Abstract
Many studies highlighted that a bidirectional communication between the gut and the central nervous system (CNS) exists. A vigorous immune response to antigens must be avoided, and pathogenic organisms crossing the gut barrier must be detected and killed. For this reason, the immune system developed fine mechanisms able to maintain this delicate balance. The microbiota is beneficial to its host, providing protection against pathogenic bacteria. It is intimately involved in numerous aspects of host physiology, from nutritional status to behavior and stress response. In the last few years, the implication of the gut microbiota and its bioactive microbiota-derived molecules in the progression of multiple diseases, as well as in the development of neurodegenerative disorders, gained increasing attention. The purpose of this review is to provide an overview of the gut microbiota with particular attention toward neurological disorders and mast cells. Relevant roles are played by the mast cells in neuroimmune communication, such as sensors and effectors of cytokines and neurotransmitters. In this context, the intake of beneficial bacterial strains as probiotics could represent a valuable therapeutic approach to adopt in combination with classical therapies. Further studies need to be performed to understand if the gut bacteria are responsible for neurological disorders or if neurological disorders influence the bacterial profile.
1. Introduction
The gastrointestinal (GI) tract is an organ system responsible for transporting, absorbing, digesting, and excreting food and waste, as well as monitoring the hydrosaline balance. The GI tract produces and releases enzymes and peptide hormones, including gastrin, secretin, cholecystokinin, gastric inhibitory peptide (GIP), and motilin in order to help the digestive process, as well as local factors such as prostaglandins, histamine, and other molecules, released into the interstitial fluid. These molecules coordinate the response to local pH variation, presence of chemical substances, or physical stimuli [1,2,3,4,5,6,7]. When homeostasis is challenged by pathogen or injury, inflammation occurs and the GI tract switches the balance from an absorptive state to a secretory status [5,6].
The internal milieu of the GI tract is the mucosal epithelium that secretes a viscous fluid (mucus) acting as a barrier against most microorganisms by coating and preventing them adhering to the epithelium [8,9,10,11,12]. Most of the microorganisms that manage to cross the epithelial surfaces are efficiently removed by the innate immune response in the underlying tissues.
The intestinal barrier is composed of cellular structural components, including enterocytes, goblet cells, Paneth cells, and enterochromaffin cells (ECs). In the gut, ECs are the most abundant neuroendocrine cells and are considered to be the first “sensors” of the luminal content. They are the primary source of neurotransmitter 5-hydroxytryptamine (5-HT) or serotonin (5-HT) within the body, synthetized by the hydroxylation and decarboxylation of tryptophan.
Enterocytes and goblet cells both produce mucin glycoproteins, forming mucus that physically separates the microbiota from the epithelium (although some bacteria can elude it), whereas Paneth cells are responsible for the production of antimicrobial peptides (AMPs), including defensins, lysozymes, and cathelicidins that limit bacterial growth, shaping the microbial population.
The first contact with luminal antigens and commensal and/or pathogenic microorganisms takes place through enterocytes [10,13], considered themselves as part of the intestinal immune system’s innate response. Enterocytes permanently interact with intestinal lumen contents and enter in close contact with a large number of antigens, harmful bacteria, or molecules. Enterocytes can function as unprofessional antigen-presenting cells (APC), presenting partially processed antigens to T cells in the lamina propria. They can also secrete a variety of cytokines and chemokines that trigger the recruitment of immune cells from different intestinal sites, inducing an active immune response [13]. The GI tract is the major immune organ equipped with the largest pool of immune cells.
The GI tract is under control of the enteric nervous system (ENS). ENS contains different types of neurons located along the length of the GI tract. In particular, the most important are the enteric neurons and enteric glial cells (EGCs), where the latter are found within the smooth muscle layer and the lamina propria of the mucosa [1,4,7,14,15,16,17,18,19,20,21,22,23,24,25]. Hence, changes in GI physiology and the environment that are associated with nutrition or the establishment of a luminal microbial population and the maturation of the mucosal immune system are likely engaged to affect the post-natal phase of ENS development [17,18,19,20,21,22,23,24,25,26,27]. Although the GI normally communicates with the central nervous system (CNS) through the parasympathetic and sympathetic (via the prevertebral ganglia) nervous systems [16,17,21,27], the ENS works as an intrinsic nervous system capable of controlling most physiological functions of the GI (such as reflexes, motility, secretion, micro-circulation, immune function, and the inflammatory process) in an independent manner [17,18,19,20,21,22,23,24,25,26]. These evidences led to defining the concept of a “brain in the gut”. Nevertheless, both the ENS and the CNS communicate and influence each other. In particular, vagal afferents send diverse GI signals to the CNS that reflect food intake, nutrient content, and overall energy stores [16,17,21,27].
2. 90% Microbes and 10% Human Cells: The Human Gut Microbiota
Most of the gut is heavily colonized by trillions of commensal microorganisms, which live in symbiosis (eubiosis) with their host, established across hundreds of years of co-evolution [28,29,30,31,32,33]. Such an intestinal community of bacteria is termed the microbiota. This abundance of different bacterial species sets up a unique relationship with the host, contributing to beneficial effects in many ways. The microbiota plays a critical role in GI physiology as it contributes to the breakdown of undigested or indigested nutrients, synthesis of endogenous vitamins (vitamin K and most of the components of the vitamin B complex), epithelial integrity and barrier function, angiogenesis, and maturation of the mucosal immune system [34,35] (Figure 1). Moreover, it affects the normal development of the ENS in early life and, in adulthood, it interacts with the ENS [36,37,38,39,40]. The microbiota colonizes virtually every surface of the human body that is exposed to the external environment. The microbiota distribution along the length of the GI tract is not homogeneous. The prevalence of bacteria in different parts of the gut appears to be dependent on several factors, such as pH, intestinal peristalsis, redox potential, bacterial adhesion, bacterial cooperation, mucus secretion, mucosal integrity, nutrient availability, diet, and bacterial antagonism [34,35,40,41,42,43,44,45,46]. In particular, the density of bacteria increases exponentially from the upper portion to the lower intestine [28,34]. The low pH and the relatively swift peristalsis through the stomach make it hard for bacteria to colonize this tract. In the colon, instead, with decreased peristalsis and acidity, as well as lower redox potentials, a more diverse microbiota is maintained and a higher bacterial population is present [29,35]. Studies suggested that the colonization of gut begins prior to birth in the fetal stage [47]. Microbiome changes in the newborn and substantial changes in composition occur during the first years of life, remain relatively constant until adult age, and then decrease in old age. The microbiota acts as a physical barrier. Microorganisms occupy all available habitats at the mucosal level, competing for receptor sites and for metabolic/nutrient substrates with exogenous pathogenic bacteria. This mechanism is known as colonization resistance [29,30,31,32,33,34,35,47].
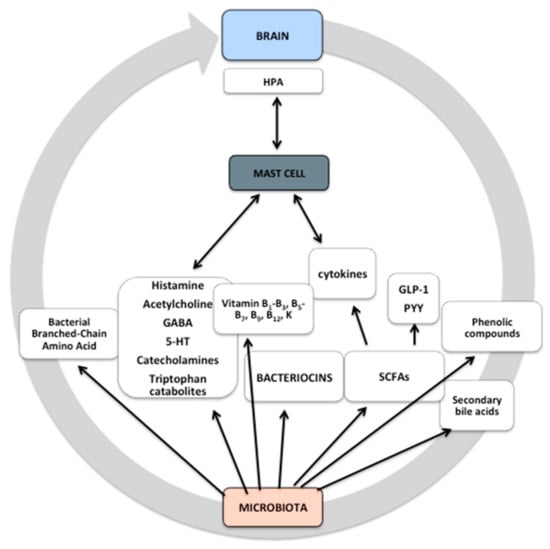
Figure 1.
Microbiota-derived molecules. Microbiota-derived molecules are produced as intermediates or end products of microbial metabolism. These molecules can be derived directly from bacteria or from modification of other molecules, such as bile acids, and they influence fundamental biological functions. Some microbial products may pass through the blood–brain barrier (BBB), thereby affecting it. The microbiota and its metabolites can control events both in the peripheral and the central nervous system (CNS) through nerve activation, cytokine production, neurotransmitters, and short-chain fatty acid (SCFA) release, as well as via systemic circulation. Some signals may activate mast cells that are both sensors and effectors of cytokines and neuropeptides/neurotransmitters, affecting hypothalamic–pituitary–adrenal (HPA) activity. The exact mechanism remains unknown. GABA, gamma-aminobutyric acid; 5-HT, 5-hydroxytryptamine.
Various commensal bacteria can influence the number of microbiota-derived molecules. The microbiota can influence the energy balance of the host by exploiting energy derived from the metabolism of sugars and proteins (fermentation) or through the transformation of indigestible polysaccharides in volatile substances, such as carbon dioxide, hydrogen sulfide, and short-chain fatty acids (SCFAs) (Figure 1). The latter include acetic acid (reabsorbed by the intestinal wall and used as a substrate by various tissues, including the adipose tissue for lipogenesis), butyric acid (energy source for the same intestinal cells), and propionic acid (reabsorbed in the gut and used by the liver for the gluconeogenic metabolism) [35,36,37,38,39]. Propionic and butyric acid were shown to exert anti-inflammatory and immunomodulatory effects [35]. They stimulate enteroendocrine cells to produce various neuropeptides, including neuropeptide Y and substance P (SP), which gain access to circulation and/or receptors affecting ENS neurons or vagal innervation [46].
