Abstract
The design of appropriate thermally responsive fragrance carrier systems is of significant importance for the application of fragrance in the food and tobacco industries. In this study, we investigate the potential of sorbitan monostearate and guar gum for the stabilization of menthol under ambient conditions and the thermally-induced release of menthol. Our results show that the sorbitan monostearate carrier could well stabilize the menthol for at least up to 15 days with neglectable menthol loss due to the favorable binding of menthol on the sorbitan monostearate carrier. In addition, rapid and controlled release of menthol could take place at a temperature of 80 °C in the sorbitan monostearate carrier system. As a comparison, guar gum could not stabilize menthol as a result of its poor compatibility. Our results suggest that sorbitan monostearate can be an ideal carrier material for the support of fragrance. In addition, our results also provide a useful guide for the tailored design of thermally responsive fragrance carriers.
1. Introduction
Fragrance is widely used in daily care and food products, such as soap, detergent, cosmetics, drinks, candy, and so on. The key to the successful application of fragrance in commercial products is the protection of fragrance under storage conditions and the controlled release of fragrance under working conditions [1]. The use of a proper carrier for fragrance is an ideal approach that can potentially stabilize and release fragrance on-demand, simultaneously [2,3,4,5,6,7,8,9,10,11,12]. Among all the fragrance carriers, thermal-responsive fragrance carriers are an important type of carrier material commonly used in a wide range of fields such as the tobacco industry [13].
For the practical applications of fragrance carriers, a number of factors should be considered. For example, a suitable carrier should be non-toxic to secure its use in daily products. In addition, the carrier should also have an intermediate binding strength with the fragrance, which is particularly important for the on-demand perseverance and release of the fragrance. Furthermore, an ideal carrier material should be cost-competitive to enable large-scale application. Additionally, the carrier should be processable and can be easily integrated into the industrial process. The constraints above make an appropriate choice of carrier material challenging.
Polymeric materials are the prevailing thermal-responsive carrier for fragrance due to the good combability with fragrance and low cost. A number of thermal-responsive polymers have been designed as potential fragrance carriers. For example, nanoparticles formed by thermal-responsive poly(stearyl acrylate) have been fabricated and used for the thermal-induced release of fragrance [14]. The loaded fragrance can be released upon gentle heating near the temperature of human skin due to the reversible transition of the polymer chain from the ordered state to the disordered state at ~45 °C.
Poly(n-isopropyl acrylamide-costyrene)/SiO2 composite hollow spheres have also been used [15]. The thermo-responsive property of the composite is inherited from the poly (n-isopropyl acrylamide-costyrene) component, which undergoes a temperature-dependent transition of the polymer structure. The controlled thermal induced release of cyclamen aldehyde was demonstrated in ethanol. While many thermal-responsive polymers have been reported so far, the composition and microstructure of the reported polymers has to be finely tailored, which leads to a high cost that may hinder their application. More importantly, these synthetic polymers might contain contaminants, such as unreacted monomer and residual solvents, thus limiting their application in food industries.
In order to realize the controllable thermal triggered release of fragrance from carriers, two representative thermal-responsive polymers were chosen for the loading of menthol, which is a widely used fragrance for generation of the sense of refreshment. The compatability of menthol with the carrier substrate is studied with both theoretical and experimental investigations to provide an insight into the interaction between the fragrance and carrier. The stabilization effect and capability of the on-demand thermal menthol release of the carriers are evaluated to screen a possible carrier for practical applications and establish the structure-performance relation of the carriers.
2. Materials and Methods
Loading of menthol: Two polymer carriers, which are sorbitan monostearate and guar gum (abbreviated as SM and GG, respectively), are used to load menthol. First of all, the polymer carrier was heated to 80 °C in a beaker to yield a liquid or viscous state. Then, menthol was quickly added into the beaker, followed by rapid stirring in a closed environment and natural cooling to the ambient temperature.
Measurement of the melting point: The melting point of the polymer or polymer carrier system was characterized with a microscopic melting point meter (X-4, SGW, Shanghai Wuguang, Shanghai, China). The reported melting point is the average value measured from three individual tests.
