Abstract
For the very first time, a study on the crystallization growth of zinc silicate glass and glass-ceramics was done, in which white rice husk ash (WRHA) was used as the silicon source. In this study, zinc silicate glass was fabricated by using melt–quenching methods based on the composition (ZnO)0.55(WRHA)0.45, where zinc oxide (ZnO) and white rice husk ash were used as the raw materials. The control crystallization technique was used in which the sample was sintered at 700–950 °C; then, the physical, structural, and optical properties of the glass and glass-ceramics were investigated by using a densitometer, linear shrinkage, X-ray diffraction (XRD), Fourier transform infrared radiation (FTIR), field-emission scanning electron microscopy (FESEM), and photoluminescence spectroscopy (PL). The density and linear shrinkage increased as the crystallinity increased and the XRD results showed the progression of the crystal formation, in which the sample was still in an amorphous state at 27 °C and 700 °C; the crystalline phase started at 750 °C. Based on the FTIR spectra, all samples showed sharpened absorption bands as the sintering temperature was increased, and the FESEM image showed the progression of crystal growth, indicating the formation of zinc silicate glass-ceramics. Lastly, the PL spectra emitted three emission peaks, at 529, 570, and 682 nm for the green, yellow, and red emission, respectively.
1. Introduction
Over the past years, in countries that produce rice, like India, China, Bangladesh, Brazil, USA, Cambodia, Vietnam, Myanmar, and the rest of Southeast Asia, a massive amount of agricultural waste was obtained and sometimes had been discarded as waste product [1]. The production of rice in paddies all over the world is approximated to be 600 million tons a year, where 20% of the crop yield is the husk-to-paddy ratio, and 18% is the ash-to-husk ration, making the overall ash production 21 million tons per year [2]. However, nowadays, people found a path to use the rice husk (RH) in a more beneficial way, instead of it being a waste product. Several research studies have focused on the properties of rice husk, finding that, among all the countries around the world that produce rice, their factories had started to use the rice husk (RH) as an energy source, especially in milling operations and also for household lighting in the countryside areas [3]. This is because rice husk ash has several properties that, depending on the combustion method, makes it beneficial in many ways.
Burning of the RH at higher temperatures produces white rice husk ash (WRHA). In previous studies, it has been found that combusted WRHA contains 95.60% SiO2 [4]. This finding of a huge amount of silica in the combusted WRHA makes it a precious material, since it can be used as the silica precursor to produce silicate glasses and many other products, such as ceramic tiles. In fact, it had been proven by Bondoli and co-researchers that by using the wasted WRHA as the silica source, an exact same product of industrial glass that has the same glassy characteristic can be produced [2]. Apart from that, silicate glass from WRHA had recently been researched as a potential luminescence material. In previous research, Khaidir and co-researchers conducted research regarding the europium oxide-doped zinc silicate glasses derived from WRHA. From the result obtained, when doping zinc silicate glass with europium oxide, the photoluminescence revealed red emission at ~722 nm and the emission occurred due to the 4f-4f transition of the europium ions [5]. Besides, another study regarding the luminescence properties of zinc silicate glass doped with cobalt oxide derived from WRHA also had been carried out by Wahab and co-researchers. In 2018, Wahab and co-researchers had found that zinc silicate glass and glass-ceramics from WRHA doped with cobalt oxide emitted a red emission under UV light. The peak was attributed to the d-d transition of the cobalt oxide ions [6]. However, a luminescence study of undoped zinc silicate glass and glass-ceramics derived from WRHA is rare to be found. Hence, for this high composition of silica in the WRHA and its potential as a luminescence material, a comprehensive study regarding the fabrication and characterization of zinc silicate (willemite) will be reported.
Zinc silicate (Zn2SiO4) or willemite is well known among researchers as a supreme and most suitable host matrix for glass phosphor and other optoelectronic materials, since it has a quite huge band gap around 5.5 eV, high chemical compound stability, and a great glass transparency [7]. Besides, it also has another special feature—a large excitation binding energy—allowing it to be used as an enhancer for triggering the luminescence inside the neon discharged lamps, fluorescent lamps, black-and-white televisions, colour televisions, oscilloscopes, laser technology, optical communications, optical fibre amplifiers, waveguides, and light emitting diodes (LED) [8,9,10]. Nowadays, the production of low-cost zinc silicate glass and glass-ceramics is a topic of interest among researchers. In this present work, the fabrication of a novel zinc silicate glass and glass-ceramics fabricated from WRHA by using conventional melt and quenching methods is reported. The best part of this work is the utilization of WRHA as the silicon source instead of using pure silica, which is very expensive. Thus, this will reduce the cost of production of zinc silicate glass and glass-ceramics; it is also very environmentally friendly, economically safe, and has low energy consumption. In this context, the major purpose of this work is to produce, characterize, and study the luminescence of zinc silicate glass and glass-ceramics that had been produced from waste materials (WRHA), as a potential material to be used in optical application.
