Comparing Graphene Oxide and Reduced Graphene Oxide as Blending Materials for Polysulfone and Polyvinylidene Difluoride Membranes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Fabrication of GO and rGO
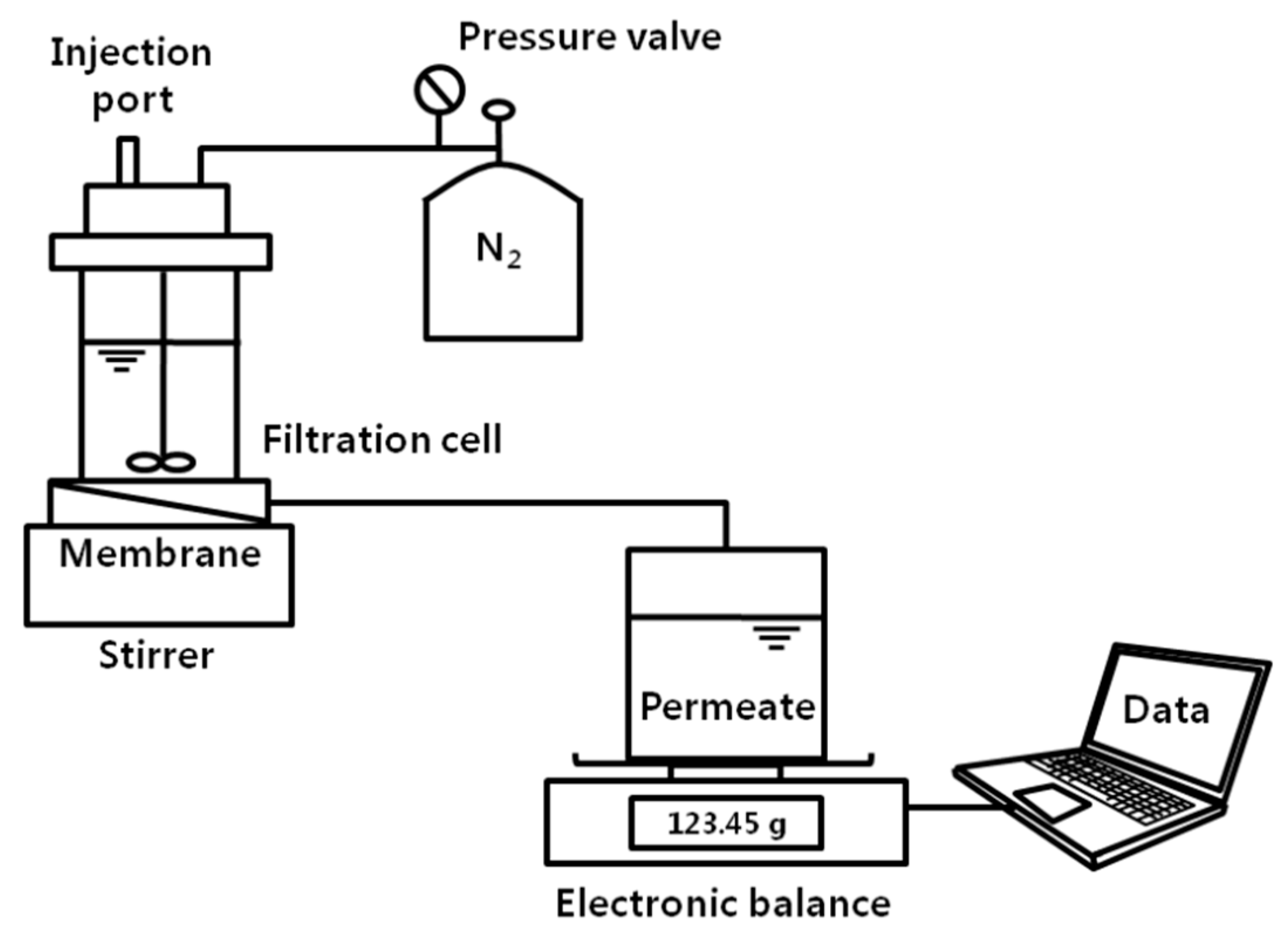
2.3. Preparation of Membranes and Permeation Test
2.4. Characterization Techniques
2.5. Analytical Methods in Rejection Test
3. Results and Discussion
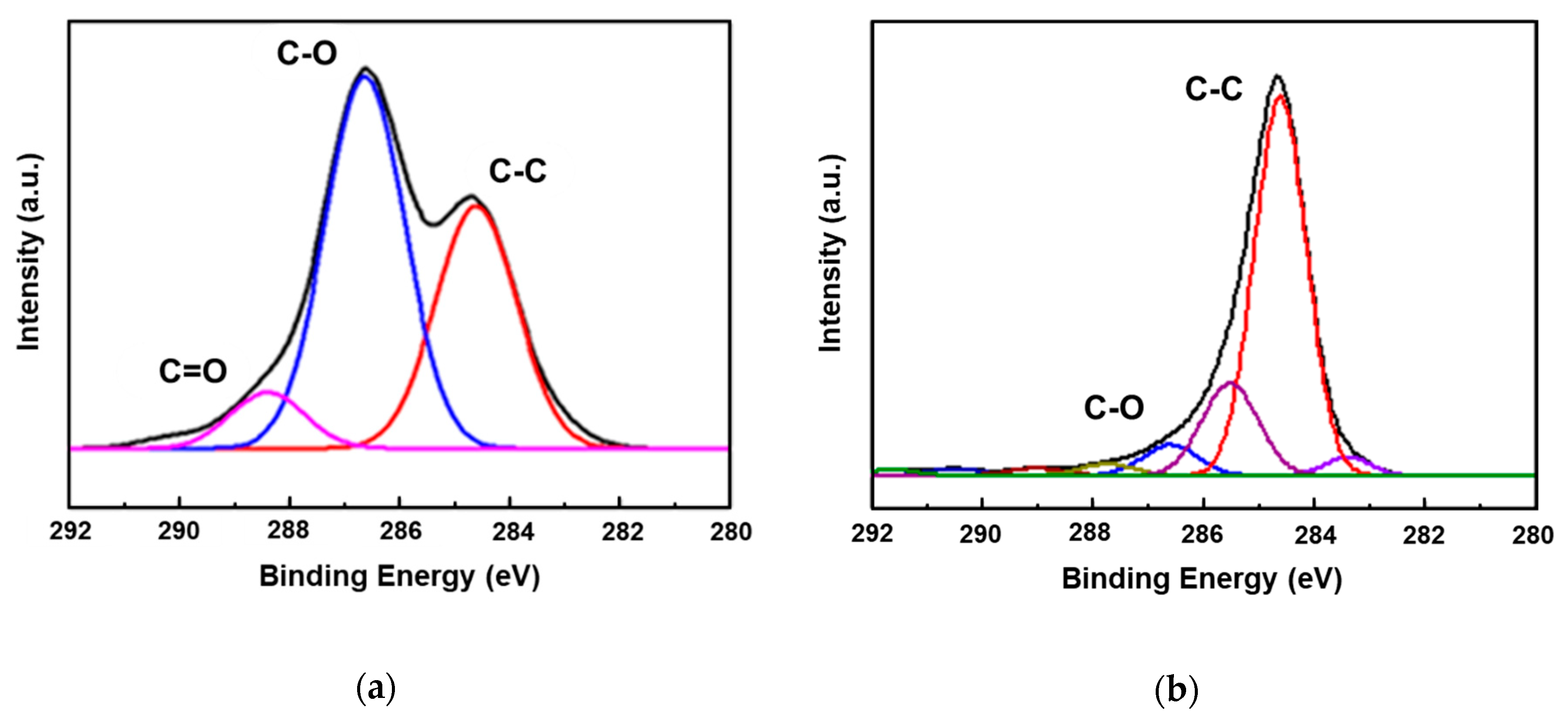
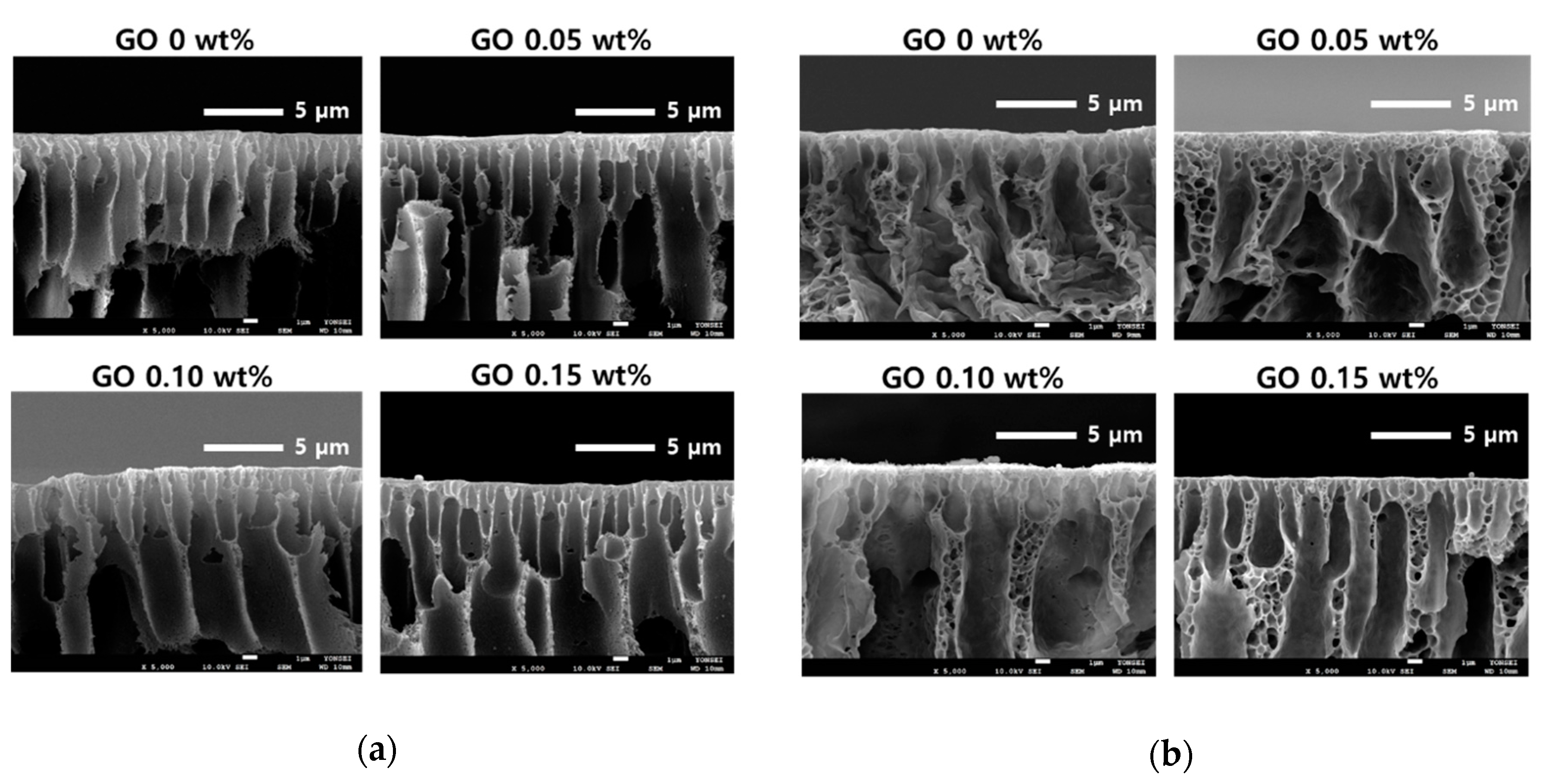
3.1. Characterization of Graphenes and Membranes
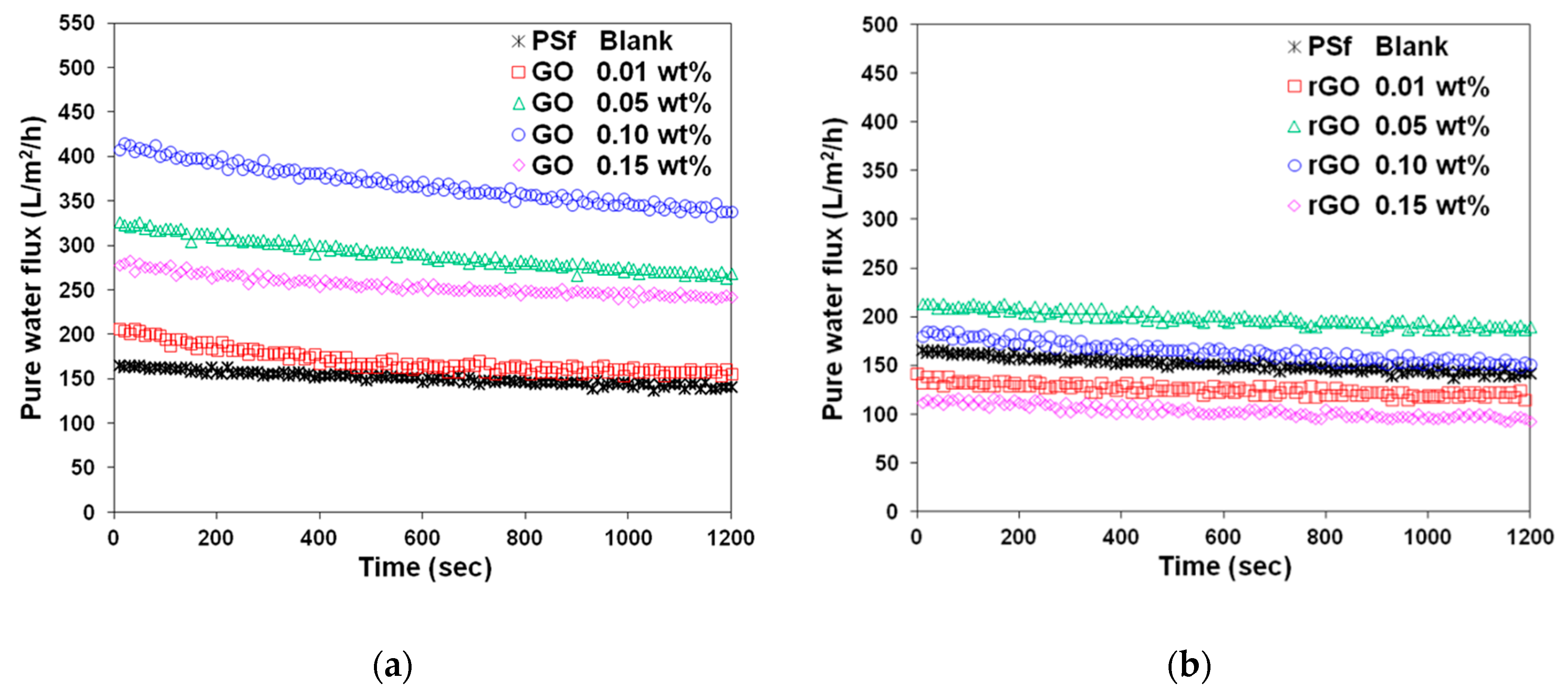
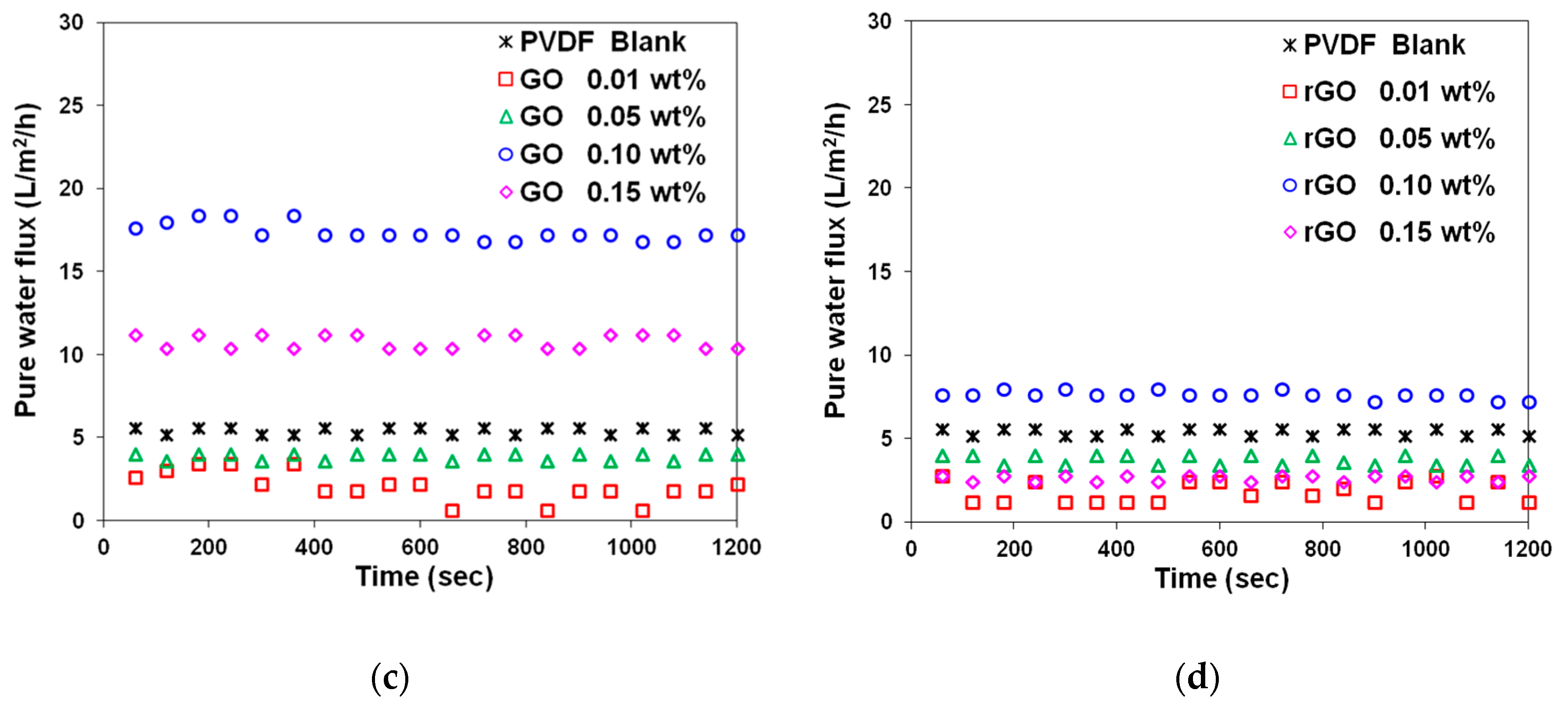
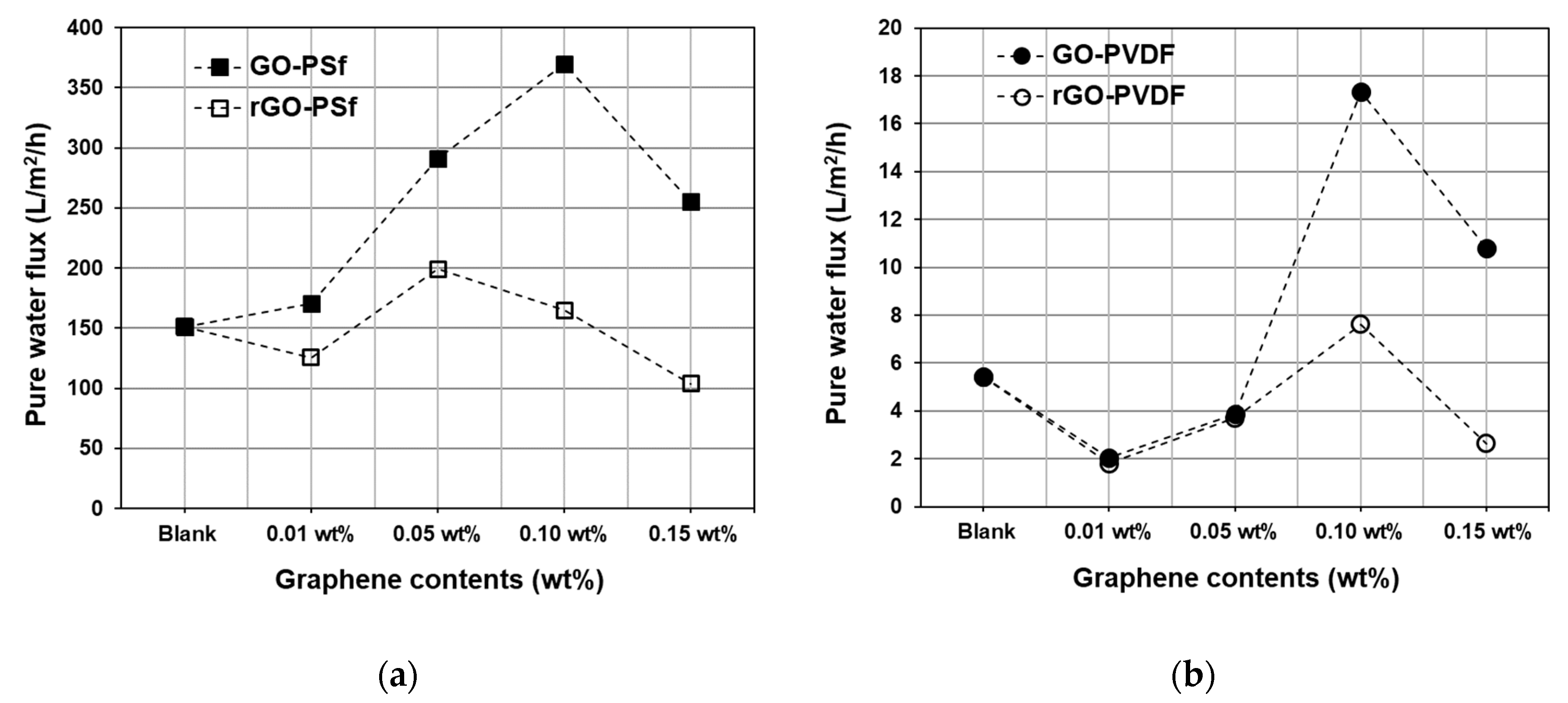
3.2. Effect of GO and rGO on Membrane Flux Increase
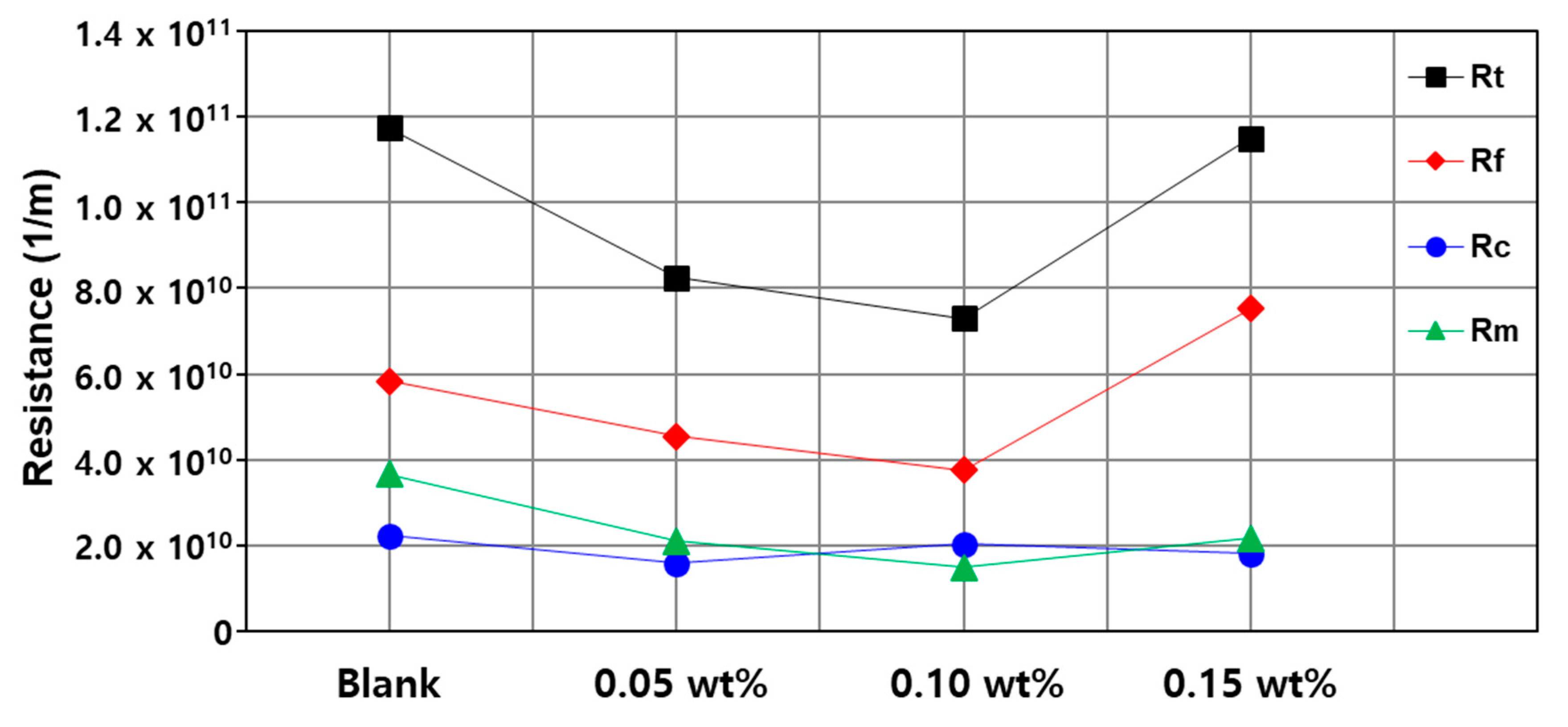
3.3. Fouling and Rejection Test
