Application of Computational Fluid Dynamics Analysis after Bimaxillary Orthognathic Surgery
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
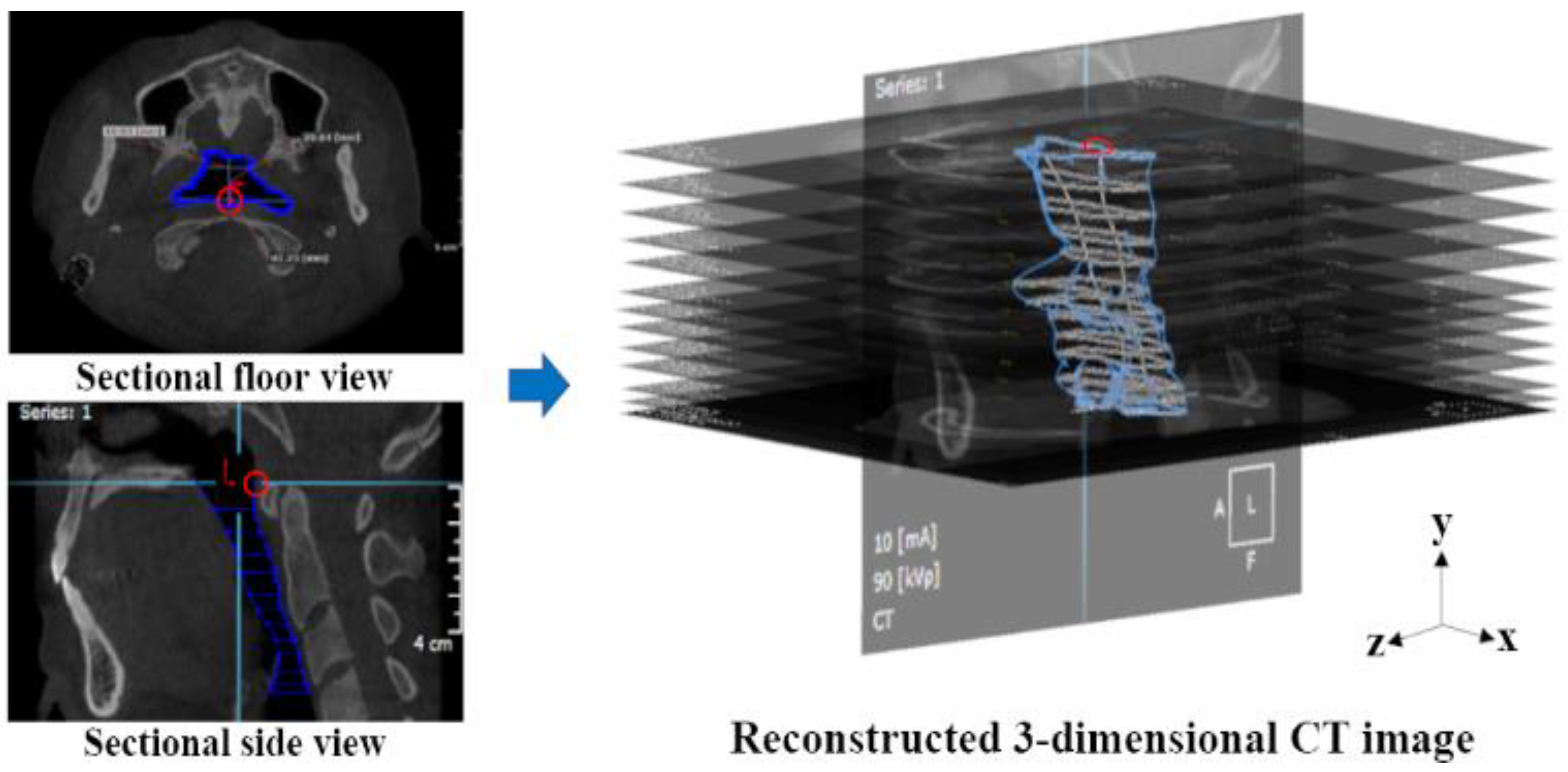
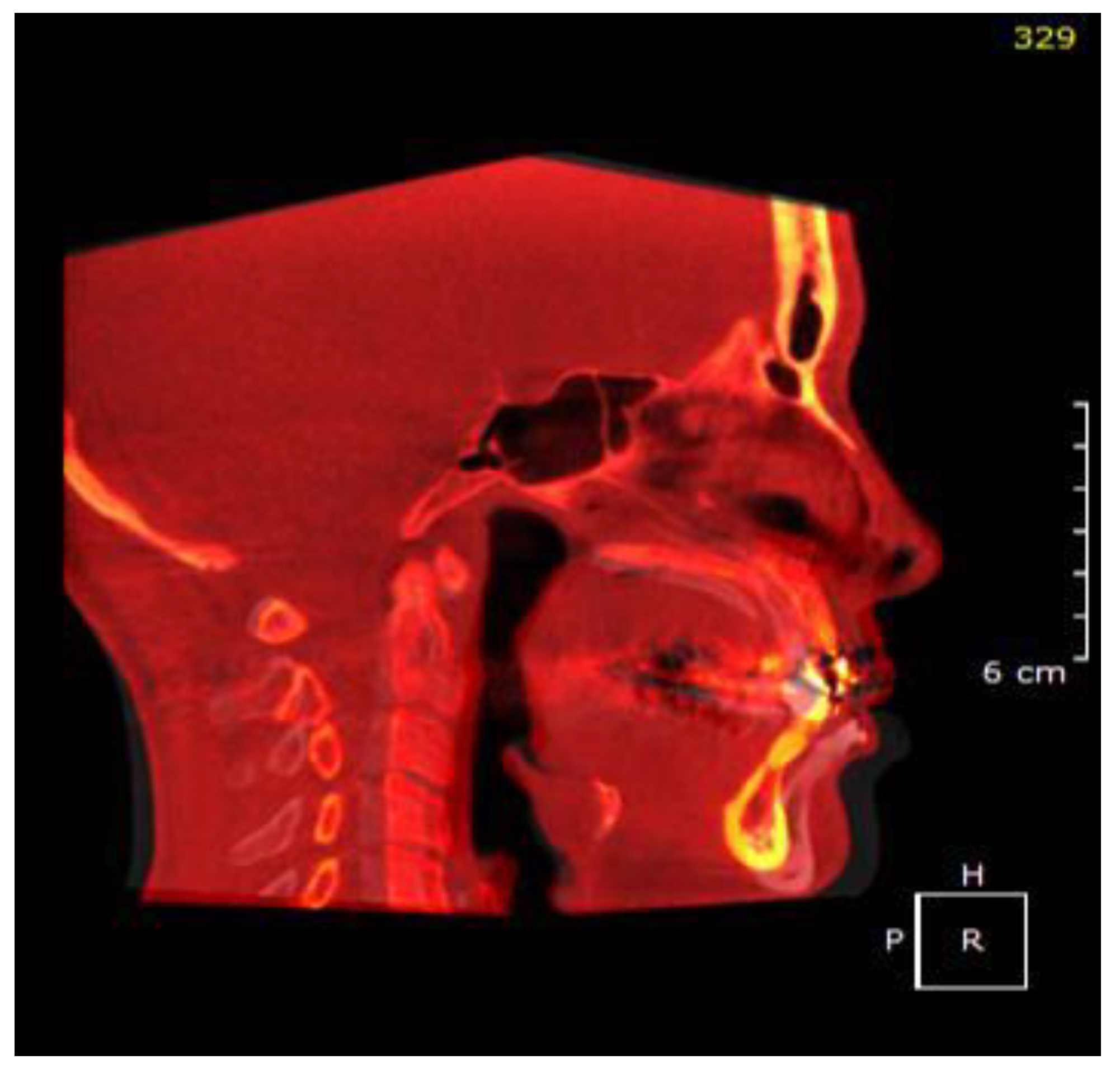
2.2. Airway Modeling
2.3. Numerical Simulations
3. Results
3.1. Volumetric Analysis
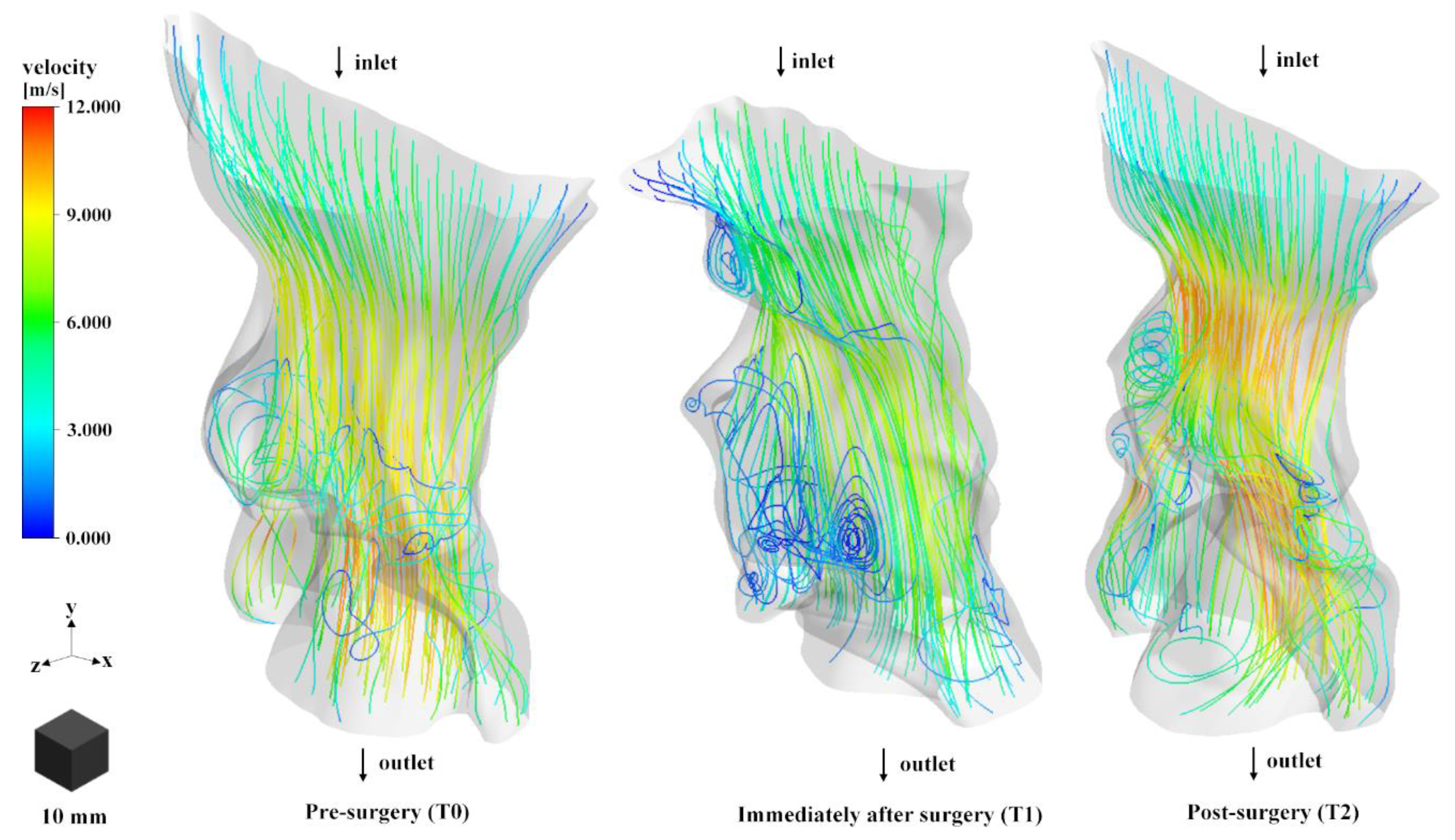
3.2. Airflow Stream Analysis
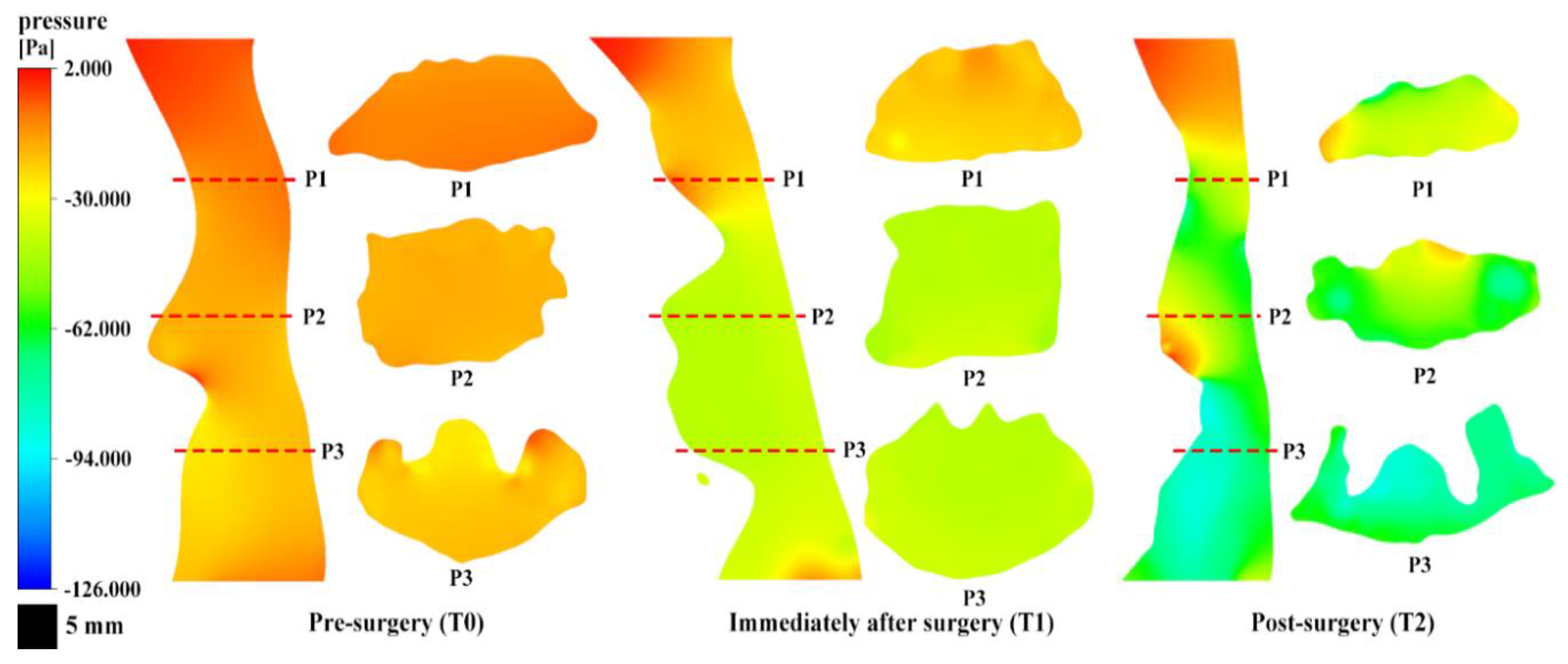
3.3. Airway Pressure Analysis
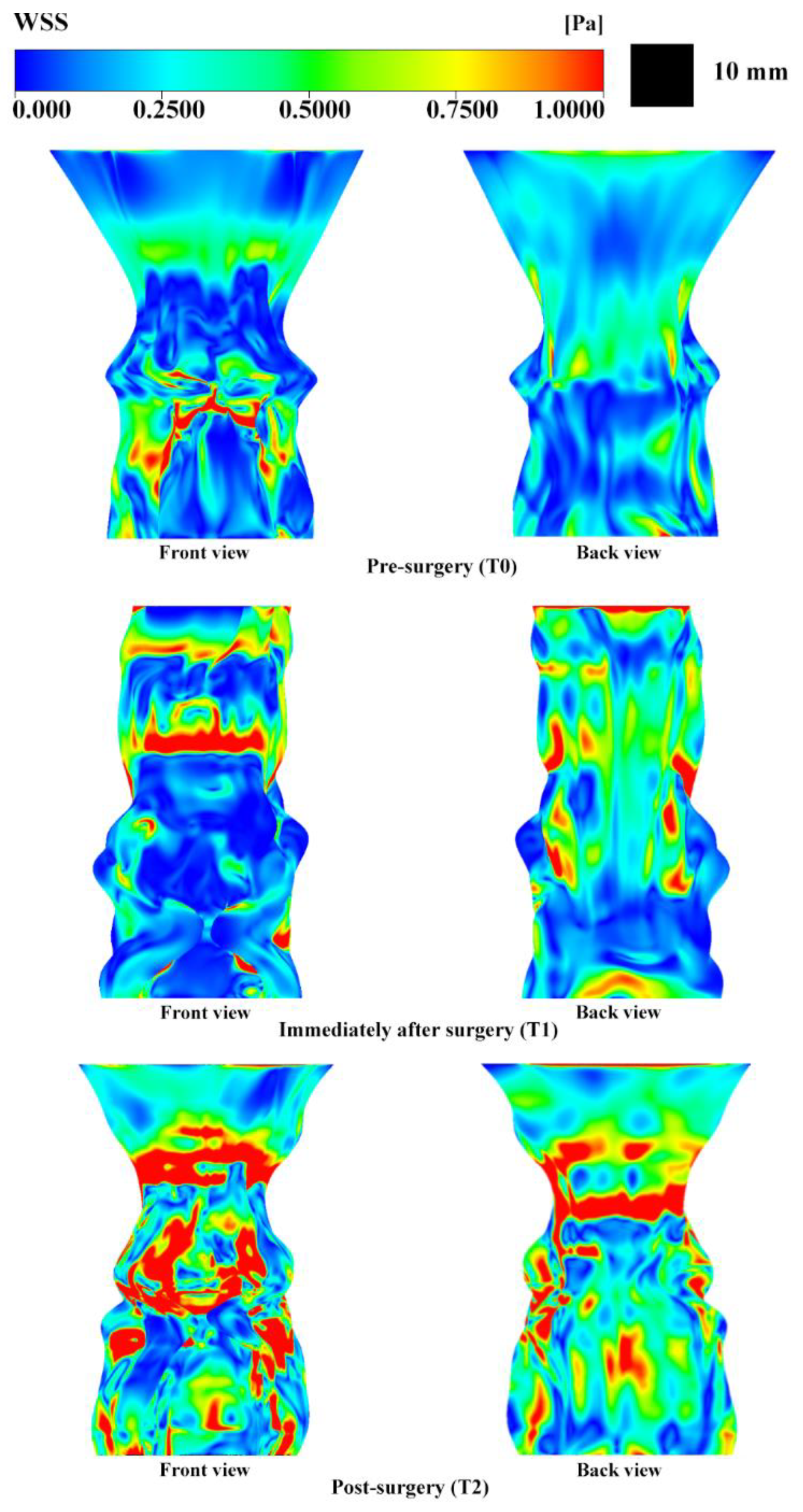
3.4. Wall Shear Stress Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ngan, P.; Moon, W. Evolution of Class III treatment in orthodontics. Am. J. Orthod. Dentofac. Orthop. 2015, 148, 22–36. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.; Ahn, S.J.; Sonnesen, L. Ethnic differences in craniofacial and upper spine morphology between European and Asian children with skeletal Class III malocclusion. Am. J. Orthod. Dentofac. Orthop. 2019, 156, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, M.; Yamamoto, K.; Fujimoto, M.; Ohgi, K.; Inoue, M.; Kirita, T. Changes in tongue and hyoid positions, and posterior airway space following mandibular setback surgery. J. Cranio Maxillofac. Surg. 2005, 33, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Becker, O.E.; Avelar, R.L.; Goelzer, J.G.; Dolzan Ado, N.; Haas, O.L., Jr.; De Oliveira, R.B. Pharyngeal airway