2. Materials and Methods
The RH used was collected from a local rice factory located at Tanjung Karang, Selangor, Malaysia, where the species of the RH is recognized as Oryza sative (Asian rice) [4]. At an earlier state, after being milled, the RH was kept indoor inside the factory to reduce the chances of contamination from the surroundings. The RH was washed numerous times by using pipe water to remove stains and impurities. By using a large basin, the RH was soaked in water and the sands and soils submerged to the bottom of the basin. After that, only the RH that floated on the surface of the water was collected. Then, the washed RH was dried in an oven at 120 °C for 8 h. The dried RH was then burned in the electrical furnace at 1000 °C for 2 h at a heating rate 10 °C/min to obtain the WRHA. Subsequently, the obtained WRHA was ground by using a mortar and pestle. In order to obtain the fine powder, the WRHA was then sieved into the size of 45 µm. The obtained WRHA was then mixed with ZnO.
A zinc silicate glass with a weight composition of (ZnO)0.55(WRHA)0.45 was prepared by using the solid state method [2]. In this research, the raw material used was zinc oxide (ZnO) (99.9%, Sigma Aldrich, Subang Jaya, Malaysia) and WRHA was extracted as mentioned previously. The ZnO and WRHA were weighed by using an electronic balance for about ~16.5 g and ~13.5 g, respectively, to give the total amount of ~30 g of the whole mixture. The mixture was then dry milled by using a milling machine at 80 rpm for one hour to ensure that the composition was well mixed together. Right after the mixture was milled together, it was carefully poured into a cylindrical alumina crucible of 47 mm in height and with a wall thickness of 2.5 mm, which has the ability to withstand high temperatures of up to 1600 °C; then, the mixture was melted in a closed electrical furnace at 1450 °C for 3 h. In the meantime, a pail of water with a glass collector at the bottom of the pail was prepared. After the melting process was done, a flowy molten glass was obtained inside the alumina crucible and it was poured into the pail of water. As a result, transparent glass frits were formed. The collected glass frits were left to dry at room temperature and ground by using a plunger, mortar, and pestle to obtain the fine powder, and then sieved at 45 µm. In order to get the pallet for the purpose of ceramization, a palleting procedure was carried out. The powder was mixed with polyvinyl alcohol (PVA) to bind it together and later was put into the stainless-steel mould that was 13 mm in diameter and 2 mm in thickness. Lastly, the powder was pressed by using a uniaxial pressing pallet with an applied load of 3 tons for 10 min. The pallet was then subjected to the sintering process at various sintering temperatures, ranging from 700 to 950 °C, with an increment of 50 °C for each sample in a 4 h duration. The zinc silicate glass and glass-ceramics were characterized for their physical, structural and optical properties.
The densities of the zinc silicate glass and glass-ceramics were measured by using an MD-300S densitometer in which Archimedes’ principle was applied. The results obtained were compared with true density that was measured by using a micromeritics AccuPyc II 1340 Gas Pycnometer. For bulk density by using Archimedes principle, at the beginning stage, the samples were weighed by using the weighing machine in the air, giving their weight in air (Wa). Then, it was followed by the submersion of the sample into liquid, to obtain the weight of the displaced fluid. Then, the density of the sample was calculated by using the following formula:
where ρsample is the density of the sample, wa is the weight of the sample measured in the air, and wb is the weight of the sample measured in the reference liquid (distilled water was used in this experiment). On the other hand, the true density was determined by using Boyle’s law:
where P1 is the pressure 1, V1 is the volume 1, P2 is the pressure 2, and V2 is the volume 2. When the testing was carried out, the calculation of the sample’s volume was calculated by the program in the machine by using the rearranged Boyle’s law as below:
where Vs is the volume of the sample, Vc is the volume of the sample’s cell, and VE is the volume of the expansion cell. After that, the true density of the sample was calculated by using the formula below:
where ρt is the true density and m is the mass of the sample. As for the true density, it measures just the volume of the solid materials and exclude the volume of all open pores. Contrarily, in bulk density (using Archimedes’ principle), it includes the volume of all pores both open and close within the sample. Meanwhile, linear shrinkage was determined by calculating the change in the percentage of the diameter of the sample by using an electronic vernier calliper. The structural properties of the zinc silicate glasses were examined by X-ray techniques, with an X’pert PRO MPD diffractometer (PANalytical, Philips, Amelo, The Netherlands and Malvern (UK), carried out with Ni-filtered Cu-Ka radiation = 1.5405° positioned from 20° to 80°. The molecular vibrations of the precursor glasses were observed by using a Fourier transform infrared spectrometer (FTIR Spectrum 100, Perkin Elmer, Waltham, MA, USA), ranging from 400 to 2000 cm−1. The microstructure of the sample was performed by using field-emission scanning electron microscopy (FESEM), using a FEI NOVA NanoSEM 230, Hillsboro, OR, USA. Lastly, the photoluminescence spectra of the zinc silicate glass were measured by using a spectrometer, in this case the Perkin Elmer LS 55 Fluorescence Spectrometer instrument.
