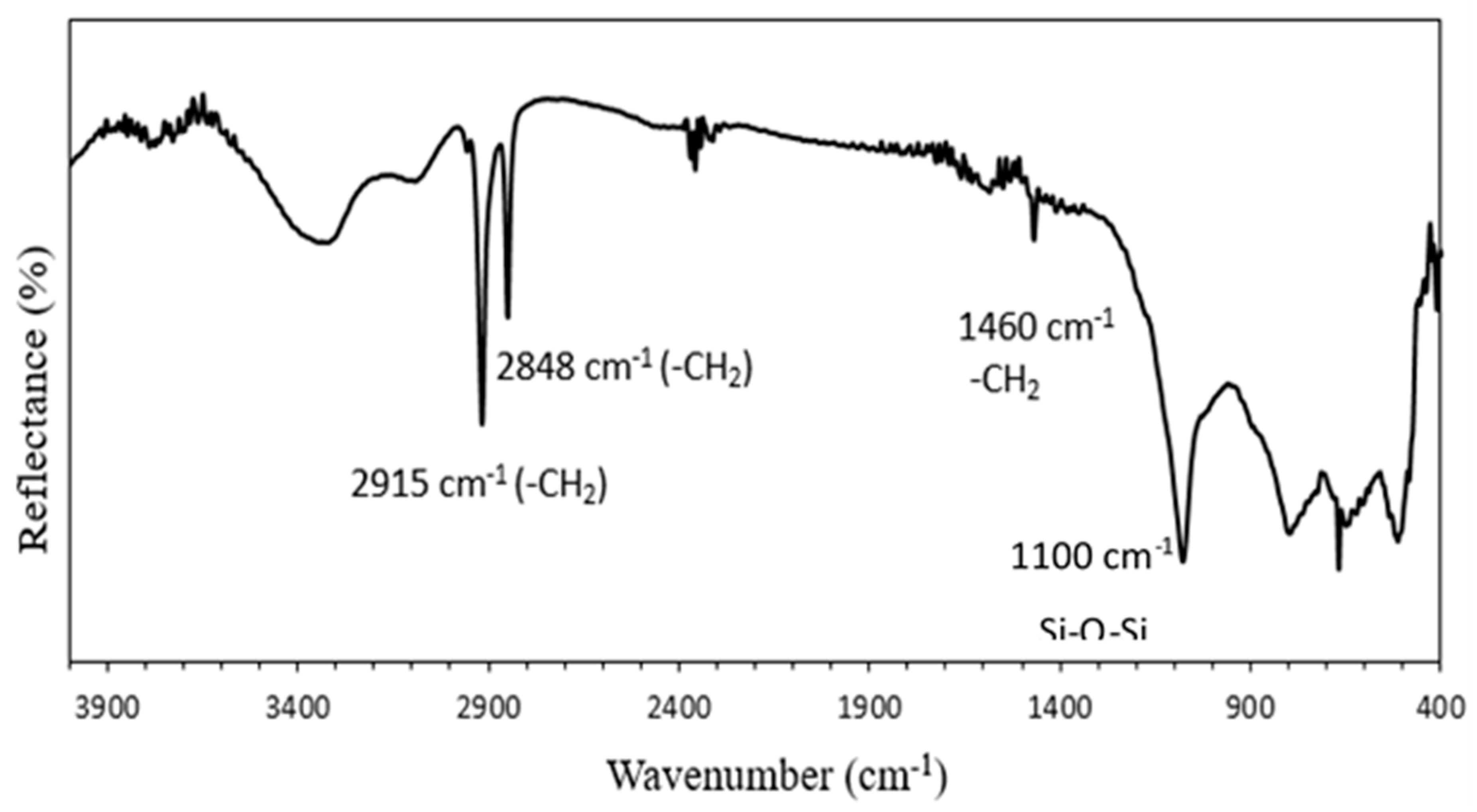
3.2. Element Analysis
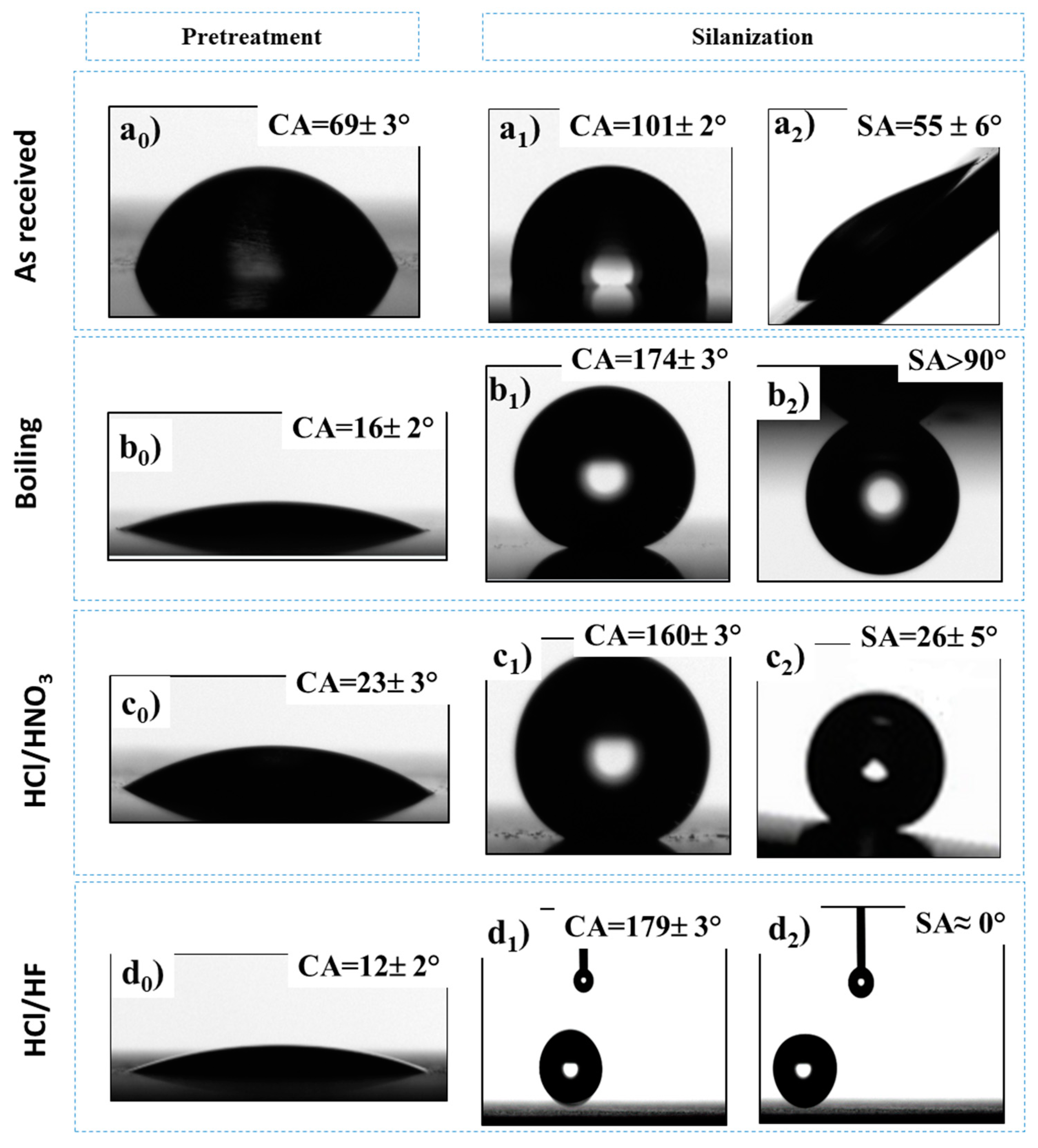
3.3. Wettability Behavior
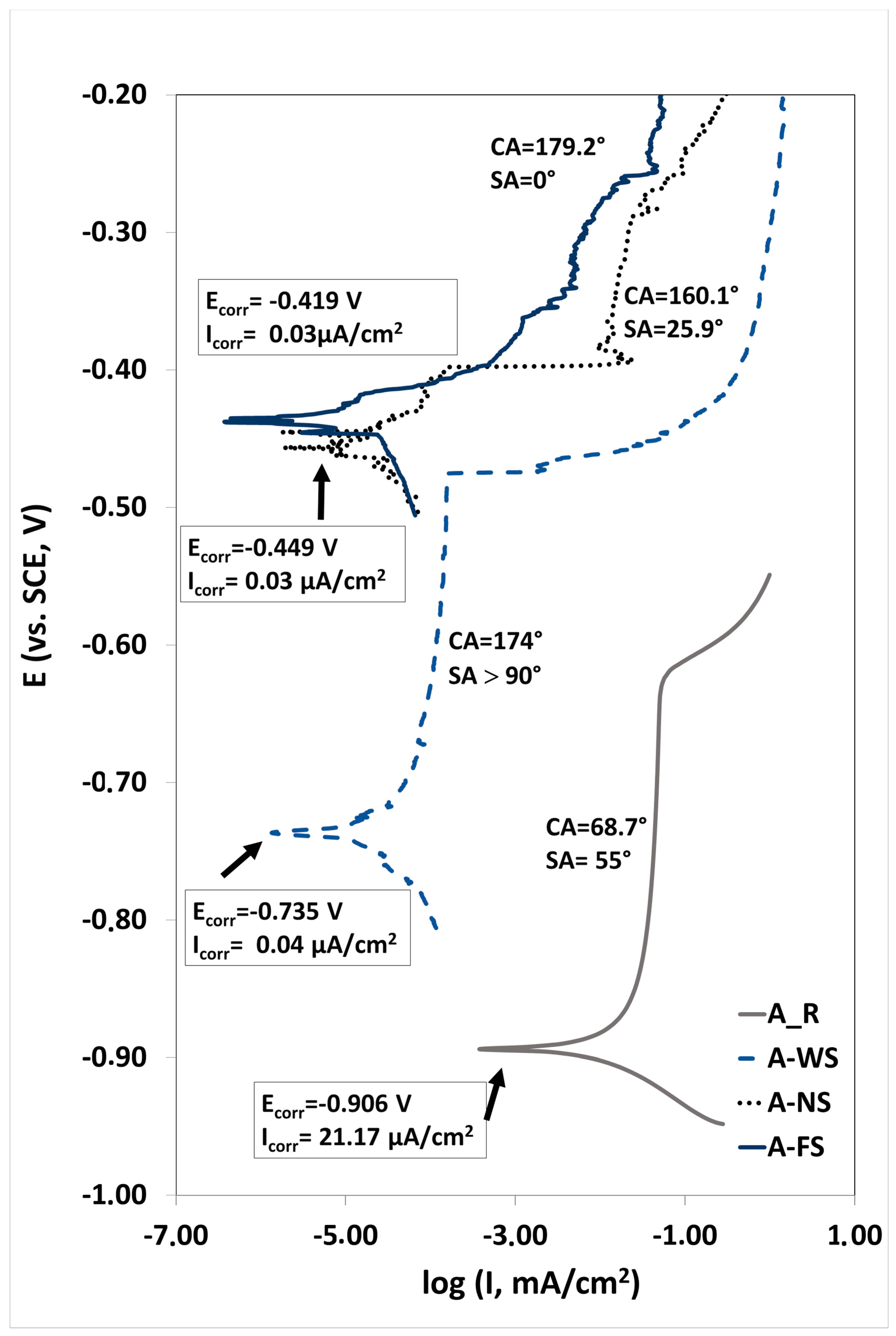
3.4. Corrosion Resistance
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Simpson, J.T.; Hunter, S.R.; Aytug, T. Superhydrophobic materials and coatings: A review. Rep. Prog. Phys. 2015, 78, 086501. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Chen, Z.; Yang, H.; Hou, K.; Zeng, X.; Zheng, Y.; Cheng, J. Nature-Inspired Strategy toward Superhydrophobic Fabrics for Versatile Oil/Water Separation. ACS Appl. Mater. Interfaces 2017, 9, 9184–9194. [Google Scholar] [CrossRef] [PubMed]
- Vazirinasab, E.; Jafari, R.; Momen, G. Application of superhydrophobic coatings as a corrosion barrier: A review. Surf. Coat. Technol. 2018, 341, 40–56. [Google Scholar] [CrossRef]
- Ran, M.; Zheng, W.; Wang, H. Fabrication of superhydrophobic surfaces for corrosion protection: A review. Mater. Sci. Tech. 2019, 35, 313–326. [Google Scholar] [CrossRef]
- Zheng, S.; Li, C.; Fu, Q.; Hu, W.; Xiang, T.; Wang, Q.; Du, M.; Liu, X.; Chen, Z. Development of stable superhydrophobic coatings on aluminum surface for corrosion-resistant, self-cleaning, and anti-icing applications. Mater. Des. 2016, 93, 261–270. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, J.; Zhang, J. Bioinspired one step hydrothermal fabricated superhydrophobic aluminum alloy with favorable corrosion resistance. Colloids Surf. A Physicochem. Eng. Asp. 2020, 589, 124469. [Google Scholar] [CrossRef]