changes in Class III patients treated with double jaw orthognathic surgery-maxillary advancement and mandibular setback. J. Oral Maxillofac. Surg. 2012, 70, e639–e647. [Google Scholar] [CrossRef] [PubMed]
- Tatullo, M.; Marrelli, M.; Scacco, S.; Lorusso, M.; Doria, S.; Sabatini, R.; Auteri, P.; Cagiano, R.; Inchingolo, F. Relationship between oxidative stress and “burning mouth syndrome” in female patients: A scientific hypothesis. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 1218–1221. [Google Scholar] [PubMed]
- Chen, F.; Terada, K.; Hua, Y.; Saito, I. Effects of bimaxillary surgery and mandibular setback surgery on pharyngeal airway measurements in patients with Class III skeletal deformities. Am. J. Orthod. Dentofac. Orthop. 2007, 131, 372–377. [Google Scholar] [CrossRef]
- Degerliyurt, K.; Ueki, K.; Hashiba, Y.; Marukawa, K.; Simsek, B.; Okabe, K.; Nakagawa, K.; Yamamoto, E. The effect of mandibular setback or two-jaws surgery on pharyngeal airway among different genders. Int. J. Oral Maxillofac. Surg. 2009, 38, 647–652. [Google Scholar] [CrossRef]
- Collins, T.P.; Tabor, G.R.; Young, P.G. A computational fluid dynamics study of inspiratory flow in orotracheal geometries. Med. Biol. Eng. Comput. 2007, 45, 829–836. [Google Scholar] [CrossRef]
