Green Pepper (Piper nigrum L.) Extract Suppresses Oxidative Stress and LPS-Induced Inflammation via Regulation of JNK Signaling Pathways
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction
2.3. Color
2.4. High-Performance Liquid Chromatography-Diode Array Detector (HPLC-DAD) Analysis
2.5. Chlorophyll Contents
2.6. Total Flavonoids and Total Phenolics
2.7. Radical Scavenging Effect
2.8. Cell Culture
2.9. Cell Viability
2.10. Nitrite Assay
2.11. Western Blot Analysis
2.12. Immunofluorescence
2.13. Statistical Analysis
3. Results
3.1. Color and Chlorophyll Content Analysis
3.2. Total Flavonoids and Total Phenolic Contents and Radical Scavenging Effects
3.3. Quantification of the Major Compounds in Pepper by HPLC
3.4. Effect of GPEs on LPS-Induced Nitrite Production, iNOS Expression, and Cell Viability of RAW 264.7 Cells
3.5. Effect of GPEs on LPS-Induced NF-κB and MAPK Signaling Pathways in RAW 264.7 Cells
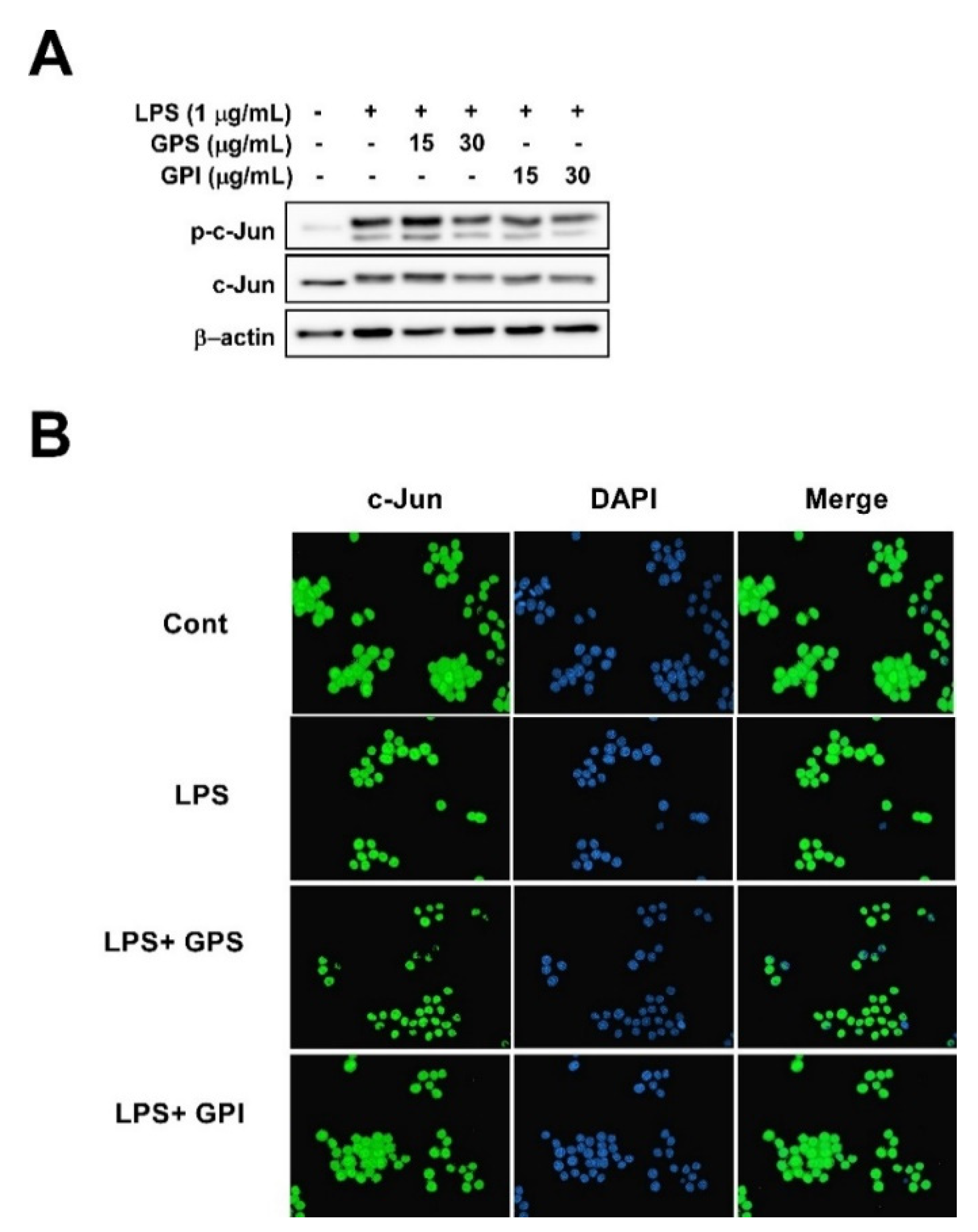
3.6. Effect of GPE on LPS-Induced Phosphorylation of c-Jun and c-Jun Translocation from the Cytosol to the Nucleus in RAW 264.7 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Glennon-Alty, L.; Hackett, A.P.; Chapman, E.A.; Wright, H.L. Neutrophils and redox stress in the pathogenesis of autoimmune disease. Free Radic. Biol. Med. 2018, 125, 25–35. [Google Scholar] [CrossRef] [PubMed]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell. Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef] [PubMed]
- Brglez Mojzer, E.; Knez Hrncic, M.; Skerget, M.; Knez, Z.; Bren, U. Polyphenols: Extraction methods, antioxidative action, bioavailability and anticarcinogenic effects. Molecules 2016, 21. [Google Scholar] [CrossRef]
- Brewer, M.S. Natural antioxidants: Sources, compounds, mechanisms of action, and potential applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Daeron, M. Innate myeloid cells under the control of adaptive immunity: The example of mast cells and basophils. Curr. Opin. Immunol. 2016, 38, 101–108. [Google Scholar] [CrossRef]
- Barrachina, M.D.; Panes, J.; Esplugues, J.V. Role of nitric oxide in gastrointestinal inflammatory and ulcerative diseases: Perspective for drugs development. Curr. Pharm. Des. 2001, 7, 31–48. [Google Scholar] [CrossRef]
- Dominguez, P.M.; Ardavin, C. Differentiation and function of mouse monocyte-derived dendritic cells in steady state and inflammation. Immunol. Rev. 2010, 234, 90–104. [Google Scholar] [CrossRef]
