Applications and Limits of Time-to-Energy Mapping of Protein Crystal Diffraction Using Energy-Chirped Polychromatic XFEL Pulses
Abstract
1. Introduction
2. Results
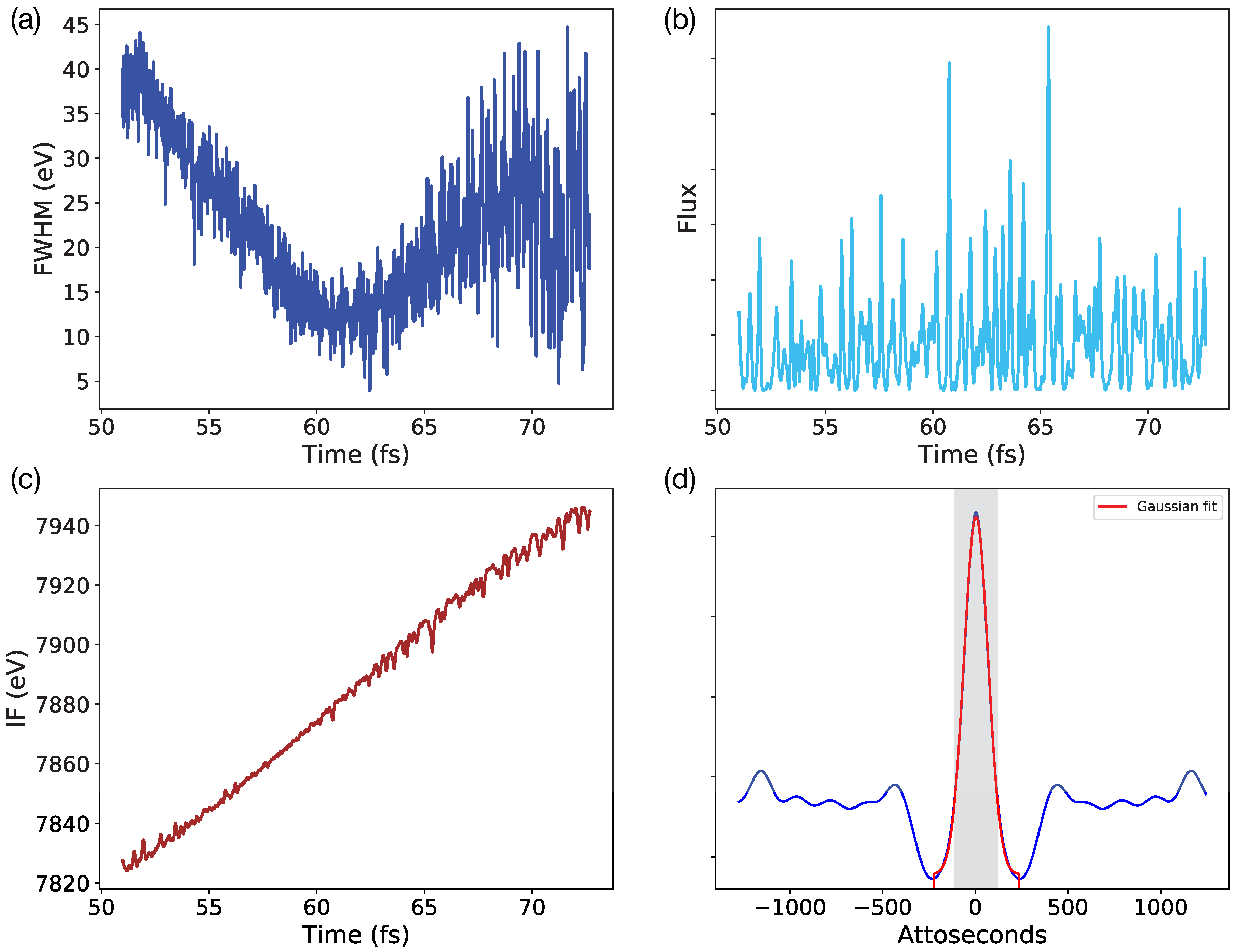
2.1. SwissFEL Chirped Broadband Simulations
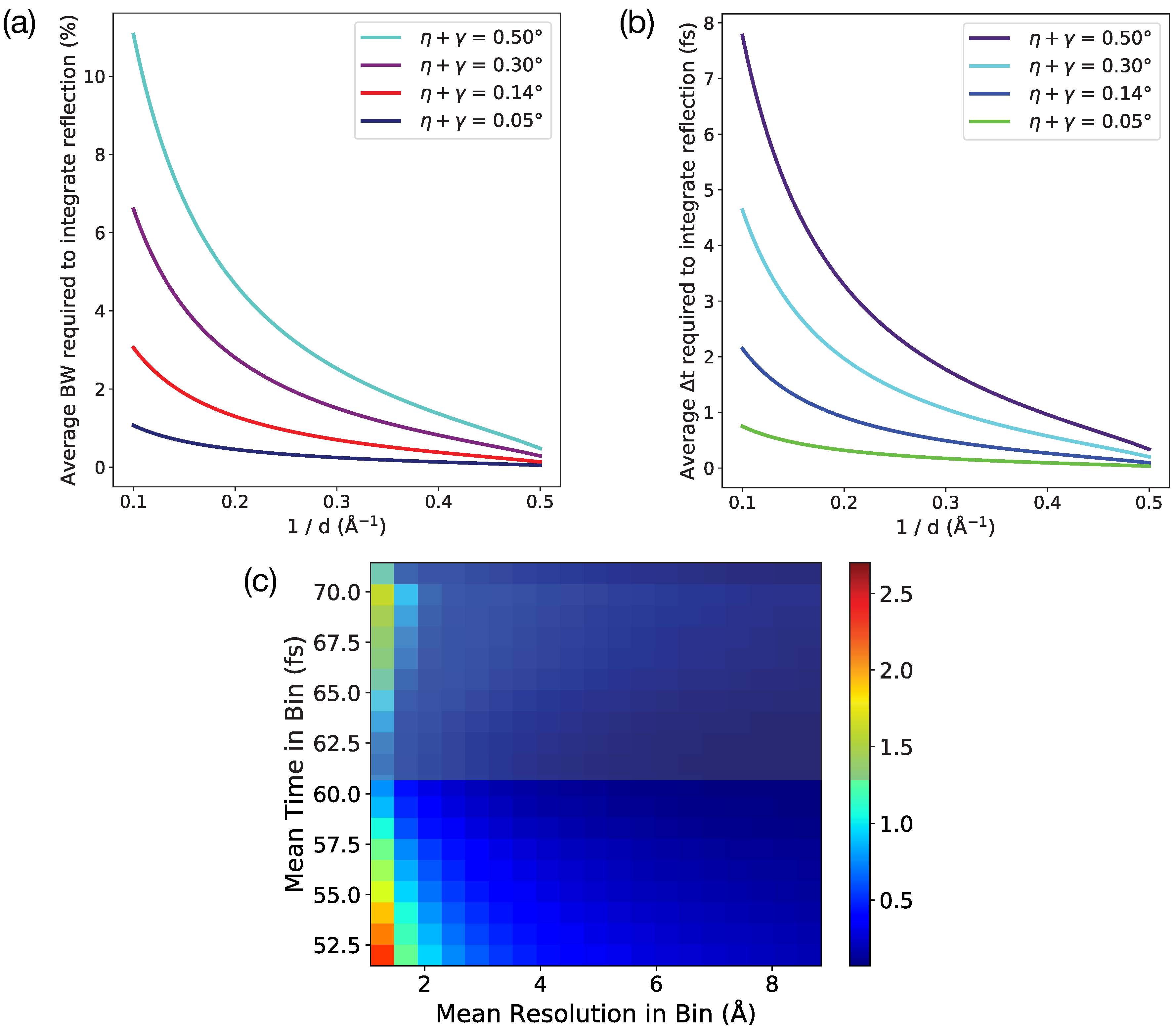
2.2. Determination of
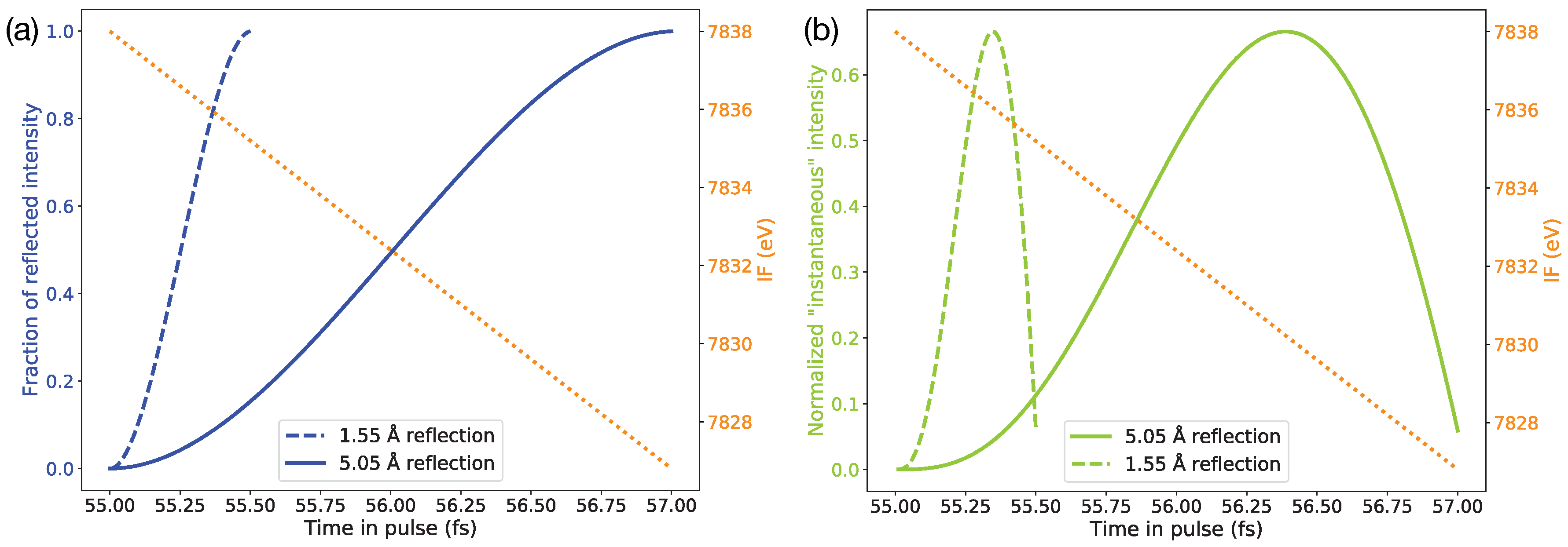
2.3. Ray-Tracing Simulation and Time Mapping
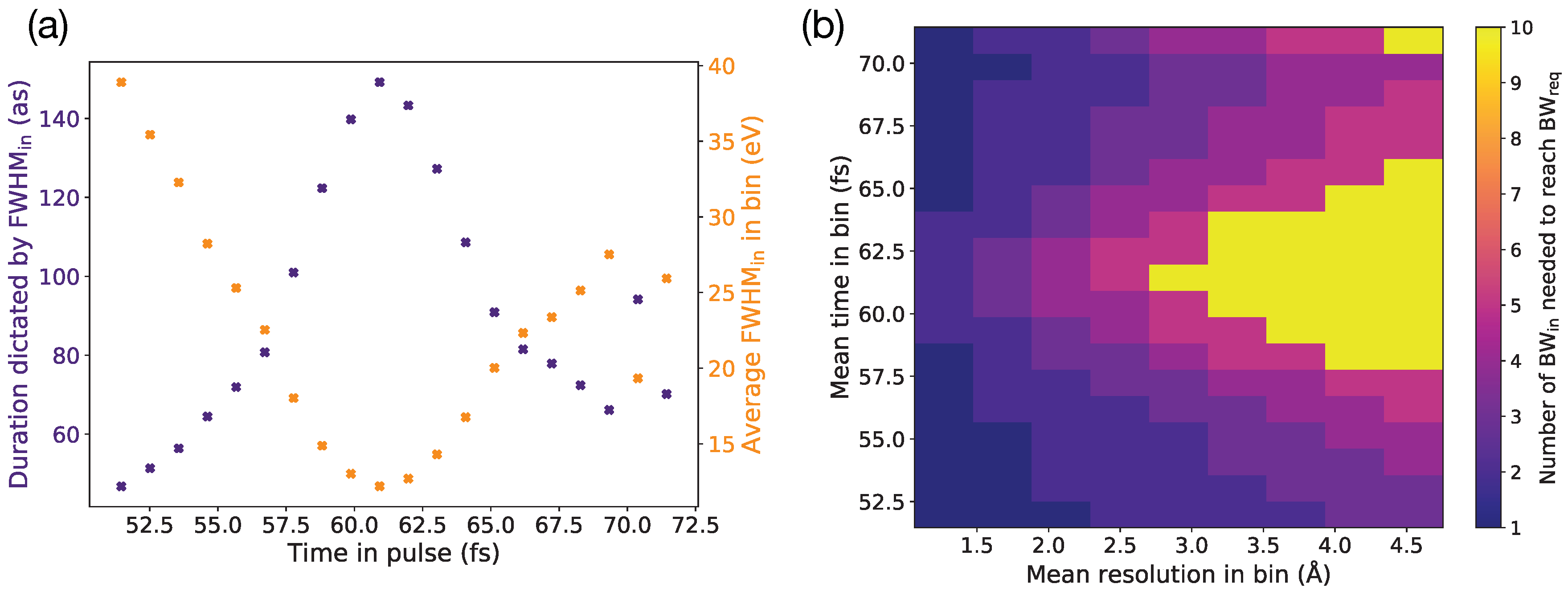
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pande, K.; Hutchison, C.D.; Groenhof, G.; Aquila, A.; Robinson, J.S.; Tenboer, J.; Basu, S.; Boutet, S.; DePonte, D.P.; Liang, M.; et al. Femtosecond structural dynamics drives the trans/cis isomerization in photoactive yellow protein. Science 2016, 352, 725–729. [Google Scholar] [CrossRef] [PubMed]
- Young, I.D.; Ibrahim, M.; Chatterjee, R.; Gul, S.; Fuller, F.D.; Koroidov, S.; Brewster, A.S.; Tran, R.; Alonso-Mori, R.; Kroll, T.; et al. Structure of photosystem II and substrate binding at room temperature. Nature 2016, 540, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Suga, M.; Akita, F.; Sugahara, M.; Kubo, M.; Nakajima, Y.; Nakane, T.; Yamashita, K.; Umena, Y.; Nakabayashi, M.; Yamane, T.; et al. Light-induced structural changes and the site of O=O bond formation in PSII caught by XFEL. Nature 2017, 543, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Coquelle, N.; Sliwa, M.; Woodhouse, J.; Schirò, G.; Adam, V.; Aquila, A.; Barends, T.R.; Boutet, S.; Byrdin, M.; Carbajo, S.; et al. Chromophore twisting in the excited state of a photoswitchable fluorescent protein captured by time-resolved serial femtosecond crystallography. Nat. Chem. 2018, 10, 31–37. [Google Scholar] [CrossRef]
- Arnlund, D.; Johansson, L.C.; Wickstrand, C.; Barty, A.; Williams, G.J.; Malmerberg, E.; Davidsson, J.; Milathianaki, D.; DePonte, D.P.; Shoeman, R.L.; et al. Visualizing a protein quake with time-resolved X-ray scattering at a free-electron laser. Nat. Methods 2014, 11, 923–926. [Google Scholar] [CrossRef]
- Nango, E.; Royant, A.; Kubo, M.; Nakane, T.; Wickstrand, C.; Kimura, T.; Tanaka, T.; Tono, K.; Song, C.; Tanaka, R.; et al. A three-dimensional movie of structural changes in bacteriorhodopsin. Science 2016, 354, 1552–1557. [Google Scholar] [CrossRef]
- Nogly, P.; Weinert, T.; James, D.; Carbajo, S.; Ozerov, D.; Furrer, A.; Gashi, D.; Borin, V.; Skopintsev, P.; Jaeger, K.; et al. Retinal isomerization in bacteriorhodopsin captured by a femtosecond X-ray laser. Science 2018, 361, 1–15. [Google Scholar] [CrossRef]
- Dunne, M. LCLS Strategic Facility Development Plan; Technical Report; SLAC National Accelerator Laboratory: Menlo Park, CA, USA, 2019. [Google Scholar]
- Schneidmiller, E.A.; Yurkov, M. Photon Beam Properties at the European XFEL; Technical Report Report No. XFEL.EU TR-2011-006; Deutsches Elektronen-Synchrotron (DESY): Hamburg, Germany, 2011. [Google Scholar] [CrossRef]