- Ruan, M.; Li, W.; Wang, B.; Luo, Q.; Ma, F.; Yu, Z. Optimal conditions for the preparation of superhydrophobic surfaces on al substrates using a simple etching approach. Appl. Surf. Sci. 2012, 258, 7031–7035. [Google Scholar] [CrossRef]
- Chen, Z.; Guo, Y.; Fang, S. A facial approach to fabricate superhydrophobic aluminum surface. Surf. Interface Anal. 2010, 42, 1–6. [Google Scholar] [CrossRef]
- Shi, Y.; Xiao, X.; Zhang, W. Facile fabrication of superhydrophobic surface with needle-like microflower structure on aluminum substrate. J. Coat. Technol. Res. 2015, 12, 1143–1151. [Google Scholar] [CrossRef]
- Dou, W.; Wu, J.; Gu, T.; Wang, P.; Zhang, D. Preparation of super-hydrophobic micro-needle CuO surface as a barrier against marine atmospheric corrosion. Corros. Sci. 2018, 131, 156–163. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Q.; Guo, Z.; Shi, T.; Yu, J.; Tang, M.; Huang, X. Fabrication of superhydrophobic surface with improved corrosion inhibition on 6061 aluminum alloy substrate. Appl. Surf. Sci. 2015, 342, 76–83. [Google Scholar] [CrossRef]
- Feng, L.; Yan, Z.; Qiang, X.; Liu, Y.; Wang, Y. Facile formation of superhydrophobic aluminum alloy surface and corrosion-resistant behavior. Appl. Phys. A Mater. Sci. Process. 2016, 122, 122. [Google Scholar] [CrossRef]
- Fadeev, A.Y.; McCarthy, T.J. Self-assembly is not the only reaction possible between alkyltrichlorosilanes and surfaces: Monomolecular and oligomeric covalently attached layers of dichloro- and trichloroalkylsilanes on silicon. Langmuir 2000, 16, 7268–7274. [Google Scholar] [CrossRef]
- Saleema, N.; Sarkar, D.K.; Paynter, R.W.; Chen, X.-G. Superhydrophobic Aluminum Alloy Surfaces by a Novel One-Step Process. Appl. Mater. Interfaces 2010, 2, 2500–2502. [Google Scholar] [CrossRef]