(Fourier Transform Infrared) FTIR measurement: FTIR spectra of the samples was recorded on an AVATAR FT-IR370 meter (ThermoNicolet, U.S.) in the wavenumber rage of 400 to 4000 cm−1.
Stability and release performance of the carrier/fragrance system: For the stability test, the samples were evenly spread in a petri dish which was placed on a hot plate heated to 40 °C under an open condition. The mass of the samples was recorded after certain time intervals and the changes in the mass of the sample were used to reflect the stability of the fragrance carrier system. The setup of the fragrance release experiment was identical to that of the stability test, except that the temperature of the hot plat was set to 80 °C.
Density Functional Theory (DFT) analysis: The optimized geometric adsorptive configuration was obtained by using density functional theory in Vienna Ab-initio Simulation Package (VASP) passphrase. The exchange correlation interaction in the calculation was described as general gradient approximation (GGA) in the form of Perdew–Burke–Ernzerhof functional (PBE). A cut-off energy of 400 eV for plain-wave basis sets was used, and the convergence criteria of energy and force was set to be 10−5 eV and 0.002 eV Å−1, respectively. Gamma point 1 × 1 × 1 was used to model the Brillouin zone. In addition, the physical van der Waals (vdW) interaction was included in the calculation in the simulation using the vdw-DF (density function). The affinity of the fragrance and polymer carried was described with the binding energy, which was calculated with the Equation:
in which Etotal is the total energy after the system after adsorption, and Esub and EI represent the energy of substrate and the menthol after molecular optimization, respectively.
Eb = Etotal − Esub − EI,
3. Results
First, the affinity of the menthol with SM and guar was investigated theoretically with DFT investigations. The adsorptive configuration of menthol on SM and guar was optimized and the binding energy was calculated accordingly (Figure 1). Guar is a high molecular weight galactomannan polysaccharide composed by linear D-mannose units joined by 1,4-β-glycosidic linkages. To generate the adsorption model with guar gum, we built a block composed of galactose and mannose to mimic the molecule structure of guar gum (Figure 1B). According to the calculation results, the binding energy of menthol on SM and guar gum was −0.915 eV and −0.05 eV, respectively. These results suggest that menthol favorably binds with the SM and the affinity of menthol with SM was higher than that for guar gum. Such a high affinity between the fragrance and polymeric substrate helps to evenly distribute and stabilize the fragrance in the polymer carrier.
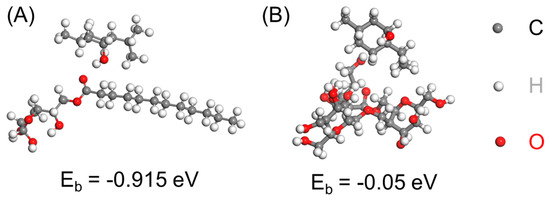
Figure 1.
(A) Optimized configuration showing the adsorption of menthol on (A) sorbitan monostearate and on (B) a model polymer block mimicking guar.
Thermal induced fragrance release below 100 °C is important in food and tobacco applications. Therefore, it is important to identify possible polymer carrier for typical fragrances, which could realize sustainable and controllable fragrance release at a temperature below 100 °C. As the thermal release performance of the carrier system is closely related to the thermal stability of the carrier, we first evaluated the melting behavior of the polymer carrier. The melting point of SM was measured to be 63 °C. For guar gum, no distinct melting behavior was observed, indicating the low crystallinity of the material. The fragrance was loaded into the carrier material through mixing of the fragrance and polymer carrier at 80 °C, under which temperature the mixture exists as fluid. After mixing, the carrier system was naturally cooled down to room temperature. To demonstrate the successful loading of the fragrance into the polymer carrier, an FTIR measurement was performed (Figure 2). Except the typical bands from alkyl chains, characteristic bands associated with the unique functional groups of the polymer carrier were observed. For example, an absorption band at 1735 cm−1 (C=O, stretching vibration) was present in the FTIR spectra of SM, menthol-SM, and menthol-SM + GG; an absorption band at 1650 cm−1 (C=C, stretching vibration) was recorded in the spectra of GG, menthol-GG, and menthol-SM + GG. The successful loading of menthol into the polymer carrier was evidenced by the presence of characteristic bands from menthol in the spectra of menthol-SM, menthol-GG, and menthol-SM + GG (e.g., 1345 cm−1 for the in-plane wagging vibration of CH and 1025 cm−1 for the stretching vibration of C-O).