- Yabashi, M.; Tanaka, H.; Ishikawa, T. Overview of the SACLA facility. J. Synchrotron Radiat. 2015, 22, 477–484. [Google Scholar] [CrossRef]
- Madey, J.M. Stimulated emission of bremsstrahlung in a periodic magnetic field. J. Appl. Phys. 1971, 42, 1906–1913. [Google Scholar] [CrossRef]
- Deacon, D.A.G.; Elias, L.R.; Madey, J.M.J.; Ramian, G.J.; Schwettman, H.A.; Smith, T.I. First Operation of a Free-Electron Laser. Phys. Rev. Lett. 1977, 38, 892–894. [Google Scholar] [CrossRef]
- Bonifacio, R.; Pellegrini, C.; Narducci, L.M. Collective instabilities and high-gain regime in a free electron laser. Opt. Commun. 1984, 50, 373–378. [Google Scholar] [CrossRef]
- Kondratenko, A.M.; Saldin, E.L. Generation of Coherent Radiation by a Relativistic Electron Beam in an Ondulator. Part. Accel. Print 1980, 10, 207–216. [Google Scholar]
- Kim, K.J. Three-Dimensional Analysis of Coherent Amplification and Self-Amplified Spontaneous Emission in Free-Electron Lasers. Phys. Rev. Lett. 1986, 57, 1871–1874. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.Y.; Kang, H.S.; Choi, J. Coherence and saturation of self-amplified spontaneous emission free electron laser. Jpn. J. Appl. Phys. Part 1 Regul. Pap. Short Notes Rev. Pap. 2007, 46, 3120–3122. [Google Scholar] [CrossRef]
- Chapman, H.N.; Fromme, P.; Barty, A.; White, T.A.; Kirian, R.A.; Aquila, A.; Hunter, M.S.; Schulz, J.; Deponte, D.P.; Weierstall, U.; et al. Femtosecond X-ray protein nanocrystallography. Nature 2011, 470, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Kirian, R.A.; Wang, X.; Weierstall, U.; Schmidt, K.E.; Spence, J.C.H.; Hunter, M.; Fromme, P.; White, T.; Chapman, H.N.; Holton, J. Femtosecond protein nanocrystallography—Data analysis methods. Opt. Express 2010, 18, 5713–5723. [Google Scholar] [CrossRef]
- White, T.A.; Kirian, R.A.; Martin, A.V.; Aquila, A.; Nass, K.; Barty, A.; Chapman, H.N. CrystFEL: A software suite for snapshot serial crystallography. J. Appl. Cryst. 2012, 45, 335–341. [Google Scholar] [CrossRef]
- Rossmann, M.G.; Leslie, A.G.W.; Abdel-Meguid, S.S.; Tsukihara, T.; IUCr. Processing and post-refinement of oscillation camera data. J. Appl. Crystallogr. 1979, 12, 570–581. [Google Scholar] [CrossRef]
- Winkler, F.K.; Schutt, C.E.; Harrison, S.C. The oscillation method for crystals with very large unit cells. Acta Crystallogr. Sect. A 1979, 35, 901–911. [Google Scholar] [CrossRef]
- White, T.A. Post-refinement method for snapshot serial crystallography. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130330. [Google Scholar] [CrossRef]
- Kabsch, W. Processing of X-ray snapshots from crystals in random orientations. Acta Crystallogr. Sect. D Biol. Crystallogr. 2014, 70, 2204–2216. [Google Scholar] [CrossRef] [PubMed]