- Pantoja, M.; Abenojar, J.; Martinez, M.A. Influence of the type of solvent on the development of superhydrophobicity from silane-based solution containing nanoparticles. Appl. Surf. Sci. 2017, 397, 87–94. [Google Scholar] [CrossRef]
- Khodaei, M.; Shadmani, S. Superhydrophobicity on aluminum through reactive-etching and TEOS/GPTMS/nano-Al2O3 silane-based nanocomposite coating. Surf. Coat. Technol. 2019, 374, 1078–1090. [Google Scholar] [CrossRef]
- Calabrese, L.; Khaskhoussi, A.; Patanè, S.; Proverbio, E. Assessment of Super-Hydrophobic Textured Coatings on AA6082 Aluminum Alloy. Coatings 2019, 9, 352. [Google Scholar] [CrossRef]
- Song, X.; Zhai, J.; Wang, Y.; Jiang, L. Fabrication of Superhydrophobic Surfaces by Self-Assembly and Their Water-Adhesion Properties. J. Phys. Chem. B 2005, 109, 4048–4052. [Google Scholar] [CrossRef]
- Gittins, D.I.; Bethell, D.; Nichols, R.J.; Schiffrin, D.J. Diode-like electron transfer across nanostructured films containing a redox ligand. J. Mater. Chem. 2000, 10, 79–83. [Google Scholar] [CrossRef]
- RIZZO, H.F.; BIDWELL, L.R. Formation and Structure of SiB4. J. Am. Ceram. Soc. 1960, 43, 550–552. [Google Scholar] [CrossRef]
- McArthur, E.A.; Ye, T.; Cross, J.P.; Petoud, S.; Borguet, E. Fluorescence Detection of Surface-Bound Intermediates Produced from UV Photoreactivity of Alkylsiloxane SAMs. J. Am. Chem. Soc. 2004, 126, 2260–2261. [Google Scholar] [CrossRef] [PubMed]
- Fadeev, A.Y.; Helmy, R.; Marcinko, S. Self-assembled monolayers of organosilicon hydrides supported on titanium, zirconium, and hafnium dioxides. Langmuir 2002, 18, 7521–7529. [Google Scholar] [CrossRef]