3. Gut Microbiota and Immune Protection
An important interplay between the microbiota and immune response exists because the microbiota controls the development and function of the immune response, which in turn regulates the composition and the function of microbiota [35,40,41,42,43,44,45,46,47]
The central role of the gut microbiota in the development of mucosal immunity is not surprising considering that (A) the intestinal mucosa represents the largest surface area in contact with the antigens of the external environment, and (B) the dense carpet of the gut microbiota overlying the mucosa normally accounts for the largest proportion of antigens presented to the resident immune cells. The importance of the gut microbiota in the development of the systemic immune system was validated by a series of studies with a germ-free (GF) mouse model, born and maintained in sterile conditions, which presented various immune disorders. They contained abnormal numbers of several immune cell types and altered cytokines, as well as deficits in local and systematic lymphoid structure. GF animals showed significant differences in a variety of ENS/CNS diseases, such as neurodegenerative disorders, anxiety, depression, reduction of brain-derived neurotrophic factor (BDNF) gene expression in the amygdala and hippocampus, increased permeability of the blood–brain barrier (BBB), hypothalamic–pituitary–adrenal axis (HPA) hyperactivation, and dysfunction of the microglia [48,49].
4. Alteration of Gut–Brain Axis and Neurodegeneration
The gut microbiota plays an important role in the development of brain regions, and it affects human behavior, as well as brain function. Recent evidence points to a causative link between alteration of the gut microbiota and neurodegenerative/neuroinflammatory diseases. Several psychiatric and neurological disorders were found to be associated with changes in the gut microbiota. Studies showed that the gut–brain axis may have a significant impact on anxiety, stress, depression, chronic pain, autism, and cognitive function. The central, autonomic, and enteric nervous systems together with the immune system and the endocrine system constantly ensure the proper functioning of the gut–brain axis.
Dysregulation of the gut–brain axis through a complex bidirectional communication system is associated with the pathogenesis of several neurodegenerative diseases [50].
Gut microbiome dysbiosis is responsible for the development of local and systemic inflammation, resulting in disintegration of the gut epithelial membrane, hyperpermeability, invasion of bacteria and viruses within the brain parenchyma, and, ultimately, neuroinflammation and dysfunction of neuronal cells. Several studies using GF animal models were conducted to investigate the interaction between gut microbiota and brain, and many of them showed increased BBB permeability compared to animals with normal gut flora [51,52,53].
Bacteria secrete and consume a plethora of neuromodulators and neurotransmitters, including 5-HT, dopamine, gamma-aminobutyric acid (GABA), epinephrine, and norepinephrine, which are identical to those produced by humans. Accumulating evidence suggests that manipulation of these neurotransmitters by bacteria may have an impact on host physiology, and preliminary human studies showed that microbiota-based interventions can also alter neurotransmitter levels involved in synaptic plasticity, such as BDNF, as well as modify the activity of N-methyl-d-aspartate (NMDA) and 5-HT receptors [54].
Although the gut microbiota produces neuroactive compounds, other toxins and metabolites can be released. These molecules act through the ENS by modulating the brain signaling pathways that regulate mood, cognition, social behavior, and memory [55,56,57,58,59,60].
A key factor of the most neurodegenerative diseases is the formation of insoluble protein aggregates within neurons. Gut dysbiosis can result in the accumulation of toxic misfolded proteins with β-sheet conformation, leading to cellular cell dysfunction, loss of synaptic connections, and neurodegenerative disorders [61]. A representative scheme is illustrated in Figure 2.
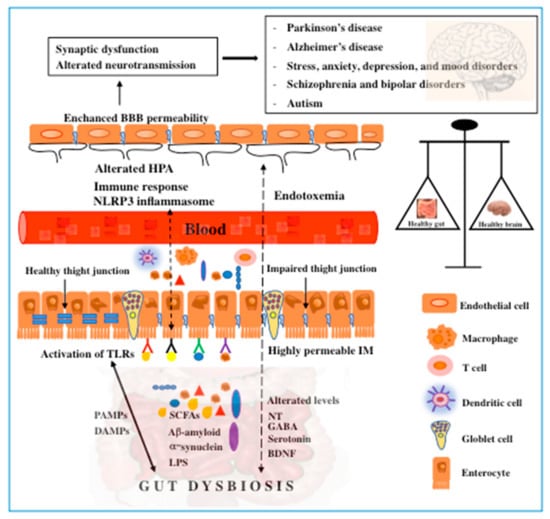
Figure 2.
Schematic representation of the role of the gut microbiota in the pathogenesis of neurodegenerative and mental disorders and vice versa. A growing body of evidence suggests the bidirectional signaling between the gut microbiota and the brain in mediating brain diseases. Alterations in gut microbiota composition or microbial-derived products play an important role in modulating the gut–brain axis. Prolonged activation of Toll-like receptors (TLRs) by damage-associated molecular patterns (DAMPs) or pathogen-associated molecular patterns (PAMPs), SCFAs, α-synuclein, etc. represents an important contributory factor to the development of neurodegenerative diseases through mechanisms which involve the immune response and inflammation. The disruption of tight junctions leads to the leakage of microbial products, including neurotransmitters, SCFAs, and neuroactive molecules into the system circulation. Endotoxemia associated with the NOD-, LRR-, and pyrin domain-containing protein 3 (NLRP3) inflammasome, alteration of the HPA, and hyperpermeability of BBB further contributes to neuroinflammation and brain disorders. Gut dysbiosis alters the levels of neurotransmitters and neurotrophins, thereby exacerbating the pathological state. In turn, neurodegenerative disorders could affect gastrointestinal functions by creating a vicious circle. NT, neurotransmitters; IM, intestinal membrane; SCFAs, short-chain fatty acids.
4.1. Gut Microbiota in Parkinson’s Disease: The Non-Motor Symptoms
Parkinson’s disease (PD) is a neurodegenerative disorder characterized by motor and non-motor symptoms (NMSs). NMSs include neuropsychiatric manifestation such as anxiety, depression, sleep disorders, cognitive impairment, and autonomic dysfunctions involving the whole GI tract, which represent one of the most common NMSs in patients with PD. The precise mechanisms underlying the gut microbiota and PD are still poorly understood, and it is still unclear whether the alteration of microbial composition is the cause or the consequence of PD. Evidence supports the hypothesis that PD may initiate in the gut since GI dysfunctions appear many years before motor impairments. The reciprocal influence between neural function and the gut microbiota can contribute to the pathogenesis of PD, and the ENS is a gateway for the bidirectional interaction between the brain and the gut [61].
The pathological hallmark of PD is represented by the toxic accumulation of α-synuclein (α-syn) in both the CNS and the ENS of PD patients [62]. This α-syn accumulation was detected in gastric, duodenal, and colonic biopsies several years prior to the onset of the motor symptoms of PD [63]. Using mice that overexpress α-syn, it was demonstrated that gut dysbiosis is required for motor deficits, microglia activation, and α-syn pathology, and enteric α-syn correlates with higher intestinal permeability and inflammation [64,65]. These findings suggest a centripetal spread of α-syn pathology from the ENS to the CNS.
In addition, Gram-negative bacteria are abundant in PD patients and produce lipopolysaccharide (LPS), the main endotoxin that, through inflammatory pathways, contributes to α-syn aggregation, dopaminergic neuronal death, reduction of dopamine levels, and, therefore, motor impairments [66].
Mast cells are reported to surround neuronal cells, including astrocytes and microglia, and they may play a role in PD pathogenesis [67]. The ENS alteration could “prime” these cells to respond to different stimuli and become activated, releasing neuromodulators, cytokines, and other inflammatory mediators that trigger a bidirectional communication able to damage the dopaminergic neurons. The intake of probiotics could modify the composition of the gut microbiota and modulate the mast-cell activation, leading to an improvement in levodopa adsorption, thereby inducing neuroprotection.
4.2. Gut Microbiota in Alzheimer’s Disease: Role of β-Amyloid
Increased permeability of the gut and BBB can contribute to the pathogenesis of Alzheimer’s disease (AD) [68]. AD is characterized by an accumulation of aggregate amyloid fibrils such as β-amyloid (Aβ) protein and neurofibrillary tangles. Amyloid precursor protein (APP) from which Aβ derives is expressed by enteric neurons and glia, thus suggesting a role of the ENS in the pathogenesis of AD [69]. The continuous deposition of the Aβ peptide in the extracellular space provokes an inflammatory response that culminates with the constant release of pro-inflammatory cytokines, worsening the cognitive functions and dementia observed in AD. Furthermore, a vicious cycle between Aβ deposition and microglia activation in the brain is generated. An analysis of the gut microbiota composition of AD patients demonstrated a predominance of Firmicutes and Bacteroidetes phyla. An in vitro study showed that valeric acid and butyric acid produced by anti-inflammatory bacteria are able to inhibit the conversion of Aβ40 monomers to pathological Aβ fibrils, thereby preventing their accumulation in the brain [70]. Misfolding of proteins is a hallmark of AD, and modification of the neuronal autophagy flux through the gut microbiota or metabolites could contribute to the balance between production and clearance of proteins in the brain.
Interestingly, the gut microbiota produces high levels of amyloids to form biofilms with resistance to infections. However, the chronic accumulation of Aβ protein may trigger an immune response, enhancing the release of inflammatory cytokines and the transport of amyloid deposits to the brain [71]. Finally, high levels of LPS were observed in the hippocampal and temporal lobe of AD patients. The abundance of LPS in the human GI tract could affect the immune system, contributing to AD neurodegeneration [72].