- Dludla, P.V.; Nkambule, B.B.; Jack, B.; Mkandla, Z.; Mutize, T.; Silvestri, S.; Orlando, P.; Tiano, L.; Louw, J.; Mazibuko-Mbeje, S.E. Inflammation and oxidative stress in an obese state and the protective effects of gallic acid. Nutrients 2018, 11. [Google Scholar] [CrossRef]
- Peake, J.M.; Suzuki, K.; Coombes, J.S. The influence of antioxidant supplementation on markers of inflammation and the relationship to oxidative stress after exercise. J. Nutr. Biochem. 2007, 18, 357–371. [Google Scholar] [CrossRef]
- Roman-Blas, J.A.; Jimenez, S.A. Targeting NF-kappaB: A promising molecular therapy in inflammatory arthritis. Int. Rev. Immunol. 2008, 27, 351–374. [Google Scholar] [CrossRef]
- Cazzola, R.; Camerotto, C.; Cestaro, B. Anti-oxidant, anti-glycant, and inhibitory activity against alpha-amylase and alpha-glucosidase of selected spices and culinary herbs. Int. J. Food Sci. Nutr. 2011, 62, 175–184. [Google Scholar] [CrossRef]
- Shan, B.; Cai, Y.Z.; Sun, M.; Corke, H. Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. J. Agric. Food Chem. 2005, 53, 7749–7759. [Google Scholar] [CrossRef]
- Gu, F.; Tan, L.; Wu, H.; Fang, Y.; Wang, Q. Analysis of the blackening of green pepper (Piper nigrum Linnaeus) berries. Food Chem. 2013, 138, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Aziz, N.S.; Sofian-Seng, N.S.; Mohd Razali, N.S.; Lim, S.J.; Mustapha, W.A. A review on conventional and biotechnological approaches in white pepper production. J. Sci. Food Agric. 2019, 99, 2665–2676. [Google Scholar] [CrossRef] [PubMed]
- Asha, S.; Soniya, E.V. Transfer RNA derived small RNAs targeting defense responsive genes are induced during phytophthora capsici infection in Black Pepper (Piper nigrum L.). Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.T.; Piao, C.H.; Hyeon, E.; Fan, Y.; Van Nguyen, T.; Jung, S.Y.; Choi, D.W.; Lee, S.Y.; Shin, H.S.; Song, C.H.; et al. The protective role of Piper nigrum fruit extract in an ovalbumin-induced allergic rhinitis by targeting of NFkappaBp65 and STAT3 signalings. Biomed. Pharmacother. 2019, 109, 1915–1923. [Google Scholar] [CrossRef]
- Pathak, N.; Khandelwal, S. Cytoprotective and immunomodulating properties of piperine on murine splenocytes: An in vitro study. Eur. J. Pharmacol. 2007, 576, 160–170. [Google Scholar] [CrossRef]
- Singh, A.; Rao, A.R. Evaluation of the modulatory influence of black pepper (Piper nigrum, L.) on the hepatic detoxication system. Cancer Lett. 1993, 72, 5–9. [Google Scholar] [CrossRef]