- Yin, L.; Wang, Y.; Ding, J.; Wang, Q.; Chen, Q. Water condensation on superhydrophobic aluminum surfaces with different low-surface-energy coatings. Appl. Surf. Sci. 2012, 258, 4063–4068. [Google Scholar] [CrossRef]
- Feng, L.; Che, Y.; Liu, Y.; Qiang, X.; Wang, Y. Fabrication of superhydrophobic aluminium alloy surface with excellent corrosion resistance by a facile and environment-friendly method. Appl. Surf. Sci. 2013, 283, 367–374. [Google Scholar] [CrossRef]
- Kumar, A.; Gogoi, B. Development of durable self-cleaning superhydrophobic coatings for aluminium surfaces via chemical etching method. Tribol. Int. 2018, 122, 114–118. [Google Scholar] [CrossRef]
- Zhang, B.; Zeng, Y.; Wang, J.; Sun, Y.; Zhang, J.; Li, Y. Superamphiphobic aluminum alloy with low sliding angles and acid-alkali liquids repellency. Mater. Des. 2020, 188, 108479. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, H.; Wang, Z.; Liu, Y. Superhydrophobic aluminum alloy surface: Fabrication, structure, and corrosion resistance. Colloids Surf. A Physicochem. Eng. Asp. 2014, 441, 319–325. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, J.; Yu, X.; Wu, H. Low-cost one-step fabrication of superhydrophobic surface on Al alloy. Appl. Surf. Sci. 2011, 257, 7928–7931. [Google Scholar] [CrossRef]
- Singh, D.D.N.; Chaudhary, R.S.; Agarwal, C.V. Corrosion Characteristics of Some Aluminum Alloys in Nitric Acid. J. Electrochem. Soc. 1982, 129, 1869–1874. [Google Scholar] [CrossRef]
- Qian, B.; Shen, Z. Fabrication of superhydrophobic surfaces by dislocation-selective chemical etching on aluminum, copper, and zinc substrates. Langmuir 2005, 21, 9007–9009. [Google Scholar] [CrossRef]
