Postural Control in Children with Cerebellar Ataxia
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Protocol
2.3. Recordings
2.4. Data Processing
3. Results
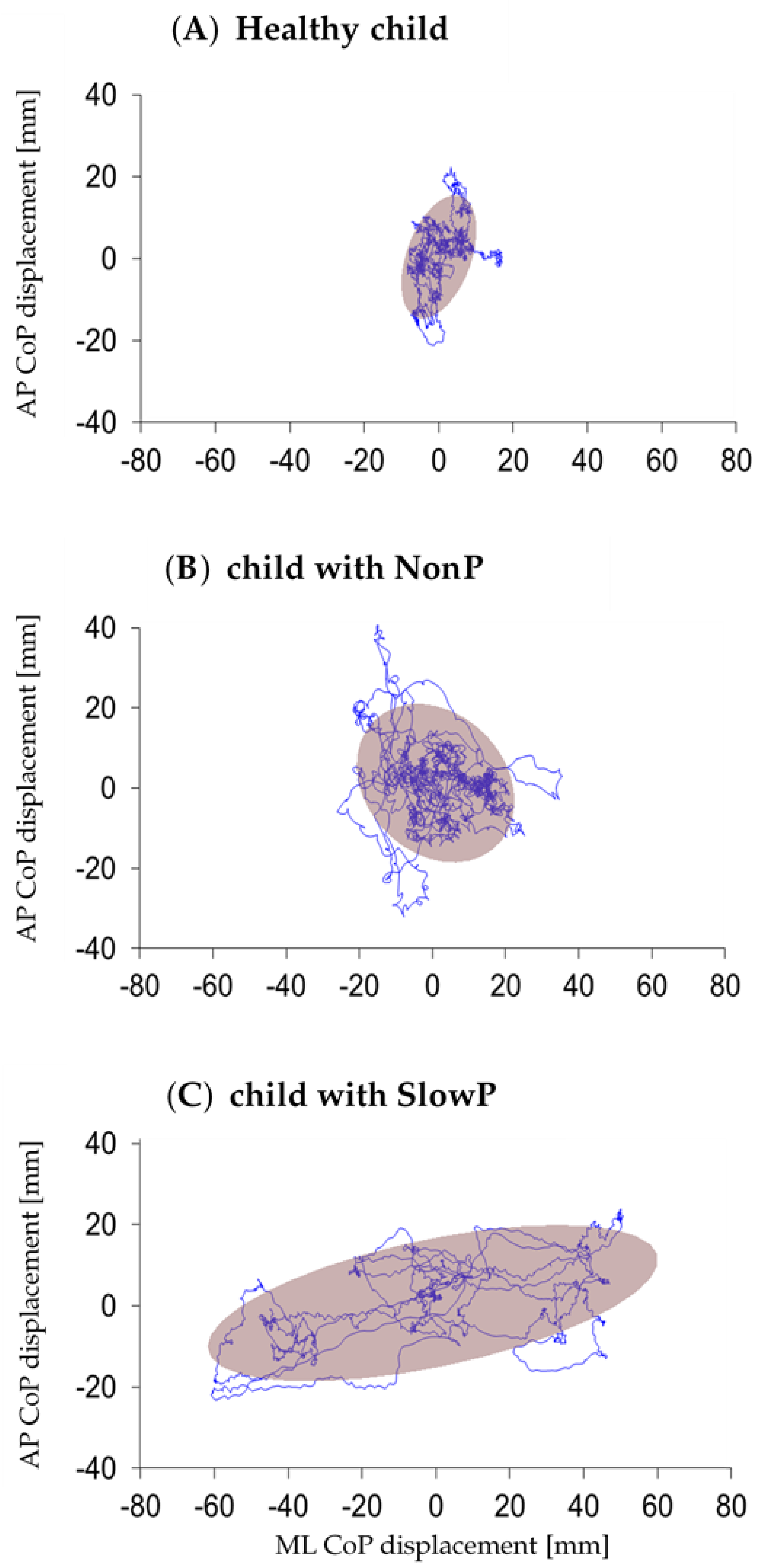
3.1. Postural Parameters
3.2. Gait Initiation Parameters
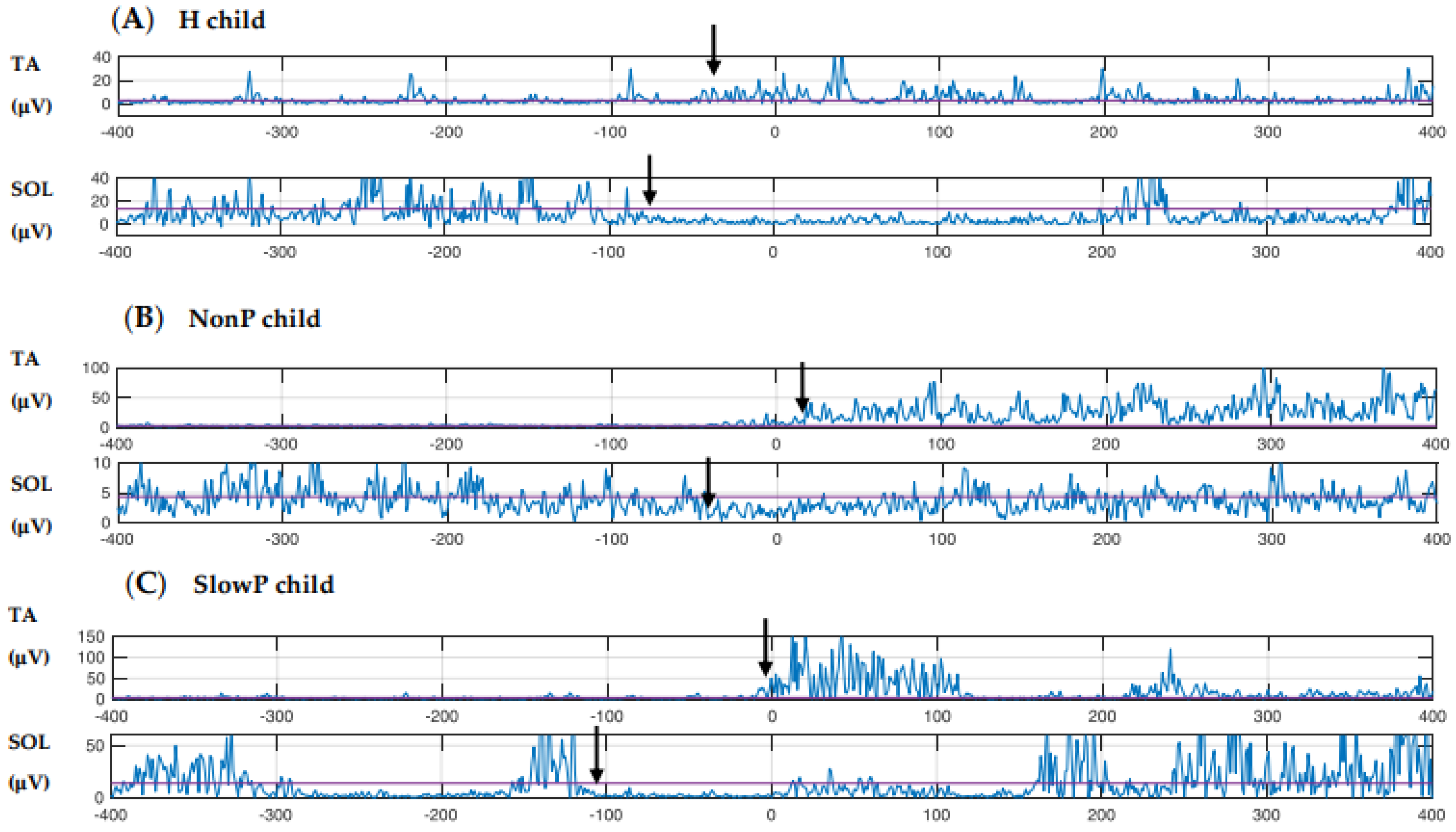
3.3. EMG
4. Discussion
4.1. Disease Progression and Postural Behavior
4.2. Putative Compensatory Network
4.3. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Aruin, A.S.; Latash, M.L. The role of motor action in anticipatory postural adjustments studied with self-induced and externally triggered perturbations. Exp. Brain Res. 1995, 106, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Azuma, T.; Yamashita, N. Anticipatory control related to the upward propulsive force during the rising on tiptoe from an upright standing position. Eur. J. Appl. Physiol. 2004, 92, 10–186. [Google Scholar] [CrossRef] [PubMed]
- Bouisset, S.; Zattara, M. Biomechanical study of the programming of anticipatory postural adjustments associated with voluntary movement. J. Biomech. 1987, 20, 735–742. [Google Scholar] [CrossRef]
- Cordo, P.J.; Nashner, L.M. Properties of postural adjustments associated with rapid arm movements. J. Neurophysiol. 1982, 47, 287–302. [Google Scholar] [CrossRef]
- Marsden, C.D.; Merton, P.A.; Morton, H.B. Human postural responses. Brain 1981, 104, 513–534. [Google Scholar] [CrossRef]
- Massion, J. Movement, posture and equilibrium: Interaction and coordination. Prog. Neurobiol. 1992, 35–56. [Google Scholar] [CrossRef]
- Crenna, P.; Frigo, C. A motor programme for the initiation of forward-oriented movements in humans. J. Physiol. 1991, 437, 635–653. [Google Scholar] [CrossRef]
- Nissan, M.; Whittle, M.W. Initiation of gait in normal subjects: A preliminary study. J. Biomed. Eng. 1990, 12, 165–171. [Google Scholar] [CrossRef]