P1V1 = P2V2
ρt = m/Vs
3. Results
3.1. Density and Linear Shrinkage
The Archimedes density (density that is measured by using Archimedes’ principle) accompanied by the linear shrinkage as a function of the sintering temperature was plotted as portrayed in Figure 1. Then, the Archimedes density was also compared with the true density as in Table 1. From Figure 1, the densities of the samples were increased from 2.9118 to 3.4138 g/cm3 as the sintering temperature increased. Meanwhile, the true density also increased as the sintering temperature increased from 2.3734 to 3.4472 g/cm3. Besides, from Figure 1, it can be observed that the zinc silicate glass and glass-ceramics shrinkage increased from 0% to 7.5%.
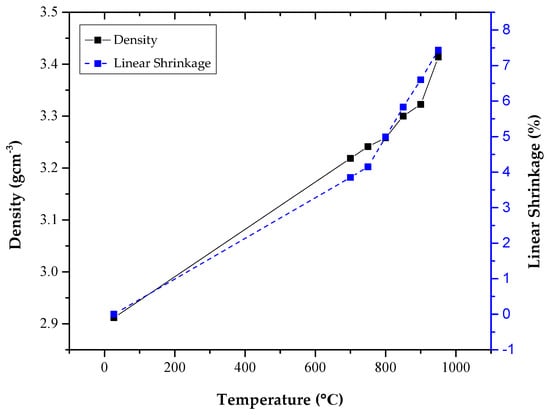
Figure 1.
Density accompanied by the linear shrinkage of the zinc silicate glass and glass-ceramics that were heat treated at various sintering temperatures.

Table 1.
Comparison of the values measured from the Archimedes density and true density.
3.2. X-ray Diffraction
The XRD patterns of the zinc silicate glass and glass-ceramic heat treated at different temperature ranging from 700 to 950 °C for 4 h are discretely presented in Figure 2. At room temperature and 700 °C, the XRD patterns showed a broad amorphous characteristic, indicating that the samples are still glass in nature. However, as the sintering temperature increased to 750 °C, a sharp peak started to appear and the β phase of the zinc silicate (β-Zn2SiO4) with a JCPDS no. of 14-0653 was detected to be formed. β-Zn2SiO4 is known to be the thermodynamically metastable phases of zinc silicate (willemite) that has an orthorhombic crystal system (from a recent study) [11,12]. In Figure 2, thirteen major diffraction peaks of β-Zn2SiO4 at 2θ = 22.04°, 25.23°, 27.10°, 31.20°, 36.23°, 37.84°, 42.44°, 44.90°, 47.84°, 56.19°, 61.22°, and 65.38°, corresponding to planes (210), (020), (12-1), (112), (221), (022), (231), (420), (40-2), (322), (511), (332), and (33-3), respectively, were detected and indexed as shown in Figure 2. This β-Zn2SiO4 continued to exist until the sintering temperature reached 850 °C.
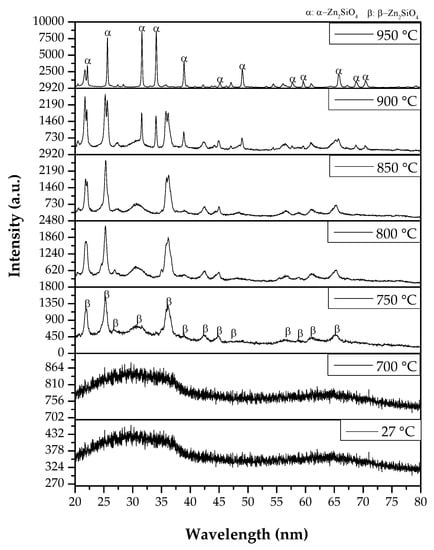
Figure 2.
X-ray diffraction patterns of the zinc silicate glass and glass-ceramics that were heat treated at various sintering temperatures.