- Kim, J.H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Realization of superhydrophobic aluminum surfaces with novel micro-terrace nano-leaf hierarchical structure. Appl. Surf. Sci. 2018, 451, 207–217. [Google Scholar] [CrossRef]
- Lee, Y.; Ju, K.Y.; Lee, J.K. Stable biomimetic superhydrophobic surfaces fabricated by polymer replication method from hierarchically structured surfaces of al templates. Langmuir 2010, 26, 14103–14110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.S.; Endrino, J.L.; Anders, A. Comparative surface and nano-tribological characteristics of nanocomposite diamond-like carbon thin films doped by silver. Appl. Surf. Sci. 2008, 255, 2551–2556. [Google Scholar] [CrossRef]
- Horváth, R.; Czifra, Á.; Drégelyi-Kiss, Á. Effect of conventional and non-conventional tool geometries to skewness and kurtosis of surface roughness in case of fine turning of aluminium alloys with diamond tools. Int. J. Adv. Manuf. Technol. 2015, 78, 297–304. [Google Scholar] [CrossRef]
- Kim, D.; Sasidharanpillai, A.; Yun, K.H.; Lee, Y.; Yun, D.J.; Park, W.I.; Bang, J.; Lee, S. Assembly mechanism and the morphological analysis of the robust superhydrophobic surface. Coatings 2019, 9, 472. [Google Scholar] [CrossRef]
- Sun, W.; Wang, L.; Yang, Z.; Li, S.; Wu, T.; Liu, G. Fabrication of polydimethylsiloxane-derived superhydrophobic surface on aluminium via chemical vapour deposition technique for corrosion protection. Corros. Sci. 2017, 128, 176–185. [Google Scholar] [CrossRef]