- Xia, G.; Tawhai, M.H.; Hoffman, E.A.; Lin, C.L. Airway wall stiffening increases peak wall shear stress: A fluid-structure interaction study in rigid and compliant airways. Ann. Biomed. Eng. 2010, 38, 1836–1853. [Google Scholar] [CrossRef]
- Sittitavornwong, S.; Waite, P.D.; Shih, A.M.; Cheng, G.C.; Koomullil, R.; Ito, Y.; Cure, J.K.; Harding, S.M.; Litaker, M. Computational fluid dynamic analysis of the posterior airway space after maxillomandibular advancement for obstructive sleep apnea syndrome. J. Oral Maxillofac. Surg. 2013, 71, 1397–1405. [Google Scholar] [CrossRef]
- Shah, D.H.; Kim, K.B.; McQuilling, M.W.; Movahed, R.; Shah, A.H.; Kim, Y.I. Computational fluid dynamics for the assessment of upper airway changes in skeletal Class III patients treated with mandibular setback surgery. Angle Orthod. 2016, 86, 976–982. [Google Scholar] [CrossRef] [PubMed]
- Na, J.S.; Jung, H.D.; Cho, H.J.; Choi, Y.J.; Lee, J.S. Computational analysis of airflow dynamics for predicting collapsible sites in the upper airways: A preliminary study. J. Appl. Physiol. 2019, 126, 330–340. [Google Scholar] [CrossRef]
- Mylavarapu, G.; Murugappan, S.; Mihaescu, M.; Kalra, M.; Khosla, S.; Gutmark, E. Validation of computational fluid dynamics methodology used for human upper airway flow simulations. J. Biomech. 2009, 42, 1553–1559. [Google Scholar] [CrossRef] [PubMed]