- Ginn, H.M.; Brewster, A.S.; Hattne, J.; Evans, G.; Wagner, A.; Grimes, J.M.; Sauter, N.K.; Sutton, G.; Stuart, D.I. A revised partiality model and post-refinement algorithm for X-ray free-electron laser data. Acta Crystallogr. Sect. D Biol. Crystallogr. 2015, 71, 1400–1410. [Google Scholar] [CrossRef] [PubMed]
- White, T.A.; Mariani, V.; Brehm, W.; Yefanov, O.; Barty, A.; Beyerlein, K.R.; Chervinskii, F.; Galli, L.; Gati, C.; Nakane, T.; et al. Recent developments in CrystFEL. J. Appl. Crystallogr. 2016, 49, 680–689. [Google Scholar] [CrossRef]
- Ginn, H.M.; Evans, G.; Sauter, N.K.; Stuart, D.I. On the release of cppxfel for processing X-ray free-electron laser images. J. Appl. Crystallogr. 2016, 49, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Sauter, N.K. XFEL diffraction: Developing processing methods to optimize data quality. J. Synchrotron Radiat. 2015, 22, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Meents, A.; Wiedorn, M.O.; Srajer, V.; Henning, R.; Sarrou, I.; Bergtholdt, J.; Barthelmess, M.; Reinke, P.Y.A.; Dierksmeyer, D.; Tolstikova, A.; et al. Pink-beam serial crystallography. Nat. Commun. 2017, 8, 1281. [Google Scholar] [CrossRef]
- Tolstikova, A.; Levantino, M.; Yefanov, O.; Hennicke, V.; Fischer, P.; Meyer, J.; Mozzanica, A.; Redford, S.; Crosas, E.; Opara, N.L.; et al. 1 kHz fixed-target serial crystallography using a multilayer monochromator and an integrating pixel detector. IUCrJ 2019, 6, 927–937. [Google Scholar] [CrossRef]
- Hernandez, A.S.; Prat, E.; Bettoni, S.; Beutner, B.; Reiche, S. Generation of large-bandwidth X-ray free-electron-laser pulses. Phys. Rev. Accel. Beams 2016, 19, 1–9. [Google Scholar] [CrossRef]
- Prat, E.; Dijkstal, P.; Ferrari, E.; Reiche, S. Demonstration of Large Bandwidth Hard X-Ray Free-Electron Laser Pulses at SwissFEL. Phys. Rev. Lett. 2020, 124, 074801. [Google Scholar] [CrossRef]
- Guetg, M.W.; Bane, K.L.F.; Brachmann, A.; Fisher, A.S.; Harrison, M.A.; Huang, Z.; Krejcik, P.; Lutman, A.A.; Maxwell, T.J.; Novokhatski, A.; et al. Commissioning of the RADIABEAM/SLAC dechirper. In Proceedings of the IPAC2016, Busan, Korea, 8–13 May 2016; pp. 1–4. [Google Scholar]
- Lutman, A.A.; Bane, K.; Ding, Y.; Emma, C.; Guetg, M.W.; Guo, Z.; Hemsing, E.; Huang, Z.; Krzywinski, J.; Macarthur, J.P.; et al. XFEL Operational Flexibility due to the Dechirper System. In Proceedings of the 10th International Partile Accelerator Conference (IPAC2019), Melbourne, Australia, 19–24 May 2019; pp. 2219–2223. [Google Scholar] [CrossRef]
- Moffat, K. The frontiers of time-resolved macromolecular crystallography: Movies and chirped X-ray pulses. Faraday Discuss. 2002, 122, 65–77. [Google Scholar] [CrossRef]
- Reiche, S. GENESIS 1.3: A fully 3D time-dependent FEL simulation code. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 1999, 429, 243–248. [Google Scholar] [CrossRef]
