Optics Based Label-Free Techniques and Applications in Brain Monitoring
Abstract
1. Introduction
2. FNIRS in Human Brain Imaging
2.1. Current Clinical Applications of fNIRS
2.2. Towards Wearable Brain Monitoring Using fNIRS
3. DCS Technology Used Currently in Human Brain Imaging
3.1. Current Clinical Applications of DCS
3.2. Wearable DCS Devices And the Commercial Ones
3.3. Animal Studies
4. Current PAI Technology Used in Brain Imaging
Brain Studies Utilising PAI
5. OCT in Brain Imaging
5.1. OCT Studies on the Brain
5.2. Current Clinical Trials Using Integrated OCT in Human
6. Summary
Author Contributions
Funding
Conflicts of Interest
Abbreviation
| APD | Avalanche Photodiode |
| AR-PAM | Acoustic-resolution PA microscopy |
| B-NIRS | Broadband NIRS |
| BBB | Blood-brain barrier |
| BBBD | Blood-brain barrier disruption |
| BCI | Brain-computer interface |
| BFI | Blood flow index |
| BMSCs | bone mesenchymal stem cells |
| CA | Cerebral aneurysm |
| CBF | Cerebral blood flow |
| CBFi | Cerebral blood flow index |
| CBV | Cerebral blood volume |
| CCD | Closed-caption device |
| CMRO2 | Cerebral oxygen metabolism |
| CP_NPs | conjugated polymer nanoparticles |
| DCS | Diffuse correlations spectroscopy |
| EEG | Electro encephalography |
| fcPAT | functional PAT |
| FD-OCT | Fourier-domain Optical coherence tomography |
| fDCS | functional DCS |
| FF-OCT | full-field Optical coherence tomography |
| fMRI | functional magnetic resonance imaging |
| fNIRS | functional near-infrared spectroscopy |
| FUS | focused ultrasound |
| HbO | Oxy-hemoglobin |
| HbR | Deoxy-hemoglobin |
| HbT | Total hemoglobin |
| ICP | Intracranial pressure |
| iOCT | Integrated optical coherence tomography |
| IR | Infrared |
| LD | Laser diode |
| LED | Light emitting diode |
| MEG | magnetic encephalography |
| NIRS | Near-infrared spectroscopy |
| OCT | Optical coherence tomography |
| OCTA | Optical Coherence Tomography based Angiography |
| OMAG | Optical Coherence Tomography based Microangiography |
| OR-PAM | Optical resolution PA microscopy |
| oxCCO | Oxidation state of cytochrome c oxidase |
| PACT | Photoacoustic computing tomography |
| PAI | Photoacoustic imaging |
| PD | Photo diode |
| PET | positron emission tomography |
| PMTs | photomultiplier tubes |
| PoC | proof of concept |
| PZT | Piezoelectric Transducer |
| SAS | Subarachnoid space |
| SD-OCT | Spectral-domain Optical coherence tomography |
| SiPD | Silicon PD |
| SPECT | Single-photon emission computed tomography |
| SS-OCT | Swept-source Optical coherence tomography |
| StO2 | Cerebral tissue oxygenation |
| TBI | Traumatic Brain Injury |
| TD-OCT | Time Domain Optical coherence tomography |
| tDCS | transcranial direct electrical stimulation |
| TMS | Transcranial magnetic stimulation |
| TR-fNIRS | Time-resolved fNIRS |
| VSD | voltage-sensitive dye |
References
- Myllylä, T.; Zacharias, N.; Korhonen, V.; Zienkiewicz, A.; Hinrichs, H.; Kiviniemi, V.; Walter, M. Multimodal brain imaging with magnetoencephalography: A method for measuring blood pressure and cardiorespiratory oscillations. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hämäläinen, M.; Hari, R.; Ilmoniemi, R.J.; Knuutila, J.; Lounasmaa, O.V. Magnetoencephalography theory, instrumentation, and applications to noninvasive studies of the working human brain. Rev. Mod. Phys. 1993, 65, 413–497. [Google Scholar] [CrossRef]
- Ogawa, S.; Lee, T.M.; Kay, A.R.; Tank, D.W. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc. Natl. Acad. Sci. USA 1990, 87, 9868–9872. [Google Scholar] [CrossRef] [PubMed]
- Hugger, T.; Zahneisen, B.; LeVan, P.; Lee, K.J.; Lee, H.-L.; Zaitsev, M.; Hennig, J. Fast Undersampled Functional Magnetic Resonance Imaging Using Nonlinear Regularized Parallel Image Reconstruction. PLoS ONE 2011, 6, e28822. [Google Scholar] [CrossRef]
- Assländer, J.; Zahneisen, B.; Hugger, T.; Reisert, M.; Lee, H.L.; LeVan, P.; Hennig, J. Single shot whole brain imaging using spherical stack of spirals trajectories. Neuroimage 2013, 73, 59–70. [Google Scholar] [CrossRef]
- Tuchin, V. Tissue Optics and Photonics: Light-Tissue Interaction II. J. Biomed. Photonics Eng. 2016, 2, 30201. [Google Scholar] [CrossRef]
- Lareau, E.; Lesage, F.; Pouliot, P.; Nguyen, D.; Le Lan, J.; Sawan, M. Multichannel wearable system dedicated for simultaneous electroencephalography/near-infrared spectroscopy real-time data acquisitions. J. Biomed. Opt. 2011, 16, 96014. [Google Scholar] [CrossRef]
- Tuchin, V. Tissue Optics: Light Scattering Methods and Instruments for Medical Diagnosis, 3rd ed.; SPIE Press: Bellingham, WA, USA, 2015. [Google Scholar]
- Korhonen, V.O.; Myllylä, T.S.; Kirillin, M.Y.; Popov, A.P.; Bykov, A.V.; Gorshkov, A.V.; Sergeeva, E.A.; Kinnunen, M.; Kiviniemi, V. Light propagation in NIR spectroscopy of the human brain. IEEE J. Sel. Top. Quantum Electron. 2014, 20, 289–298. [Google Scholar] [CrossRef]
- Madsen, S.J.; Wilson, B.C. Optical Methods and Instrumentation in Brain Imaging and Therapy; Springer: New York, NY, USA, 2013. [Google Scholar]
- Ferrari, M.; Quaresima, V. A brief review on the history of human functional near-infrared spectroscopy (fNIRS) development and fields of application. Neuroimage 2012, 63, 921–935. [Google Scholar] [CrossRef]
- Carp, S.A.; Dai, G.P.; Boas, D.A.; Franceschini, M.A.; Kim, Y.R. Validation of diffuse correlation spectroscopy measurements of rodent cerebral blood flow with simultaneous arterial spin labeling MRI; towards MRI-optical continuous cerebral metabolic monitoring. Biomed. Opt. Express 2010, 1, 553–565. [Google Scholar] [CrossRef]
- Wang, L.V.; Yao, J. A practical guide to photoacoustic tomography in the life sciences. Nat. Methods 2016, 13, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, J.M. Optical Coherence Tomography (OCT): A review. IEEE J. Sel. Top. Quantum Electron. 1999, 5, 1205–1215. [Google Scholar] [CrossRef]
- Durduran, T. Noninvasive Measurements of Tissue Hemodynamics with Hybrid Diffuse Optical Methods. Ph.D. Thesis, University of Pennsylvania, Philadelphia, PA, USA, 2004. [Google Scholar]
- Li, J.; Dietsche, G.; Iftime, D.; Skipetrov, S.E.; Maret, G.; Elbert, T.; Rockstroh, B.; Gisler, T. Noninvasive detection of functional brain activity with near-infrared diffusing-wave spectroscopy. J. Biomed. Opt. 2005, 10, 44002. [Google Scholar] [CrossRef] [PubMed]
- Roche-Labarbe, N.; Carp, S.A.; Surova, A.; Patel, M.; Boas, D.A.; Grant, P.E.; Franceschini, M.A. Noninvasive optical measures of CBV, StO2, CBF index, and rCMRO2 in human premature neonates’ brains in the first six weeks of life. Hum. Brain Mapp. 2010, 31, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.; Roskosky, M.; Freedman, B.; Schuler, M.S. Depth Penetration of Near Infrared Spectroscopy in the Obese. J. Trauma Treat. 2014, 4, 2167-1222. [Google Scholar]
- Piper, S.K.; Krueger, A.; Koch, S.P.; Mehnert, J.; Habermehl, C.; Steinbrink, J.; Obrig, H.; Schmitz, C.H. A wearable multi-channel fNIRS system for brain imaging in freely moving subjects. Neuroimage 2014, 85, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Sutin, J.; Zimmerman, B.; Tyulmankov, D.; Tamborini, D.; Wu, K.C.; Selb, J.; Gulinatti, A.; Rech, I.; Tosi, A.; Boas, D.A.; et al. Time-domain diffuse correlation spectroscopy. Optica 2016, 3, 1006. [Google Scholar] [CrossRef]
- Durduran, T.; Choe, R.; Baker, W.B.; Yodh, A.G. Diffuse optics for tissue monitoring and tomography. Rep. Prog. Phys. 2010, 73, 76701. [Google Scholar] [CrossRef]
- Huang, C.; Seong, M.; Morgan, J.P.; Mazdeyasna, S.; Kim, J.G.; Hastings, J.T.; Yu, G. Low-cost compact diffuse speckle contrast flowmeter using small laser diode and bare charge-coupled-device. J. Biomed. Opt. 2016, 21, 80501. [Google Scholar] [CrossRef]