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, Z.H.; Yu, H.R.; Xia, J.F.; Zhang, F.F.; Li, F.; Xia, Y.Z.; Li, Y.H. Novel GO-blended PVDF ultrafiltration membranes. Desalination 2012, 299, 50–54. [Google Scholar] [CrossRef]
- Guo, F.; Silverberg, G.; Bowers, S.; Kim, S.P.; Datta, D.; Shenoy, V.; Hurt, R.H. Graphene-Based Environmental Barriers. Environ. Sci. Technol. 2012, 46, 7717–7724. [Google Scholar] [CrossRef] [PubMed]
- Zularisam, A.W.; Ismail, A.F.; Salim, R. Behaviours of natural organic matter in membrane filtration for surface water treatment—A review. Desalination 2006, 194, 211–231. [Google Scholar] [CrossRef]
- Zhao, C.; Xu, X.; Chen, J.; Yang, F. Effect of graphene oxide concentration on the morphologies and antifouling properties of PVDF ultrafiltration membranes. J. Environ. Chem. Eng. 2013, 1, 349–354. [Google Scholar] [CrossRef]
- Kim, E.S.; Yu, Q.S.; Deng, B.L. Plasma surface modification of nanofiltration (NF) thin-film composite (TFC) membranes to improve anti organic fouling. Appl. Surf. Sci. 2011, 257, 9863–9871. [Google Scholar] [CrossRef]
- Rahimpour, A.; Madaeni, S.S.; Zereshki, S.; Mansourpanah, Y. Preparation and characterization of modified nano-porous PVDF membrane with high antifouling property using UV photo-grafting. Appl. Surf. Sci. 2009, 255, 7455–7461. [Google Scholar] [CrossRef]
- Wang, P.P.; Ma, J.; Wang, Z.H.; Shi, F.M.; Liu, Q.L. Enhanced Separation Performance of PVDF/PVP-g-MMT Nanocomposite Ultrafiltration Membrane Based on the NVP-Grafted Polymerization Modification of Montmorillonite (MMT). Langmuir 2012, 28, 4776–4786. [Google Scholar] [CrossRef]