- Saldin, E.L.; Schneidmiller, E.A.; Yurkov, M.V. FAST: A three-dimensional time-dependent FEL simulation code. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 1999, 429, 233–237. [Google Scholar] [CrossRef]
- Campbell, L.T.; McNeil, B.W. Puffin: A three dimensional, unaveraged free electron laser simulation code. Phys. Plasmas 2012, 19. [Google Scholar] [CrossRef]
- Tanaka, T. SIMPLEX: Simulator and postprocessor for free-electron laser experiments. J. Synchrotron Radiat. 2015, 22, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.; Wurtele, J. TDA—A three-dimensional axisymmetric code for free-electron-laser (FEL) simulation. Comput. Phys. Commun. 1989, 54, 263–272. [Google Scholar] [CrossRef]
- Reiche, S. Update on the FEL code Genesis 1.3. In Proceedings of the 36th International Free-Electron Laser Conference, Basel, Switzerland, 25–28 August 2014; p. 403. [Google Scholar]
- Dejoie, C.; Mccusker, L.B.; Baerlocher, C.; Abela, R.; Patterson, B.D.; Kunz, M.; Tamura, N. Using a non-monochromatic microbeam for serial snapshot crystallography. J. Appl. Cryst. 2013, 46, 791–794. [Google Scholar] [CrossRef]
- Lovell, B.C.; Boashash, B.; Williamson, R.C. The Relationship Between Instantaneous Frequency and Time-Frequency Representations. IEEE Trans. Signal Process. 1993, 41, 1458–1461. [Google Scholar] [CrossRef][Green Version]
- Stankovic, L. A Method for Time-Frequency Analysis. IEEE Trans. Signal Process. 1994, 42, 225–229. [Google Scholar] [CrossRef]
- Ville, J. Theorie et Application de la Notion de Signal Analytic; Cables et Transmissions: Paris, France, 1948; pp. 61–74. [Google Scholar]
- Wigner, E. On the quantum correction for thermodynamic equilibrium. Phys. Rev. 1932, 40, 749–759. [Google Scholar] [CrossRef]
- Cohen, L. Instantaneous bandwidth. Proc. SPIE 1992, 9476, 1168–1171. [Google Scholar] [CrossRef]
- Hassan, M.T.; Luu, T.T.; Moulet, A.; Raskazovskaya, O.; Zhokhov, P.; Garg, M.; Karpowicz, N.; Zheltikov, A.M.; Pervak, V.; Krausz, F.; et al. Optical attosecond pulses and tracking the nonlinear response of bound electrons. Nature 2016, 530, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Ding, Y.; Feng, Y.; Hemsing, E.; Huang, Z.; Krzywinski, J.; Lutman, A.A.; Marinelli, A.; Maxwell, T.J.; Zhu, D. Generating Single-Spike Hard X-Ray Pulses with Nonlinear Bunch Compression in Free-Electron Lasers. Phys. Rev. Lett. 2017, 119, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Li, B. X-Ray Photon Temporal Diagnostics for the European XFEL; Technical Report; European X-Ray Free-Electron Laser Facility GmbH: Hamburg, Germany, 2012. [Google Scholar]
- Helliwell, J.R. Single-crystal X-ray techniques. In International Tables for Crystallography, 3rd ed.; Prince, E., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands; Boston, MA, USA; London, UK, 2004; Chapter 2.2; Volume C, pp. 26–41. [Google Scholar]