Meanwhile, when the sintering temperature of the sample increased to 900 °C, the β-Zn2SiO4 phase transformed into α-Zn2SiO4 phases (JCPDS no. 37-1485). The α-Zn2SiO4 phase is a nesosilicate, or previously known as an orthosilicate, consisting of isolated SiO44- tetrahedrons and ZnO46- [13,14]. It is the most common practical crystalline phase of zinc silicate. At 900 °C, there were still a few of the β-Zn2SiO4 phases being traced. However, as the temperature increased to 950 °C, only a single phase of α-Zn2SiO4 was seen. There were twelve major diffraction peaks found, as indicated in Figure 1, where the diffraction peaks positioned at 2θ = 22.12°, 25.59°, 31.53°, 34.07°, 38.88°, 45.10°, 46.84°, 49.01°, 57.85°, 59.77°, 65.73°, 68.77°, and 70.45° corresponded to planes (330), (220), (113), (410), (223), (060), (431), (333), (8-70), (006), (8-73), (636), and (416), respectively. Therefore, it can be concluded that, as the sintering temperature increases, the glass gradually changes into glass-ceramics, where the crystallization of the β-Zn2SiO4 occurred first at the lower temperature, and then gradually was converted into the thermodynamically stable state of α-Zn2SiO4.
3.3. Field-Emission Scanning Electron Microscopy (FESEM)
The FESEM micrographs of the zinc silicate glass and glass-ceramics sintered at 700–950 °C with 10,000x magnification are presented in Figure 3. There was no grain growth observed at 27 °C and 700 °C. Then, upon further sintering, the sample started to aggregate, showing the growth effect of the sintering temperature.
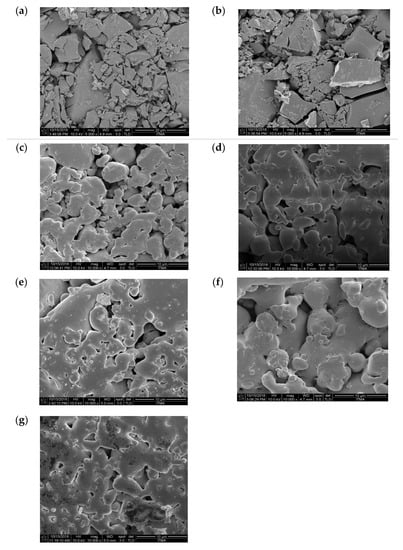
Figure 3.
The surface morphology of the zinc silicate glass and glass-ceramics that were heat treated at various sintering temperature: (a) 27 °C, (b) 700 °C, (c) 750 °C, (d) 800 °C, (e) 850 °C, (f) 900 °C, and (g) 950 °C.
3.4. Fourier Transform Infrared Radiation (FTIR)
Figure 4 shows the transmittance IR spectra of the zinc silicate glass and glass-ceramics in the region from 400 to 2000 cm−1. The band assignation of the IR spectra is tabulated in Table 2.
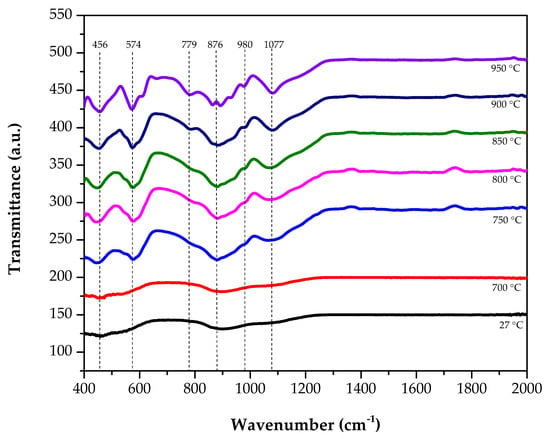
Figure 4.
FTIR spectra of the zinc silicate glass and glass-ceramics that were heat treated at various sintering temperatures.

Table 2.
FTIR assignment bands for the zinc silicate glass and glass ceramic after sintering [8].
3.5. Photoluminescence (PL)
Figure 5 shows the emission intensity of the zinc silicate glass and glass-ceramics that were heat treated at 700, 750, 800, 850, 900, and 950 °C, respectively. The samples were excited at a wavelength of 375 nm. Based on Figure 5, it was evident that three broad emission peaks at 529, 570, and 682 nm appeared.