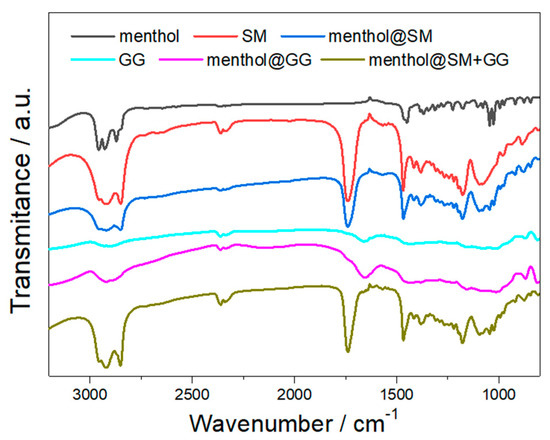
Figure 2.
FTIR spectra of menthol, sorbitan monostearate (SM), guar gum (GG), menthol-SM, menthol-GG, and menthol-SM + GG.
The incorporation of the fragrance into the polymer carrier influences the melting behavior of the carrier system. As discussed above, the pristine SM had a melting point of 63 °C. The melting point of the fragrance carrier system with fragrance (menthol): polymer carrier (SM) ratios of 1:10, 2:10, and 4:10 were determined to be 55, 52, and 42 °C. When further increasing the menthol content in the carrier system (above 4:10), the carrier system tends to behave like a semi-liquid above 30 °C. The fragrance carrier system at a semi-liquid state may not be suitable for most of the practical applications. Thus, we chose a fragrance: polymer carrier ratio of 4:10 for subsequent studies.
The release of fragrance from the polymer carriers was first characterized with a visualized time-lapse experiment with the melting point meter (Figure 3). At a set temperature of 80 °C, no obvious phase changes occurred on menthol-GG. Only a small portion of melted substance, which could be menthol (melting point 45 °C), was observed in some parts of the material. This observation confirmed that GG was able to release menthol through the melting of menthol upon heating. However, it was also shown from the image that the region of the melting was only limited in certain regions of the composite, which indicated that the distribution of menthol was not uniform in GG. The non-uniform distribution of menthol in GG was understandable due to the low affinity between menthol and GG, as suggested by the DFT analysis.
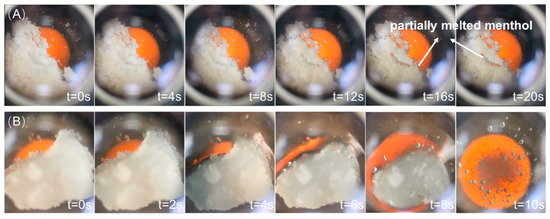
Figure 3.
Time-lapse optical image showing the fragrance release behavior of (A) menthol-GG and (B) menthol-SM at 70 °C.
The menthol-SM composite exhibited a homogeneous melting behavior upon heating (Figure 3B). In the whole melting process, no phase separation was seen and the whole composite was able to fully melt upon heating for 10 s. This rapid and uniform melting process may enable an efficient fragrance release process. Furthermore, the high homogeneity of the carrier system is beneficial for the close wrapping of fragrance within the carrier polymer and thus the high stability of the composite.
One important function of the polymer carrier is to stabilize the fragrance under storage conditions. Therefore, we characterized the mass change of the carrier system at 40 °C in open conditions. For a comprehensive understanding of the stability of the carrier system, composite polymer carriers composed of SM and GG were also investigated. As shown in Figure 4, pristine menthol had a rapid mass loss under the testing conditions. After 2 days and 15 days storage, the weight loss of pristine menthol was up to 5.0% and 62.1%. Such a high weight loss indicates that the direct use of menthol for applications in which long-term storage is required is inappropriate.