- Paduano, F.; Marrelli, M.; Alom, N.; Amer, M.; White, L.J.; Shakesheff, K.M.; Tatullo, M. Decellularized bone extracellular matrix and human dental pulp stem cells as a construct for bone regeneration. J. Biomater. Sci. Polym. Ed. 2017, 28, 730–748. [Google Scholar] [CrossRef] [PubMed]
- Kerativitayanan, P.; Tatullo, M.; Khariton, M.; Joshi, P.; Perniconi, B.; Gaharwar, A.K. Nanoengineered osteoinductive and elastomeric scaffolds for bone tissue engineering. ACS Biomater. Sci. Eng. 2017, 3, 590–600. [Google Scholar] [CrossRef]
- Tatullo, M.; Codispoti, B.; Pacifici, A.; Palmieri, F.; Marrelli, M.; Pacifici, L.; Paduano, F. Potential Use of Human Periapical Cyst-Mesenchymal Stem Cells (hPCy-MSCs) as a Novel Stem Cell Source for Regenerative Medicine Applications. Front. Cell Dev. Biol. 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Özbek, M.M.; Miyamoto, K.; Lowe, A.A.; Fleetham, J.A. Natural head posture, upper airway morphology and obstructive sleep apnoea severity in adults. Eur. J. Orthod. 1998, 20, 133–143. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Wang, Y.; Hu, H.; Liao, Q.; Zhang, W.; Xiang, X.; Fan, X. Impact on the upper airway space of different types of orthognathic surgery for the correction of skeletal class III malocclusion: A systematic review and meta-analysis. Int. J. Surg. 2017, 38, 31–40. [Google Scholar] [CrossRef]