- Burleigh, A.L.; Horak, F.B.; Malouin, F. Modification of postural responses and step initiation: Evidence for goal-directed postural interactions. J. Neurophysiol. 1994, 72, 2892–2902. [Google Scholar] [CrossRef]
- Burleigh, A.; Horak, F. Influence of instruction, prediction, and afferent sensory information on the postural organization of step initiation. J. Neurophysiol. 1996, 75, 1619–1628. [Google Scholar] [CrossRef]
- McIlroy, W.E.; Maki, B.E. Do anticipatory postural adjustments precede compensatory stepping reactions evoked by perturbation? Neurosci. Lett. 1993, 164, 199–202. [Google Scholar] [CrossRef]
- Yiou, E.; Caderby, T. Balance control during gait initiation: State-of-the-art and research perspectives. World J. Orthop. 2017, 8, 815–828. [Google Scholar] [CrossRef] [PubMed]
- Crenna, P.; Carpinella, I.; Rabuffetti, M.; Rizzone, M.; Lopiano, L.; Lanotte, M.; Ferrarin, M. Impact of subthalamic nucleus stimulation on the initiation of gait in Parkinson’s disease. Exp. Brain Res. 2006, 172, 519–532. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zatsiorsky, V.M.; Latash, M.L. Muscle synergies involved in shifting the center of pressure while making a first step. Exp. Brain Res. 2005, 167, 196–210. [Google Scholar] [CrossRef] [PubMed]
- Ledebt, A.; Bril, B.; Brenière, Y. The build-up of anticipatory behaviour: An analysis of the development of gait initiation in children. Exp. Brain Res. 1998, 120, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Assaiante, C.; Woollacott, M.; Amblard, B. Development of postural adjustment during gait initiation: Kinematic and EMG analysis. J. Mot. Behav. 2009, 32, 211–226. [Google Scholar] [CrossRef]
- Malouin, F.; Richards, C.L. Preparatory adjustments during gait initiation in 4–6-year-old children. Gait Posture 2000, 11, 239–253. [Google Scholar] [CrossRef]