- Ren, T.; Yang, M.; Xiao, M.; Zhu, J.; Xie, W.; Zuo, Z. Time-dependent inhibition of carbamazepine metabolism by piperine in anti-epileptic treatment. Life Sci. 2019, 218, 314–323. [Google Scholar] [CrossRef]
- Vurmaz, A.; Duman, R.; Sabaner, M.C.; Ertekin, T.; Bilir, A. Antioxidant effects of piperine in in-vivo chick embryo cataract model induced by steroids. Cutan. Ocul. Toxicol. 2019, 38, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Yoo, E.S.; Choo, G.S.; Kim, S.H.; Woo, J.S.; Kim, H.J.; Park, Y.S.; Kim, B.S.; Kim, S.K.; Park, B.K.; Cho, S.D.; et al. Antitumor and apoptosis-inducing effects of piperine on human melanoma cells. Anticancer Res. 2019, 39, 1883–1892. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.W.; Chiou, W.F.; Chao, S.H.; Lee, M.H.; Chen, C.C.; Tsai, Y.C. Ligustilide prevents LPS-induced iNOS expression in RAW 264.7 macrophages by preventing ROS production and down-regulating the MAPK, NF-kappaB and AP-1 signaling pathways. Int. Immunopharmacol. 2011, 11, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- Yeh, J.L.; Hsu, J.H.; Hong, Y.S.; Wu, J.R.; Liang, J.C.; Wu, B.N.; Chen, I.J.; Liou, S.F. Eugenolol and glyceryl-isoeugenol suppress LPS-induced iNOS expression by down-regulating NF-kappaB AND AP-1 through inhibition of MAPKS and AKT/IkappaBalpha signaling pathways in macrophages. Int. J. Immunopathol. Pharmacol. 2011, 24, 345–356. [Google Scholar] [CrossRef]
- Santosh, M.; Shaila, D.; Rajyalakshmi, I.; Rao, I.S. RP-HPLC method for determination of piperine from Piper longum Linn. and Piper nigrum Linn. J. Chem. 2005, 2, 131–135. [Google Scholar] [CrossRef]
- Barman, U.; Choudhury, R.D.; Saud, A.; Dey, S.; Pratim, M.; Gunjan, B. Estimation of chlorophyll using image processing. Int. J. Recent Sci. Res. 2018, 9, 24850–24853. [Google Scholar] [CrossRef]
- Meyers, K.J.; Watkins, C.B.; Pritts, M.P.; Liu, R.H. Antioxidant and antiproliferative activities of strawberries. J. Agric. Food Chem. 2003, 51, 6887–6892. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Byrne, D.H. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Gu, F.; Huang, F.; Wu, G.; Zhu, H. Contribution of polyphenol oxidation, chlorophyll and vitamin C degradation to the blackening of Piper nigrum L. Molecules 2018, 23. [Google Scholar] [CrossRef]