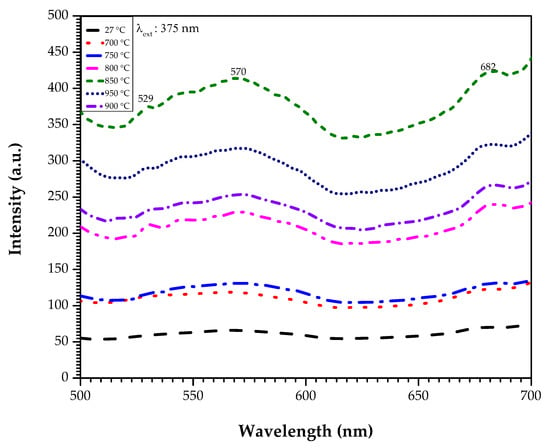
Figure 5.
Emission spectra of the zinc silicate glass and glass-ceramics heat treated at various sintering temperatures.
4. Discussion
Density and shrinkage of the zinc silicate glass and glass-ceramics were investigated to observe the physical changes that occurred when the sintering process was carried out. From Figure 1, the density increased from 2.9118 to 3.4138 g/cm3 as the sintering temperature increased, suggesting a rapid densification. The observation of this densification occurred due to the structure compactness and enhancement of the crystallization phase as the sintering temperature increased. When the structure of the sample became compact, the sample’s shrinkage became clear; this can be observed from Figure 1, where the shrinkage increased from 0% to 7.5%. Apart from that, at a higher temperature, the microstructure and grain size of the sample increased to obtain a denser packing. Consequently, this will lead to the decrement of the total fractional porosity; hence, causing the density to increase. Besides, as the heat was subjected to the sample, it applied pressure on the sample. When pressure was applied, it resulted in a decrease in the volume of the sample. Volume is subjected to be inversely proportional to the density of a material. The lower the volume, the higher the density. In conclusion, the higher the sintering temperature, the higher the density and linear shrinkage of the zinc silicate glass and glass-ceramics.
By looking at the diffraction peaks in Figure 2, it can be observed that, as the sintering temperature increases, the intensity of the XRD peak also increases. The effect of sintering has improved the crystallinity of the zinc silicate [15]. Besides, with the progression of the sintering temperature, the diffraction peaks of the zinc silicate glass-ceramics become sharper and the full width at half maxima (FWHM) is decreased. In the XRD analysis, generally the smaller the crystallite sizes, the broader the peaks; in turn, the larger the crystallite sizes, the sharper the peaks [11,12,15]. This is because at a higher sintering temperature, the diffusion of the ions increased, hence making the crystal growth of the sample accelerated, resulting in a larger crystal being produced [12]. This supports the result that was obtained, where it can be observed that the diffraction peak becomes sharper as the sintering temperature increased. The formation of zinc silicate glass and glass-ceramics was further confirmed by using the FESEM and FTIR analysis.
In order to determine the size of the crystal, the type of crystal, and the surface morphology of the zinc silicate glass and glass-ceramics samples, the FESEM analysis was done. From our observations, at the lower temperatures of 27 °C and 700 °C there were no grain growth, showing a clear glassy surface that tallies with the XRD result where at 27 °C and 700 °C the sample was still in an amorphous phase [16]. Meanwhile, as the sintering temperature increased to 750 °C and 800 °C, the microstructure of the sample still did not show a regular shape (irregular) and started to aggregate among each other. The formation of crystal growth on the surface of the zinc silicate glass and glass-ceramics proved that at 750 °C and 800 °C, the crystallization started to occur as in the XRD result. Then, after further sintering, the glass-ceramics appeared to have a greater grain size, indicating an increase in crystallinity. At the highest temperature, the glass-ceramics become densely packed, with strong necking between each other.