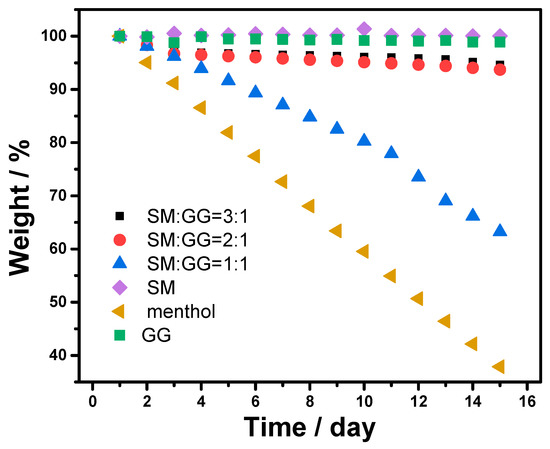
Figure 4.
Stability of the carrier systems with different carriers.
The loading of menthol into SM significantly improved the stability of menthol. Little weight change was observed for the menthol-SM carrier system for a storage period up to 15 days, which directly proved that the use of the SM carrier could effectively prevent the unwanted loss of fragrance molecules. The incorporation of the GG component into the carrier led to the loss of menthol under identical conditions. With the increase in the amount of GG, the menthol tended to loss at a higher rate. The menthol loss associated with the incorporation of GG can be explained by the low affinity of GG with menthol, which in turn results in the inhomogeneous distribution of menthol in the carrier system and the rapid loss of menthol.
Aside from the good stability, rapid release of fragrance under working conditions is also essential for an ideal fragrance carrier system. To assess the fragrance release performance, the fragrance carrier system was heated to 80 °C in an open environment and the weight of the carrier system was recorded continuously (Figure 5). It was shown that rapid weight change occurred as the experiment started. All the carrier systems could effectively deliver the fragrance upon heating and the incorporation of GG into SM did not change the thermal release behavior of the system. This is because a temperature of 80 °C is above the melting temperature of both menthol and SM. In addition, the softening of GG at this temperature also allows for the free migration of menthol in the composite and the release of menthol from the composite.
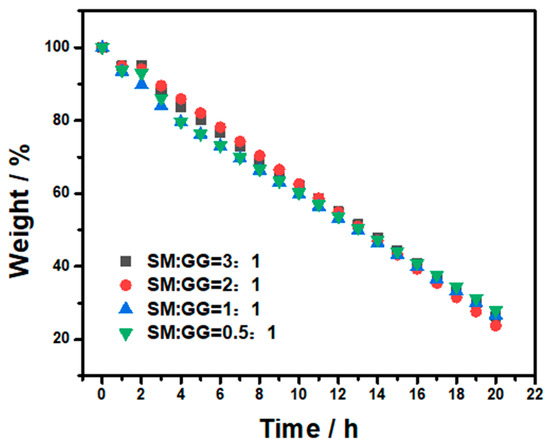
Figure 5.
Fragrance release from the carrier systems with different ratios of SM and GG.
4. Conclusions
To summarize, sorbitan monostearate and guar gum are selected as model thermal responsive polymers as the carrier of menthol. The thermal stability and menthol release performance of both carrier systems was evaluated. Our results reveal that sorbitan monostearate is an ideal carrier for enhancing the stability of the menthol under storage conditions and to simultaneously achieve the rapid release of menthol under working conditions. Through comparative studies and DFT investigations, we demonstrate that guar gum is less favorable for the loading of menthol. This study not only successfully identifies sorbitan monostearate as an effective fragrance carrier but also shows that the affinity between the carrier and fragrance is an important criterion in the design of applicable carriers for fragrance molecules.
Author Contributions
Conceptualization, J.L.; methodology, M.W.; investigation, X.S., X.P., R.L., C.C., X.D.; writing—review and editing, J.L.; supervision, J.L.; project administration, M.W. All authors have read and agreed to the published version of the manuscript.