- Isaias, I.U.; Dipaola, M.; Michi, M.; Marzegan, A.; Volkmann, J.; Roidi, M.L.R.; Frigo, C.A.; Cavallari, P. Gait initiation in children with rett syndrome. PLoS ONE 2014, 9, e92736. [Google Scholar] [CrossRef]
- Dipaola, M.; Frigo, C.A.; Cavallari, P.; Iasias, I.U. Alterations of load transfer mechanism during gait initiation in Parkinson’s disease. In Proceedings of the EHB 2017 IEEE International Conference on e-Health and Bioengineering, Sinaia, Romania, 22–24 June 2017; pp. 579–582. [Google Scholar] [CrossRef]
- Timmann, D.; Horak, F.B. Perturbed step initiation in cerebellar subjects 1. Modifications of postural responses. Exp. Brain Res. 1998, 119, 73–84. [Google Scholar] [CrossRef]
- Timmann, D.; Horak, F.B. Perturbed step initiation in cerebellar subjects: 2. Modification of anticipatory postural adjustments. Exp. Brain Res. 2001, 141, 110–120. [Google Scholar] [CrossRef]
- Martino, G.; Ivanenko, Y.P.; Serrao, M.; Ranavolo, A.; D’Avella, A.; Draicchio, F.; Conte, C.; Casali, C.; Lacquaniti, F. Locomotor patterns in cerebellar ataxia. J. Neurophysiol. 2014, 112, 2810–2821. [Google Scholar] [CrossRef] [PubMed]
- Ilg, W.; Golla, H.; Thier, P.; Giese, M.A. Specific influences of cerebellar dysfunctions on gait. Brain 2007, 130, 786–798. [Google Scholar] [CrossRef] [PubMed]
- Asaka, T.; Wang, Y. Feedforward postural muscle modes and multi-mode coordination in mild cerebellar ataxia. Exp. Brain Res. 2011, 210, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Serrao, M.; Pierelli, F.; Ranavolo, A.; Draicchio, F.; Conte, C.; Don, R.; Di Fabio, R.; Lerose, M.; Padua, L.; Sandrini, G.; et al. Gait pattern in inherited cerebellar ataxias. Cerebellum 2012, 11, 194–211. [Google Scholar] [CrossRef] [PubMed]