Other than that, by carrying out FTIR analysis, the functional groups of the respective zinc silicate glass and glass-ceramics that could appear in the corresponding materials can be obtained. At a lower sintering temperature, there were four broad SiO4 and ZnO4 bands, whose presence was attributed to the bending modes of the O-Si-O and Si-O-Si bonds, Zn-O stretching vibrational bond, vibrations of Zn-O in the ZnO4 tetrahedral, the decreasing of the Si-O stretching bonds, and the stretching vibrations of the SiO4 and ZnO4 tetrahedron [17]. This four broad SiO4 and ZnO4 bands indicated that there was no formation of zinc silicate glass-ceramics, which agrees with the XRD pattern, where all samples were in an amorphous form at 27 °C and 700 °C. While, for the IR spectrum of the zinc silicate glass-ceramics, there were eight types of absorption bands located at the 459 cm−1 asymmetric deformation of the SiO4, symmetric stretching of ZnO4 at 576 cm−1, asymmetric stretching of ZnO4 at 615 cm−1, bond vibration of SiO4 at 697 cm−1, symmetric stretching of SiO4 at 865 cm−1, and asymmetric stretching of SiO4 at 905 cm−1, 932 cm−1, and 978 cm−1, respectively [18]. From Figure 4, after the sintering temperature reached 750 °C up to 950 °C, a sharp band at 456 cm−1 was spotted at the IR region, showing the asymmetric deformation of the Si-O bond in the SiO4 units. Then, a band at 574 cm−1 associated with the Zn-O symmetric stretching vibration in the ZnO4− was also spotted. While, for the asymmetric stretching of the Zn-O during vibration of the ZnO4 group was located at 615 cm−1. The bands positioned at 779 cm−1 are accredited to the Si-O bond vibration and the bands at 876 cm−1 are subjected to Si-O symmetric stretching vibration [6,19]. Lastly, the band that appeared at 980 cm−1 was transmitted due to the Si-O asymmetric stretching vibration. The band assignation of the IR spectra was tabulated in Table 2. Considering all the IR spectra, it was found that, at first, there were only four broad bands present, proving the formation of the zinc silicate glass. Later, as the sintering temperature increased, there was an additional, sharp band at the IR region, indicating the formation of zinc silicate glass-ceramics, which supports the XRD result.
The photoluminescence spectrometer was cast-off to analyse and prove the luminescence properties of the zinc silicate glass and glass-ceramics by looking at the emission spectra of the sample as in Figure 5, where three broad emission peaks at 529, 570, and 682 nm appeared. The glass-ceramics reveals green, yellow, and red emission. It was found that the origin of these emission peaks might originate from the intrinsic or native defects in ZnO [20]. According to Lima and co-researchers, these emissions can be attributed to the following transitions: Zni+ → Vzn− at 529 nm, Vzn− → Vzn− at 570 nm, and CB → Vo+ / Vo+ → VB at 682 nm [21]. The green emission happened due to the electron-hole recombination, in which the electron was trapped at the singly ionized oxygen vacancy (Vo) centre; then, it recombined with the hole in the valance band (VB) [22]. The recombination process between the electron and hole releases the green emission. Moreover, based on study by Ramanachalam and co-researchers, it has been concluded that the centre/origin of the green luminescence in ZnO can be either the zinc interstitial (Zni) and/or Vo [20]. Apart from that, it has been discussed that the Zn2+ ions are the ones that caused the green emission to occur and appear in zinc silicate glass and glass-ceramics [22,23,24]. The green emission of the zinc silicate glass and glass-ceramics was attributed to the radiative decay of the electronic defect that happened in the forbidden band. Meanwhile, the broad yellow emission at 570 nm was probably due to the intrinsic or excess of oxygen [25]. Previously, a few researchers had proposed that the centre origin of yellow luminescence could be the zinc vacancy (Vzn) oxygen interstitial (Oi) and other defects [20,26,27,28]. According to Lui and co-researchers, the yellow luminescence of high purity ZnO may happen due to the intrinsic and excess of oxygen. Moreover, Ramanchalam and co-researchers had discussed that the Oi may be the yellow luminescence centre of the pure ZnO and ZnO varistor [20]. In the meantime, the red emission at 682 nm may be emitted due to the Oi or Vo. Based on the study by Alvi and co-researchers, it has been found that the origin of the ZnO-nanotubes red emission in the range of 620 nm to 690 nm was attributed to the Oi; meanwhile, red emission in the range of 690 nm to 750 nm was due to Vo [29]. Therefore, since the emission occurred at 682 nm, there might be a chance that the emission occurred due to Vo. Other than that, from Figure 5, it was also observed that the emission peak intensity increased as the sintering temperature increased. This increment occurred as a result from the improvement of the crystallinity inside the zinc silicate glass and glass-ceramics sample [30]. When heat is applied to the zinc silicate sample, this will cause changes in the donor and the acceptor levels of the electron, in which it is interrelated with the number of electronic defects. The presence of the zinc interstitial defect lead to the increase in the emission intensity. Besides, according to Fujihara and co-researchers, the photoluminescence intensity of the materials depends on the amount of ZnO provided due to the oxygen defect, and is dependent on the heat given to the sample [22]. Hence, as was discussed above, it can be concluded that the sintering temperature also affects the intensity of the zinc silicate glass and glass-ceramics emission.