4.3. Gut Microbiota in Stress, Anxiety, and Depression
The link between chronic inflammation and mental health is well documented. The gut microbiota influences the neuroendocrine HPA axis and the central stress response system. Stress can increase intestinal permeability and induce cytokine release, which initiate an immune response named “endotoxemia” that spreads to the brain to initiate a neuroinflammatory state that could culminate in anxiety and depression [73] BDNF was reported to mediate the therapeutic action of antidepressants, especially in the hippocampus [74,75]. Enteric microbial by-products, such as SCFAs, are able to able to cross the BBB and induce BDNF expression in the hippocampus and reduce anxiety in humans [75]. Several species of enteric microbes, such as Lactobacillus, Bifidobacterium, Escherichia, Bacillus, and Saccharomyces, were found to produce neurotransmitters such as GABA, 5-HT, and dopamine. Unlike 5-HT, tryptophan produced by gut microbiota is permeable with respect to the BBB and produces positive effects on mood through an increase in 5-HT levels in the brain.
Hormonal influences in early puberty may influence the microbial enteric profile and, therefore, the symptoms of stress, anxiety, and depression [76].
The intake of specific probiotics and prebiotics seems to prevent the HPA dysregulation in response to stress, and it results in the normalization of corticosterone levels and reduction of intestinal permeability [77]. Neural pathways can be directly activated by enteric microbiota or more probably by endotoxins and pro-inflammatory cytokines released in response to local and systemic inflammatory response [78]. Additional clinical studies might aid in understanding the precise role of the gut microbiota in depression in humans.
4.4. Schizophrenia, Bipolar Disorder, and Gut Dysbiosis
Schizophrenia and bipolar disorder are severe and complex mental disorders with multifactorial etiology which start many years before the appearance of numerous psychotic symptoms, mainly characterized by hallucinations, delusion, negative thinking, mania, depression, and memory and attention problems. Although these disorders have a strong genetic component, environmental factors may play an important role. Recent studies support the hypothesis that an imbalance of the gut microbiota might contribute to schizophrenia and that individuals affected by schizophrenia display an abundance of specific bacterial genera, including Clostridium and Lactobacillus, as well as members of the Veillonellaceae and Lachnospiraceae families [52,79]. In schizophrenic patients, dysfunction of the metabolic pathway of tryptophan was found. Toxic metabolites released during microbe fermentation decrease the bioavailability of phenylalanine, tyrosine, and other aromatic amino acids, including tryptophan precursors of neurotransmitters such as 5-HT, dopamine, and noradrenaline [80]. High levels of kynurenic acid, an endogenous antagonist of NMDA and α7 nicotinic acetylcholine receptors produced during tryptophan degradation, was found in schizophrenia patients [81]. An aberrant gut microbiota was also considered to be a possible mediator of bipolar disorder. A study conducted by McIntyre et al. showed that Clostridiaceae resulted more abundant in individuals affected by bipolar disorder than in healthy controls [82]. Many studies need to be performed to understand if the gut bacteria are responsible for mental disorders or if mental disorders influence the bacterial profile.
4.5. Gut Microbiota and Autism
Studies reported an alteration of the gut microbiota in individuals with autism spectrum disorders (ASDs) [83]. ASDs are complex behavioral disabilities growing worldwide, particularly in the United States. They manifest as impaired neurodevelopment, repetitive behaviors, and the incapacity of affected children to communicate and interact with others.
Although the etiology of ASD is poorly understood, genetic and environmental factors, nutrition, viral infections, and immune system dysfunction are associated with the pathology.
ASD patients display increased microflora and reduced microbial diversity associated with higher intestinal permeability and higher levels of butyric, propionic, acetic, and valeric acids compared to controls [84]. The overgrowth of harmful bacterial species, due to the high intake of antibiotics, produces virulent factors that can contribute to the pathogenesis of ASD [85].
The transplantation of gut microbiota from human donors with ASD in GF mice proved to be sufficient to induce the appearance of behavioral symptoms associated with ASD [86]. Similarly, microbiota transplantation from healthy to ASD children proved effective in improving GI symptoms [87]. Animal and epidemiological/clinical studies suggest that the treatment of an ASD with neuroactive microbial metabolites improves behavior and affects the brain activity.
5. Gut Microbiota: Role of Toll-Like Receptors
Commensal microbiota and microbial metabolites are sensed by Toll-like receptors (TLRs), the most extensively characterized class of pattern-recognition receptors (PRRs), via which the innate immune system in the gut recognizes pathogen-associated molecular patterns (PAMPs) and endogenous molecules generated by damaged cell and tissues, named damage-associated molecular patterns (DAMPs) [88].
About 10 TLRs (TLR1–10) were identified in neurons and glial cells of human CNS and gut. Their expression undergoes modifications following microbial infections and during sterile inflammation. Multifaceted TLR signaling influences brain function and immune-mediated processes both in the gut and in the brain. Gut epithelial cells express minimal TLRs under physiological conditions, and the gut has a high tolerance to TLR ligands [89]. However, an altered gut microbiota releases a variety of TLRs ligands which can activate downstream signaling pathways in glial cells, initiating an inflammatory response which involves myeloid differentiation primary gene 88 response adaptor protein and downstream nuclear factor kappa B (NF-κB), interferon regulatory factor, and mitogen-activated protein kinase pathways, which culminate in the release of chemokine, cytokine, and type I interferon production [90]. This activation cascade triggers a vicious circle leading to an increase in proinflammatory cytokines, oxidative and nitrosative stress, and finally neuronal death. Enteric neurons express TLRs to detect Gram-negative bacterial LPS, an outer-membrane component of all Gram-negative bacteria.
TLRs, mainly TLR2 and TLR4, were shown to play a key role in several aspects of neurodegenerative diseases including PD. Reports showed that misfolded α-syn released by damaged neurons can act as a DAMP for TLR4, promoting microglial and astroglia activation, which culminates in proinflammatory cytokine overproduction and oxidative stress [91]. Animal studies revealed that TLR4 affects the cerebral biochemical changes in a mouse model of PD [92], and the ablation of this receptor modulates the dopaminergic cell number and α-syn accumulation [93]. Intestinal TLR4 dysregulation may play a role in the pathology of PD. Furthermore, extracellular α-syn was found to be an effective agonist of TLR2 and downstream neurotoxic signals [94].
Increasing evidence suggests that TLRs can modulate the priming of NOD-, LRR-, and pyrin domain-containing protein 3 (NLRP3) inflammasome in the gut. Moreover, Zhang and coworkers showed that NLRP3 is associated with depression, and the gut microbiota composition of NLRP3-deficient mice was significantly altered compared to control animals. In particular, an increased Firmicutes/Bacteroidetes ratio was found in the NLRP3 knockout (KO) group, suggesting that microbiota may be a factor contributing to behavioral characteristics [95].
Nevertheless, it is important to point out that the precise role of TLR2 and TLR4 in neurodegeneration is controversial since they can trigger neurotoxicity but might be essential for the clearance of misfolded α-synuclein, thus exhibiting neuroprotective effects [96]. TLR2 and TLR4 also seem to play a dual role in the pathogenesis and progression of AD. In AD patients, the population of pro-inflammatory bacteria increases [97]. However, on one hand, microglial TLR4 mediates Aβ-induced neurotoxicity [98]. On the other hand, TLR4-mutant AD mice had less microglial activation [99].
Intriguingly, α-syn in PD can cause intestinal permeability as well as translocation of bacteria and their toxic products [100,101]; in turn, extracellular fibers and SCFAs produced by microbes in the GI tract may affect α-syn aggregation and motor dysfunction [102].
Clostridia or botulinum toxins spread to multiple organs, including the CNS, affecting synaptic neurotransmission. On the other hand, multiple bacterial species, as well as lactobacilli and bifidobacterial strains of the gut, produce neurotransmitters that actively regulate neural circuits.
A growing body of evidence suggests that the administration of probiotics and diets rich in n-3 polyunsaturated fatty acids and docosahexaenoic acid may modulate the TLR signaling pathways and improve brain function, preventing several mental disorders including anxiety and depression [103]. Taken together, these findings suggest that the identification of novel enteric products released by the microbiota able to activate specific TLRs and their downstream signaling pathways could help in understanding the precise role of the microbiota in neurodegeneration. A representative scheme is illustrated in Figure 2.
6. Overview on Mast Cells
In the scenario of neuroimmune communication, greater interest is placed in the role of mast cells (MCs) in the maintenance of the inflammatory cascade, as well as their effects on motility of the GI tract [104,105,106,107,108,109]. Mast cells are immune sentinels at the environment/host interface, strategically positioned in close proximity to blood, lymphatic vessels, and nerves to respond to various allergens, pathogens, and other agents that can be ingested, inhaled, or encountered for their putative functions in host defense after disruption of the epithelial barrier. The physiological role of MCs extends far beyond allergy. They are involved in physiological processes such as tissue repair, wound healing, and angiogenesis [106,107]. Nevertheless, just as the entire immune system is a double-edged sword, so are MCs, whose dysregulated activation contributes to the pathology of autoimmune disorders and cancer. MCs are involved in homeostatic regulation of the intestinal barrier, controlling intestinal epithelial ion transport, vascular and epithelial permeability, peristalsis, fibrosis and tissue repair, bacterial defense, and chemiotaxis, as well as modulating both the innate and the adaptive immune response [104,105,106,107,108,109,110]. For this purpose, MCs are armed with a large repertoire of receptors, including TLRs. In addition to the classical immunoglobulin E (IgE)-dependent mode of stimulation, MCs react to a multitude of other stimuli, including cytokines, hormones, neuropeptides, and neurotransmitters [106]. Upon the activation of MCs, there are two possible outcomes: release of preformed mediators stored in the granules through degranulation or de novo synthesis of mediators. The variety of these mediators, individually or in aggregate, can have many different effects on immune or structural cells present in mucosal tissue. In small quantities, MCs are also present in some areas of the brain, such as the postrema area, thalamus and hypothalamus parenchyma, and leptomeninges, as well as in dura mater of the spinal cord. In the brain, MCs are located on the abluminal side of the blood vessels, where they interact with neurons, glia, and endothelial cells. Although in small numbers, MCs are able to release a number of inflammatory mediators that can affect the integrity of the BBB and activate glia and neurons [106]. In addition, MCs release molecules such as corticotropin-releasing factor in stress and neuroinflammation conditions, suggesting their role in the pathogenesis of stress-related neurodegeneration and neuroinflammation [106,109].