- Morton, S.M.; Bastian, A.J. Cerebellar control of balance and locomotion. Neuroscientist 2004, 10, 247–259. [Google Scholar] [CrossRef]
- Bastian, A.J. Learning to predict the future: The cerebellum adapts feedforward movement control. Curr. Opin. Neurobiol. 2006, 645–649. [Google Scholar] [CrossRef]
- Stoodley, C.J. The cerebellum and neurodevelopmental disorders. The Cerebellum 2017, 15, 34–37. [Google Scholar] [CrossRef]
- Valence, S.; Cochet, E.; Rougeot, C.; Garel, C.; Chantot-Bastaraud, S.; Lainey, E.; Afenjar, A.; Barthez, M.A.; Bednarek, N.; Doummar, D.; et al. Exome sequencing in congenital ataxia identifies two new candidate genes and highlights a pathophysiological link between some congenital ataxias and early infantile epileptic encephalopathies. Genet. Med. 2019, 21, 553–563. [Google Scholar] [CrossRef]
- Romani, M.; Micalizzi, A.; Valente, E.M. Joubert syndrome: Congenital cerebellar ataxia with the molar tooth. Lancet Neurol. 2013, 12, 894–905. [Google Scholar] [CrossRef]
- Prieto, T.E.; Myklebust, J.B.; Hoffmann, R.G.; Lovett, E.G.; Myklebust, B.M. Measures of postural steadiness: Differences between healthy young and elderly adults. IEEE Trans. Biomed. Eng. 1996, 43, 956–966. [Google Scholar] [CrossRef]
- Rocchi, L.; Chiari, L.; Horak, F.B. The effects of deep brain stimulation and levodopa on postural sway in subjects with Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2002, 73, 240. [Google Scholar] [CrossRef] [PubMed]
- Schmitz-Hübsch, T.; Du Montcel, S.T.; Baliko, L.; Berciano, J.; Boesch, S.; Depondt, C.; Giunti, P.; Globas, C.; Infante, J.; Kang, J.S.; et al. Scale for the assessment and rating of ataxia: Development of a new clinical scale. Neurology 2006, 66, 1717–1720. [Google Scholar] [CrossRef] [PubMed]
- Orphanet. Available online: https://www.orpha.net (accessed on 27 January 2020).