- Huang, C.; Gu, Y.; Chen, J.; Bahrani, A.A.; Jawdeh, E.G.A.; Bada, H.S.; Saatman, K.; Yu, G.; Chen, L. A Wearable Fiberless Optical Sensor for Continuous Monitoring of Cerebral Blood Flow in Mice. IEEE J. Sel. Top. Quantum Electron. 2019, 25, 6900108. [Google Scholar] [CrossRef]
- Fantini, S.; Franceschini, M.A.; Fishkin, J.B.; Barbieri, B.; Gratton, E. Quantitative determination of the absorption spectra of chromophores in strongly scattering media: A light-emitting-diode based technique. Appl. Opt. 1994, 33, 5204–5213. [Google Scholar] [CrossRef] [PubMed]
- Tromberg, B.J.; Coquoz, O.; Fishkin, J.B.; Pham, T.; Anderson, E.R.; Butler, J.; Cahn, M.; Gross, J.D.; Venugopalan, V.; Pham, D. Non-invasive measurements of breast tissue optical properties using frequency-domain photon migration. Philos. Trans. R. Soc. B Biol. Sci. 1997, 352, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Delpy, D.T.; Cope, M.; Van Der Zee, P.; Arridge, S.; Wray, S.; Wyatt, J. Estimation of optical pathlength through tissue from direct time of flight measurement. Phys. Med. Biol. 1988, 33, 1433–1442. [Google Scholar] [CrossRef] [PubMed]
- Patterson, M.S.; Chance, B.; Wilson, B.C. Time resolved reflectance and transmittance for the noninvasive measurement of tissue optical properties. Appl. Opt. 1989, 28, 2331–2336. [Google Scholar] [CrossRef]
- Yul, G.; Durduran, T.; Zhou, C.; Zhu, T.C.; Finlay, J.C.; Busch, T.M.; Malkowicz, S.B.; Hahn, S.M.; Yodh, A.G. Real-time ln Situ Monitoring of Human Prostate Photodynamic Therapy with Diffuse Light. Photochem. Photobiol. 2006, 82, 1279–1280. [Google Scholar]
- Yu, G. Diffuse Correlation Spectroscopy (DCS): A Diagnostic Tool for Assessing Tissue Blood Flow in Vascular-Related Diseases and Therapies. Curr. Med. Imaging Rev. 2012, 8, 194–210. [Google Scholar] [CrossRef]
- Nasiriavanaki, M.; Xia, J.; Wan, H.; Bauer, A.Q.; Culver, J.P.; Wang, L.V. High-resolution photoacoustic tomography of resting-state functional connectivity in the mouse brain. Proc. Natl. Acad. Sci. USA 2014, 111, 21–26. [Google Scholar] [CrossRef]
- Tang, J.; Dai, X.; Jiang, H. Wearable scanning photoacoustic brain imaging in behaving rats. J. Biophotonics 2016, 9, 570–575. [Google Scholar] [CrossRef]
- Zhang, P.; Li, L.; Lin, L.; Hu, P.; Shi, J.; He, Y.; Zhu, L.; Zhou, Y.; Wang, L.V. High-resolution deep functional imaging of the whole mouse brain by photoacoustic computed tomography in vivo. J. Biophotonics 2018, 11, e201700024. [Google Scholar] [CrossRef]
- Hu, S. Listening to the Brain With Photoacoustics. IEEE J. Sel. Top. Quantum Electron. 2016, 22, 117–126. [Google Scholar] [CrossRef]
- Yao, J.; Wang, L.V. Photoacoustic brain imaging: From microscopic to macroscopic scales. Neurophotonics 2014, 1, 11003. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.; Cai, X.; Maslov, K.; Garcia-Uribe, A.; Anastasio, M.A.; Wang, L. V Photoacoustic tomography through a whole adult human skull with a photon recycler. J. Biomed. Opt. 2012, 17, 110506. [Google Scholar] [CrossRef] [PubMed]
- Petrov, I.Y.; Petrov, Y.; Prough, D.S.; Richardson, C.J.; Fonseca, R.A.; Robertson, C.S.; Asokan, C.V.; Agbor, A.; Esenaliev, R.O. Transmission (forward) mode, transcranial, noninvasive optoacoustic measurements for brain monitoring, imaging, and sensing. In Photons Plus Ultrasound: Imaging and Sensing 2016; Oraevsky, A.A., Wang, L.V., Eds.; International Society for Optics and Photonics: Bellingham, WA, USA, 2016; p. 97084P. [Google Scholar]
- Wang, X.; Chamberland, D.L.; Xi, G. Noninvasive reflection mode photoacoustic imaging through infant skull toward imaging of neonatal brains. J. Neurosci. Methods 2008, 168, 412–421. [Google Scholar] [CrossRef]
- Ibne Mokbul, M. Optical Coherence Tomography: Basic Concepts and Applications in Neuroscience Research. J. Med. Eng. 2017, 2017, 3409327. [Google Scholar] [CrossRef] [PubMed]
- Myllylä, T.; Korhonen, V.; Kiviniemi, V.; Tuchin, V. Experimental studies with selected light sources for NIRS of brain tissue: Quantifying tissue chromophore concentration. In Proceedings of the Optical Techniques in Neurosurgery, Neurophotonics, and Optogenetics II, San Francisco, CA, USA, 7–10 February 2015; SPIE: Bellingham, WA, USA, 2015; Volume 9305, p. 93051S. [Google Scholar]
- Myllylä, T.; Korhonen, V.; Suraźynski, Ł.; Zienkiewicz, A.; Sorvoja, H.; Myllylä, R. Measurement of cerebral blood flow and metabolism using high power light-emitting diodes. Measurement 2014, 58, 387–393. [Google Scholar] [CrossRef]
- Dean Kurth, C.; Thayer, W.S. A multiwavelength frequency-domain near-infrared cerebral oximeter. Phys. Med. Biol. 1999, 44, 727–740. [Google Scholar] [CrossRef] [PubMed]
- Scholkmann, F.; Kleiser, S.; Metz, A.J.; Zimmermann, R.; Mata Pavia, J.; Wolf, U.; Wolf, M. A review on continuous wave functional near-infrared spectroscopy and imaging instrumentation and methodology. Neuroimage 2014, 85, 6–27. [Google Scholar] [CrossRef]
- Re, R.; Contini, D.; Turola, M.; Spinelli, L.; Zucchelli, L.; Caffini, M.; Cubeddu, R.; Torricelli, A. Multi-channel medical device for time domain functional near infrared spectroscopy based on wavelength space multiplexing. Biomed. Opt. Express 2013, 4, 2231. [Google Scholar] [CrossRef]
- Pifferi, A.; Contini, D.; Mora, A.D.; Farina, A.; Spinelli, L.; Torricelli, A. New frontiers in time-domain diffuse optics, a review. J. Biomed. Opt. 2016, 21, 91310. [Google Scholar] [CrossRef]
- Di Sieno, L.; Nissinen, J.; Hallman, L.; Martinenghi, E.; Contini, D.; Pifferi, A.; Kostamovaara, J.; Mora, A.D. Miniaturized pulsed laser source for time-domain diffuse optics routes to wearable devices. J. Biomed. Opt. 2017, 22, 85004. [Google Scholar] [CrossRef]
- Re, R.; Martinenghi, E.; Mora, A.D.; Contini, D.; Pifferi, A.; Torricelli, A. Probe-hosted silicon photomultipliers for time-domain functional near-infrared spectroscopy: Phantom and in vivo tests. Neurophotonics 2016, 3, 45004. [Google Scholar] [CrossRef] [PubMed]
- Gatto, R.; Hoffman, W.; Mueller, M.; Flores, A.; Valyi-Nagy, T.; Charbel, F.T. Frequency domain near-infrared spectroscopy technique in the assessment of brain oxygenation: A validation study in live subjects and cadavers. J. Neurosci. Methods 2006, 157, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Giacalone, G.; Zanoletti, M.; Contini, D.; Re, R.; Spinelli, L.; Roveri, L.; Torricelli, A. Cerebral time domain-NIRS: Reproducibility analysis, optical properties, hemoglobin species and tissue oxygen saturation in a cohort of adult subjects. Biomed. Opt. Express 2017, 8, 4987. [Google Scholar] [CrossRef] [PubMed]
- Tachtsidis, I.; Papaioannou, A. Investigation of frontal lobe activation with fNIRS and systemic changes during video gaming. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2013; Volume 789, pp. 89–95. [Google Scholar]
- Lee, C.W.; Cooper, R.J.; Austin, T. Diffuse optical tomography to investigate the newborn brain. Pediatr. Res. 2017, 82, 376–386. [Google Scholar] [CrossRef]
- Liao, S.M. High-density diffuse optical tomography of term infant visual cortex in the nursery. J. Biomed. Opt. 2012, 17, 81414. [Google Scholar] [CrossRef]
- Shoaib, Z.; Ahmad Kamran, M.; Mannan, M.M.N.; Jeong, M.Y. Approach to optimize 3-dimensional brain functional activation image with high resolution: A study on functional near-infrared spectroscopy. Biomed. Opt. Express 2019, 10, 4684. [Google Scholar] [CrossRef]
- Wolf, M.; Ferrari, M.; Quaresima, V. Progress of near-infrared spectroscopy and topography for brain and muscle clinical applications. J. Biomed. Opt. 2007, 12, 62104. [Google Scholar]
- Hamamatsu photonics K.K Near infrared oxygenation monitor C10448-20. Available online: https://www.hamamatsu.com/eu/en/product/type/C10448-20/index.html (accessed on 8 December 2019).