Increasing evidence indicates that MCs are critical for the pathogenesis of inflammatory disease. The increase in the number of MCs in the terminal ileum and colon of inflammatory bowel disease (IBD) patients is relevant. IBD represents a group of inflammatory conditions of the colon and small intestine, generally assumed as a result of an uncontrolled immune response in genetically predisposed subjects following a change in microbiota (dysbiosis) [110]. The close morphological relationship between MC and afferent nerve endings creates a bidirectional communication network between the GI and ENS–CNS. Specifically, MC mediators activate nerves that in turn release neurotransmitters able to enhance MC activity. Histamine (H) is the major mediator released by MCs, and it is a potent vasodilator, as well as one of the main culprits in mediating allergic responses [111,112,113,114,115,116]. Once activated, the MC tends to perpetuate the inflammatory state through a positive feedback loop [106,113]. Under stress conditions, MCs release tryptase and histamine into the intestinal lumen and increase intestinal permeability [109,117,118,119]. In IBD, the expression of tight junction (TJ) proteins is reduced in correlation with MC activation.
Mast cells are important mediators in the GI tract and the mucosal immune system, as well as a crucial link between ENS and CNS. As reported, in the gut, MCs live in close proximity to GI mucosal sensory nerve fibers containing neuropeptides, including visceral afferents expressing transient receptor potential vanilloid 1 (TRPV1) receptors. Activation of TRPV1 channels on primary sensory neurons contributes to neurogenic inflammation [109,120]. Indeed, afferent innervation of enteric MCs can trigger the release of histamine and protease—mediators that, in a paracrine manner, elevate the sensitivity of spinal afferent terminals. A brain–MC interaction is one plausible mechanism linking stress and GI symptoms with the involvement of the vagal nerve pathway [121,122,123]. The CNS influences intestinal MC degranulation through extrinsic sensory vagal nerves, while degranulation is prevented by sympathetic activation. If the ENS has a direct influence on MCs, the opposite is also true. Interestingly, MCs operate as a sensory cell activated by immune and non-immune stimuli, while they act as effector cells by releasing biologically activate mediators [113].
7. Probiotics as New Therapy
According to Hippocrates, “all disease begins in the gut.”
It is increasingly recognized that the gut microbiota composition influences the intestinal epithelial barrier integrity and plays a key role in regulating neuronal cell function. Probiotics exert their biological actions in different manners. Firstly, they are able to produce and secrete bacterio-toxins, such as bacteriocins, which can block pathogen adhesion to epithelial cells and inhibit bacterial invasion. Members of the Lactobacillus genus produce lactic acid, which, in addition to creating an inhibitory environment for the growth of many bacteria, potentiate the antimicrobial activity of the host lysozyme by disrupting the bacterial outer membrane [49]. Secondly, probiotics compete with potential pathogenic bacteria for binding sites and for nutrients [35]. Thirdly, probiotics and their derivatives exert a trophic effect on the intestinal mucosa, increasing mucus production and enhancing barrier integrity [36,37]; Lastly, probiotics affect epithelial cell cytokine secretion, such as interleukin (IL)-1β, IL-6, IL-8, and tumor necrosis factor (TNF)-α. They seem to be able to promote host defense against infection while reducing hypersensitivity reactions to commensal bacteria and food. It was shown that some specific probiotic strains show various interesting properties, including antigenotoxic, antioxidant, and anti-inflammatory activity [124,125,126,127,128,129]. In conclusion, probiotics could represent a promising therapy to balance dysbiosis and facilitate the remission of diseases, as well as attenuate the symptoms of neurological disorders and mucosal compartments.
7.1. Probiotics and Microbiota-Derived Molecules
The beneficial impact of probiotics or even prebiotics or symbiotics on HPA and MCs was reported [109]. In particular, evidence indicates that the administration of a probiotic combined formulation of Lactobacillus helveticus and Bifidobacterium longum attenuates HPA axis activity and the autonomic nervous system (ANS), with a decrease in plasma cortisol and catecholamine levels [38]. L. rhamnosus reduces hippocampal expression of the GABA receptor gene, suggesting a modulation of the balance of inhibition/excitation to control responses to stress, anxiety, and depression [130]. Several preclinical studies demonstrated the potential beneficial effects of Lactobacillus and Bifidobacteria species on the intestinal mucosal barrier. For example, L helveticus and B. longum restore the TJ integrity and the protection of the intestinal barrier [38]. L. rhamnosus and B. breve increase the production of mucins, the induction and production of defensins (human-β-defensin-2), and the secretion of immunoglobulin A, as well as the reduction of mucosal permeability through action on epithelial TJs. B. infantis was able to reduce the systemic proinflammatory cytokine profile along with symptom improvement in patients with IBD [131]. The ENS represents the target of bacterial metabolites; one of the main products of bacterial metabolism constitutes SCFAs, which are able to stimulate the sympathetic nervous system and mucosal 5-HT release.
SCFAs are produced via the fermentation of complex carbohydrates by bacteria. In particular, propionate and acetate are produced by Bacteroides phylum, and butyrate is mainly produced by Firmicutes. SCFAs are critical for both enterocytes and colonocytes [132].
SCFAs exert their effects through the activation of membrane G-protein-coupled receptors (GPRs), including GPR41, GPR43, and GPR109, expressed on a wide range of cell types, such as intestinal epithelial cells, macrophages, and dendritic cells. In intestinal endocrine-L cells, GPR43 mediates the release of glucagon-like peptide 1 and peptide YY, suggesting a role in insulin resistance and obesity [133].
SCFA can directly control the immune response, sustaining an anti-inflammatory environment.
Butyrate is a histone deacetylases inhibitor regulating epithelial cell gene expression and function through an epigenetic mechanism. In this manner, butyrate can induce regulatory T cell (Treg) differentiation, acting as an anti-inflammatory molecule [132,134].
In addition, propionate and butyrate can modulate immune system cells, causing apoptosis of neutrophils and affecting cytokine production. SCFA propionic acid increases the number of regulatory T cells associated with an increase in remyelinization. It can protect BBB integrity from oxidative stress through the nuclear factor erythroid 2-related factor 2 (Nrf2) signaling pathway. Butyrate plays an immunomodulatory role in macrophages, leading to a reduction in inflammation. Diet-derived SCFAs are implicated in neuroinflammatory and neurodegenerative disesases [135].
It was reported that the combination of SCFAs with the probiotic B. breve M-16V reduced MC degranulation without significantly reducing allergen-specific IgE levels. SCFAs also have an effect on MC activation. Sodium butyrate decreased the percentage of degranulated MCs and their inflammatory mediator content in weaned pigs. SCFAs are crucial in improving the barrier activity of intestinal epithelial cells, acting on TJs [136].
Sodium butyrate could improve intestinal barrier function and inflammatory mediator production through modulation of mitogen-activated protein kinase (MAPK) signaling pathways.
In addition, sodium butyrate reduces the expression of MC-specific tryptase, TNF-α, and IL-6 messenger RNA (mRNA). A butyrate-producing probiotic (Clostridium butyricum) restored the intestinal epithelial barrier integrity through the regulation of tandem of pore domains in a weak inward rectifying K+ channel and by reducing the allergic response [137].
7.2. Probiotics and Mast Cells
Live probiotic bacteria have species-specific effects on human MCs. The ability of specific strains of probiotic bacteria to influence MC function and their activation was studied [138]. In particular, it was reported that L. rhamnosus GG and some other probiotic strains reduce the number of MC [138,139], whereas L. casei reduces intestinal barrier dysfunction via a TLR signaling-mediated MC pathway [140]. L. rhamnosus GG, L. rhamnosus Lc705, and B. animalis ssp. lactis Bb12 induce significant changes in MC gene expression including high-affinity IgE receptor subtype α (FCεR1A) and HRH4 (HRH4) FCεR1G, as well as changes in immunological responses. In particular, 24-h stimulation of MCs downregulated the expression of the FCεR1A and HRH4 genes [139]. FCεR1 plays a key role in mediating the allergy-related IgE-dependent activation and degranulation of MCs. After FCεR1 aggregation, inflammatory mediators are released, such as histamine, which is a potent modulator of immune responses. By suppressing the expression of FCεR1 and HRH4 genes, Lactobacillus could attenuate MC activation and the release of allergy-related mediators. Moreover, the expression of the phospholipase C gene, involved in the FCεR signaling pathway, is significantly downregulated, suggesting the suppression of the release of intracellular calcium and MC degranulation. In a mouse model, the combination of Bacillus subtilis and Enterococcus faecium could inhibit MC degranulation [141]. Lactobacillus paracasei CNCM I-1518 affects the immune response by inhibiting IgE-dependent human basophil and mouse MC activation L. paracasei inhibited MC granule formation, and L. rhamnosus downregulated the expression of high-affinity IgE receptor and histamine receptor HRH4 genes [142].