- Hiraoka, K.; Hatanaka, R.; Nikaido, Y.; Jono, Y.; Nomura, Y.; Tani, K.; Chujo, Y. Asymmetry of anticipatory postural adjustment during gait initiation. J. Hum. Kinet. 2014, 42, 7–14. [Google Scholar] [CrossRef][Green Version]
- Ferrari, A.; Benedetti, M.G.; Pavan, E.; Frigo, C.; Bettinelli, D.; Rabuffetti, M.; Crenna, P.; Leardini, A. Quantitative comparison of five current protocols in gait analysis. Gait Posture 2008, 28, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Hof, A.L. Scaling gait data to body size. Gait Posture 1996, 222–223. [Google Scholar] [CrossRef]
- Marchese, S.M.; Esposti, R.; Bolzoni, F.; Cavallari, P. Transcranial Direct Current Stimulation on Parietal Operculum Contralateral to the Moving Limb Does Not Affect the Programming of Intra-Limb Anticipatory Postural Adjustments. Front. Physiol. 2019, 10, 1–9. [Google Scholar] [CrossRef]
- Mummel, P.; Timmann, D.; Krause, U.W.H.; Boering, D.; Thilmann, A.F.; Diener, H.C.; Horak, F.B. Postural responses to changing task conditions in patients with cerebellar lesions. J. Neurol. Neurosurg. Psychiatry 1998, 65, 734–742. [Google Scholar] [CrossRef]
- Ivry, R.B.; Keele, S.W. Timing functions of the cerebellum. J. Cogn. Neurosci. 1989, 1, 136–152. [Google Scholar] [CrossRef]
- Ivry, R.B.; Spencer, R.M.; Zelaznik, H.N.; Diedrichsen, J. The cerebellum and event timing. Ann. N. Y. Acad. Sci. 2002, 302–317. [Google Scholar] [CrossRef]
- Cerri, G.; Esposti, R.; Locatelli, M.; Cavallari, P. Coupling of hand and foot voluntary oscillations in patients suffering cerebellar ataxia: Different effect of lateral or medial lesions on coordination. Prog. Brain Res. 2005, 148, 227–241. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, E. Rebuilding cerebellar network computations from cellular neurophysiology. Front. Cell. Neurosci. 2010, 4, 131. [Google Scholar] [CrossRef]
- Bareš, M.; Apps, R.; Avanzino, L.; Breska, A.; D’Angelo, E.; Filip, P.; Gerwig, M.; Ivry, R.B.; Lawrenson, C.L.; Louis, E.D.; et al. Consensus paper: Decoding the contributions of the cerebellum as a time machine. from neurons to clinical applications. Cerebellum 2019, 18, 266–286. [Google Scholar] [CrossRef] [PubMed]
- Bruttini, C.; Esposti, R.; Bolzoni, F.; Vanotti, A.; Mariotti, C.; Cavallari, P. Temporal disruption of upper-limb anticipatory postural adjustments in cerebellar ataxic patients. Exp. Brain Res. 2015, 233, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Bolzoni, F.; Esposti, R.; Marchese, S.M.; Pozzi, N.G.; Ramirez-Pasos, U.E.; Isaias, I.U.; Cavallari, P. Disrupt of intra-limb APA pattern in Parkinsonian patients performing index-finger flexion. Front. Physiol. 2018, 9, 1745. [Google Scholar] [CrossRef] [PubMed]
- Marquer, A.; Barbieri, G.; Pérennou, D. The assessment and treatment of postural disorders in cerebellar ataxia: A systematic review. Ann. Phys. Rehabil. Med. 2014, 57, 67–78. [Google Scholar] [CrossRef]
- Titomanlio, L.; Romaniello, R.; Borgatti, R. Cerebellar agenesis. Handb. Cereb. Cereb. Disord. 2005, 1855–1872. [Google Scholar] [CrossRef]
- Lu, C.; Huffmaster, S.L.A.; Harvey, J.C.; MacKinnon, C.D. Anticipatory postural adjustment patterns during gait initiation across the adult lifespan. Gait Posture 2017, 57, 182–187. [Google Scholar] [CrossRef]
- Bostan, A.C.; Dum, R.P.; Strick, P.L. The basal ganglia communicate with the cerebellum. Proc. Natl. Acad. Sci. USA 2010, 107, 8452–8456. [Google Scholar] [CrossRef]