- Zhao, H.; Cooper, R.J. Review of recent progress toward a fiberless, whole-scalp diffuse optical tomography system. Neurophotonics 2017, 5, 11012. [Google Scholar] [CrossRef]
- Biopac fNIR Funcitonal Near Infrared Optical Brain Imaging System 2019. Available online: https://www.biopac.com/product-category/education/fnir-optical-brain-imaging-education/ (accessed on 15 January 2020).
- NIRx NIRSport 2 | fNIRS Systems | NIRS Devices | NIRx. Available online: https://nirx.net/nirsport (accessed on 15 January 2020).
- Rogue Research Inc. NIRS Specifications | Rogue Research. Available online: https://www.rogue-research.com/nirs/specifications/ (accessed on 15 January 2020).
- ETG 4100—Hitachi Medical Systems. Available online: http://www.hitachi-medical-systems.eu/products-and-services/optical-topography/etg-4100.html (accessed on 16 January 2020).
- CW6 | NIRSOptix by TechEn, Inc. Available online: https://www.nirsoptix.com/CW6.html (accessed on 24 February 2020).
- Artinis Medical Systems | fNIRS devices | NIRS devices-Home. Available online: https://www.artinis.com/ (accessed on 4 March 2020).
- Silverlineresearch Starstim fNIRS. Available online: https://static1.squarespace.com/static/54ddd9b0e4b0e11f3685f546/t/5c8269d8b208fc7a2f962f12/1552050649366/Starstim+fNIRS+leafet.pdf (accessed on 24 February 2020).
- ISS Functional Brain Imaging System. Available online: http://www.iss.com/biomedical/instruments/imagent.html (accessed on 10 March 2020).
- Obrig, H. NIRS in clinical neurology—A “promising” tool? Neuroimage 2014, 85, 535–546. [Google Scholar] [CrossRef]
- Saxena, V.; Gonzalez-Gomez, I.; Laug, W.E. A noninvasive multimodal technique to monitor brain tumor vascularization. Phys. Med. Biol. 2007, 52, 5295–5308. [Google Scholar] [CrossRef]
- Vishwanath, K.; Klein, D.; Chang, K.; Schroeder, T.; Dewhirst, M.W.; Ramanujam, N. Quantitative optical spectroscopy can identify long-term local tumor control in irradiated murine head and neck xenografts. J. Biomed. Opt. 2009, 14, 54051. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, A.; Tremblay, J.; Vannasing, P. Language mapping in children using resting-state functional connectivity: Comparison with a task-based approach. J. Biomed. Opt. 2016, 21, 125006. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Uzuka, T.; Aoki, H.; Natsumeda, M.; Oishi, M.; Fukuda, M.; Fujii, Y. Near-infrared spectroscopic study and the Wada test for presurgical evaluation of expressive and receptive language functions in glioma patients: With a case report of dissociated language functions. Neurosci. Lett. 2012, 510, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Sunar, U.; Quon, H.; Durduran, T.; Zhang, J.; Du, J.; Zhou, C.; Yu, G.; Choe, R.; Kilger, A.; Lustig, R.; et al. Noninvasive diffuse optical measurement of blood flow and blood oxygenation for monitoring radiation therapy in patients with head and neck tumors: A pilot study. J. Biomed. Opt. 2006, 11, 64021. [Google Scholar] [CrossRef] [PubMed]
- Rizki, E.E.; Uga, M.; Dan, I.; Dan, H.; Tsuzuki, D.; Yokota, H.; Oguro, K.; Watanabe, E. Determination of epileptic focus side in mesial temporal lobe epilepsy using long-term noninvasive fNIRS/EEG monitoring for presurgical evaluation. Neurophotonics 2015, 2, 25003. [Google Scholar] [CrossRef] [PubMed]
- Pouliot, P.; Tremblay, J.; Robert, M.; Vannasing, P.; Lepore, F.; Lassonde, M.; Sawan, M.; Nguyen, D.K.; Lesage, F. Nonlinear hemodynamic responses in human epilepsy: A multimodal analysis with fNIRS-EEG and fMRI-EEG. J. Neurosci. Methods 2012, 204, 326–340. [Google Scholar] [CrossRef]
- Adorni, R.; Gatti, A.; Brugnera, A.; Sakatani, K.; Compare, A. Could fNIRS Promote Neuroscience Approach in Clinical Psychology? Front. Psychol. 2016, 7, 456. [Google Scholar] [CrossRef]
- Maidan, I.; Nieuwhof, F.; Bernad-Elazari, H.; Reelick, M.F.; Bloem, B.R.; Giladi, N.; Deutsch, J.E.; Hausdorff, J.M.; Claassen, J.A.H.; Mirelman, A. The Role of the Frontal Lobe in Complex Walking Among Patients With Parkinson’s Disease and Healthy Older Maidan, I., Nieuwhof, F., Bernad-Elazari, H., Reelick, M.F., Bloem, B.R., Giladi, N., … Mirelman, A. (2016). The Role of the Frontal Lobe in Comple. Neurorehabil. Neural Repair 2016, 30, 963–971. [Google Scholar] [CrossRef]
- Nieuwhof, F.; Reelick, M.F.; Maidan, I.; Mirelman, A.; Hausdorff, J.M.; Olde Rikkert, M.G.M.; Bloem, B.R.; Muthalib, M.; Claassen, J.A.H.R. Measuring prefrontal cortical activity during dual task walking in patients with Parkinson’s disease: Feasibility of using a new portable fNIRS device. Pilot Feasibility Stud. 2016, 2, 59. [Google Scholar] [CrossRef]
- Rea, M.; Rana, M.; Lugato, N.; Terekhin, P.; Gizzi, L.; Brötz, D.; Fallgatter, A.; Birbaumer, N.; Sitaram, R.; Caria, A. Lower Limb Movement Preparation in Chronic Stroke: A Pilot Study Toward an fNIRS-BCI for Gait Rehabilitation. Neurorehabil. Neural Repair 2014, 28, 564–575. [Google Scholar] [CrossRef]