- Kulkarni, S.A.; Ogale, S.B.; Vijayamohanan, K.P. Tuning the hydrophobic properties of silica particles by surface silanization using mixed self-assembled monolayers. J. Colloid Interface Sci. 2008, 318, 372–379. [Google Scholar] [CrossRef]
- Larkin, P. Infrared and Raman Spectroscopy: Principles and Spectral Interpretation; Elsevier: Oxford, UK, 2011. [Google Scholar]
- Wenzel, R.N. Surface roughness and contact angle. J. Phys. Colloid Chem. 1949, 53, 1466–1467. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Zhang, H.; Yin, L.; Shi, S.; Liu, X.; Wang, Y.; Wang, F. Facile and fast fabrication method for mechanically robust superhydrophobic surface on aluminum foil. Microelectron. Eng. 2015, 141, 238–242. [Google Scholar] [CrossRef]
- Nie, H.Y. Self-assembled monolayers of octadecylphosphonic acid and polymer films: Surface chemistry and chemical structures studied by time-of-flight secondary ion mass spectrometry. Surf. Interface Anal. 2017, 49, 1431–1441. [Google Scholar] [CrossRef]
- Nie, H.Y.; Walzak, M.J.; McIntyre, N.S. Delivering octadecylphosphonic acid self-assembled monolayers on a Si wafer and other oxide surfaces. J. Phys. Chem. B 2006, 110, 21101–21108. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, L.; Bonaccorsi, L.; Caprì, A.; Proverbio, E. Electrochemical behavior of hydrophobic silane–zeolite coatings for corrosion protection of aluminum substrate. J. Coat. Technol. Res. 2014, 11, 883–898. [Google Scholar] [CrossRef]
- Calabrese, L.; Bonaccorsi, L.; Proverbio, E. Corrosion protection of aluminum 6061 in NaCl solution by silane-zeolite composite coatings. J. Coat. Technol. Res. 2012, 9, 597–607. [Google Scholar] [CrossRef]