In particular, TLR2 is a receptor for Lactobacillus, which triggers the nuclear factor κB signaling cascade, which leads to the expression of different cytokines in human primary macrophages. The paradox of the ability of probiotics to diminish MC activation but enhance the MC immune response could be regulated through the same TLR2 [139,140].
8. Conclusions and Future Directions
In the last few years, the implications of the gut microbiota and its bioactive microbiota-derived molecules in the progression of multiple diseases, as well as in the development of neurodegenerative disorders, gained increasing attention. Microbial products may influence the host cellular pathways involved in proliferation, differentiation, and maturation functions.
It is essential to fully understand the mechanisms that govern the precise dialogue between the gut, brain, and MCs.
Finally, it would be interesting to investigate the effect of different probiotic strains and their metabolites on the modulation of specific signaling pathways.
Author Contributions
Conceptualization, G.T.; writing—original draft preparation, C.C.; M.S.; G.T.; writing—review and editing, C.C.; G.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We thanks Paola Paolotti for reference editing.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| GABA | gamma-aminobutyric acid |
| AD | Alzheimer’s disease |
| α-syn | α-synuclein |
| APP | amyloid precursor protein |
| APC | Antigen-presenting cells |
| ANS | autonomic nervous system |
| ASD | autism spectrum disorder |
| (Aβ) | amyloid-beta |
| BDNF | brain-derived neurotrophic factor |
| CNS | central nervous system |
| DAMPs | damage-associated molecular patterns |
| EGCs | enteric glial cells |
| ENS | enteric nervous system |
| GIP | gastric inhibitory peptide |
| GI | gastrointestinal |
| GPRs | G-protein-coupled receptors |
| GF | germ-free |
| HPA | hypothalamic–pituitary–adrenal |
| (IBD) | inflammatory bowel disease |
| LPS | lipopolysaccharide |
| MC | mast cell |
| MLN | mesenteric lymph nodes |
| MAPK | mitogen-activated protein kinase |
| NLRP3 | NOD-, LRR-, and pyrin domain-containing protein 3 |
| NMDA | N-methyl-d-aspartate |
| NMS | non-motor symptoms |
| PAMPs | pathogen-associated molecular patterns |
| PD | Parkinson’s disease |
| PMD | piecemeal degranulation |
| PRRs | pattern-recognition receptors |
| SCFAs | short-chain fatty acids |
| 5-HT | 5-hydroxytryptamine, serotonin |
| TLRs. | Toll-like receptors |
References
- Costa, M.; Brookes, S.; Hennig, G. Anatomy and physiology of the enteric nervous system. Gut 2000, 47, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Chaudhri, O.; Small, C.; Bloom, S. Gastrointestinal hormones regulating appetite. Phil. Trans. R. Soc. B 2006, 361, 1187–1209. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.J. Gastrointestinal hormones and receptors. In Textbook of Gastroenterology, 3rd ed.; Yamada, T., Ed.; Lipincott Williams & Wilkins: Philadelphia, PA, USA, 1999; pp. 35–66. [Google Scholar]
- Konturek, S.J.; Zabielski, R.; Konturek, J.W.; Czarnecki, J. Neuroendocrinology of the pancreas; role of brain–gut axis in pancreatic secretion. Eur. J. Pharm. 2003, 481, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Amin, K. The role of mast cells in allergic inflammation. Respir. Med. 2012, 106, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Stone, K.D.; Prussin, C.; Metcalfe, D.D. IgE, mast cells, basophils, and eosinophils. J. Allergy Clin. Immunol. 2010, 125, S73–S80. [Google Scholar] [CrossRef] [PubMed]
- Trowers, E.; Tischler, M. Form and function: The physiological implications of the anatomy of the gastrointestinal system. In Gastrointestinal Physiology; Springer: Berlin/Heidelberg, Germany, 2014; pp. 9–35. [Google Scholar]
- Brandtzaeg, P.; Baekkevold, E.S.; Farstad, I.N.; Jahnsen, F.L.; Johansen, F.E.; Nilsen, E.M.; Yamanaka, T. Regional specialization in the mucosal immune system: What happens in the microcompartments? Immunol. Today 1999, 20, 141–151. [Google Scholar] [CrossRef]
- Giorgetti, G.; Brandimarte, G.; Fabiocchi, F.; Ricci, S.; Flamini, P.; Sandri, G.; Trotta, M.C.; Elisei, W.; Penna, A.; Lecca, P.G.; et al. Interactions Between Innate Immunity, Microbiota, And Probiotics. J. Immunol. Res. 2015, 2015, 501361. [Google Scholar] [CrossRef]
- Campbell, N.; Yio, X.Y.; So, L.P.; Li, Y.; Mayer, L. The intestinal epithelial cell: Processing and presentation of antigen to the mucosal immune system. Immunol. Rev. 1999, 172, 315–324. [Google Scholar] [CrossRef]
- Sirisinha, S. The mucosal immune system. Asian Pac. J. Allergy Immunol. 1984, 2, 281–288. [Google Scholar]
- Corr, S.C.; Gahan, C.C.G.M.; Hill, C. M-cells: Origin, morphology and role in mucosal immunity and microbial pathogenesis. FEMS Immunol. Med. Microbiol. 2008, 52, 2–12. [Google Scholar] [CrossRef]
- Jung, C.; Hugot, J.P.; Barreau, F. Peyer’s Patches: The Immune Sensors of the Intestine. Int. J. Inflamm. 2010, 2010, 823710. [Google Scholar] [CrossRef] [PubMed]
- Nezami, B.G.; Srinivasan, S. Enteric Nervous System in the Small Intestine: Pathophysiology and Clinical Implications. Curr. Gastroenterol. Rep. 2010, 12, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Kabouridis, P.S.; Pachnis, V. Emerging roles of gut microbiota and the immune system in the development of the enteric nervous system. J. Clin. Invest. 2015, 125, 956–964. [Google Scholar] [CrossRef]
- Sasselli, V.; Pachnis, V.; Burns, A.J. The Enteric Nervous System. Dev. Biol. 2012, 366, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Langness, S.; Coimbra, R.; Costantini, T.J. Complexities of the Enteric Nervous System: In Reply to Fujita. J. Am. Coll. Surg. 2016, 222, 105–106. [Google Scholar] [CrossRef] [PubMed]
- Furness, J.B. Types of neurons in the enteric nervous system. J. Auton. Nerv. Syst. 2000, 81, 87–96. [Google Scholar] [CrossRef]
- Campbell, I. Gut motility and its control. Anaesth. Intens. Care Med. 2015, 16, 40–42. [Google Scholar] [CrossRef]
- Brookes, S.J.H. Classes of enteric nerve cells in the guinea-pig small intestine. Anat. Rec. 2001, 262, 58–70. [Google Scholar] [CrossRef]
- Goldstein, A.M.; Hofstra, R.M.W.; Burns, A.J. Building a brain in the gut: Development of the enteric nervous system. Clin. Genet. 2013, 83, 307–316. [Google Scholar] [CrossRef]
- Young, H.M.; Bergner, A.J.; Anderson, R.B.; Enomoto, H.; Milbrandt, J.; Newgreen, D.F.; Whitington, P.M. Dynamics of neural crest-derived cell migration in the embryonic mouse gut. Dev. Biol. 2004, 70, 455–473. [Google Scholar] [CrossRef]
- Boesmans, W.; Lasrado, R.; Vanden Berghe, P.; Pachnis, V. Heterogeneity and phenotypic plasticity of glial cells in the mammalian enteric nervous system. Glia 2015, 63, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Burns, A.J. Migration of neural crest-derived enteric nervous system precursor cells to and within the gastrointestinal tract. Int. J. Dev. Biol. 2005, 49, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Lake, J.I.; Heuckeroth, R.O. Enteric Nervous System Development: Migration, Differentiation, and Disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 305, G1–G24. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.; Borojevic, R.; Verdu, E.F.; Huizinga, J.D.; Ratcliffe, E.M. Intestinal Microbiota Influence The Early Postnatal Development Of The Enteric Nervous System. Neurogastroenterol. Motil. 2014, 26, 98–107. [Google Scholar] [CrossRef]
- Grund, D.; Schemann, M. Enteric Nervous System. Curr. Opin. Gastroenterol. 2007, 23, 121–126. [Google Scholar] [CrossRef]
- Sekirov, I.; Russell, S.L.; Antunes, L.C.; Finlay, B.B. Gut microbiota in health and disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef]
- Matamoros, S.; Gras-Leguen, C.; Le Vacon, F.O.; Potel, G.; De La Cochetiere, M.F. Human Microbiome Development Of Intestinal Microbiota In Infants And Its Impact On Health. Trends Microbiol. 2013, 21, 167–173. [Google Scholar] [CrossRef]
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery Mode Shapes The Acquisition And Structure Of The Initial Microbiota Across Multiple Body Habitats In Newborns. PNAS 2010, 107, 11971–11975. [Google Scholar] [CrossRef]