- Nguyen, T.; Babawale, O.; Kim, T.; Jo, H.J.; Liu, H.; Kim, J.G. Exploring brain functional connectivity in rest and sleep states: A fNIRS study. Sci. Rep. 2018, 8, 16144. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Gu, Y.; Duan, X.; Cheng, L.; Liang, S.; Tong, Y.; Li, X. Design of multichannel functional near-infrared spectroscopy system with application to propofol and sevoflurane anesthesia monitoring. Neurophotonics 2016, 3, 45001. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Meza, G.; Izzetoglu, M.; Osbakken, M.; Green, M.; Abubakar, H.; Izzetoglu, K. Investigation of optical neuro-monitoring technique for detection of maintenance and emergence states during general anesthesia. J. Clin. Monit. Comput. 2018, 32, 147–163. [Google Scholar] [CrossRef] [PubMed]
- Altvater-Mackensen, N.; Grossmann, T. The role of left inferior frontal cortex during audiovisual speech perception in infants. Neuroimage 2016, 133, 14–20. [Google Scholar] [CrossRef]
- Vannasing, P.; Florea, O.; González-Frankenberger, B.; Tremblay, J.; Paquette, N.; Safi, D.; Wallois, F.; Lepore, F.; Béland, R.; Lassonde, M.; et al. Distinct hemispheric specializations for native and non-native languages in one-day-old newborns identified by fNIRS. Neuropsychologia 2016, 84, 63–69. [Google Scholar] [CrossRef]
- Urakawa, S.; Takamoto, K.; Ishikawa, A.; Ono, T.; Nishijo, H. Selective Medial Prefrontal Cortex Responses During Live Mutual Gaze Interactions in Human Infants: An fNIRS Study. Brain Topogr. 2015, 28, 691–701. [Google Scholar] [CrossRef]
- Lloyd-Fox, S.; Széplaki-Köllod, B.; Yin, J.; Csibra, G. Are you talking to me? Neural activations in 6-month-old infants in response to being addressed during natural interactions. Cortex 2015, 70, 35–48. [Google Scholar] [CrossRef]
- Zaidi, A.D.; Munk, M.H.J.; Schmidt, A.; Risueno-Segovia, C.; Bernard, R.; Fetz, E.; Logothetis, N.; Birbaumer, N.; Sitaram, R. Simultaneous epidural functional near-infrared spectroscopy and cortical electrophysiology as a tool for studying local neurovascular coupling in primates. Neuroimage 2015, 120, 394–399. [Google Scholar] [CrossRef]
- Roche-Labarbe, N.; Zaaimi, B.; Mahmoudzadeh, M.; Osharina, V.; Wallois, A.; Nehlig, A.; Grebe, R.; Wallois, F. NIRS-measured oxy- and deoxyhemoglobin changes associated with EEG spike-and-wave discharges in a genetic model of absence epilepsy: The GAERS. Epilepsia 2010, 51, 1374–1384. [Google Scholar] [CrossRef] [PubMed]
- Abookasis, D.; Shochat, A.; Mathews, M.S. Monitoring hemodynamic and morphologic responses to closed head injury in a mouse model using orthogonal diffuse near-infrared light reflectance spectroscopy. J. Biomed. Opt. 2013, 18, 45003. [Google Scholar] [CrossRef]
- Myllylä, T.S.; Kaakinen, M.; Zienkiewicz, A.; Vihriala, E.; Korhonen, V.; Kuittinen, O.; Eklund, L.; Kiviniemi, V.; Jukkola, J. Cardiovascular effects of mannitol infusion: A comparison study performed on mouse and human. Biophotonics Photonic Solut. Better Heal. Care VI 2018, 10685, 106854A. [Google Scholar]
- Jessen, N.A.; Munk, A.S.F.; Lundgaard, I.; Nedergaard, M. The Glymphatic System: A Beginner’s Guide. Neurochem. Res. 2015, 40, 2583–2599. [Google Scholar] [CrossRef] [PubMed]
- Myllylä, T.; Harju, M.; Korhonen, V.; Bykov, A.; Kiviniemi, V.; Meglinski, I. Assessment of the dynamics of human glymphatic system by near-infrared spectroscopy. J. Biophotonics 2018, 11, e201700123. [Google Scholar] [CrossRef] [PubMed]
- Kiviniemi, V.; Korhonen, V.; Kortelainen, J.; Rytky, S.; Keinänen, T.; Tuovinen, T.; Isokangas, M.; Sonkajärvi, E.; Siniluoto, T.; Nikkinen, J.; et al. Real-time monitoring of human blood-brain barrier disruption. PLoS ONE 2017, 12, e0174072. [Google Scholar] [CrossRef]
- Saikia, M.J.; Besio, W.G.; Mankodiya, K. WearLight: Toward a Wearable, Configurable Functional NIR Spectroscopy System for Noninvasive Neuroimaging. IEEE Trans. Biomed. Circuits Syst. 2019, 13, 91–102. [Google Scholar] [CrossRef]
- Chitnis, D.; Cooper, R.J.; Dempsey, L.; Powell, S.; Quaggia, S.; Highton, D.; Elwell, C.; Hebden, J.C.; Everdell, N.L. Functional imaging of the human brain using a modular, fibre-less, high-density diffuse optical tomography system. Biomed. Opt. Express 2016, 7, 4275–4288. [Google Scholar] [CrossRef]
- Funane, T.; Numata, T.; Sato, H.; Hiraizumi, S.; Hasegawa, Y.; Kuwabara, H.; Hasegawa, K.; Kiguchi, M. Rearrangeable and exchangeable optical module with system-on-chip for wearable functional near-infrared spectroscopy system. Neurophotonics 2017, 5, 11007. [Google Scholar] [CrossRef]
- Chitnis, D.; Airantzis, D.; Highton, D.; Williams, R.; Phan, P.; Giagka, V.; Powell, S.; Cooper, R.J.; Tachtsidis, I.; Smith, M.; et al. Towards a wearable near infrared spectroscopic probe for monitoring concentrations of multiple chromophores in biological tissue in vivo. Rev. Sci. Instrum. 2016, 87, 65112. [Google Scholar] [CrossRef]