5. Conclusions
In conclusion, the effect of sintering on the structural and optical properties of zinc silicate glass and glass-ceramics derived from WRHA by the melt and quenching technique is demonstrated. The density is increased as the sintering temperature increased and linearly increased with the sample shrinkage. Meanwhile, from the XRD result, it was obtained that the crystallinity of the zinc silicate glass-ceramics increased as the sintering temperature increased. Besides, the β-Zn2SiO4 phase of the zinc silicate was formed at 750 °C until 850 °C. Then it thermodynamically changed into the α-Zn2SiO4 phase of zinc silicate as the temperature reached 900 °C and 950 °C. The surface morphology has revealed the glass and glass-ceramics formations; thus, the FTIR spectra showed the formation of the zinc silicate glass and glass-ceramics, supporting the finding from the XRD analysis. Lastly, the photoluminescence spectroscopy revealed a green, yellow, and red emission indicating the luminescence zinc silicate glass and glass-ceramics. The luminescence properties of the zinc silicate portrayed in this study suggest that this zinc silicate is suitable for optical applications.
Author Contributions
S.A.A.W., K.A.M. and M.H.M.Z. participated in the planning and design of the study, as well as prepared the manuscript; M.M.A.K. and S.H.A.A. joined as the project observer and conceptualization; R.A.T. was the project monitor for the density measurement; A.Z.K.A., R.E.M.K., M.Z.A.K. and N.E. helped conduct the experiment, collected data, and analysed the data. All authors have read and agreed to the published version of the manuscript.
Funding
The author gracefully acknowledged the financial support from Ministry of Science, Technology and Innovation, Malaysia and Universiti Putra Malaysia (UPM), each under the Fundamental Research Grant Scheme (FRGS-5540163), Inisiatif Putra Berimpak (IPM-9531300) and Inisiatif Putra Siswazah (IPS-9627500).
Conflicts of Interest
The authors declare that there is no conflict of interests regarding the publication of this article.
References
- Pode, R. Potential applications of rice husk ash waste from rice husk biomass power plant. Renew. Sustain. Energy Rev. 2016, 53, 1468–1485. [Google Scholar] [CrossRef]
- Bondioli, F.; Barbieri, L.; Ferrari, A.M.; Manfredini, T. Characterization of rice husk ash and its recycling as quartz substitute for the production of ceramic glazes. J. Am. Ceram. Soc. 2010, 93, 121–126. [Google Scholar] [CrossRef]
- Pode, R.; Diouf, B.; Pode, G. Sustainable rural electrification using rice husk biomass energy: A case study of Cambodia. Renew. Sustain. Energy Rev. 2015, 44, 530–542. [Google Scholar] [CrossRef]
- Lee, C.S.; Matori, K.A.; Ab Aziz, S.H.; Kamari, H.M.; Ismail, I.; Zaid, M.H.M. Fabrication and characterization of glass and glass-ceramics from rice husk ash as a potent material for opto-electronic applications. J. Mater. Sci. Mater. Electron. 2017, 28, 17611–17621. [Google Scholar] [CrossRef]
- Khaidir, R.E.M.; Fen, Y.W.; Zaid, M.H.M.; Matori, K.A.; Omar, N.A.S.; Anuar, M.F.; Wahab, S.A.A.; Azman, A.Z.K. Exploring Eu3+-doped ZnO-SiO2 glass derived by recycling renewable source of waste rice husk for white-LEDs application. Results Phys. 2019, 15, 102596. [Google Scholar] [CrossRef]
- Wahab, S.A.A.; Matori, K.A.; Aziz, S.H.A.; Zaid, M.H.M.; Kechik, M.M.A.; Azman, A.Z.K.; Khiri, M.Z.A.; Effendy, N. Synthesis of cobalt oxide Co3O4 doped zinc silicate based glass-ceramics derived for LED applications. Optik 2019, 179, 919–926. [Google Scholar] [CrossRef]
- Feldmann, C.; Jüstel, T.; Ronda, C.R.; Schmidt, P.J. Inorganic luminescent materials: 100 years of research and application. Adv. Funct. Mater. 2003, 13, 511–516. [Google Scholar] [CrossRef]