- Correa-Borroel, A.L.; Gutierrez, S.; Arce, E.; Cabrera-Sierra, R.; Herrasti, P. Organosilanes and polypyrrole as anticorrosive treatment of aluminium 2024. J. Appl. Electrochem. 2009, 39, 2385–2395. [Google Scholar] [CrossRef]
- Olawuni, E.O.; Drowoju, M.O.; Asafa, T.B.; Mudashiru, L.O. Development of Piston Materials from Discarded Aluminum Piston Alloyed with Zirconium Diboride and Snailshells. Int. J. Adv. Sci. Eng. 2018, 5, 818. [Google Scholar] [CrossRef]
- Liu, T.; Chen, S.; Cheng, S.; Tian, J.; Chang, X.; Yin, Y. Corrosion behavior of super-hydrophobic surface on copper in seawater. Electrochim. Acta 2007, 52, 8003–8007. [Google Scholar] [CrossRef]
- Mohamed, A.M.A.; Abdullah, A.M.; Younan, N.A. Corrosion behavior of superhydrophobic surfaces: A review. Arab. J. Chem. 2015, 8, 749–765. [Google Scholar] [CrossRef]
- Liu, C.; Su, F.; Liang, J. Facile fabrication of a robust and corrosion resistant superhydrophobic aluminum alloy surface by a novel method. RSC Adv. 2014, 4, 55556–55564. [Google Scholar] [CrossRef]
- He, T.; Wang, Y.; Zhang, Y.; Lv, Q.; Xu, T.; Liu, T. Super-hydrophobic surface treatment as corrosion protection for aluminum in seawater. Corros. Sci. 2009, 51, 1757–1761. [Google Scholar] [CrossRef]
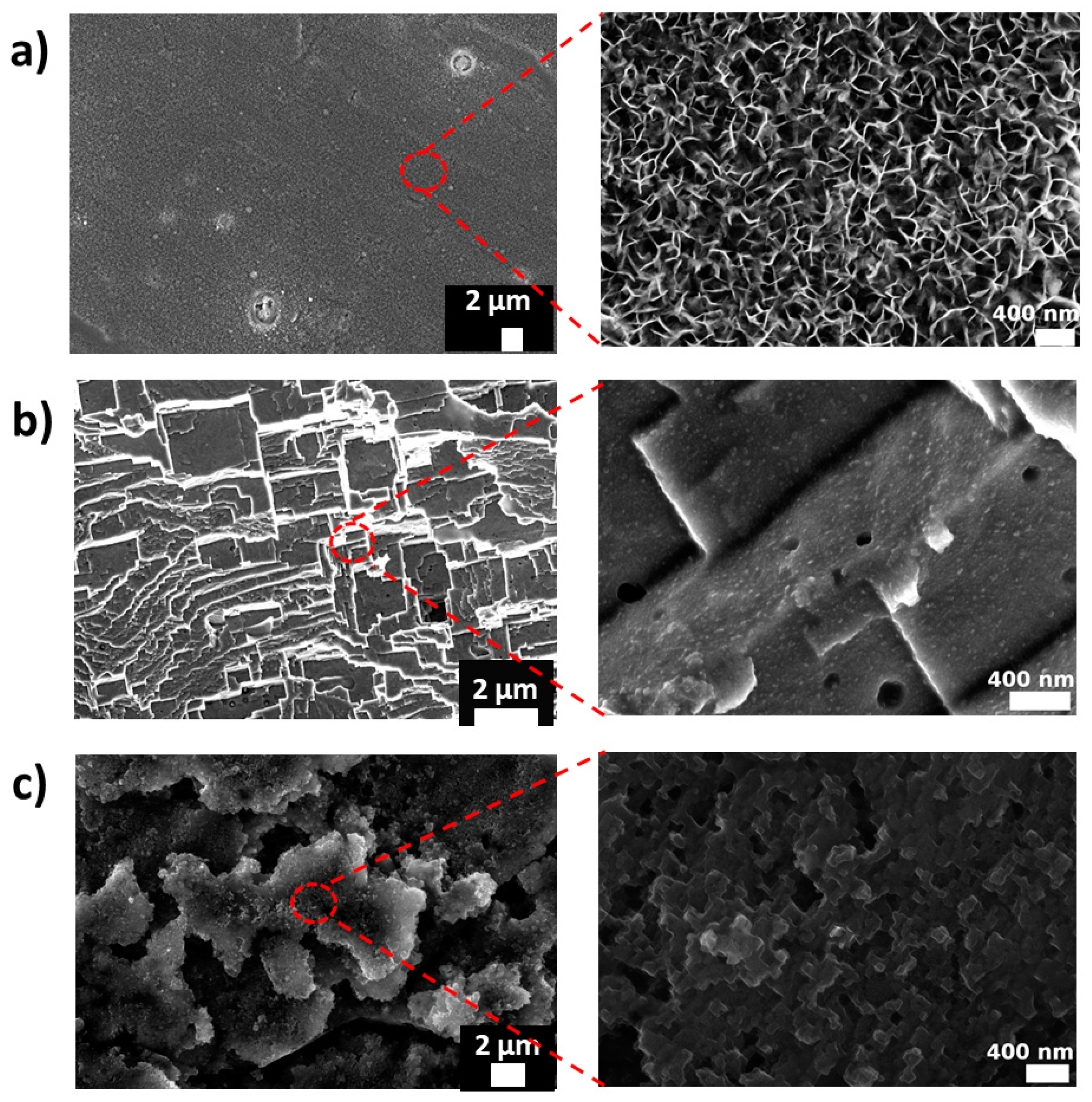
| Code | Step 1 | Step 2 |
|---|---|---|
| A_R | – | – |
| A_RS | – | Silane |
| A_W | Boiling water | – |
| A_WS | Boiling water | Silane |
| A_N | HNO3/HCl | – |
| A_NS | HNO3/HCl | Silane |
| A_F | HF/HCl | – |
| A_FS | HF/HCl | Silane |
| Samples | Arithmetic Roughness Ra (μm) | Root-Mean-Square Roughness Rq (μm) | Skewness (Sku) |
|---|---|---|---|
| A_R | 0.254 | 0.301 | 0.256 |
| A_WS | 0.297 | 0.382 | 1.063 |