- Inubushi, Y.; Tono, K.; Togashi, T.; Sato, T.; Hatsui, T.; Kameshima, T.; Togawa, K.; Hara, T.; Tanaka, T.; Tanaka, H.; et al. Determination of the Pulse Duration of an X-Ray Free Electron Laser Using Highly Resolved Single-Shot Spectra. Phys. Rev. Lett. 2012, 109, 144801. [Google Scholar] [CrossRef] [PubMed]
- Altarelli, M.; Brinkmann, R.; Chergui, M.; Decking, W.; Dobson, B.; Düsterer, S.; Grübel, G.; Graeff, W.; Graafsma, H.; Hajdu, J.; et al. The European X-Ray Free-Electron Laser Technical Design Report X FEL X-Ray Free-Ele Ctron Laser; Technical Report; Deutsches Elektronen-Synchrotron: Hamburg, Germany, 2007. [Google Scholar]
- Follath, R.; Flechsig, U.; Milne, C.; Szlachetko, J.; Ingold, G.; Patterson, B.; Patthey, L.; Abela, R. Optical design of the ARAMIS-beamlines at SwissFEL. AIP Conf. Proc. 2016, 1741, 020009. [Google Scholar] [CrossRef]
- Liang, M.; Williams, G.J.; Messerschmidt, M.; Seibert, M.M.; Montanez, P.A.; Hayes, M.; Milathianaki, D.; Aquila, A.; Hunter, M.S.; Koglin, J.E.; et al. The Coherent X-ray Imaging instrument at the Linac Coherent Light Source. J. Synchrotron Radiat. 2015, 22, 514–519. [Google Scholar] [CrossRef]
- Sierra, R.G.; Batyuk, A.; Sun, Z.; Aquila, A.; Hunter, M.S.; Lane, T.J.; Liang, M.; Yoon, C.H.; Alonso-Mori, R.; Armenta, R.; et al. The Macromolecular Femtosecond Crystallography Instrument at the Linac Coherent Light Source. J. Synchrotron Radiat. 2019, 26, 346–357. [Google Scholar] [CrossRef]
- Van Thor, J.J.; Warren, M.M.; Lincoln, C.N.; Chollet, M.; Lemke, H.T.; Fritz, D.M.; Schmidt, M.; Tenboer, J.; Ren, Z.; Srajer, V.; et al. Signal to noise considerations for single crystal femtosecond time resolved crystallography of the Photoactive Yellow Protein. Faraday Discuss. 2014, 171, 439–455. [Google Scholar] [CrossRef]
- Diederichs, K. Simulation of X-ray frames from macromolecular crystals using a ray-tracing approach. Acta Crystallogr. Sect. D Biol. Crystallogr. 2009, 65, 535–542. [Google Scholar] [CrossRef]
- Van Thor, J.J.; Madsen, A. A split-beam probe-pump-probe scheme for femtosecond time resolved protein X-ray crystallography. Struct. Dyn. 2015, 2, 014102. [Google Scholar] [CrossRef]
- Helliwell, J.R.; Habash, J.; Cruickshank, D.W.J.; Harding, M.M.; Greenhough, T.J.; Campbell, J.W.; Clifton, I.J.; Elder, M.; Machin, P.A.; Papiz, M.Z.; et al. The recording and analysis of synchrotron X-radiation Laue diffraction photographs. J. Appl. Crystallogr. 1989, 22, 483–497. [Google Scholar] [CrossRef]
- Szebenyi, D.M.E.; Bilderback, D.H.; LeGrand, A.; Moffat, K.; Schildkamp, W.; Smith Temple, B.; Teng, T. Quantitive analysis of Laue diffraction patterns recorded with a 120 ps exposure from an X-ray undulator. J. Appl. Crystallogr. 1992, 25, 414–423. [Google Scholar] [CrossRef]