- Staderini, E.; Patini, R.; De Luca, M.; Gallenzi, P. Three-dimensional stereophotogrammetric analysis of nasolabial soft tissue effects of rapid maxillary expansion: A systematic review of clinical trials. Acta Otorhinolaryngol. Ital. 2018, 38, 399–408. [Google Scholar] [CrossRef]
- Patini, R.; Arrica, M.; Di Stasio, E.; Gallenzi, P.; Cordaro, M. The use of magnetic resonance imaging in the evaluation of upper airway structures in paediatric obstructive sleep apnoea syndrome: A systematic review and meta-analysis. Dentomaxillofac. Radiol. 2016, 45, 20160136. [Google Scholar] [CrossRef]
- Hochban, W.; Schurmann, R.; Brandenburg, U.; Conradt, R. Mandibular setback for surgical correction of mandibular hyperplasia--does it provoke sleep-related breathing disorders? Int. J. Oral Maxillofac. Surg. 1996, 25, 333–338. [Google Scholar] [CrossRef]
- Canellas, J.V.; Barros, H.L.; Medeiros, P.J.; Ritto, F.G. Sleep-disordered breathing following mandibular setback: A systematic review of the literature. Sleep Breath 2016, 20, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Y.; Huon, L.K.; Iwasaki, T.; Yoon, A.; Riley, R.; Powell, N.; Torre, C.; Capasso, R. Efficacy of Maxillomandibular Advancement Examined with Drug-Induced Sleep Endoscopy and Computational Fluid Dynamics Airflow Modeling. Otolaryngol. Head Neck Surg. 2016, 154, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, T.M.; Bariani, R.C.; Iafigliola, S.G.; Guimaraes, C.M.; Chaves