- Munyaka, P.M.; Khafipour, E.; Ghia, J.E. External Influence of Early Childhood Establishment of Gut Microbiota and Subsequent Health Implications. Front. Pediatr. Neonatol. 2014, 2, 1–9. [Google Scholar] [CrossRef]
- Rodríguez, J.M.; Murphy, K.; Stanton, C.; Ross, R.P.; Kober, O.I.; Juge, N.; Avershina, E.; Rudi, K.; Narbad, A.; Jenmalm, M.C.; et al. The Composition of the Gut Microbiota throughout Life, With an Emphasis on Early Life. Microb. Ecol. Health Dis. 2015, 26, 26050. [Google Scholar] [CrossRef]
- Goulet, O. Potential Role of the Intestinal Microbiota in Programming Health and Disease. Nutr. Rev. 2015, 73, 32–40. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, J.B.; Milani, C.; de Giori, G.S.; Sesma, F.; Sinderen, D.; Ventura, M. Bacteria as vitamin suppliers to their host: A gut microbiota perspective. Curr. Opin. Biotech. 2013, 24, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Amaretti, A.; Raimondi, S. Folate production by probiotic bacteria. Nutrients 2011, 3, 118–134. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.W.; Yan, L.; Ao, X.; Zhou, T.X.; Wang, J.P.; Lee, J.H.; Kim, I.H. Influence of Probiotics in Different Energy and Nutrient Density Diets On Growth Performance, Nutrient Digestibility, Meat Quality, And Blood Characteristics in Growing-Finishing Pigs. J. Anim. Sci. 2010, 88, 3320–3326. [Google Scholar] [CrossRef]
- Neunlist, M.; Schemann, M. Nutrient-Induced Changes in the Phenotype and Function of the Enteric Nervous System. J. Physiol. 2014, 592, 2959–2965. [Google Scholar] [CrossRef]
- Plaza-Diaz, J.; Gomez-Llorente, C.; Fontana, L.; Gil, A. Modulation of Immunity and Inflammatory Gene Expression in the Gut, In Inflammatory Diseases of the Gut and In the Liver by Probiotics. World J. Gastroenterol. 2014, 20, 15632–15649. [Google Scholar] [CrossRef]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the Human Intestinal Microbial Flora. Science 2005, 308, 1635–1638. [Google Scholar]
- Björkstén, B. The gastrointestinal flora and the skin—Is there a link? Pediatr. Allergy Immunol. 2001, 12, 51–55. [Google Scholar] [CrossRef]
- Lee, Y.K.; Mazmanian, S.K. Has the microbiota played a critical role in the evolution of the adaptive immune system? Science 2010, 330, 1768–1773. [Google Scholar] [CrossRef]
- Guarner, F.; Malagelada, J.R. Gut flora in health and disease. Lancet 2003, 361, 512–519. [Google Scholar] [CrossRef]
- Duerkop, B.A.; Vaishnava, S.; Hooper, L.V. Immune Responses to the Microbiota at the Intestinal Mucosal Surface. Immunity 2009, 18, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Rakoff-Nahoum, S.; Paglino, J.; Eslami-Varzaneh, F.; Edberg, S.; Medzhitov, R. Recognition of Commensal Microflora by Toll-Like Receptors Is Required for Intestinal Homeostasis. Cell 2004, 118, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.H.; Thomassen, M.T.; Madsen, M.L.; Kern, T.; Bak, E.G.; Kashani, A.; Allin, K.H.; Hansen, T.; Pedersen, O. The effect of drinking water pH on the human gut microbiota and glucose regulation: results of a randomized controlled cross-over intervention. Sci. Rep. 2018, 8, 16626. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Knauf, C. How gut microbes talk to organs: The rôle of endocrine and nervous routes. Mol. Metab. 2016, 5, 743–752. [Google Scholar] [CrossRef]
- Nuriel-Ohayon, M.; Neuman, H.; Koren, O. Microbial Changes during Pregnancy, Birth, and Infancy. Front. Microbiol. 2016, 7, 1031. [Google Scholar] [CrossRef]
- Erny, D.; Hrabe de Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Sandhu, K.; Peterson, V.; Dinan, T.G. The gut microbiome in neurological disorders. Lancet Neurol. 2019. [Google Scholar] [CrossRef]
- König, J.; Wells, J.; Cani, P.D.; García-Ródenas, C.L.; MacDonald, T.; Mercenier, A.; Whyte, J.; Troost, F.; Brummer, R.-J. Human Intestinal Barrier Function in Health and Disease. Clin. Transl. Gastroenterol. 2016, 7, e196. [Google Scholar] [CrossRef]
- Neufeld, K.M.; Kang, N.; Bienenstock, J.; Foster, J.A. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol. Motil. 2011, 13, 255–e119. [Google Scholar] [CrossRef]
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Tóth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Kundu, P.; et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014, 6, 263ra158. [Google Scholar] [CrossRef]
- Horne, R.; Foster, J.A. Metabolic and microbiota measures as peripheral biomarkers in major depressive disorder. Front. Psychiatry 2018, 9, 513. [Google Scholar] [CrossRef] [PubMed]
- Strandwitz, P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018, 1693, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.J.; Chiu, J.I.M. Bacterial Signaling to the Nervous System via Toxins and Metabolites. Mol. Biol. 2017, 429, 587–605. [Google Scholar] [CrossRef] [PubMed]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef]
- Peirce, J.M.; Alviña, K. The role of inflammation and the gut microbiome in depression and anxiety. J. Neurosci. Res. 2019, 97, 1223–1241. [Google Scholar] [CrossRef]
- Cho, I.; Blaser, M.J. The human microbiome: At the interface of health and disease. Nat. Rev. Genet. 2012, 13, 260–270. [Google Scholar] [CrossRef]
- Chalazonitis, A.; Rao, M. Enteric nervous system manifestations of neurodegenerative disease. Brain Res. 2018, 1693, 207–213. [Google Scholar] [CrossRef]
- Mayer, E.A.; Tillisch, K.; Gupta, A. Gut/brain axis and the microbiota. J. Clin. Invest. 2015, 125, 926–938. [Google Scholar] [CrossRef]
- Quigley, E.M.M. Microbiota-brain-gut axis and neurodegenerative diseases. Curr. Neurol. Neurosci. Rep. 2017, 17, 94. [Google Scholar] [CrossRef]
- Beach, T.G.; Adler, C.H.; Sue, L.I.; Vedders, L.; Lue, L.; White Iii, C.L.; Akiyama, H.; Caviness, J.N.; Shill, H.A.; Sabbagh, M.N.; et al. Arizona Parkinson’s Disease Consortium. Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol. 2010, 119, 689–702. [Google Scholar] [CrossRef]
- Shannon, K.M.; Keshavarzian, A.; Dodiya, H.B.; Jakate, S.; Kordower, J.H. Is alpha-synuclein in the colon a biomarker for premotor Parkinson’s disease? Evidence from 3 cases. Mov. Disord. 2012, 27, 716–719. [Google Scholar] [CrossRef] [PubMed]
- Forsyth, C.B.; Shannon, K.M.; Kordower, J.H.; Voigt, R.M.; Shaikh, M.; Jaglin, J.A.; Estes, J.D.; Dodiya, H.B.; Keshavarzian, A. Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson’s disease. PLoS ONE 2011, 6, e28032. [Google Scholar] [CrossRef] [PubMed]
- Sampson, T.R.; Debelius, J.W.; Thron, T.; Janssen, S.; Shastri, G.G.; Ilhan, Z.E.; Challis, C.; Schretter, C.E.; Rocha, S.; Gradinaru, V.; et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell 2016, 167, 1469–1480. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Nehru, B. Characterization of the lipopolysaccharide induced model of Parkinson’s disease: Role of oxidative stress and neuroinflammation. Neurochem. Int. 2015, 87, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Kempuraj, D.; Selvakumar, G.P.; Zaheer, S.; Thangavel, R.; Ahmed, M.E.; Raikwar, S.; Govindarajan, R.; Iyer, S.; Zaheer, A. Cross-talk between glia, neurons and mast cells in neuroinflammation associated with Parkinson’s disease. J. Neuroimmune Pharmacol. 2018, 13, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Li, G.; Huang, P.; Liu, Z.; Zhao, B. The Gut Microbiota and Alzheimer’s Disease. J. Alzheimers Dis. 2017, 58, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Arai, H.; Lee, V.M.; Messinger, M.L.; Greenberg, B.D.; Lowery, D.E.; Trojanowski, J.Q. Expression patterns of beta-amyloid precursor protein (beta-APP) in neural and nonneural human tissues from Alzheimer’s disease and control subjects. Ann. Neurol. 1991, 30, 686–693. [Google Scholar] [CrossRef]
- Bostanciklioglu, M. The role of gut microbiota in pathogenesis of Alzheimer’s disease. J. Appl. Microbiol. 2019, 127, 954–967. [Google Scholar] [CrossRef]
- Friedland, R.P.; Chapman, M.R. The role of microbial amyloid in neurodegeneration. PLoS Pathog. 2017, 13, e1006654. [Google Scholar] [CrossRef]
- Zhao, Y.; Jaber, V.L.W. Secretory products of the human GI tract microbiome and their potential impact on Alzheimer’s disease (AD): Detection of lipopolysaccharide (LPS) in AD hippocampus. Front. Cell. Infect. Microbiol. 2017, 7, 318. [Google Scholar] [CrossRef]