- Ren, Z.; Moffat, K. Deconvolution of Energy Overlaps in Laue Diffraction. J. Appl. Crystallogr. 1995, 28, 482–494. [Google Scholar] [CrossRef]
- Arzt, S.; Campbell, J.W.; Harding, M.M.; Hao, Q.; Helliwell, J.R. LSCALE—The new normalization, scaling and absorption correction program in the Daresbury Laue software suite. J. Appl. Crystallogr. 1999, 32, 554–562. [Google Scholar] [CrossRef]
- Šrajer, V.; Crosson, S.; Schmidt, M.; Key, J.; Schotte, F.; Anderson, S.; Perman, B.; Ren, Z.; Teng, T.; Bourgeois, D.; et al. Extraction of accurate structure-factor amplitudes from Laue data: Wavelength normalization with wiggler and undulator X-ray sources. J. Synchrotron Radiat. 2000, 7, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Gonzalez, A.; Johnson, A.S.; Fitzpatrick, A.; Hutchison, C.D.M.; Fare, C.; Cordon-Preciado, V.; Dorlhiac, G.; Ferreira, J.L.; Morgan, R.M.; Marangos, J.P.; et al. Coincidence timing of femtosecond optical pulses in an X-ray free electron laser. J. Appl. Phys. 2017, 122, 203105. [Google Scholar] [CrossRef]
- Harmand, M.; Coffee, R.; Bionta, M.R.; Chollet, M.; French, D.; Zhu, D.; Fritz, D.M.; Lemke, H.T.; Medvedev, N.; Ziaja, B.; et al. Achieving few-femtosecond time-sorting at hard X-ray free-electron lasers. Nat. Photonics 2013, 7, 215–218. [Google Scholar] [CrossRef]
- Lemke, H.T.; Weaver, M.; Chollet, M.; Robinson, J.; Glownia, J.M.; Zhu, D.; Bionta, M.R.; Cammarata, M.; Harmand, M.; Coffee, R.N.; et al. Femtosecond optical/hard X-ray timing diagnostics at an FEL: Implementation and performance. Adv. X Ray Free Electron Lasers II Instrum. 2013, 8778, 87780S. [Google Scholar] [CrossRef]
- Nisoli, M.; De Silvestri, S.; Svelto, O. Generation of high energy 10 fs pulses by a new pulse compression technique. Appl. Phys. Lett. 1996, 68, 2793–2795. [Google Scholar] [CrossRef]
- Böhle, F.; Kretschmar, M.; Jullien, A.; Kovacs, M.; Miranda, M.; Romero, R.; Crespo, H.; Morgner, U.; Simon, P.; Lopez-Martens, R.; et al. Compression of CEP-stable multi-mJ laser pulses down to 4 fs in long hollow fibers. Laser Phys. Lett. 2014, 11, 6–11. [Google Scholar] [CrossRef]
- Jacqmin, H.; Jullien, A.; Mercier, B.; Hanna, M.; Druon, F.; Papadopoulos, D.; Lopez-Martens, R. Passive coherent combining of CEP-stable few-cycle pulses from a temporally divided hollow fiber compressor. Opt. Lett. 2015, 40, 709–712. [Google Scholar] [CrossRef]
- Hwang, S.I.; Park, S.B.; Mun, J.; Cho, W.; Nam, C.H.; Kim, K.T. Generation of a single-cycle pulse using a two-stage compressor and its temporal characterization using a tunnelling ionization method. Sci. Rep. 2019, 9, 1613. [Google Scholar] [CrossRef] [PubMed]
- Eilzer, S.; Wedel, B. Hollow core optical fibers for industrial ultra short pulse laser beam delivery applications. Fibers 2018, 6, 80. [Google Scholar] [CrossRef]