Junior, C.M.; Ferraz, O.; Cappellette Junior, M.; Abraao-Cunha, T.C.; Dal-Fabbro, C.; Rossi, R.; et al. Cone beam computed tomography in assessment on pharynx effects of orthopedic-surgical treatment—A review of the literature. Sleep Sci. 2019, 12, 106–109. [Google Scholar] [CrossRef]
- Song, B.; Li, Y.; Sun, J.; Qi, Y.; Li, P.; Li, Y.; Gu, Z. Computational fluid dynamics simulation of changes in the morphology and airflow dynamics of the upper airways in OSAHS patients after treatment with oral appliances. PLoS ONE 2019, 14, e0219642. [Google Scholar] [CrossRef]
- Suga, H.; Iwasaki, T.; Mishima, K.; Nakano, H.; Ueyama, Y.; Yamasaki, Y. Evaluation of the effect of oral appliance treatment on upper-airway ventilation conditions in obstructive sleep apnea using computational fluid dynamics. Cranio 2019, 1–9. [Google Scholar] [CrossRef]
- Huang, H.; Yin, H.; Wang, Y.; Chen, N.; Huang, D.; Luo, X.; Yin, X.; Zheng, Q.; Shi, B.; Li, J. Computational Fluid Dynamic Analysis of Different Velopharyngeal Closure Patterns. Ann. Otol. Rhinol. Laryngol. 2020, 129, 157–163. [Google Scholar] [CrossRef]
- Yajima, Y.; Oshima, M.; Iwai, T.; Kitajima, H.; Omura, S.; Tohnai, I. Computational fluid dynamics study of the pharyngeal airway space before and after mandibular setback surgery in patients with mandibular prognathism. Int. J. Oral Maxillofac. Surg. 2017, 46, 839–844. [Google Scholar] [CrossRef]