- Liu, R.T. The microbiome as a novel paradigm in studying stress and mental health. Am. Psychol. 2017, 72, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Chen, Z.Y. The role of BDNF in depression on the basis of its location in the neural circuitry. Acta Pharmacol. Sin. 2011, 32, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.M.; Surette, M.; Bercik, P. The interplay between the intestinal microbiota and the brain. Nat. Rev. Microbiol. 2012, 10, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Rea, K.; Dinan, T.G.; Cryan, J.F. The microbiome: A key regulator of stress and neuroinflammation. Neurobiol. Stress 2016, 4, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K.; Cowen, P.J.; Harmer, C.J.; Tzortzis, G.; Errington, S.; Burnet, P.W.J. Prebiotic intake reduces the waking cortisol response and alters emotional bias in healthy volunteers. Psychopharmacology 2015, 232, 1793–1801. [Google Scholar] [CrossRef]
- Bonaz, B.; Bazin, T.; Pellissier, S. The Vagus Nerve at the Interface of the Microbiota-Gut-Brain Axis. Front. Neurosci. 2018, 12, 49. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Xu, J.; Li, Z.; Huang, Y.; Yuan, Y.; Wang, J.; Zhang, M.; Hu, S.; Liang, Y. Analysis of gut microbiota diversity and auxiliary diagnosis as a biomarker in patients with schizophrenia: A cross-sectional study. Schizophr. Res. 2018, 197, 470–477. [Google Scholar] [CrossRef]
- Severance, E.G.; Prandovszky, E.; Castiglione, J.; Yolken, R.H. Gastroenterology issues in schizophrenia: Why the gut matters. Curr. Psychiatry Rep. 2015, 17, 27. [Google Scholar] [CrossRef]
- Erhardt, S.; Schwieler, L.; Nilsson, L.; Linderholm, K.; Engberg, G. The kynurenic acid hypothesis of schizophrenia. Physiol. Behav. 2007, 92, 203–209. [Google Scholar] [CrossRef]
- McIntyre, R.S.; Subramaniapillai, M.; Shekotikhina, M.; Carmona, N.E.; Lee, Y.; Mansur, R.B.; Brietzke, E.; Fus, D.; Coles, A.S.; Iacobucci, M.; et al. Characterizing the gut microbiota in adults with bipolar disorder: A pilot study. Nutr. Neurosci. 2019, 28, 1–8. [Google Scholar] [CrossRef]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota–gut–brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Christophersen, C.T.; Sorich, M.J.; Gerber, J.P.; Angley, M.T.; Conlon, M.A. Elevated fecal short chain fatty acid and ammonia concentrations in children with autism spectrum disorder. Dig. Dis. Sci. 2012, 57, 2096–2102. [Google Scholar] [CrossRef] [PubMed]
- Emanuele, E.; Orsi, P.; Boso, M.; Broglia, D.; Brondino, N.; Barale, F.; di Nemi, S.U.; Politi, P. Low-grade endotoxemia in patients with severe autism. Neurosci. Lett. 2010, 471, 162–165. [Google Scholar] [CrossRef]
- Sharon, G.; Cruz, N.J.; Kang, D.W.; Gandal, M.J.; Wang, B.; Kim, Y.M.; Zink, E.M.; Casey, C.P.; Taylor, B.C.; Lane, C.J.; et al. Human Gut Microbiota from Autism Spectrum Disorder Promote Behavioral Symptoms in Mice. Cell 2019, 177, 1600–1618.e17. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Adams, J.B.; Coleman, D.; Pollard, E.L.; Maldonado, J.; McDonough-Means, S.; Caporaso, J.G.; Krajmalnik-Brown, R. Long-term benefit of Microbiota Transfer Therapy on autism symptoms and gut microbiota. Sci. Rep. 2019, 9, 5821. [Google Scholar] [CrossRef]
- Caputi, V.; Giron, M.C. Microbiome-Gut-Brain Axis and Toll-Like Receptors in Parkinson’s Disease. Int. J. Mol. Sci. 2018, 19, 1689. [Google Scholar] [CrossRef]
- Kubinak, J.L.; Round, J.L. Toll-like receptors promote mutually beneficial commensal-host interactions. PLoS Pathog. 2012, 8, e1002785. [Google Scholar] [CrossRef]
- Dowling, J.K.; Mansell, A. Toll-like receptors: The swiss army knife of immunity and vaccine development. Clin. Transl. Immunol. 2016, 5, e85. [Google Scholar] [CrossRef]
- Fellner, L.; Irschick, R.; Schanda, K.; Reindl, M.; Klimaschewski, L.; Poewe, W.; Wenning, G.K.; Stefanova, N. Toll-like receptor 4 is required for alpha-synuclein dependent activation of microglia and astroglia. Glia 2013, 61, 349–360. [Google Scholar] [CrossRef]
- Mariucci, G.; Pagiotti, R.; Galli, F.; Romani, L.; Conte, C. The potential role of toll-like receptor 4 in mediating dopaminergic cell loss and alpha-synuclein expression in the acute MPTP mouse model of Parkinson’s disease. J. Mol. Neurosci. 2018, 64, 611–618. [Google Scholar] [CrossRef]
- Conte, C.; Roscini, L.; Sardella, R.; Mariucci, G.; Scorzoni, S.; Beccari, T.; Corte, L. Toll Like Receptor 4 Affects the Cerebral Biochemical Changes Induced by MPTP Treatment. Neurochem. Res. 2017, 42, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Daniele, S.G.; Beraud, D.; Davenport, C.; Cheng, K.; Yin, H.; Maguire-Zeiss, K.A. Activation of MyD88-dependent TLR1/2 signaling by misfolded alpha-synuclein, a protein linked to neurodegenerative disorders. Sci. Signal. 2015, 8, ra45. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, R.; Cheng, M.; Wang, L.; Chao, J.; Li, J.; Zheng, P.; Xie, P.; Zhang, Z.; Yao, H. Gut microbiota from NLRP3-deficient mice ameliorates depressive-like behaviors by regulating astrocyte dysfunction via circHIPK2. Microbiome 2019, 7, 116. [Google Scholar] [CrossRef] [PubMed]
- Rietdijk, C.D.; van Wezel, R.J.; Garssen, J.; Kraneveld, A.D. Neuronal toll-like receptors and neuro-immunity in Parkinson’s disease, Alzheimer’s disease and stroke. Neuroimmunol. Neuroinflamm. 2016, 3, 27–37. [Google Scholar] [CrossRef]
- Lin, C.; Zhao, S.; Zhu, Y.; Fan, Z.; Wang, J.; Zhang, B.; Chen, Y. Microbiota-gut-brain axis and toll-like receptors in Alzheimer’s disease. Comput. Struct. Biotechnol. J. 2019, 17, 1290–1308. [Google Scholar] [CrossRef]
- Walter, S.; Letiembre, M.; Liu, Y.; Heine, H.; Penke, B.; Hao, W.; Bode, B.; Manietta, N.; Walter, J.; Schulz-Schuffer, W.; et al. Role of the toll-like receptor 4 in neuroinflammation in Alzheimer’s disease. Cell. Physiol. Biochem. 2007, 20, 947–956. [Google Scholar] [CrossRef]
- Song, M.; Jin, J.; Lim, J.E.; Kou, J.; Pattanayak, A.; Rehman, J.A.; Kim, H.-D.; Tahara, K.; Lalonde, R.; Fukuchi, K. TLR4 mutation reduces microglial activation, increases Abeta deposits and exacerbates cognitive deficits in a mouse model of Alzheimer’s disease. J. Neuroinflamm. 2011, 8, 92. [Google Scholar] [CrossRef]
- Wakabayashi, K.; Takahashi, H.; Takeda, S.; Ohama, E.; Ikuta, F. Parkinson’s disease: The presence of Lewy bodies in Auerbach’s and Meissner’s plexuses. Acta Neuropathol. 1988, 76, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, E.; Murphy, S.; Holly Martinson, H.A. Alpha-Synuclein Pathology and the Role of the Microbiota in Parkinson’s Disease. Front. Neurosci. 2019. [Google Scholar] [CrossRef]
- Sampson, T.R.; Challis, C.; Jain, N.; Moiseyenko, A.; Ladinsky, M.S.; Shastri, G.G.; Thron, T.; Needham, M.D.; Horvath, I.; Debelius, J.W.; et al. A gut bacterial amyloid promotes α-synuclein aggregation and motor impairment in mice. Microbiol. Infect. Dis. Neurosci 2020. [Google Scholar] [CrossRef]
- Hwang, D.H.; Kim, J.A.; Lee, J.Y. Mechanisms for the activation of toll-like receptor 2/4 by saturated fatty acids and inhibition by docosahexaenoic acid. Eur. J. Pharmacol. 2016, 785, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Wouters, M.M.; Vicario, M.; Santos, J. The role of mast cells in functional GI disorders. Gut 2016, 65, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Reber, L.L.; Sibilano, R.; Mukai, K.; Galli, S.J. Potential Effector and Immunoregulatory Functions of Mast Cells in Mucosal Immunity. Mucosal Immunol. 2015, 8, 444–463. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.N.; Brown, M.A. Mast Cells Multifaceted Immune Cells with Diverse Roles in Health and Disease. Ann. N. Y. Acad. Sci. 2008, 1143, 83–104. [Google Scholar] [CrossRef]
- Dahlin, J.S.; Hallgren, J. Mast cell progenitors: Origin, development and migration to tissues. Mol. Immunol. 2015, 63, 9–17. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Krämer, S. Human mast cells, bacteria, and intestinal immunity. Immunol. Rev. 2007, 217, 329–337. [Google Scholar] [CrossRef]