- Ciappina, M.F.; Perez-Hernandez, J.A.; Landsman, A.S.; Okell, W.A.; Zherebtsov, S.; Forg, B.; Schotz, J.; Seiffert, L.; Fennel, T.; Shaaran, T.; et al. Attosecond physics at the nanoscale. Rep. Prog. Phys. 2017, 80, 054401. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, C.S.; Silva, A.S.; Navas, D.; Miranda, M.; Silva, F.; Crespo, H.; Schmool, D.S. A Dual-Colour Architecture for Pump-Probe Spectroscopy of Ultrafast Magnetization Dynamics in the Sub-10-femtosecond Range. Sci. Rep. 2016, 6, 22872. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.T.; Zürch, M.; Kraus, P.M.; Borja, L.J.; Neumark, D.M.; Leone, S.R. Simultaneous generation of sub-5-femtosecond 400 nm and 800 nm pulses for attosecond extreme ultraviolet pump–probe spectroscopy. Opt. Lett. 2016, 41, 5365. [Google Scholar] [CrossRef]
- Kohmoto, T.; Fukui, Y.; Furue, S.; Nakayama, K.; Fukuda, Y. Propagation of femtosecond light pulses in a dye solution: Nonadherence to the conventional group velocity. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2006, 74, 1–5. [Google Scholar] [CrossRef]
- Hutchison, C.D.; Van Thor, J.J. Populations and coherence in femtosecond time resolved X-ray crystallography of the photoactive yellow protein. Int. Rev. Phys. Chem. 2017, 36, 117–143. [Google Scholar] [CrossRef]
- Walmsley, I.; Waxer, L.; Dorrer, C. The role of dispersion in ultrafast optics. Rev. Sci. Instrum. 2001, 72, 1–29. [Google Scholar] [CrossRef]
- VanEngen Spivey, A.G.; Seid, N. Group velocity dispersion of dyes in solution measured with white-light interferometry. Appl. Opt. 2011, 50, 194–202. [Google Scholar] [CrossRef][Green Version]
- Coello, Y.; Xu, B.; Miller, T.L.; Lozovoy, V.V.; Dantus, M. Group-velocity dispersion measurements of water, seawater, and ocular components using multiphoton intrapulse interference phase scan. Appl. Opt. 2007, 46, 8394–8401. [Google Scholar] [CrossRef]
- Roessler, C.; Agarwal, R.; Allaire, M.; Alonso-Mori, R.; Andi, B.; Bachega, J.; Bommer, M.; Brewster, A.; Browne, M.; Chatterjee, R.; et al. Acoustic Injectors for Drop-On-Demand Serial Femtosecond Crystallography. Structure 2016, 24, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Cole, T.; Kathman, A.; Koszelak, S.; Mcpherson, A. Determination of Local Refractive Index for Protein and Virus Crystals in Solution by Mach-Zehnder Interferometry. Anal. Biochem. 1995, 231, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Fredericks, W.; Hammonds, M.; Howard, S.; Rosenberger, F. Density, thermal expansivity, viscosity and refractive index of lysozyme solutions at crystal growth concentrations. J. Cryst. Growth 1994, 141, 183–192. [Google Scholar] [CrossRef]
- Polynkin, P.; Kolesik, M. Critical power for self-focusing in the case of ultrashort laser pulses. Phys. Rev. A 2013, 87, 53829. [Google Scholar] [CrossRef]