- Wyser, D.; Lambercy, O.; Scholkmann, F.; Wolf, M.; Gassert, R. Wearable and modular functional near-infrared spectroscopy instrument with multidistance measurements at four wavelengths. Neurophotonics 2017, 4, 41413. [Google Scholar] [CrossRef]
- Zhang, Y.; Brooks, D.H.; Franceschini, M.A.; Boas, D.A. Eigenvector-based spatial filtering for reduction of physiological interference in diffuse optical imaging. J. Biomed. Opt. 2005, 10, 11014. [Google Scholar] [CrossRef]
- Santosa, H.; Jiyoun Hong, M.; Kim, S.P.; Hong, K.S. Noise reduction in functional near-infrared spectroscopy signals by independent component analysis. Rev. Sci. Instrum. 2013, 84, 73106. [Google Scholar] [CrossRef] [PubMed]
- Izzetoglu, M.; Chitrapu, P.; Bunce, S.; Onaral, B. Motion artifact cancellation in NIR spectroscopy using discrete Kalman filtering. Biomed. Eng. Online 2010, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Bray, S.; Reiss, A.L. Functional near infrared spectroscopy (NIRS) signal improvement based on negative correlation between oxygenated and deoxygenated hemoglobin dynamics. Neuroimage 2010, 49, 3039–3046. [Google Scholar] [CrossRef] [PubMed]
- Molavi, B.; Dumont, G.A. Wavelet-based motion artifact removal for functional near-infrared spectroscopy. Physiol. Meas. 2012, 33, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Scholkmann, F.; Spichtig, S.; Muehlemann, T.; Wolf, M. How to detect and reduce movement artifacts in near-infrared imaging using moving standard deviation and spline interpolation. Physiol. Meas. 2010, 31, 649–662. [Google Scholar] [CrossRef]
- Kassab, A.; Le Lan, J.; Tremblay, J.; Vannasing, P.; Dehbozorgi, M.; Pouliot, P.; Gallagher, A.; Lesage, F.; Sawan, M.; Nguyen, D.K. Multichannel wearable fNIRS-EEG system for long-term clinical monitoring. Hum. Brain Mapp. 2018, 39, 7–23. [Google Scholar] [CrossRef]
- Pinti, P.; Aichelburg, C.; Lind, F.; Power, S.; Swingler, E.; Merla, A.; Hamilton, A.; Gilber, S.; Burgess, P.; Tachtsidis, I. Using fiberless, wearable fnirs to monitor brain activity in real-world cognitive tasks. J. Vis. Exp. 2015, 2015, e53336. [Google Scholar] [CrossRef]
- Quaresima, V.; Ferrari, M. Functional Near-Infrared Spectroscopy (fNIRS) for Assessing Cerebral Cortex Function During Human Behavior in Natural/Social Situations: A Concise Review. Organ. Res. Methods 2019, 22, 46–68. [Google Scholar] [CrossRef]
- Funane, T. Wearable near-infrared spectroscopy neuroimaging and its applications. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; pp. 4025–4028. [Google Scholar]
- Kotozaki, Y.; Takeuchi, H.; Sekiguchi, A.; Yamamoto, Y.; Shinada, T.; Araki, T.; Takahashi, K.; Taki, Y.; Ogino, T.; Kiguchi, M.; et al. Biofeedback-based training for stress management in daily hassles: An intervention study. Brain Behav. 2014, 4, 566–579. [Google Scholar] [CrossRef]
- Zhang, Q.; Ivkovic, V.; Hu, G.; Strangman, G.E. Twenty-four-hour ambulatory recording of cerebral hemodynamics, systemic hemodynamics, electrocardiography, and actigraphy during people’s daily activities. J. Biomed. Opt. 2014, 19, 47003. [Google Scholar] [CrossRef]
- Farzam, P.; Durduran, T. Multidistance diffuse correlation spectroscopy for simultaneous estimation of blood flow index and optical properties. J. Biomed. Opt. 2015, 20, 55001. [Google Scholar] [CrossRef][Green Version]
- Yu, G. Near-infrared diffuse correlation spectroscopy in cancer diagnosis and therapy monitoring. J. Biomed. Opt. 2012, 17, 10901. [Google Scholar] [CrossRef] [PubMed]
- Kondepati, V.R.; Heise, H.M.; Backhaus, J. Recent applications of near-infrared spectroscopy in cancer diagnosis and therapy. Anal. Bioanal. Chem. 2008, 390, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Durduran, T.; Zhou, C.; Wang, H.W.; Putt, M.E.; Saunders, H.M.; Sehgal, C.M.; Glatstein, E.; Yodh, A.G.; Busch, T.M. Noninvasive monitoring of murine tumor blood flow during and after photodynamic therapy provides early assessment of therapeutic efficacy. Clin. Cancer Res. 2005, 11, 3543–3552. [Google Scholar] [CrossRef] [PubMed]
- Durduran, T.; Choe, R.; Yu, G.; Zhou, C.; Tchou, J.C.; Czerniecki, B.J.; Yodh, A.G. Diffuse optical measurement of blood flow in breast tumors. Opt. Lett. 2005, 30, 2915. [Google Scholar] [CrossRef]
- Zhou, C.; Choe, R.; Shah, N.; Durduran, T.; Yu, G.; Durkin, A.; Hsiang, D.; Mehta, R.; Butler, J.; Cerussi, A.; et al. Diffuse optical monitoring of blood flow and oxygenation in human breast cancer during early stages of neoadjuvant chemotherapy. J. Biomed. Opt. 2007, 12, 51903. [Google Scholar] [CrossRef]
- Boas, D.; Sutin, J.; Franceschini, M.A. System and Method for Non-Invasively Monitoring Intracranial Pressure 2016. International Application No. PCT/US2016/026920, 11 April 2016. [Google Scholar]
