Gold/QDs-Embedded-Ceria Nanoparticles: Optical Fluorescence Enhancement as a Quenching Sensor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Materials Characterization
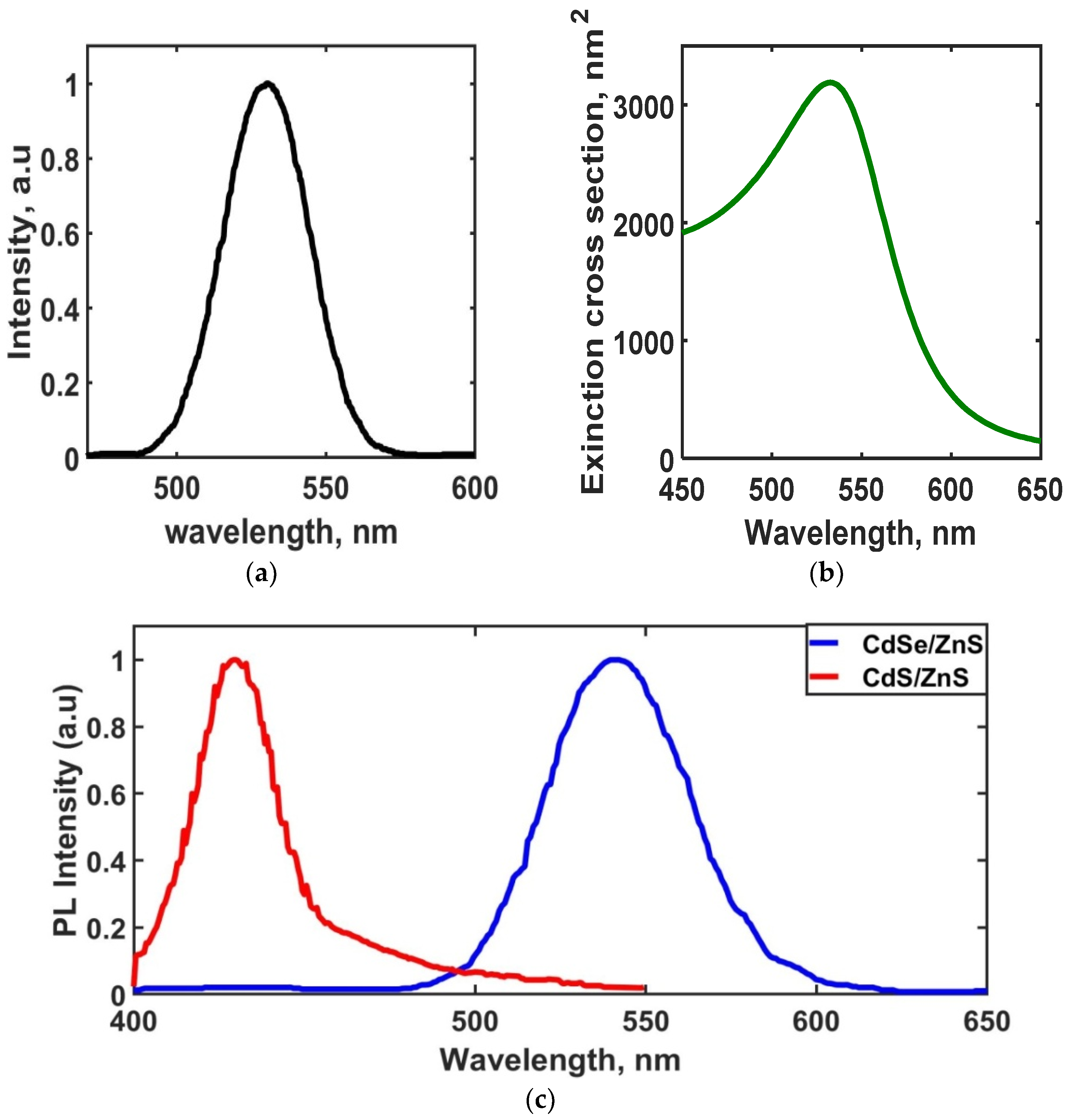
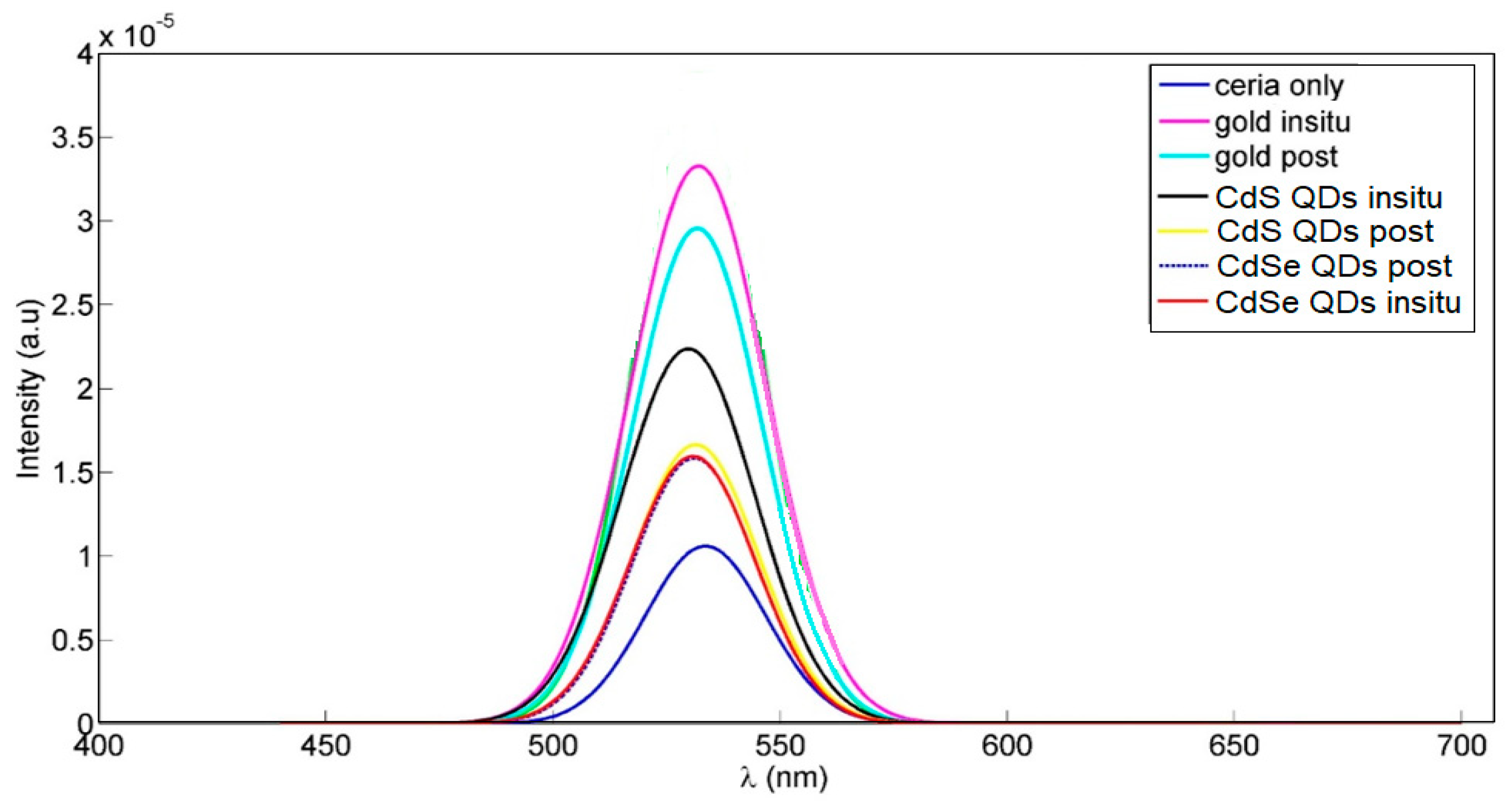
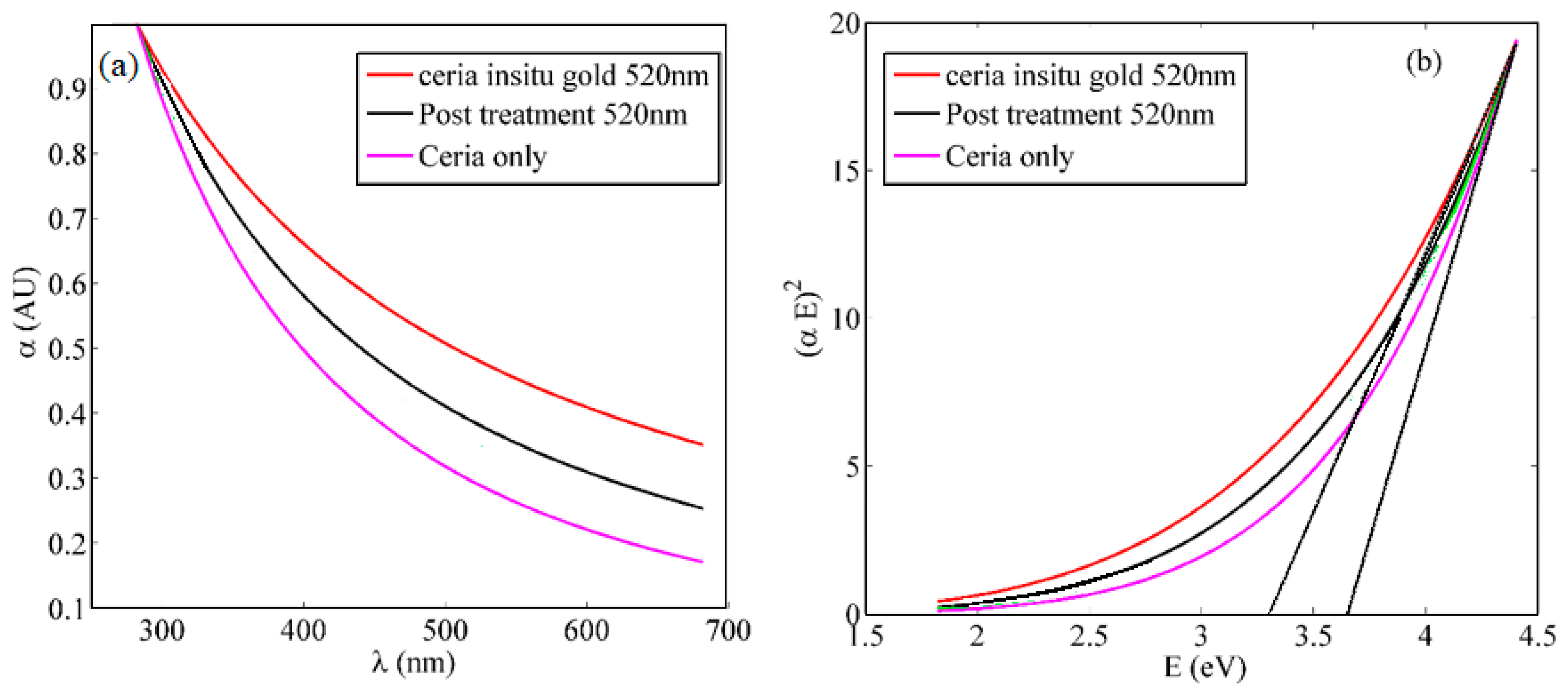
2.2.1. Optical Characterizations
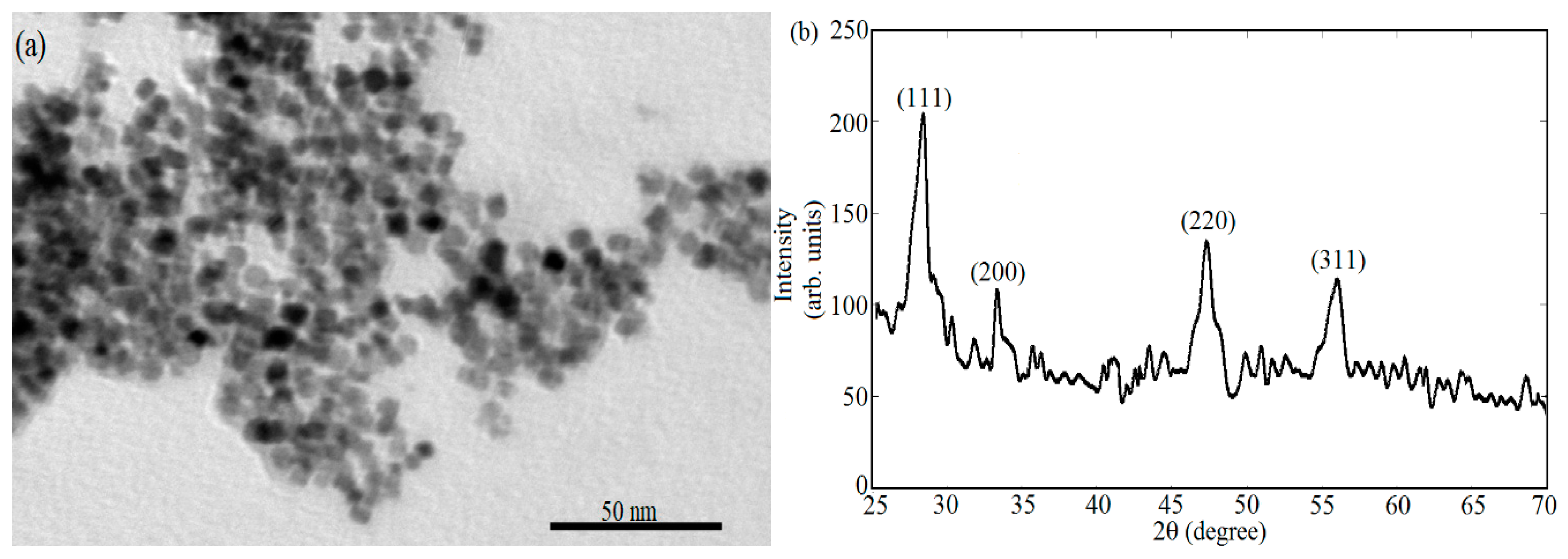
2.2.2. Morphological Analysis
2.3. Optical Sensing Application
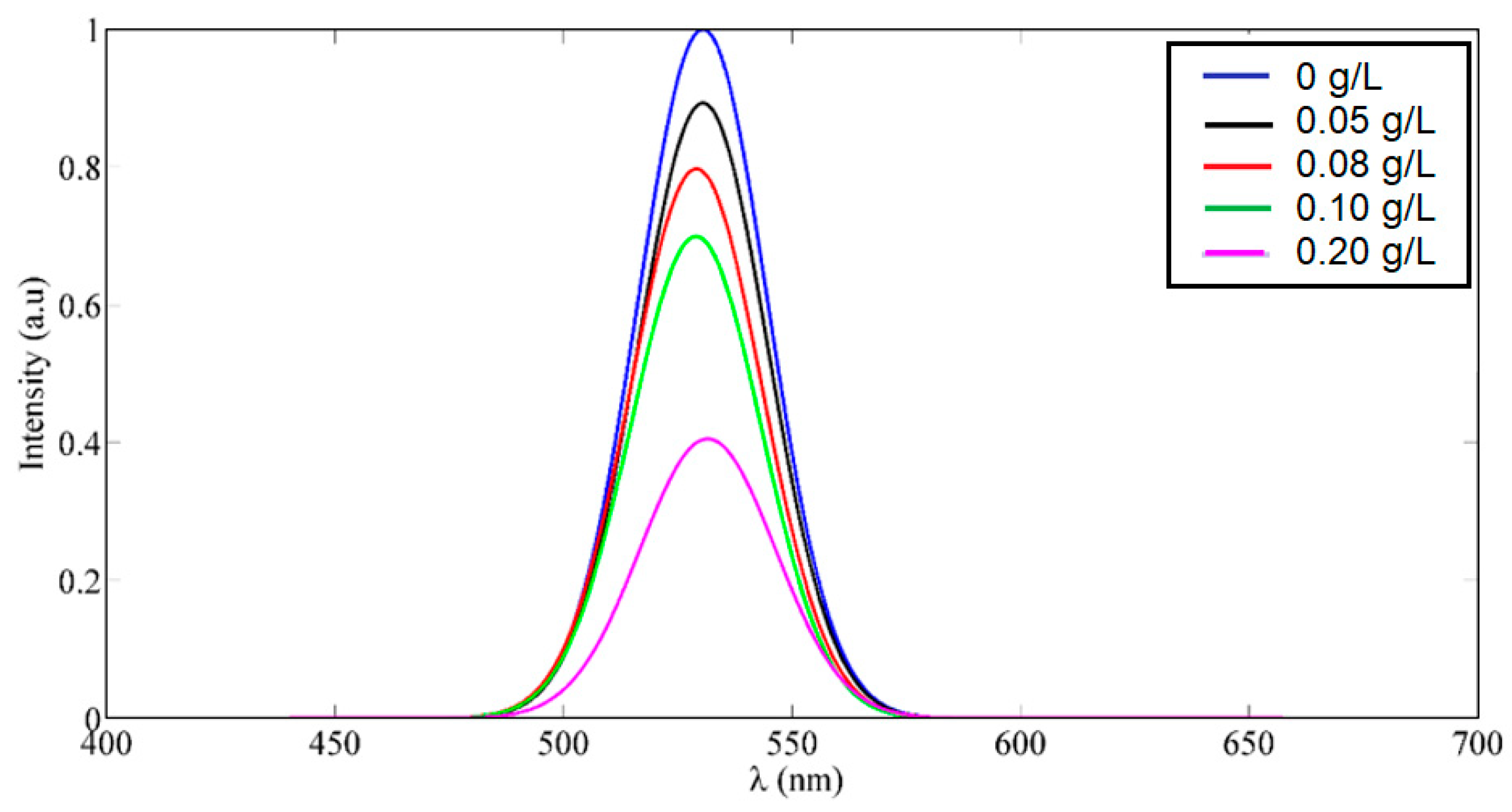
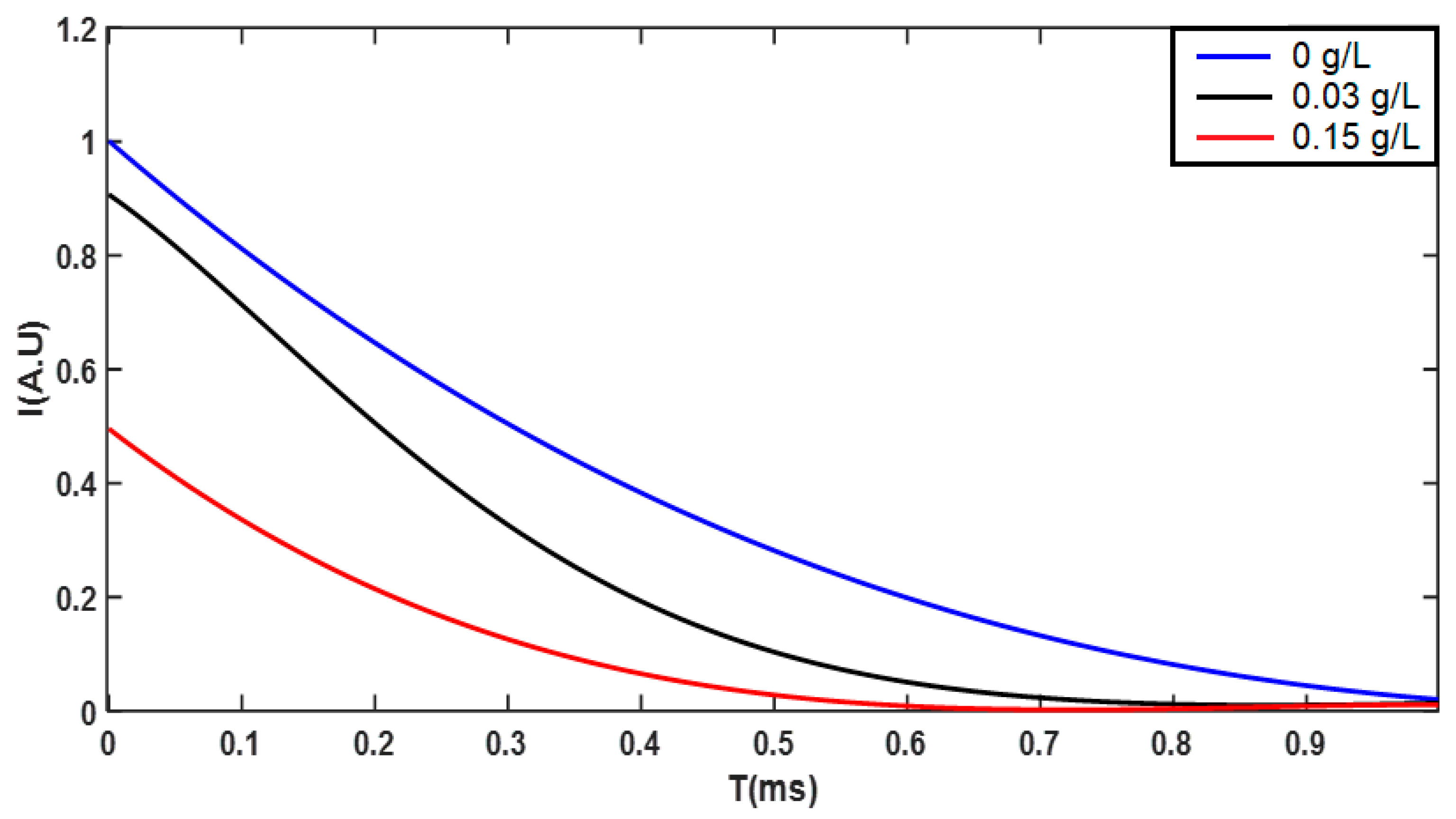
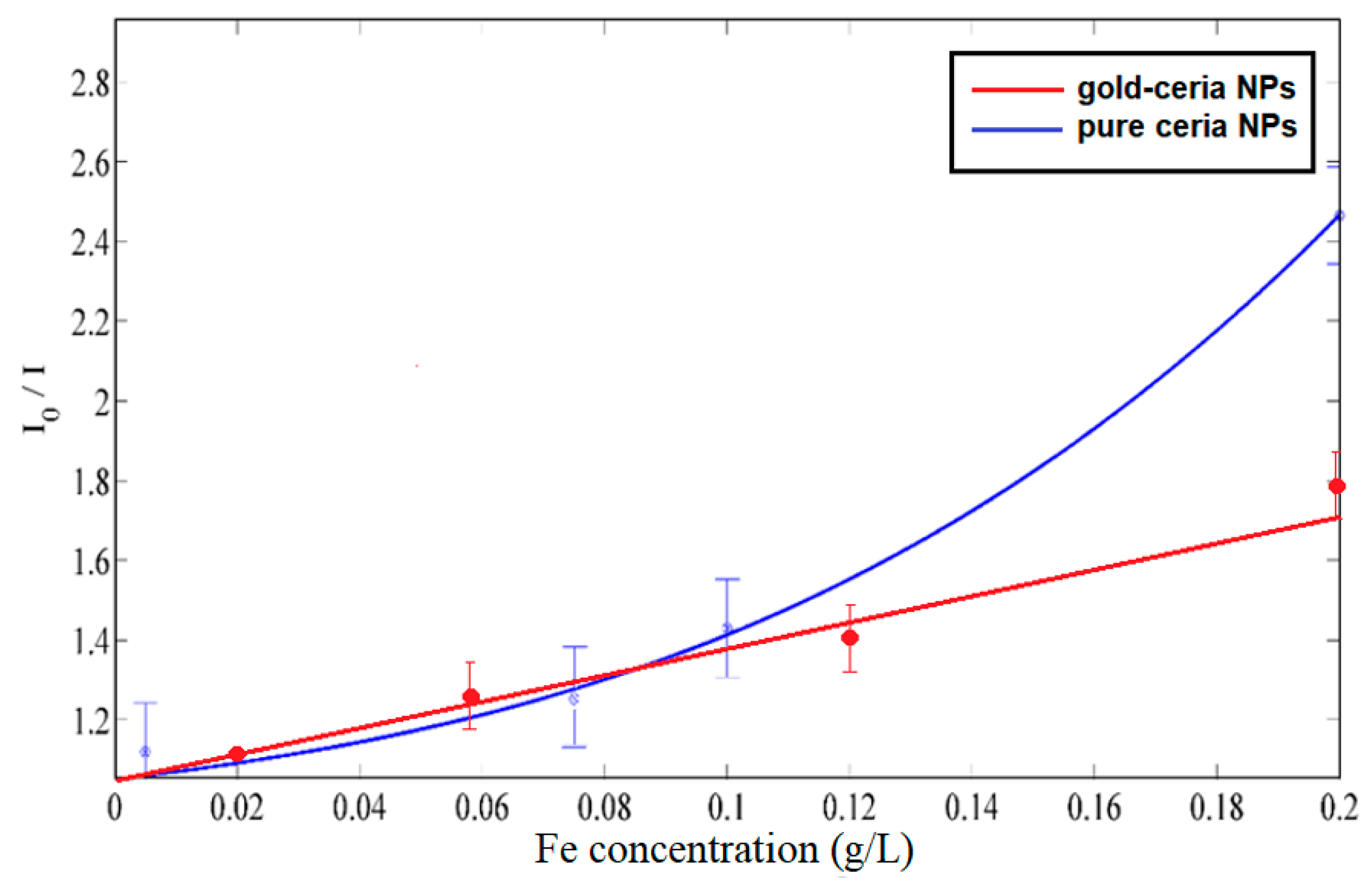
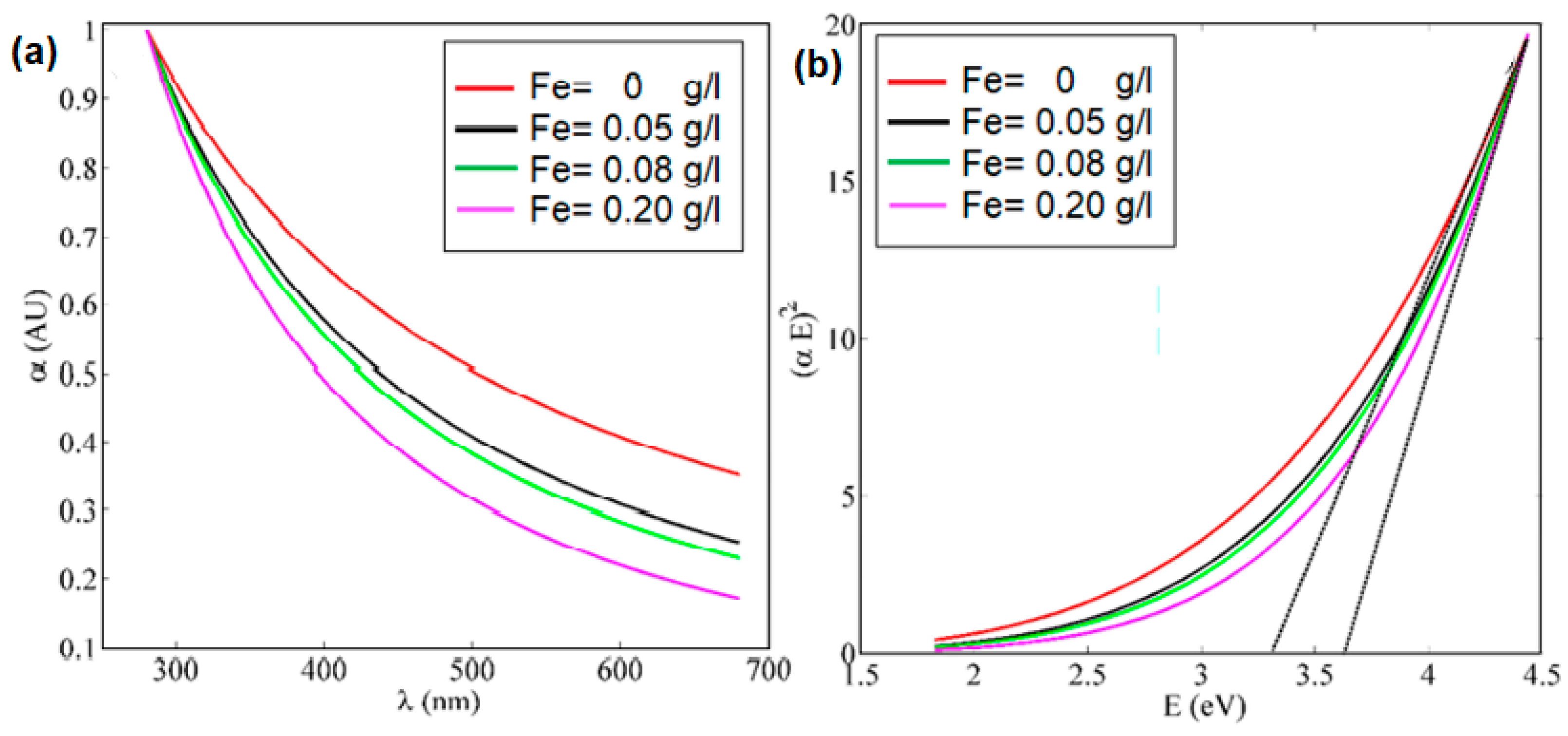