- Xia, T.; Yao, J.; Zhang, J.; Duan, W.; Zhang, B.; Xie, X.; Xia, M.; Song, J.; Zheng, Y.; Wang, M. Evaluation of nutritional compositions, bioactive compounds, and antioxidant activities of Shanxi aged vinegars during the aging process. J. Food Sci. 2018, 83, 2638–2644. [Google Scholar] [CrossRef]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total phenolic content, flavonoid content and antioxidant potential of wild vegetables from western Nepal. Plants 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Chonpathompikunlert, P.; Wattanathorn, J.; Muchimapura, S. Piperine, the main alkaloid of Thai black pepper, protects against neurodegeneration and cognitive impairment in animal model of cognitive deficit like condition of Alzheimer’s disease. Food Chem. Toxicol. 2010, 48, 798–802. [Google Scholar] [CrossRef] [PubMed]
- Joo, T.; Sowndhararajan, K.; Hong, S.; Lee, J.; Park, S.Y.; Kim, S.; Jhoo, J.W. Inhibition of nitric oxide production in LPS-stimulated RAW 264.7 cells by stem bark of Ulmus pumila L. Saudi J. Biol. Sci. 2014, 21, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Panieri, E.; Santoro, M.M. ROS homeostasis and metabolism: A dangerous liason in cancer cells. Cell Death Dis. 2016, 7, e2253. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shanahan, C.M. Signalling pathways and vascular calcification. Front. Biosci. 2011, 16, 1302–1314. [Google Scholar] [CrossRef]
- Steven, S.; Frenis, K.; Oelze, M.; Kalinovic, S.; Kuntic, M.; Bayo Jimenez, M.T.; Vujacic-Mirski, K.; Helmstadter, J.; Kroller-Schon, S.; Munzel, T.; et al. Vascular inflammation and oxidative stress: Major triggers for cardiovascular disease. Oxid. Med. Cell. Longev. 2019, 2019, 7092151. [Google Scholar] [CrossRef]
- Zuo, L.; Prather, E.R.; Stetskiv, M.; Garrison, D.E.; Meade, J.R.; Peace, T.I.; Zhou, T. Inflammaging and oxidative stress in human diseases: From molecular mechanisms to novel treatments. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef]
- Attanayake, R.; Rajapaksha, R.; Weerakkody, P.; Bandaranayake, P.C.G. The effect of maturity status on biochemical composition, antioxidant activity, and anthocyanin biosynthesis gene expression in a pomegranate (Punica granatum L.) cultivar with red flowers, yellow peel, and pinkish arils. J. Plant Growth Regul. 2019, 38, 992–1006. [Google Scholar] [CrossRef]
- Reichel, M.; Carle, R.; Sruamsiri, P.; Neidhart, S. Changes in flavonoids and nonphenolic pigments during on-tree maturation and postharvest pericarp browning of litchi (Litchi chinensis Sonn.) as shown by HPLC-MSn. J. Agric. Food Chem. 2011, 59, 3924–3939. [Google Scholar] [CrossRef]