- Boas, D.A.; Franceschini, M.A. Haemoglobin oxygen saturation as a biomarker: The problem and a solution. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2011, 369, 4407–4424. [Google Scholar] [CrossRef]
- Forti, R.M.; Favilla, C.G.; Cochran, J.M.; Baker, W.B.; Detre, J.A.; Kasner, S.E.; Mullen, M.T.; Messé, S.R.; Kofke, W.A.; Balu, R.; et al. Transcranial Optical Monitoring of Cerebral Hemodynamics in Acute Stroke Patients during Mechanical Thrombectomy. J. Stroke Cerebrovasc. Dis. 2019, 28, 1483–1494. [Google Scholar] [CrossRef]
- Giovannella, M.; Ibañez, D.; Gregori-Pla, C.; Kacprzak, M. Concurrent measurement of cerebral hemodynamics and electroencephalography during transcranial direct current stimulation. Neurophotonics 2018, 5, 15001. [Google Scholar] [CrossRef]
- Mesquita, R.C.; Faseyitan, O.K.; Turkeltaub, P.E.; Buckley, E.M.; Thomas, A.; Kim, M.N.; Durduran, T.; Greenberg, J.H.; Detre, J.A.; Yodh, A.G.; et al. Blood flow and oxygenation changes due to low-frequency repetitive transcranial magnetic stimulation of the cerebral cortex. J. Biomed. Opt. 2013, 18, 67006. [Google Scholar] [CrossRef]
- Kim, M.N.; Edlow, B.L.; Durduran, T.; Frangos, S.; Mesquita, R.C.; Levine, J.M.; Greenberg, J.H.; Yodh, A.G.; Detre, J.A. Continuous optical monitoring of cerebral hemodynamics during head-of-bed manipulation in brain-injured adults. Neurocrit. Care 2014, 20, 443–453. [Google Scholar] [CrossRef]
- Kim, M.N.; Durduran, T.; Frangos, S.; Edlow, B.L.; Buckley, E.M.; Moss, H.E.; Zhou, C.; Yu, G.; Choe, R.; Maloney-Wilensky, E.; et al. Noninvasive measurement of cerebral blood flow and blood oxygenation using near-infrared and diffuse correlation spectroscopies in critically brain-injured adults. Neurocrit. Care 2010, 12, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Sutin, J.; Franceschini, M.A.; Boas, D. System and Method for Monitoring Absolute Blood Flow 2016. International Application No. PCT/US2016/026925, 11 April 2016. [Google Scholar]
- Yu, G.; Huang, C.; Hastings, J.T. Compact Low-Cost Fiberless Diffuse Speckle Contrast Flow-Oximeter 2018. U.S. Patent Application No. 15/655,988, 25 January 2018. [Google Scholar]
- Carp, S.A.; Farzam, P.; Redes, N.; Hueber, D.M.; Franceschini, M.A. Combined multi-distance frequency domain and diffuse correlation spectroscopy system with simultaneous data acquisition and real-time analysis. Biomed. Opt. Express 2017, 8, 39934006. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Tamborini, D.; Carp, S.A.; Shatrovoy, O.; Zimmerman, B.; Tyulmankov, D.; Siegel, A.; Blackwell, M.; Franceschini, M.A.; Boas, D.A. Time domain diffuse correlation spectroscopy: Modeling the effects of laser coherence length and instrument response function. Opt. Lett. 2018, 43, 2756–2759. [Google Scholar] [CrossRef] [PubMed]
- Diop, M.; Verdecchia, K.; Lee, T.-Y.; Lawrence, K.S. Calibration of diffuse correlation spectroscopy with a time-resolved near-infrared technique to yield absolute cerebral blood flow measurements. Biomed. Opt. Express 2011, 2, 2068–2081. [Google Scholar] [CrossRef] [PubMed]
- Verdecchia, K.; Diop, M.; Lee, T.-Y.; St. Lawrence, K. Quantifying the cerebral metabolic rate of oxygen by combining diffuse correlation spectroscopy and time-resolved near-infrared spectroscopy. J. Biomed. Opt. 2013, 18, 27007. [Google Scholar] [CrossRef]
- Mesquita, R.C.; D’Souza, A.; Bilfinger, T.V.; Galler, R.M.; Emanuel, A.; Schenkel, S.S.; Yodh, A.G.; Floyd, T.F. Optical monitoring and detection of spinal cord ischemia. PLoS ONE 2013, 8, e83370. [Google Scholar] [CrossRef]
- Zhou, C.; Eucker, S.A.; Durduran, T.; Yu, G.; Ralston, J.; Friess, S.H.; Ichord, R.N.; Margulies, S.S.; Yodh, A.G. Diffuse optical monitoring of hemodynamic changes in piglet brain with closed head injury. J. Biomed. Opt. 2009, 14, 34015. [Google Scholar] [CrossRef]
- Herrmann, S.; Petrov, I.Y.; Petrov, Y.; Richardson, C.J.; Fonseca, R.A.; Prough, D.S.; Esenaliev, R.O. Cerebral blood oxygenation measurements in neonates with optoacoustic technique. In Photons Plus Ultrasound: Imaging and Sensing 2017; Oraevsky, A.A., Wang, L.V., Eds.; International Society for Optics and Photonics: Bellingham, WA, USA, 2017; p. 100640Q. [Google Scholar]
- Rajaram, A.; Bale, G.; Kewin, M.; Morrison, L.B.; Tachtsidis, I.; Lawrence, K.S.; Diop, M. Simultaneous monitoring of cerebral perfusion and cytochrome c oxidase by combining broadband near-infrared spectroscopy and diffuse correlation spectroscopy. Biomed. Opt. Express 2018, 9, 2588–2603. [Google Scholar] [CrossRef]
- Wang, X.; Pang, Y.; Ku, G.; Xie, X.; Stoica, G.; Wang, L. V Noninvasive laser-induced photoacoustic tomography for structural and functional in vivo imaging of the brain. Nat. Biotechnol. 2003, 21, 803–806. [Google Scholar] [CrossRef]