- Bostan, A.C.; Dum, R.P.; Strick, P.L. Cerebellar networks with the cerebral cortex and basal ganglia. Trends Cogn. Sci. 2013, 17, 241–254. [Google Scholar] [CrossRef]
- Hoshi, E.; Tremblay, L.; Féger, J.; Carras, P.L.; Strick, P.L. The cerebellum communicates with the basal ganglia. Nat. Neurosci. 2005, 8, 1491–1493. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Hallett, M. The cerebellum in Parkinson’s disease. Brain 2013, 136, 696–709. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Sternad, D.; Corcos, D.M.; Vaillancourt, D.E. Role of hyperactive cerebellum and motor cortex in Parkinson’s disease. Neuroimage 2007, 35, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Rascol, O.; Sabatini, U.; Fabre, N.; Brefel, C.; Loubinoux, I.; Celsis, P.; Senard, J.M.; Montastruc, J.L.; Chollet, F. The ipsilateral cerebellar hemisphere is overactive during hand movements in akinetic parkinsonian patients. Brain 1997, 120, 103–110. [Google Scholar] [CrossRef]
- Simioni, A.C.; Dagher, A.; Fellows, L.K. Compensatory striatal-cerebellar connectivity in mild-moderate Parkinson’s disease. NeuroImage Clin. 2016, 10, 54–62. [Google Scholar] [CrossRef]
- Martinu, K.; Monchi, O. Cortico-basal ganglia and cortico-cerebellar circuits in Parkinson’s disease: Pathophysiology or compensation? Behav. Neurosci. 2013, 127, 222–236. [Google Scholar] [CrossRef]
| Patient | Age | Gender | Molecular Diagnosis | SARA |
|---|---|---|---|---|
| SlowP_01 | 9 | M | mutation in a candidate gene | 8 |
| SlowP_02 | 8 | M | mutation in a candidate gene | 14 |
| SlowP_03 | 12 | M | EXOSC3: c.572G > A | 15 |
| SlowP_04 | 13 | F | KCNC3: c.1268G > A | 17 |
| SlowP_05 | 13 | F | to be evaluated | 13 |
| SlowP_06 | 16 | F | ADCK3: c547C > T; c1042C > T | 13 |
| SlowP_07 | 17 | M | to be evaluated | 18 |
| NonP_01 | 10 | M | NPHP1: c.1358G > T; c.1438-4C > T | 12 |
| NonP_02 | 12 | F | to be evaluated | 15 |
| NonP_03 | 18 | M | to be evaluated | 14 |
| NonP_04 | 9 | M | AHI1: c.1829G > C; c.2671C > T | 15 |
| NonP_05 | 12 | F | SUFU: c.1217T > C | 11 |
| NonP_06 | 9 | M | SUFU: c.1217T > C | 15.5 |
| SlowP | NonP | H | ||||
|---|---|---|---|---|---|---|
| CoP length (mm) | 1924 | (1222 to 2983) | 1481 | (893 to 2911) | 1594 | (895 to 2309) |
| Average CoP velocity (mm/s) | 64 | (40 to 99) | 49 | (29 to 110) | 53 | (29 to 76) |
| ML range (mm) | * 37 | (24 to 97) | 29.72 | (13 to 85) | * 19 | (11 to 33) |
| AP range (mm) | 39 | (28 to 57) | 31 | (18.39 to 77.96) | 26 | (15 to 60) |
| Ellipse area (mm2) | * 564 | (354 to 2857) | 447 | (123 to 2075) | * 208 | (54 to 609) |
| Ellipse eccentricity | 0.68 | (0.48 to 0.87) | 0.73 | (0.65 to 0.93) | 0.79 | (0.51 to 0.83) |
| SlowP | NonP | H | |||||
|---|---|---|---|---|---|---|---|
| IMBALANCE | Phase duration (s) | 0.41 | (0.25 to 1.03) | 0.42 | (0.31 to 1.90) | 0.31 | (0.25 to 0.53) |
| CoP length (mm) | 34 | (24 to 100) | 70 | (33 to 134) | 42 | (17 to 68) | |
| ML CoP shift (mm) | 15.99 | (13 to 28) | 39.80 | (−6 to 67) | 25.95 | (8 to 49) | |
| AP CoP shift (mm) | −18 | (−43 to −5) | −25 | (−43 to −11) | −14 | (−41 to −8) | |
| ML COM shift (mm) | −7.33 | (−14.67 to −2) | −6 | (−9.67 to 2) | −7 | (−13.67 to −2.33) | |
| AP COM shift (mm) | 4.33 | (−0.67 to 20.33) | 6 | (2 to 8.67) | 6 | (2.33 to 11.67) | |
| ML CoP→ COM (mm) | −25 | (−54.67 to −16.67) | −30.75 | (−49.25 to 54) | −37.33 | (−56.67 to −18) | |
| AP CoP → COM (mm) | 24.67 | (2.67 to 62.67) | 28.83 | (12 to 47.33) | 21 | (12 to 56) | |