- Grzeszczuk, M.; Salachna, P.; Meller, E. Changes in Photosynthetic Pigments, Total phenolic content, and antioxidant activity of Salvia coccinea Buc’hoz Ex Etl. induced by exogenous salicylic acid and soil salinity. Molecules 2018, 23. [Google Scholar] [CrossRef]
- Gülçin, İ. The antioxidant and radical scavenging activities of black pepper (Piper nigrum) seeds. Int. J. Food Sci. Nutr. 2005, 56, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, R.; Surya, D.; Nalini, N. Antioxidant efficacy of black pepper (Piper nigrum L.) and piperine in rats with high fat diet induced oxidative stress. Redox Rep. 2004, 9, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, H.; Abdul Manap, M.Y.; Solati, Z. Antioxidant activity of Piper nigrum L. essential oil extracted by supercritical CO(2) extraction and hydro-distillation. Talanta 2014, 121, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, I.P.; Singh, B.; Singh, G.; De Heluani, C.S.; De Lampasona, M.P.; Catalan, C.A. Chemistry and in vitro antioxidant activity of volatile oil and oleoresins of black pepper (Piper nigrum). J. Agric. Food Chem. 2009, 57, 5358–5364. [Google Scholar] [CrossRef]
- Adefegha, S.A. Functional foods and nutraceuticals as dietary intervention in chronic diseases; novel perspectives for health promotion and disease prevention. J. Diet. Suppl. 2018, 15, 977–1009. [Google Scholar] [CrossRef]
- Guillin-Amarelle, C.; Sanchez-Iglesias, S.; Mera, A.; Pintos, E.; Castro-Pais, A.; Rodriguez-Canete, L.; Pardo, J.; Casanueva, F.F.; Araujo-Vilar, D. Inflammatory myopathy in the context of an unusual overlapping laminopathy. Arch. Endocrinol. Metab. 2018, 62, 376–382. [Google Scholar] [CrossRef]
- Jain, S.; Buttar, H.S.; Chintameneni, M.; Kaur, G. Prevention of cardiovascular diseases with anti-inflammatory and anti- oxidant nutraceuticals and herbal products: An overview of pre-clinical and clinical studies. Recent Pat. Inflamm. Allergy Drug Discov. 2018, 12, 145–157. [Google Scholar] [CrossRef]
- Song, K.-M.; Ha, S.J.; Lee, J.-E.; Kim, S.-H.; Kim, Y.H.; Kim, Y.; Hong, S.P.; Jung, S.K.; Lee, N.H. High yield ultrasonication extraction method for Undaria pinnatifida sporophyll and its anti-inflammatory properties associated with AP-1 pathway suppression. LWT-Food Sci. Technol. 2015, 64, 1315–1322. [Google Scholar] [CrossRef]