Funding
We thank the State Key Laboratory of Advanced Technology for Material Synthesis and Processing (Wuhan University of Technology, 2019-KF-10) for financial support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sansukcharearnpon, A.; Wanichwecharungruang, S.; Leepipatpaiboon, N.; Kerdcharoen, T.; Arayachukeat, S. High loading fragrance encapsulation based on a polymer-blend: Preparation and release behavior. Int. J. Pharm. 2010, 391, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.H.; Wang, Y.X.; Huang, J.X.; Zhou, Z.X.; Zhao, D.; Jiang, L.M.; Shen, Y.Q. Encapsulation and controlled release of fragrances from functionalized porous metal-organic frameworks. AIChE J. 2019, 65, 491–499. [Google Scholar] [CrossRef]
- Elesini, U.S.; Svarc, J.; Sumiga, B.; Urbas, R. Melamine formaldehyde microcapsules with fragrance core material: Preparation, properties, and end use. Text Res. J. 2017, 87, 2435–2448. [Google Scholar] [CrossRef]
- Zhao, D.; Jiao, X.; Zhang, M.M.; Ye, K.; Shi, X.D.; Lu, X.H.; Qiu, G.; Shea, K.J. Preparation of high encapsulation efficiency fragrance microcapsules and their application in textiles. RSC Adv. 2016, 6, 80924–80933. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, J.; Chen, H.L. Preparation of polyacrylate/paraffin microcapsules and its application in prolonged release of fragrance. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Liao, Z.Y.; Xue, D.Q.; Li, H.; Shi, L. Fragrance-containing microcapsules based on interfacial thiol-ene polymerization. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Cao, Z.H.; Xu, C.; Ding, X.X.; Zhu, S.D.; Chen, H.N.; Qi, D.M. Synthesis of fragrance/silica nanocapsules through a sol-gel process in miniemulsions and their application as aromatic finishing agents. Colloid Polym. Sci. 2015, 293, 1129–1139. [Google Scholar] [CrossRef]
- Xiao, Z.B.; Tian, T.; Hu, J.; Wang, M.X.; Zhou, R.J. Preparation and characterization of chitosan nanoparticles as the delivery system for tuberose fragrance. Flavour Fragr. J. 2014, 29, 22–34. [Google Scholar] [CrossRef]
- Wang, Z.Z.; Johns, V.K.; Liao, Y. Controlled Release of Fragrant Molecules with Visible Light. Chem. Eur. J. 2014, 20, 14637–14640. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, Y.Q.; Fan, H.F.; Xia, Q. Heat-Resistant Sustained-Release Fragrance Microcapsules. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Ciobanu, A.; Ruellan, S.; Mallard, I.; Landy, D.; Gennequin, C.; Siffert, S.; Fourmentin, S. Cyclodextrin-intercalated layered double hydroxides for fragrance release. J. Incl. Phenom. Macrocycl. Chem. 2013, 75, 333–339. [Google Scholar] [CrossRef]
- Seemork, J.; Tree-Udom, T.; Wanichwecharungruang, S. A refillable fragrance carrier with a tuneable thermal switch. Flavour Fragr. J. 2012, 27, 386–392. [Google Scholar] [CrossRef]
- Kaur, R.; Kukkar, D.; Bhardwaj, S.K.; Kim, K.H.; Deep, A. Potential use of polymers and their complexes as media for storage and delivery of fragrances. J. Control Release 2018, 285, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Popadyuk, N.; Popadyuk, A.; Kohut, A.; Voronov, A. Thermoresponsive latexes for fragrance encapsulation and release. Int. J. Cosmet. Sci. 2016, 38, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Liu, L.Q.; Xie, Y.Y.; Wu, L.M. Facile synthesis of thermal-responsive P(NIPAM-S)/SiO2 hybrid hollow spheres and their controllable release properties for fragrance. Polym. Chem. 2013, 4, 3293–3299. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).