| UNLOADING | Phase duration (s) | 0.46 | (0.21 to 1.53) | 0.33 | (0.23 to 1.20) | 0.41 | (0.21 to 0.56) |
| CoP length (mm) | 172 | (112 to 202) | 134 | (62 to 175) | 143 | (90 to 172) | |
| ML CoP shift (mm) | −104 | (−147 to −48) | −127 | (−168 to −9) | −121 | (−152 to −76) | |
| AP CoP shift (mm) | 9 | (−12 to 28) | −1 | (−26 to 17) | −9 | (−59 to 9) | |
| ML COM shift (mm) | −34.67 | (−66.67 to −21) | −34.37 | (−49 to −17) | −35.67 | (−53.33 to −22) | |
| AP COM shift (mm) | 43 | (21.67 to 47.33) | 35.08 | (12 to 55.5) | 38.67 | (28 to 55.33) | |
| ML CoP → COM (mm) | 44.25 | (33.67 to 90.33) | 50.96 | (−7 to 62.67) | 46.67 | (23 to 61.67) | |
| AP CoP → COM (mm) | 51 | (37.33 to 94.33) | 67.75 | (29.33 to 106) | 80 | (57.33 to 108.5) | |
| SlowP | NonP | H | ||||
|---|---|---|---|---|---|---|
| First step length (%LL) | * 47.34 | (38.39 to 58.70) | 62.93 | (51.83 to 72.67) | * 72.99 | (47.46 to 83.61) |
| First step velocity (Fr) | * 0.24 | (0.12 to 0.32) | 0.31 | (0.24 to 0.39) | * 0.39 | (0.26 to 0.49) |
| SlowP | NonP | H | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| STANCE | Erector spinae | inhibition | −68 | (−85 to −52) | n = 2 | −79 | (−109 to −49) | n = 2 | −102 | (−115 to −80) | n = 3 |
| Biceps femoris | inhibition | −70 | (−70 to −70) | n = 4 | −68 | (−84 to −64) | n = 3 | −100 | (−187 to 119) | n = 7 | |
| Soleus | inhibition | −72 | (−199 to 63) | n = 6 | −98 | (−130 to −63) | n = 3 | −72 | (−146 to −25) | n = 5 | |
| Rectus femoris | excitation | 15 | (−32 to 141) | n = 4 | −35 | (−112 to 22) | n = 5 | −72 | (−110 to 57) | n = 5 | |
| Tibialis anterior | excitation | 31 | (−52 to 66) | n = 5 | −41 | (−49 to 26) | n = 5 | −31 | (−49 to 108) | n = 7 | |
| SWING | Erector spinae | inhibition | −55 | (−99 to 2) | n = 3 | −111 | (−127 to −95) | n = 2 | −90 | (−263 to 100) | n = 4 |
| Biceps femoris | inhibition | −71 | (−90 to −39) | n = 4 | −136 | (−160 to −113) | n = 2 | −94 | (−199 to 70) | n = 5 | |
| Soleus | inhibition | −98 | (−184 to −35) | n = 6 | −53 | (−100 to −24) | n = 4 | −121 | (−259 to −111) | n = 5 | |
| Rectus femoris | excitation | 3 | (−40 to 24) | n = 3 | −11 | (−83 to 162) | n = 4 | 68 | (−80 to 113) | n = 3 | |
| Tibialis anterior | excitation | −2 | (−51 to 145) | n = 4 | 6 | (−67 to 45) | n = 4 | −61 | (−101 to 6) | n = 6 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farinelli, V.; Palmisano, C.; Marchese, S.M.; Strano, C.M.M.; D’Arrigo, S.; Pantaleoni, C.; Ardissone, A.; Nardocci, N.; Esposti, R.; Cavallari, P. Postural Control in Children with Cerebellar Ataxia. Appl. Sci. 2020, 10, 1606. https://doi.org/10.3390/app10051606
Farinelli V, Palmisano C, Marchese SM, Strano CMM, D’Arrigo S, Pantaleoni C, Ardissone A, Nardocci N, Esposti R, Cavallari P. Postural Control in Children with Cerebellar Ataxia. Applied Sciences. 2020; 10(5):1606. https://doi.org/10.3390/app10051606
Chicago/Turabian StyleFarinelli, Veronica, Chiara Palmisano, Silvia Maria Marchese, Camilla Mirella Maria Strano, Stefano D’Arrigo, Chiara Pantaleoni, Anna Ardissone, Nardo Nardocci, Roberto Esposti, and Paolo Cavallari. 2020. "Postural Control in Children with Cerebellar Ataxia" Applied Sciences 10, no. 5: 1606. https://doi.org/10.3390/app10051606
APA StyleFarinelli, V., Palmisano, C., Marchese, S. M., Strano, C. M. M., D’Arrigo, S., Pantaleoni, C., Ardissone, A., Nardocci, N., Esposti, R., & Cavallari, P. (2020). Postural Control in Children with Cerebellar Ataxia. Applied Sciences, 10(5), 1606. https://doi.org/10.3390/app10051606