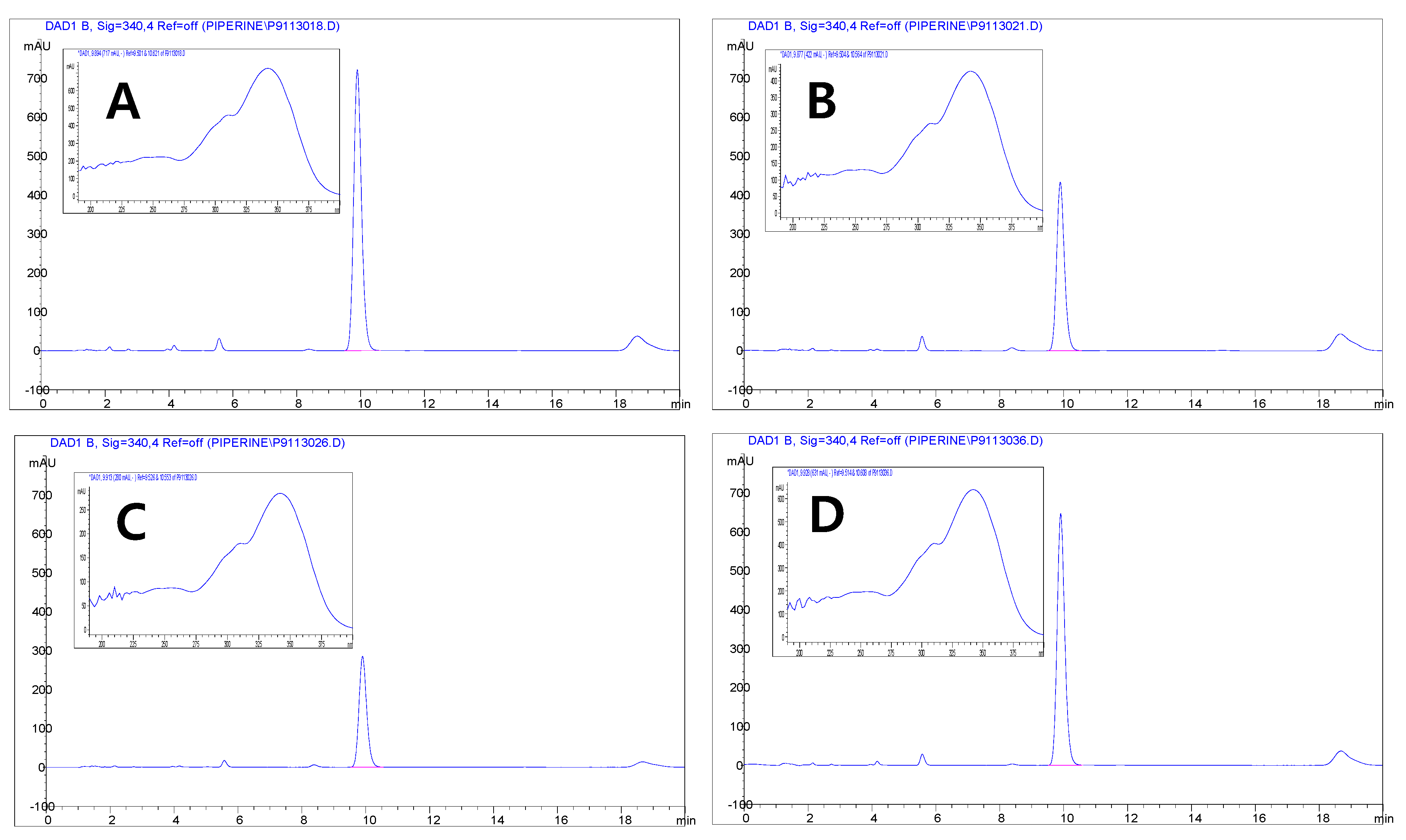


| Color | Origin | L* | a* | b* | Chlorophyll a (mg/L) | Chlorophyll b (mg/L) |
|---|---|---|---|---|---|---|
| Green | India | 48.21 ± 0.04 b | −0.95 ± 0.02 d | 19.16 ± 0.01 b | 28.97 ± 0.09 a | 15.41 ± 0.06 a |
| Sri Lanka | 49.47 ± 0.06 a | 0.27 ± 0.05 c | 19.43 ± 0.02 a | 21.92 ± 0.07 b | 8.62 ± 0.03 b | |
| Black | India | 42.03 ± 0.01 c | 3.44 ± 0.01 b | 11.43 ± 0.01 d | 13.68 ± 0.01 c | 4.08 ± 0.03 c |
| Sri Lanka | 36.66 ± 0.03 d | 3.64 ± 0.02 a | 12.35 ± 0.02 c | 7.15 ± 0.02 d | 3.12 ± 0.01 d |
| Color | Region | Total Flavonoid (mg CE/100 g) | Total Phenolic (mg GAE/100 g) | DPPH (mg VCE/100 g) | ABTS (mg VCE/100 g) |
|---|---|---|---|---|---|
| Green | India | 923.43 ± 11.43 b | 1414.63 ± 13.85 a | 522.83 ± 26.00 a | 1941.91 ± 67.44 a |
| Sri Lanka | 1083.43 ± 8.24 a | 1414.63 ± 10.56 a | 518.67 ± 7.21 a | 1902.77 ± 30.07 a | |
| Black | India | 576.97 ± 14.70 c | 985.69 ± 5.22 b | 362.59 ± 7.86 b | 1269.66 ± 84.01 b |
| Sri Lanka | 275.00 ± 10.10 d | 589.39 ± 9.19 c | 194.42 ± 5.67 c | 526.45 ± 55.89 c |
| Color | Origin | Piperine (mg/100 g) |
|---|---|---|
| Green | India | 8613.27 ± 45.86 a |
| Sri Lanka | 5087.97 ± 29.78 c | |
| Black | India | 3291.65 ± 3.53 d |
| Sri Lanka | 7388.50 ± 37.00 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.W.; Kim, M.J.; Shin, Y.; Jung, S.K.; Kim, Y.-J. Green Pepper (Piper nigrum L.) Extract Suppresses Oxidative Stress and LPS-Induced Inflammation via Regulation of JNK Signaling Pathways. Appl. Sci. 2020, 10, 2519. https://doi.org/10.3390/app10072519
Kim DW, Kim MJ, Shin Y, Jung SK, Kim Y-J. Green Pepper (Piper nigrum L.) Extract Suppresses Oxidative Stress and LPS-Induced Inflammation via Regulation of JNK Signaling Pathways. Applied Sciences. 2020; 10(7):2519. https://doi.org/10.3390/app10072519
Chicago/Turabian StyleKim, Dae Won, Min Jeong Kim, Youngjae Shin, Sung Keun Jung, and Young-Jun Kim. 2020. "Green Pepper (Piper nigrum L.) Extract Suppresses Oxidative Stress and LPS-Induced Inflammation via Regulation of JNK Signaling Pathways" Applied Sciences 10, no. 7: 2519. https://doi.org/10.3390/app10072519
APA StyleKim, D. W., Kim, M. J., Shin, Y., Jung, S. K., & Kim, Y.-J. (2020). Green Pepper (Piper nigrum L.) Extract Suppresses Oxidative Stress and LPS-Induced Inflammation via Regulation of JNK Signaling Pathways. Applied Sciences, 10(7), 2519. https://doi.org/10.3390/app10072519