| Volume (mm3) | |
|---|---|
| Pre-surgery (T0) | 21,102.38 |
| Immediately after surgery (T1) | 19,556.17 |
| Post-surgery (T2) | 14,895.94 |
| Vmax (m/s) | ΔP (Pa) | WSSmax (Pa) | |
|---|---|---|---|
| Pre-surgery (T0) | 7.038 | −7.723 | 4.214 |
| Immediately after surgery (T1) | 8.723 | −27.004 | 10.733 |
| Post-surgery (T2) | 12.054 | −53.739 | 14.323 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, J.M.; Seo, H.; Choi, N.-R.; Yeom, E.; Kim, Y.-D. Application of Computational Fluid Dynamics Analysis after Bimaxillary Orthognathic Surgery. Appl. Sci. 2020, 10, 1676. https://doi.org/10.3390/app10051676
Song JM, Seo H, Choi N-R, Yeom E, Kim Y-D. Application of Computational Fluid Dynamics Analysis after Bimaxillary Orthognathic Surgery. Applied Sciences. 2020; 10(5):1676. https://doi.org/10.3390/app10051676
Chicago/Turabian StyleSong, Jae Min, Heerim Seo, Na-Rae Choi, Eunseop Yeom, and Yong-Deok Kim. 2020. "Application of Computational Fluid Dynamics Analysis after Bimaxillary Orthognathic Surgery" Applied Sciences 10, no. 5: 1676. https://doi.org/10.3390/app10051676
APA StyleSong, J. M., Seo, H., Choi, N.-R., Yeom, E., & Kim, Y.-D. (2020). Application of Computational Fluid Dynamics Analysis after Bimaxillary Orthognathic Surgery. Applied Sciences, 10(5), 1676. https://doi.org/10.3390/app10051676