- Ellerie, J.R.; Apul, O.G.; Karanfil, T.; Ladner, D.A. Comparing graphene, carbon nanotubes, and superfine powdered activated carbon as adsorptive coating materials for microfiltration membranes. J. Hazard. Mater. 2013, 261, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.G.; Hyeon, D.H.; Chun, J.H.; Chun, B.H.; Kim, S.H. Novel thin nanocomposite RO membranes for chlorine resistance. Desalin Water Treat 2013, 51, 6338–6345. [Google Scholar] [CrossRef]
- Perreault, F.; Tousley, M.E.; Elimelech, M. Thin-Film Composite Polyamide Membranes Functionalized with Biocidal Graphene Oxide Nanosheets. Environ. Sci. Technol. Lett. 2014, 1, 71–76. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Chae, H.R.; Won, Y.J.; Lee, K.; Lee, C.H.; Lee, H.H.; Kim, I.C.; Lee, J.M. Graphene oxide nanoplatelets composite membrane with hydrophilic and antifouling properties for wastewater treatment. J. Membrane. Sci. 2013, 448, 223–230. [Google Scholar] [CrossRef]
- Gomez-Navarro, C.; Meyer, J.C.; Sundaram, R.S.; Chuvilin, A.; Kurasch, S.; Burghard, M.; Kern, K.; Kaiser, U. Atomic Structure of Reduced Graphene Oxide. Nano Lett. 2010, 10, 1144–1148. [Google Scholar] [CrossRef] [PubMed]
- McAllister, M.J.; Li, J.L.; Adamson, D.H.; Schniepp, H.C.; Abdala, A.A.; Liu, J.; Herrera-Alonso, M.; Milius, D.L.; Car, R.; Prud’homme, R.K.; et al. Single sheet functionalized graphene by oxidation and thermal expansion of graphite. Chem. Mater. 2007, 19, 4396–4404. [Google Scholar] [CrossRef]
- Moon, I.K.; Lee, J.; Ruoff, R.S.; Lee, H. Reduced graphene oxide by chemical graphitization. Nat. Commun. 2010, 1, 1–6. [Google Scholar] [CrossRef]