- Traina, G. Mast cells in gut and brain and their potential role as an emerging therapeutic target for neural diseases. Front. Cell. Neurosci. 2019, 13, 345. [Google Scholar] [CrossRef]
- Barbara, G.; Cremon, C.; Carini, G.; Bellacosa, L.; Zecchi, L.; De Giorgio, R.; Corinaldesi, R.; Stanghellini, V. The Immune System In Irritable Bowel Syndrome. J. Neurogastroenterol. Motil. 2011, 17, 349–359. [Google Scholar] [CrossRef]
- Galli, S.J.; Grimbaldeston, M.; Tsai, M. Immunomodulatory mast cells: Negative, as well as positive, regulators of innate and acquired immunity. Nat. Rev. Immunol. 2008, 8, 478–486. [Google Scholar] [CrossRef]
- Bischoff, S.C. Role of mast cells in allergic and non-allergic immune responses: Comparison of human and murine data. Nat. Rev. Immunol. 2007, 7, 93–104. [Google Scholar] [CrossRef]
- Traina, G. Mast cells in the brain—Old cells, new target. J. Int. Neurosci. 2017, 16, S69–S83. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, L.; Akdis, M.; Akdis, C.A. Regulation of the immune response and inflammation by histamine and histamine receptors. J. Allergy Clin. Immunol. 2011, 128, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jubair, S.; Levick, S.P.; Janicki, J.S. The autocrine role of tryptase in pressure overload-induced mast cell activation, chymase release and cardiac fibrosis. IJC Metab. Endocr. 2016, 10, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Akdis, A.C.; Blaser, K. Histamine in the immune regulation of allergic inflammation. J. Allergy Clin. Immunol. 2003, 112, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Novotný, M.; Klimova, B.; Valis, M. Microbiome and Cognitive Impairment: Can Any Diets Influence Learning Processes in a Positive Way? Front. Aging Neurosci. 2019, 28. [Google Scholar] [CrossRef] [PubMed]
- Barbara, G.; Stanghellini, V.; De Giorgio, R.; Cremon, C.; Cottrell, G.S.; Santini, D.; Pasquinelli, G.; Morselli-Labate, A.M.; Grady, E.F.; Bunnett, N.W.; et al. Activated Mast Cells in Proximity to Colonic Nerves Correlate with Abdominal Pain in Irritable Bowel Syndrome. Gastroenterology 2004, 126, 693–702. [Google Scholar] [CrossRef]
- De Winter, B.Y.; De Man, J.G. Interplay between Inflammation, Immune System and Neuronal Pathways: Effect on Gastrointestinal Motility. World J. Gastroenterol. 2010, 28, 5523–5535. [Google Scholar] [CrossRef]
- Barbara, G.; Wang, B.; Stanghellini, V.; De Giorgio, R.; Cremon, C.; Di Nardo, G.; Trevisani, M.; Campi, B.; Geppetti, P.; Tonini, M.; et al. Mast Cell-Dependent Excitation of Visceral-Nociceptive Sensory Neurons in Irritable Bowel Syndrome. Gastroenterology 2007, 132, 26–37. [Google Scholar] [CrossRef]
- Dong, H.; Zhang, X.; Wang, Y.; Zhou, X.; Qian, Y.; Zhang, S. Suppression of Brain Mast Cells Degranulation Inhibits Microglial Activation and Central Nervous System Inflammation. Mol. Neurobiol. 2017, 54, 997–1007. [Google Scholar] [CrossRef]
- Buhner, S.; Schemann, M. Mast Cell-Nerve Axis with a Focus on the Human Gut. Biochim. Biophys. Acta 2012, 1822, 85–92. [Google Scholar] [CrossRef]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The Gut-Brain Axis: Interactions between Enteric Microbiota, Central and Enteric Nervous Systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar] [PubMed]
- De Marco, S.; Sichetti, M.; Muradyan, D.; Piccioni, M.; Traina, G.; Pagiotti, R.; Pietrella, D. Probiotic Cell-Free Supernatants Exhibited Anti-Inflammatory and Antioxidant Activity on Human Gut Epithelial Cells and Macrophages Stimulated with LPS. Evid. Based Complement. Alternat. Med. 2018, 2018, 1756308. [Google Scholar] [CrossRef] [PubMed]
- Sichetti, M.; De Marco, S.; Pagiotti, R.; Traina, G.; Pietrella, D. Anti-inflammatory effect of multi-strain probiotics formulation (L. rhamnosus, B. lactis and B. longum). Nutrition 2018, 53, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Bellavia, M.; Rappa, F.; Lo Bello, M.; Brecchia, G.; Tomasello, G.; Leone, A.; Spatola, G.; Uzzo, M.L.; Bonaventura, G.; David, S.; et al. Lactobacillus casei and bifidobacterium lactis supplementation reduces tissue damage of intestinal mucosa and liver after 2,4,6-trinitrobenzenesulfonic acid treatment in mice. J. Biol. Reg. Homeost. Agents 2014, 28, 251–261. [Google Scholar]
- Traina, G.; Menchetti, L.; Rappa, F.; Casagrande-Proietti, P.; Barbato, O.; Leonardi, L.; Carini, F.; Piro, F.; Brecchia, G. Probiotic mixture supplementation in the preventive management of trinitrobenzenesulfonic acid-induced inflammation in a murine model. J. Biol. Reg. Homeost. Agents 2016, 30, 895–901. [Google Scholar]
- Persichetti, E.; De Michele, A.; Codini, M.; Traina, G. Antioxidative Capacity of Lactobacillus Fermentum LF31 Evaluated In Vitro by Oxygen Radical Absorbance Capacity Assay. Nutrition 2014, 30, 936–938. [Google Scholar] [CrossRef]
- Dominici, L.; Moretti, M.; Villarini, M.; Vannini, S.; Cenci, G.; Zampino, C.; Traina, G. In vivo antigenotoxic properties of a commercial probiotic supplement containing bifidobacteria. Int. J. Probiot. Prebiot. 2011, 6, 179–186. [Google Scholar]
- Wiley, N.C.; Dinan, T.G.; Ross, R.P.; Stanton, C.; Clarke, G.; Cryan, J.F. The microbiota-gut-brain axis as a key regulator of neural function and the stress response: Implications for human and animal health. J. Anim. Sci. 2017, 95, 3225–3246. [Google Scholar] [CrossRef]
- Barbara, G.; Zecchi, L.; Barbaro, R.; Cremon, C.; Bellacosa, L.; Marcellini, M.; De Giorgio, R.; Corinaldesi, R.; Stanghellini, V. Mucosal Permeability And Immune Activation As Potential Therapeutic Targets Of Probiotics In Irritable Bowel Syndrome. J. Clin. Gastroenterol. 2012, 46, S52–S55. [Google Scholar] [CrossRef]
- McNabney, S.M.; Henagan, T.M. Short Chain Fatty Acids in the Colon and Peripheral Tissues: A Focus on Butyrate, Colon Cancer, Obesity and Insulin Resistance. Nutrients 2017, 9, 1348. [Google Scholar] [CrossRef]
- Psichas, A.; Sleeth, M.L.; Murphy, K.G.; Brooks, L.; Bewicick, G.A.; Hanyaloglu, A.C.; Ghatei, M.A.; Bloom, S.R.; Frost, G. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int. J. Obes. 2015, 39, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Hirschberg, S.; Gisevius, B.; Duscha, A.; Haghikia, A. Implications of diet and the gut microbiome in neuroinflammatory and neurodegenerative diseases. Int. J. Mol. Sci. 2019, 20, 3109. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, S.; Toh, H.; Hase, K.; Oshima, K.; Nakanishi, Y.; Yoshimura, K.; Tobe, T.; Clarke, J.M.; Topping, D.L.; Suzuki, T.; et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 2011, 469, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Liu, J.Q.; Yu, Y.; Mo, L.H.; Ge, R.T.; Zhang, H.P.; Liu, Z.G.; Zheng, P.Y.; Yang, P.C. Regulation of TWIK-related potassium channel-1 (Trek1) restitutes intestinal epithelial barrier function. Cell. Mol. Immunol. 2016, 13, 110–118. [Google Scholar] [CrossRef] [PubMed]
- De Zuani, M.; Dal Secco, C.; Frossi, B. Mast cells at the crossroads of microbiota and IBD. Eur. J. Immunol. 2018, 48, 1929–1937. [Google Scholar] [CrossRef] [PubMed]
- Oksaharju, A.; Kankainen, M.; Kekkonen, R.A.; Lindstedt, K.A.; Kovanen, P.T.; Korpela, R.; Miettinen, M. Probiotic Lactobacillus Rhamnosus Downregulates Fcer1 And HRH4 Expression In Human Mast Cells. World J. Gastroenterol. 2011, 17, 750–759. [Google Scholar] [CrossRef]
- Xu, C.; Yan, S.; Guo, Y.; Qiao, L.; Ma, L.; Dou, X.; Zhang, B. Lactobacillus casei ATCC 393 alleviates Enterotoxigenic Escherichia coli K88-induced intestinal barrier dysfunction via TLRs/mast cells pathway. Life Sci. 2020, 244, 117281. [Google Scholar] [CrossRef]
- Guo, L.; Meng, M.; Wei, Y.; Lin, F.; Jiang, Y.; Cui, X.; Wang, G.; Wang, C.; Guo, X. Protective Effects of Live Combined B. subtilis and E. faecium in Polymicrobial Sepsis Through Modulating Activation and Transformation of Macrophages and Mast Cells. Front. Pharmacol. 2019, 9, 1506. [Google Scholar] [CrossRef]
- Cassard, L.; Lalanne, A.I.; Garault, P.; Cotillard, A.; Chervaux, C.; Wels, M.; Smokvina, T.; Daëron, M.; Bourdet-Sicard, R. Individual strains of Lactobacillus paracasei differentially inhibit human basophil and mouse mast cell activation. Immun. Inflamm. Dis. 2016, 4, 289–299. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).