| A_NS | 4.151 | 4.800 | −0.783 |
| A_FS | 1.427 | 1.722 | 0.243 |
| Samples | CA (°) | SA (°) |
|---|---|---|
| A_R | 69 ± 3° | – |
| A_RS | 101 ± 2° | 55 ± 6° |
| A_W | 16 ± 2° | – |
| A_WS | 174 ± 3° | >90° |
| A_N | 23 ± 3° | – |
| A_NS | 160 ± 3° | 26 ± 5° |
| A_F | 12 ± 2° | – |
| A_FS | 179 ± 3° | 0° |
| Code | OCP(V) | Ecorr (V vs. SCE) | Icorr (μA/cm2) |
|---|---|---|---|
| A_R | −0.931 | −0.906 | 21.17 |
| A_RS | −0.786 | ||
| A_W | −0.769 | ||
| A_WS | −0.698 | −0.735 | 0.04 |
| A_N | −0.645 | ||
| A_NS | −0.537 | −0.449 | 0.03 |
| A_F | −0.620 | ||
| A_FS | −0.472 | −0.419 | 0.03 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khaskhoussi, A.; Calabrese, L.; Proverbio, E. Superhydrophobic Self-Assembled Silane Monolayers on Hierarchical 6082 Aluminum Alloy for Anti-Corrosion Applications. Appl. Sci. 2020, 10, 2656. https://doi.org/10.3390/app10082656
Khaskhoussi A, Calabrese L, Proverbio E. Superhydrophobic Self-Assembled Silane Monolayers on Hierarchical 6082 Aluminum Alloy for Anti-Corrosion Applications. Applied Sciences. 2020; 10(8):2656. https://doi.org/10.3390/app10082656
Chicago/Turabian StyleKhaskhoussi, Amani, Luigi Calabrese, and Edoardo Proverbio. 2020. "Superhydrophobic Self-Assembled Silane Monolayers on Hierarchical 6082 Aluminum Alloy for Anti-Corrosion Applications" Applied Sciences 10, no. 8: 2656. https://doi.org/10.3390/app10082656
APA StyleKhaskhoussi, A., Calabrese, L., & Proverbio, E. (2020). Superhydrophobic Self-Assembled Silane Monolayers on Hierarchical 6082 Aluminum Alloy for Anti-Corrosion Applications. Applied Sciences, 10(8), 2656. https://doi.org/10.3390/app10082656