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Quaranta, M.; Borisov, S.M.; Klimant, I. Indicators for optical oxygen sensors. Bioanal. Rev. 2012, 2, 115–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skala, T.; Tsud, N.; Prince, K.C.; Matolin, V. Formation of alumina–ceria mixed oxide in model systems. Appl. Surf. Sci. 2011, 257, 3682–3687. [Google Scholar] [CrossRef]
- Shehata, N.; Meehan, K.; Hassounah, I.; Hudait, M.; Jain, N.; Clavel, M.; Elhelw, S.; Madi, N. Reduced erbium-doped ceria nanoparticles: One nano-host applicable for simultaneous optical down- and up-conversions. Nanoscale Res. Lett. 2014, 9, 231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chorvat, D.; Chorvatova, A. Multi-wavelength fluorescence lifetime spectroscopy: A new approach to the study of endogenous fluorescence in living cells and tissues. Lasers Phys. Lett. 2009, 6, 175–193. [Google Scholar] [CrossRef]
- Shehata, N.; Meehan, K.; Jain, N. Control of oxygen vacancies and Ce+3 concentrations in doped ceria nanoparticles via the selection of lanthanide element. J. Nanoparticle Res. 2012, 14, 1173–1183. [Google Scholar] [CrossRef] [Green Version]
- Feng, N.; Xie, J.; Zhang, D. Synthesis, characterization, photophysical and oxygen-sensing properties of a novel europium(III) complex. Spectrochim. Part A 2010, 77, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Rzigalinski, B.A.; Meehan, K.; Davis, R.M.; Xu, Y.; Miles, W.C.; Cohen, C.A. Radical nanomedicine. Nanomedicine 2006, 1, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Shehata, N.; Azab, M.; Kandas, I.; Meehan, K. Nano-Enriched and Autonomous Sensing Framework for Dissolved Oxygen. Sensors 2015, 15, 20193–20203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Garcia, F.J.; Yubero, F.; González-Elipe, A.; Lambert, R. Microstructural Engineering and Use of Efficient Poison Resistant Au-doped Ni-GDC Ultrathin Anodes in Methane-fed Solid Oxide Fuel Cells. Int. J. Hydrogen Energy 2018, 43, 885–893. [Google Scholar] [CrossRef]
- Wang, J.; Xiao, X.; Liu, Y.; Pan, K.; Pang, H.; Wei, S. The application of CeO2-based materials in electrocatalysis. J. Mater. Chem. A 2019, 7, 17675–17702. [Google Scholar] [CrossRef]
- Gao, Y.; Li, X.; Wu, H.; Meng, S.; Fan, X.; Huang, M.; Guo, Q.; Tung, C.; Wu, L. Exceptional Catalytic Nature of Quantum Dots for Photocatalytic Hydrogen Evolution without External Cocatalysts. Adv. Funct. Mater. 2018, 28, 1801769. [Google Scholar] [CrossRef]
- Pawlis, S.; Berth, G.; Wiedemeier, V.; Schmidt, L.; Zrenner, A.; Warneck, H.J. Oxygen sensing by fluorescence quenching of [Cu(btmgp)I]. J. Luminscence 2010, 130, 1958–1962. [Google Scholar] [CrossRef]
- Chu, C.; Lo, Y. A plastic optical fiber sensor for the dual sensing of temperature and oxygen. IEEE Photonics Technol. Lett. 2008, 20, 63–65. [Google Scholar] [CrossRef]