- Tang, J.; Zhou, J.; Carney, P.R.; Jiang, H. Non-Invasive Real-time Photoacoustic Tomography of Hemodynamics in Freely Moving Rats. In Biomedical Optics; The Optical Society: Washington, DC, USA, 2014; p. BS4A.4. [Google Scholar]
- Deán-Ben, X.L.; Sela, G.; Lauri, A.; Kneipp, M.; Ntziachristos, V.; Westmeyer, G.G.; Shoham, S.; Razansky, D. Functional optoacoustic neuro-tomography for scalable whole-brain monitoring of calcium indicators. Light Sci. Appl. 2016, 5, e16201. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Sheng, Z.; Hu, D.; Lin, X.; Xu, S.; Liu, C.; Zheng, H.; Liu, B. Biocompatible conjugated polymer nanoparticles for highly efficient photoacoustic imaging of orthotopic brain tumors in the second near-infrared window. Mater. Horiz. 2017, 4, 1151–1156. [Google Scholar] [CrossRef]
- Guo, B.; Feng, Z.; Hu, D.; Xu, S.; Middha, E.; Pan, Y.; Liu, C.; Zheng, H.; Qian, J.; Sheng, Z.; et al. Precise Deciphering of Brain Vasculatures and Microscopic Tumors with Dual NIR-II Fluorescence and Photoacoustic Imaging. Adv. Mater. 2019, 31, 1902504. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, H.; Yan, H.; Liu, Y.; Zhang, J.; Shan, W.; Lai, P.; Li, H.; Ren, L.; Li, Z.; et al. Aggregation-Induced Absorption Enhancement for Deep Near-Infrared II Photoacoustic Imaging of Brain Gliomas In Vivo. Adv. Sci. 2019, 6, 1801615. [Google Scholar] [CrossRef]
- Li, W.; Chen, R.; Lv, J.; Wang, H.; Liu, Y.; Peng, Y.; Qian, Z.; Fu, G.; Nie, L. In Vivo Photoacoustic Imaging of Brain Injury and Rehabilitation by High-Efficient Near-Infrared Dye Labeled Mesenchymal Stem Cells with Enhanced Brain Barrier Permeability. Adv. Sci. 2018, 5, 1700277. [Google Scholar] [CrossRef]
- Kang, J.; Zhang, H.K.; Kadam, S.D.; Fedorko, J.; Valentine, H.; Malla, A.P.; Yan, P.; Harraz, M.M.; Kang, J.U.; Rahmim, A.; et al. Transcranial Recording of Electrophysiological Neural Activity in the Rodent Brain in vivo Using Functional Photoacoustic Imaging of Near-Infrared Voltage-Sensitive Dye. Front. Neurosci. 2019, 13, 579. [Google Scholar] [CrossRef]
- Fry, F.J.; Barger, J.E. Acoustical properties of the human skull. J. Acoust. Soc. Am. 1978, 63, 1576–1590. [Google Scholar] [CrossRef]
- Mohammadi, L.; Manwar, R.; Behnam, H.; Tavakkoli, J.; Nasiri Avanaki, M.R. Skull’s aberration modeling: Towards photoacoustic human brain imaging. In Photons Plus Ultrasound: Imaging and Sensing 2019; International Society for Optics and Photonics: Bellingham, WA, USA, 2019; p. 215. [Google Scholar]
- Petrov, Y.; Prough, D.S.; Petrov, I.Y.; Richardson, C.J.; Fonseca, R.A.; Robertson, C.S.; Esenaliev, R.O. Optoacoustic mapping of cerebral blood oxygenation in humans. In Photons Plus Ultrasound: Imaging and Sensing 2017; Oraevsky, A.A., Wang, L.V., Eds.; International Society for Optics and Photonics: Bellingham, WA, USA, 2017; p. 100640A. [Google Scholar]
- Tavakolian, P. Potential for Photoacoustic Imaging of Neonatal Brain. Master’s Thesis, The University of Western Ontario, London, ON, Canada, 2014. [Google Scholar]
- Hariri, A.; Tavakoli, E.; Adabi, S.; Gelovani, J.; Avanaki, M.R.N. Functional photoacoustic tomography for neonatal brain imaging: Developments and challenges. In Photons Plus Ultrasound: Imaging and Sensing 2017; Oraevsky, A.A., Wang, L.V., Eds.; International Society for Optics and Photonics: Bellingham, WA, USA, 2017; p. 100642Z. [Google Scholar]
- Wang, X.; Fowlkes, J.B.; Chamberland, D.L.; Xi, G.; Carson, P.L. Reflection mode photoacoustic imaging through infant skull toward noninvasive imaging of neonatal brains. In Photons Plus Ultrasound: Imaging and Sensing 2009; Oraevsky, A.A., Wang, L.V., Eds.; International Society for Optics and Photonics: Bellingham, WA, USA, 2009; p. 717709. [Google Scholar]
- Yang, X.; Wang, L.V. Monkey brain cortex imaging by photoacoustic tomography. J. Biomed. Opt. 2008, 13, 44009. [Google Scholar] [CrossRef]
- Kang, J.; Boctor, E.M.; Adams, S.; Kulikowicz, E.; Zhang, H.K.; Koehler, R.C.; Graham, E.M. Validation of noninvasive photoacoustic measurements of sagittal sinus oxyhemoglobin saturation in hypoxic neonatal piglets. J. Appl. Physiol. 2018, 125, 983–989. [Google Scholar] [CrossRef]
- Chen, Y.; Kateb, B. Neurophotonics and Brain Mapping, 1st ed.; CRC Press: Boca Raton, FL, USA, 2017; ISBN 9781482236859. [Google Scholar]
- Rao, Y.; Sarwade, N.P.; Makkar, R. Modeling and simulation of Optical Coherence Tomography on Virtual OCT. Procedia Comput. Sci. 2015, 45, 644–650. [Google Scholar] [CrossRef]
- Chen, C.-L.; Wang, R.K. Optical coherence tomography based angiography [Invited]. Biomed. Opt. Express 2017, 8, 1056. [Google Scholar] [CrossRef] [PubMed]
- Chong, S.P.; Merkle, C.W.; Leahy, C.; Srinivasan, V.J. Cerebral metabolic rate of oxygen (CMRO_2) assessed by combined Doppler and spectroscopic OCT. Biomed. Opt. Express 2015, 6, 3941. [Google Scholar] [CrossRef] [PubMed]