- Christov, I.P.; Kapteyn, H.C.; Murnane, M.M.; Huang, C.P.; Zhou, J. Space–time focusing of femtosecond pulses in a Ti:sapphire laser. Opt. Lett. 1995, 20, 309. [Google Scholar] [CrossRef] [PubMed]
- Zozulya, A.A.; Diddams, S.A. Dynamics of self-focused femtosecond laser pulses in the near and far fields. Opt. Express 1999, 4, 336. [Google Scholar] [CrossRef]
- Crego, A.; Conejero Jarque, E.; San Roman, J. Influence of the spatial confinement on the self-focusing of ultrashort pulses in hollow-core fibers. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Kandidov, V.; Dormidonov, A.; Kosareva, O.; Chin, S.; Liu, W. Self-focusing and Filamentation of Powerful Femtosecond Laser Pulses. Top. Appl. Phys. 2008, 114, 413–438. [Google Scholar] [CrossRef]
- Gevorkov, Y.; Barty, A.; Brehm, W.; White, T.A.; Tolstikova, A.; Wiedorn, M.O.; Meents, A.; Grigat, R.R.; Chapman, H.N.; Yefanov, O. pinkIndexer—A universal indexer for pink-beam X-ray and electron diffraction snapshots. Acta Crystallogr. Sect. A Found. Adv. 2020, 76. [Google Scholar] [CrossRef]




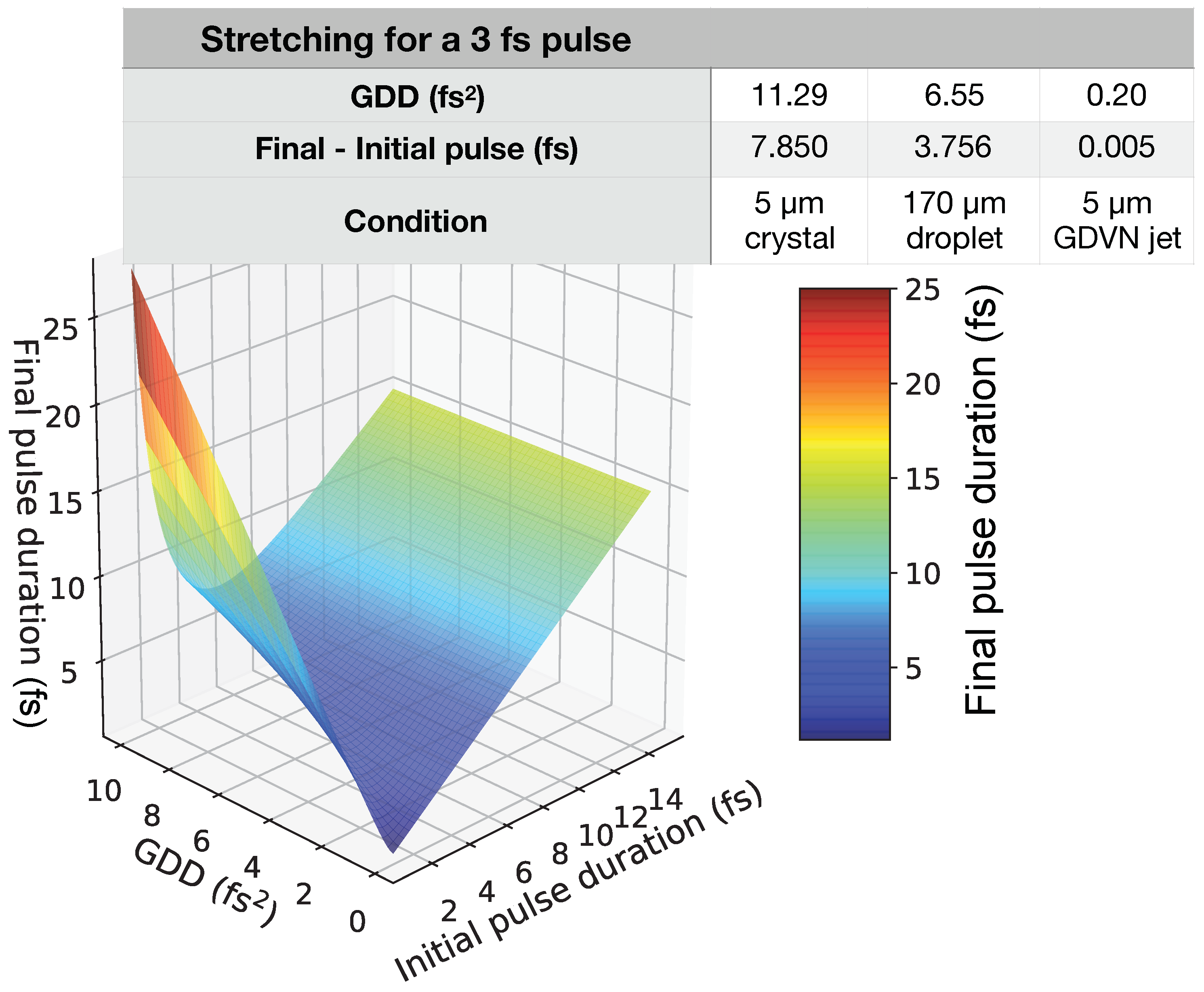
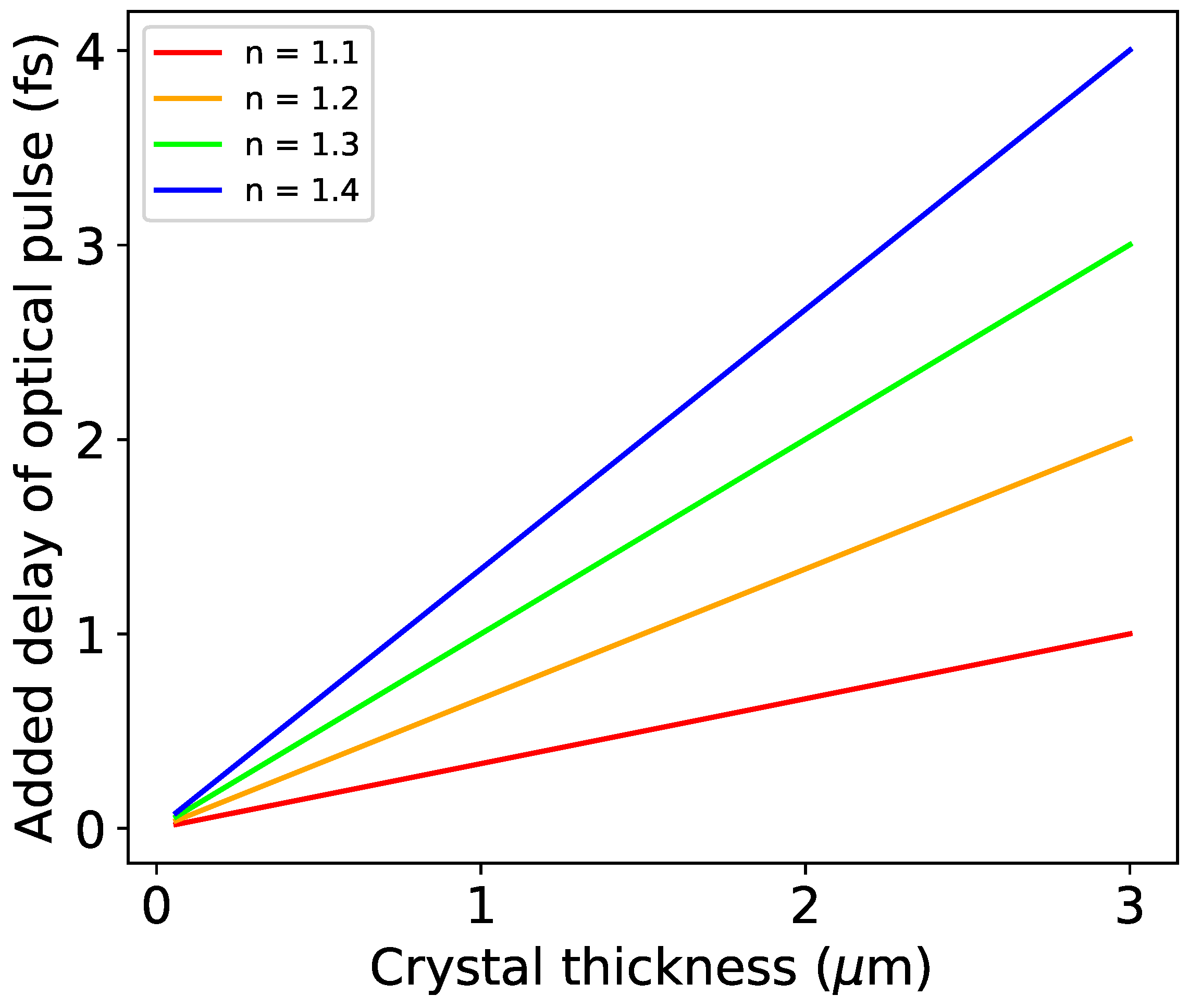
| Input Parameter | Value | |||
|---|---|---|---|---|
| UNIT_CELL_A-AXIS | −41.745625 | −19.906780 | 47.598270 | |
| UNIT_CELL_B-AXIS | 24.133776 | −43.948139 | −43.482101 | |
| UNIT_CELL_C-AXIS | 31.530138 | −7.105332 | 24.681583 | |
| EXPOSURE FACTOR | 1 | |||
| GAIN | 1 | |||
| BACKGROUND | 20 | |||
| BIG_CRYSTAL | TRUE | |||
| BEAM_STDDEV | 0.1 | 0.1 | ||
| CELL_STDDEV | 0.04 | 0.04 | 0.04 | |
| ORIENTATION_STDDEV | 0.04 | 0.04 | 0.04 | |
| OSCILLATION_RANGE | 0.01 | |||
| DETECTOR_DISTANCE | 59.1 mm | |||
| INCIDENT_BEAM_DIRECTION | 0.0 | 0.008727 | 0.99999619 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fadini, A.; Reiche, S.; Nass, K.; van Thor, J.J. Applications and Limits of Time-to-Energy Mapping of Protein Crystal Diffraction Using Energy-Chirped Polychromatic XFEL Pulses. Appl. Sci. 2020, 10, 2599. https://doi.org/10.3390/app10072599
Fadini A, Reiche S, Nass K, van Thor JJ. Applications and Limits of Time-to-Energy Mapping of Protein Crystal Diffraction Using Energy-Chirped Polychromatic XFEL Pulses. Applied Sciences. 2020; 10(7):2599. https://doi.org/10.3390/app10072599
Chicago/Turabian StyleFadini, Alisia, Sven Reiche, Karol Nass, and Jasper J. van Thor. 2020. "Applications and Limits of Time-to-Energy Mapping of Protein Crystal Diffraction Using Energy-Chirped Polychromatic XFEL Pulses" Applied Sciences 10, no. 7: 2599. https://doi.org/10.3390/app10072599
APA StyleFadini, A., Reiche, S., Nass, K., & van Thor, J. J. (2020). Applications and Limits of Time-to-Energy Mapping of Protein Crystal Diffraction Using Energy-Chirped Polychromatic XFEL Pulses. Applied Sciences, 10(7), 2599. https://doi.org/10.3390/app10072599