- Iosin, M.; Canpean, V.; Astilean, S. Spectroscopic studies on pH and thermally induced conformational changes of bovine serum albumin adsorbed ontogold nanoparticles. J. Photochem. Photobiol. 2011, 217, 395–401. [Google Scholar] [CrossRef]
- Chen, H.; Chang, H. Homogeneous precipitation of cerium dioxide nanoparticles in alcohol/water mixed solvents. Colloids Surf. A Physicochem. Eng. Asp. 2004, 242, 61–69. [Google Scholar] [CrossRef]
- Galloway, J.F.; Park, J.; Lee, K.H.; Wirtz, D.; Searson, P.C. Exploiting Nucleation and Growth in the Synthesis and Electrical Passivation of CdSe Quantum Dot. Sci. Adv. Mater. 2009, 1, 93–100. [Google Scholar] [CrossRef]
- Sabir, N.; Qayyum, W.; Hussain, S.Z.; Hussain, I.; Amin, F. Photoluminescence properties of Co and Ni co-doped CdS/ZnS core/shell nanoparticles. In Proceedings of the Colloidal Nanoparticles for Biomedical Applications XIII, San Francisco, CA, USA, 27 January–1 February 2018. [Google Scholar]
- Pankove, J. Optical Processes in Semiconductors, 1st ed.; Dover Publications Inc.: New York, NY, USA, 1971. [Google Scholar]
- Jiang, Z.; Yu, X.; Zhai, S.; Hao, Y. Ratiometric Dissolved Oxygen Sensors Based on Ruthenium Complex Doped with Silver Nanoparticles. Sensors 2017, 17, 548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shehata, N.; Samir, E.; Kandas, I. Gold/QDs-Embedded-Ceria Nanoparticles: Optical Fluorescence Enhancement as a Quenching Sensor. Appl. Sci. 2020, 10, 1236. https://doi.org/10.3390/app10041236
Shehata N, Samir E, Kandas I. Gold/QDs-Embedded-Ceria Nanoparticles: Optical Fluorescence Enhancement as a Quenching Sensor. Applied Sciences. 2020; 10(4):1236. https://doi.org/10.3390/app10041236
Chicago/Turabian StyleShehata, Nader, Effat Samir, and Ishac Kandas. 2020. "Gold/QDs-Embedded-Ceria Nanoparticles: Optical Fluorescence Enhancement as a Quenching Sensor" Applied Sciences 10, no. 4: 1236. https://doi.org/10.3390/app10041236
APA StyleShehata, N., Samir, E., & Kandas, I. (2020). Gold/QDs-Embedded-Ceria Nanoparticles: Optical Fluorescence Enhancement as a Quenching Sensor. Applied Sciences, 10(4), 1236. https://doi.org/10.3390/app10041236