- Chong, S.P.; Merkle, C.W.; Cooke, D.F.; Zhang, T.; Radhakrishnan, H.; Krubitzer, L.; Srinivasan, V.J. Noninvasive, in vivo imaging of subcortical mouse brain regions with 17 μm optical coherence tomography. Opt. Lett. 2015, 40, 4911. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Shin, J.G.; Qureshi, M.M.; Chung, E.; Eom, T.J. Deep brain optical coherence tomography angiography in mice: In vivo, noninvasive imaging of hippocampal formation. Sci. Rep. 2018, 8, 11614. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, K.; Stein, K.P.; Neyazi, B.; Sandalcioglu, I.E. First in vivo visualization of the human subarachnoid space and brain cortex via optical coherence tomography. Ther. Adv. Neurol. Disord. 2019, 12, 1756286419843040. [Google Scholar] [CrossRef]
- Hartmann, K.; Stein, K.P.; Neyazi, B.; Erol Sandalcioglu, I. Aneurysm Architecture: First in vivo Imaging of Human Cerebral Aneurysms with Extravascular Optical Coherence Tomography. Cerebrovasc. Dis. 2019, 48, 26–31. [Google Scholar] [CrossRef]
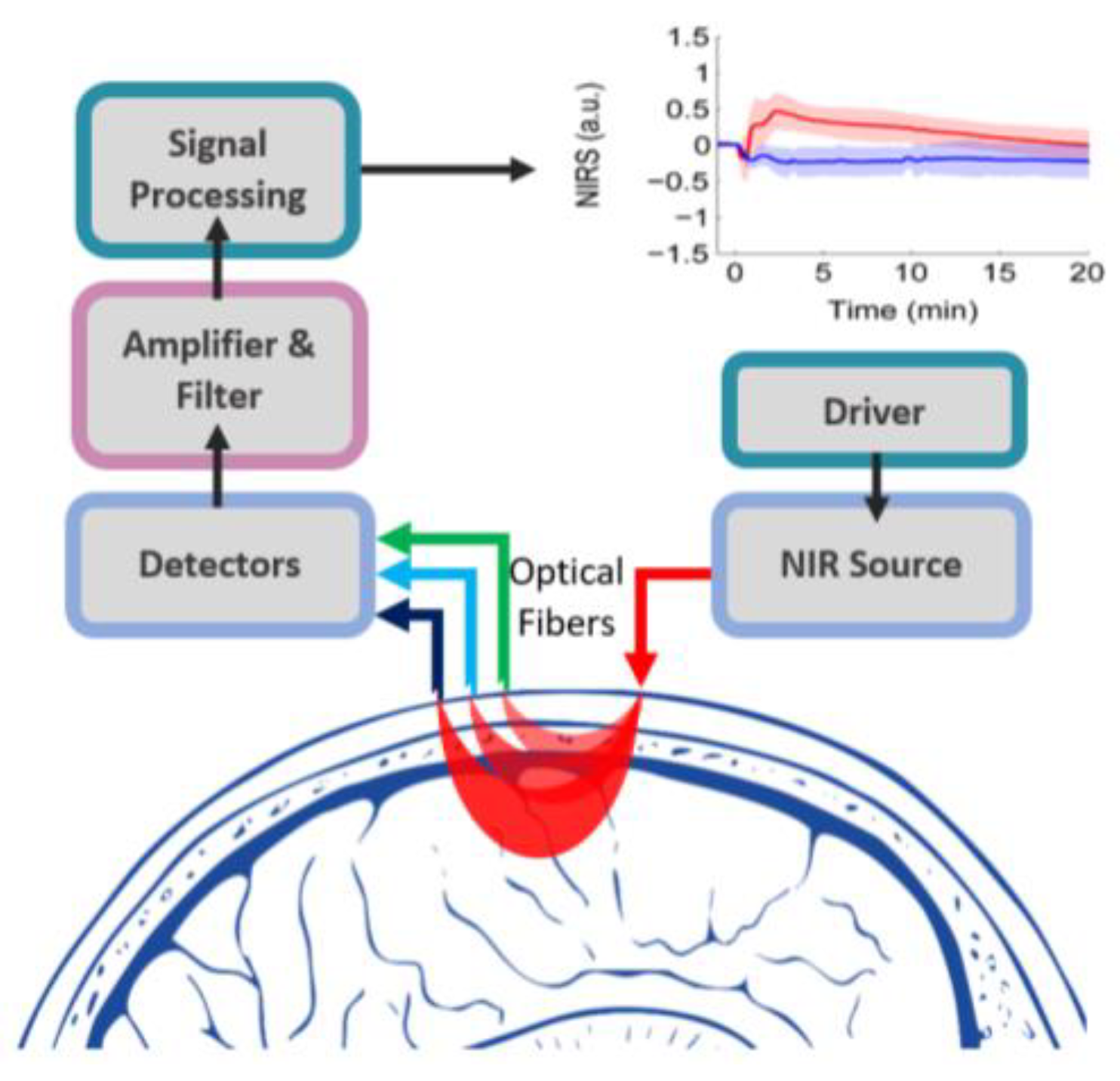
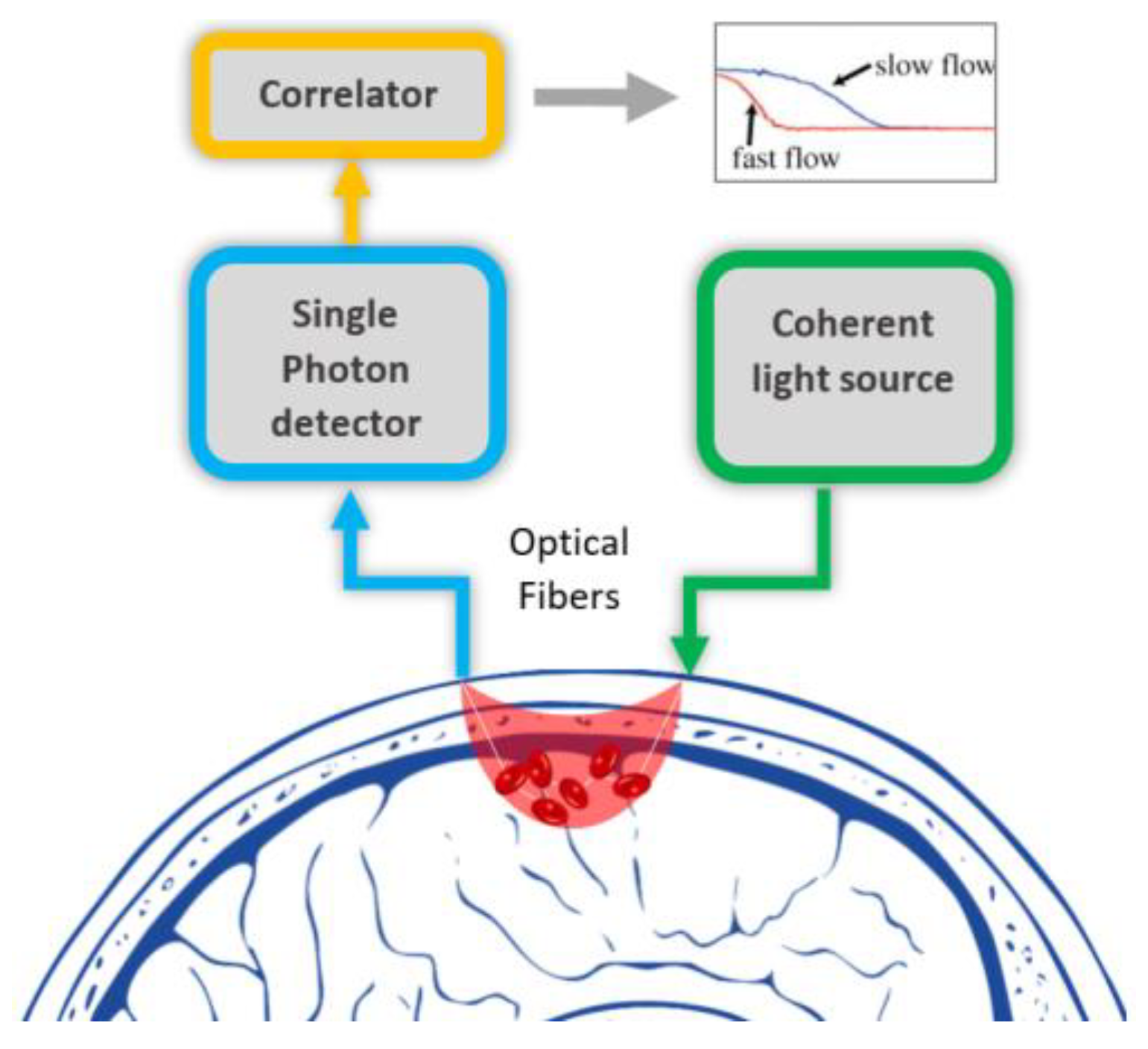
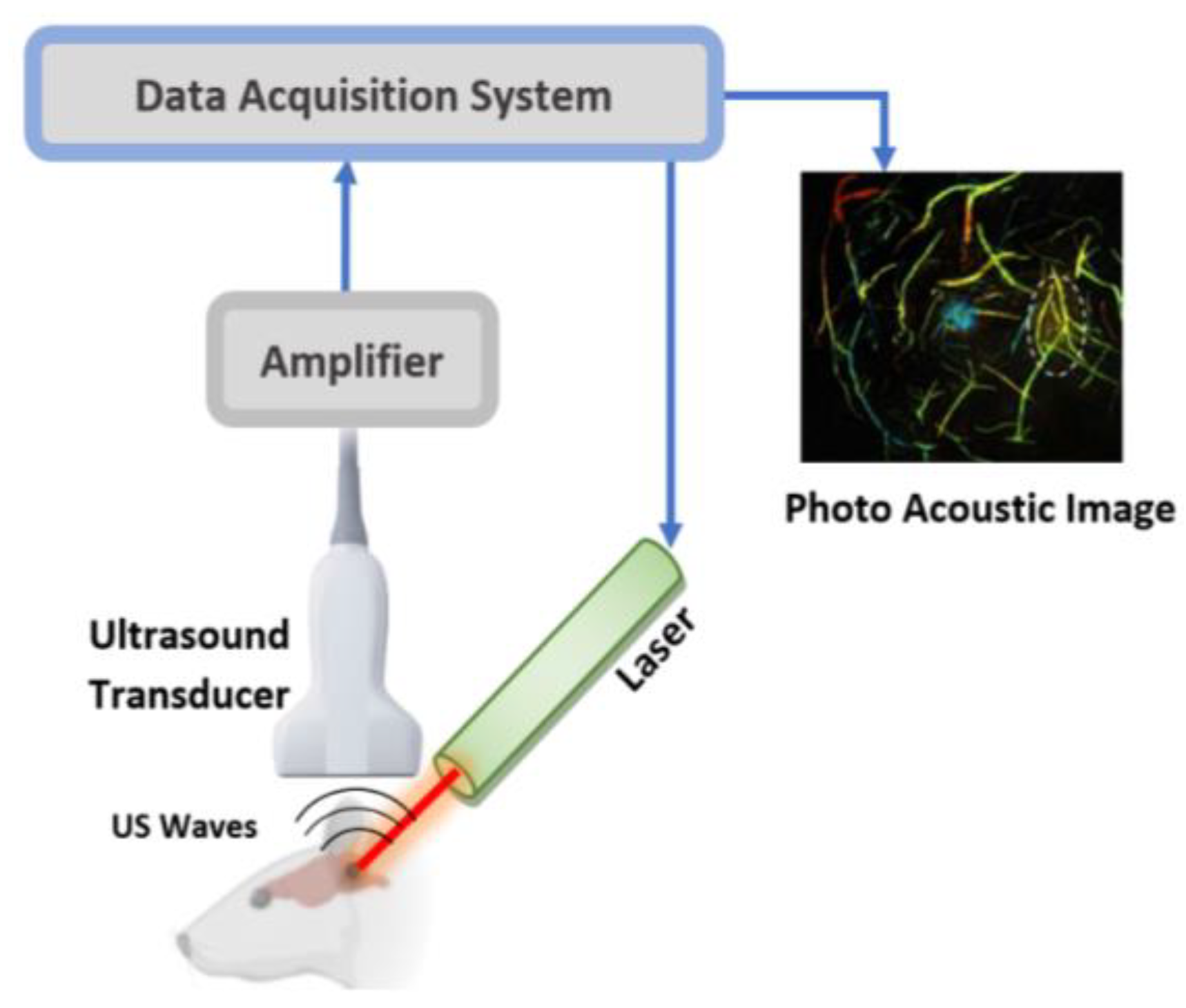
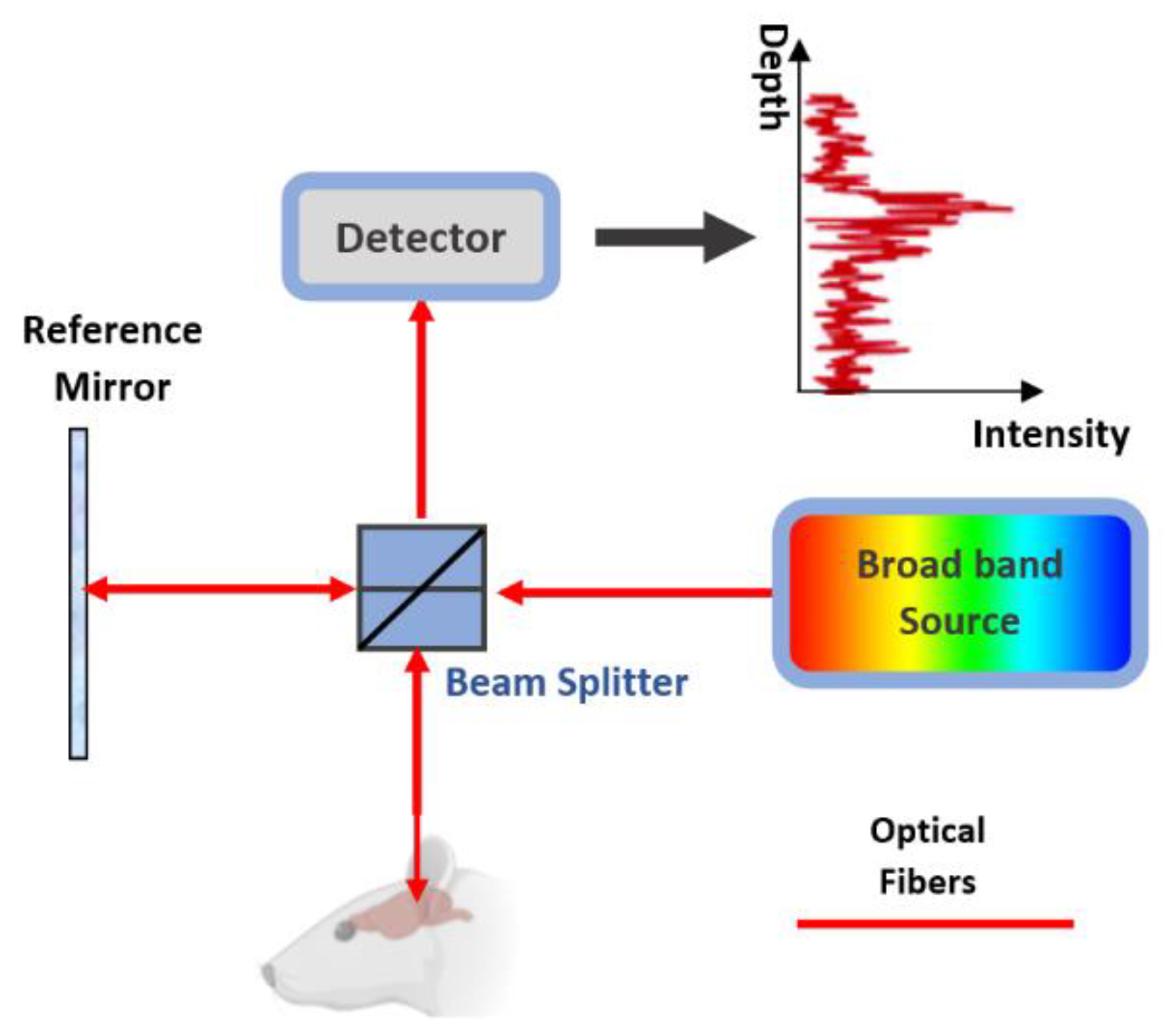
| Technology | Measurement Principle | Measuring Parameters | Wavelength (nm) | Source | Common Detector | penetration Depth | Spatial Resolution | Used as Wearable | Speed | Label Free | Cost |
|---|---|---|---|---|---|---|---|---|---|---|---|
| fNIRS | Scattering + absorption | CMRO2, HbT | 660–950 | LED, LD, Laser | PD | A few cm | ~1 cm/Fast | Yes | High | Yes | Low |
| Publications [15,16,17,18,19] | |||||||||||
| DCS | Speckle fluctuation | BFI, CMRO2 | 660–950 | Laser (coherent) | APD9 | up to ~1.5 cm | ~1 cm/ fast | Yes | High | Yes | Low |
| Publications [20,21,22,23,24,25,26,27,28,29] | |||||||||||
| PAI | PA effect | CMRO2, optical absorbers | 500–1300 | LD, Laser | PZT or array | up to 7 cm in soft tissues | Up to 1 μm | Yes | Depend on image size, Slow | Yes | High |
| Publications [13,30,31,32,33,34,35,36,37] | |||||||||||
| OCT | Light coherence properties | CBF, CMRO2 | Visible +IR | Wide band source | PD6 | up to 2 mm | Up to 1 μm | No | Depend on image size, high | Yes | Low |
| Publication [38] | |||||||||||
| Reference | Remark | Study/Application | Phase |
|---|---|---|---|
| Piper et al. [19] | 2-wavelength LED, frequency-encoded illumination, time-multiplexing detector, fixed number of channels | Self-paced left-hand gripping during outdoor bicycle riding, indoor pedaling on a training bicycle and sitting still | PoC |
| Pinti et al. [102] | 2-wavelength LED, 16 channels | Social daily life activities with cognitive tasks | PoC |
| Chitnis et al. [91] | 2-wavelength LED, time division, modular up to 128 channels | classic motor cortex stimulation experiments: movement of dominant hands and thumb-to-finger extension task | PoC |
| Funane et al. [92] | 2-wavelength LED, time division, modular up to 128 individual modules source or detector | human brain activity in the left prefrontal area of an adult male participant during a verbal fluency task | PoC |
| Chitnis et al. [93] | 8-wavelength LED, time division, modular, multidistance | visual stimulation paradigm designed to elicit a functional response in the primary visual cortex | PoC |
| Wyser et al. [94] | 4-wavelength LED, time division, modular, multidistance | Arterial occlusion and Task-evoked brain activity | PoC |
| Saikia et al. [90] | 2-wavelength LED, continuous wave, configurable up to 128 channels | Arterial occlusion and Prefrontal Cortex with various breathing task | PoC |
| Funane [104] | Continuous wave, 22 channels | Mental health | PoC in clinical use |
| Kotozaki et al. [105] | 1 channel, 810 nm | Stress management in daily hassles | PoC |
| Zhang et al. [106] | 2-wavelength laser diode, continuous wave, with ECG and acceleration sensor | Normal daily activities, e.g., walking, playing | PoC in clinical use |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karthikeyan, P.; Moradi, S.; Ferdinando, H.; Zhao, Z.; Myllylä, T. Optics Based Label-Free Techniques and Applications in Brain Monitoring. Appl. Sci. 2020, 10, 2196. https://doi.org/10.3390/app10062196
Karthikeyan P, Moradi S, Ferdinando H, Zhao Z, Myllylä T. Optics Based Label-Free Techniques and Applications in Brain Monitoring. Applied Sciences. 2020; 10(6):2196. https://doi.org/10.3390/app10062196
Chicago/Turabian StyleKarthikeyan, Priya, Sadegh Moradi, Hany Ferdinando, Zuomin Zhao, and Teemu Myllylä. 2020. "Optics Based Label-Free Techniques and Applications in Brain Monitoring" Applied Sciences 10, no. 6: 2196. https://doi.org/10.3390/app10062196
APA StyleKarthikeyan, P., Moradi, S., Ferdinando, H., Zhao, Z., & Myllylä, T. (2020). Optics Based Label-Free Techniques and Applications in Brain Monitoring. Applied Sciences, 10(6), 2196. https://doi.org/10.3390/app10062196