- Williams, G.; Seger, B.; Kamat, P.V. TiO2-graphene nanocomposites. UV-assisted photocatalytic reduction of graphene oxide. ACS Nano 2008, 2, 1487–1491. [Google Scholar] [CrossRef]
- Ganesh, B.M.; Isloor, A.M.; Ismail, A.F. Enhanced hydrophilicity and salt rejection study of graphene oxide-polysulfone mixed matrix membrane. Desalination 2013, 313, 199–207. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Park, W.K.; Kim, H.; Kim, T.; Kim, Y.; Yoo, S.; Kim, S.; Yoon, D.H.; Yang, W.S. Facile synthesis of graphene oxide in a Couette-Taylor flow reactor. Carbon 2015, 83, 217–223. [Google Scholar] [CrossRef]
- Cheryan, M.; Cheryan, M. Ultrafiltration and Microfiltration Handbook; Technomic Publisher Co.: Lancaster, PA, USA, 1998; p. 527. [Google Scholar]
- Oh, B.S.; Jang, H.Y.; Hwang, T.M.; Kang, J.W. Role of ozone for reducing fouling due to pharmaceuticals in MF (microfiltration) process. J. Membrane. Sci. 2007, 289, 178–186. [Google Scholar] [CrossRef]

| Parameters | Turbidity (NTU) | TDS (mg/L) | UVA254 (UV Absorbance at 254 nm) | TOC (mg/L) |
|---|---|---|---|---|
| Feed water | 250 | 16 | 1.057 | 10.8 |
| PSf Blank | 1.1 | 6.0 | 0.0100 | 1.1 |
| PSf/GO 0.05 wt% | 0.8 | 5.0 | 0.0047 | 0.9 |
| PSf/GO 0.10 wt% | 0.5 | 4.0 | 0.0043 | 0.8 |
| PSf/GO 0.15 wt% | 2.2 | 4.0 | 0.0290 | 1.2 |
| PSf/rGO 0.05 wt% | 1.7 | 5.0 | 0.0111 | 1.3 |
| PSf/rGO 0.10 wt% | 0.88 | 5.0 | 0.0065 | 0.97 |
| PSf/rGO 0.15 wt% | 2.54 | 5.0 | 0.0208 | 1.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, Y.; Kye, H.; Yang, W.S.; Kang, J.-W. Comparing Graphene Oxide and Reduced Graphene Oxide as Blending Materials for Polysulfone and Polyvinylidene Difluoride Membranes. Appl. Sci. 2020, 10, 2015. https://doi.org/10.3390/app10062015
Yoon Y, Kye H, Yang WS, Kang J-W. Comparing Graphene Oxide and Reduced Graphene Oxide as Blending Materials for Polysulfone and Polyvinylidene Difluoride Membranes. Applied Sciences. 2020; 10(6):2015. https://doi.org/10.3390/app10062015
Chicago/Turabian StyleYoon, Yeojoon, Homin Kye, Woo Seok Yang, and Joon-Wun Kang. 2020. "Comparing Graphene Oxide and Reduced Graphene Oxide as Blending Materials for Polysulfone and Polyvinylidene Difluoride Membranes" Applied Sciences 10, no. 6: 2015. https://doi.org/10.3390/app10062015
APA StyleYoon, Y., Kye, H., Yang, W. S., & Kang, J.-W. (2020). Comparing Graphene Oxide and Reduced Graphene Oxide as Blending Materials for Polysulfone and Polyvinylidene Difluoride Membranes. Applied Sciences, 10(6), 2015. https://doi.org/10.3390/app10062015