- Zaid, M.H.M.; Matori, K.A.; Aziz, S.H.A.; Kamari, H.M.; Wahab, Z.A.; Fen, Y.W.; Alibe, I.M. Synthesis and characterization of low cost willemite based glass–ceramic for opto-electronic applications. J. Mater. Sci. Mater. Electron. 2016, 27, 11158–11167. [Google Scholar] [CrossRef]
- Sarrigani, G.V.; Matori, K.A.; Lim, W.F.; Kharazmi, A.; Quah, H.J.; Bahari, H.R.; Hashim, M. Structural and optical properties of erbium-doped willemite-based glass-ceramics. Appl. Opt. 2015, 54, 9925–9929. [Google Scholar] [CrossRef]
- Effendy, N.; Wahab, Z.A.; Abdul Aziz, S.H.; Matori, K.A.; Zaid, M.H.M.; Rashid, S.S.A. Characterization and optical properties of erbium oxide doped ZnO–SLS glass for potential optical and optoelectronic materials. Mater. Express 2017, 7, 59–65. [Google Scholar] [CrossRef]
- Effendy, N.; Wahab, Z.A.; Kamari, H.M.; Matori, K.A.; Ab Aziz, S.H.; Zaid, M.H.M. Structural and optical properties of Er3+ -doped willemite glass-ceramics from waste materials. Optik 2016, 127, 11698–11705. [Google Scholar] [CrossRef]
- Zaid, M.H.M.; Matori, K.A.; Aziz, S.H.A.; Kamari, H.M.; Yunus, W.M.M.; Samsudin, N.F. Fabrication and crystallization of ZnO-SLS glass derived willemite glass-ceramics as a potential material for optics applications. J. Spec. 2016, 2016, 8084301. [Google Scholar]
- Takesue, M.; Hayashi, H.; Smith, R.L., Jr. Thermal and chemical methods for producing zinc silicate (willemite): A review. Prog. Cryst. Growth Charact. Mater. 2009, 55, 98–124. [Google Scholar] [CrossRef]
- Tarafder, A.; Molla, A.R.; Dey, C.; Karmakar, B. Thermal, Structural, and Enhanced Photoluminescence Properties of Eu3+-doped Transparent Willemite Glass–Ceramic Nanocomposites. J. Am. Ceram. Soc. 2013, 96, 2424–2431. [Google Scholar] [CrossRef]
- Omar, N.A.S.; Fen, Y.W.; Matori, K.A.; Aziz, S.H.A.; Alassan, Z.N.; Samsudin, N.F. Development and characterization studies of Eu3+-doped Zn2SiO4 phosphors with waste silicate sources. Procedia Chem. 2016, 19, 21–29. [Google Scholar] [CrossRef]
- Khaidir, R.E.M.; Fen, Y.W.; Zaid, M.H.M.; Matori, K.A.; Omar, N.A.S.; Anuar, M.F.; Wahab, S.A.A.; Azman, A.Z.K. Optical band gap and photoluminescence studies of Eu3+-doped zinc silicate derived from waste rice husks. Optik 2019, 182, 486–495. [Google Scholar] [CrossRef]
- Sarrigani, G.V.; Quah, H.J.; Lim, W.F.; Matori, K.A.; Mohd Razali, N.S.; Kharazmi, A.; Hashim, M.; Bahari, H.R. Characterization of waste material derived willemite-based glass-ceramics doped with erbium. Adv. Mater. Sci. Eng. 2015, 2015, 953659. [Google Scholar] [CrossRef]
- Syamimi, N.F.; Matori, K.A.; Lim, W.F.; Aziz, S.H.A.; Zaid, M.H.M. Effect of Sintering Temperature on Structural and Morphological Properties of Europium (III) Oxide Doped Willemite. J. Spec. 2014, 2014, 328931. [Google Scholar] [CrossRef]
- Azman, A.Z.K.; Matori, K.A.; Aziz, S.H.A.; Zaid, M.H.M.; Wahab, S.A.A.; Khaidir, R.E.M. Comprehensive study on structural and optical properties of Tm2O3 doped zinc silicate based glass–ceramics. J. Mater. Sci. Mater. Electron. 2018, 29, 19861–19866. [Google Scholar] [CrossRef]
- Ramanachalam, M.S.; Rohatgi, A.; Carter, W.B.; Schaffer, J.P.; Gupta, T.K. Photoluminescence study of ZnO varistor stability. J. Electron. Mater. 1995, 24, 413–419. [Google Scholar] [CrossRef]
- Lima, S.A.M.; Sigoli, F.A.; Jafelicci, M., Jr.; Davolos, M.R. Luminescent properties and lattice defects correlation on zinc oxide. Int. J. Inorg. Mater. 2001, 3, 749–754. [Google Scholar] [CrossRef]
- Fujihara, S.; Naito, H.; Kimura, T. Visible photoluminescence of ZnO nanoparticles dispersed in highly transparent MgF2 thin-films via sol–gel process. Thin Solid Films 2001, 389, 227–232. [Google Scholar] [CrossRef]
- Janotti, A.; Van de Walle, C.G. Fundamentals of zinc oxide as a semiconductor. Rep. Prog. Phys. 2009, 72, 126501. [Google Scholar] [CrossRef]
- Fu, Z.; Yang, B.; Li, L.; Dong, W.; Jia, C.; Wu, W. An intense ultraviolet photoluminescence in sol–gel ZnO–SiO2 nanocomposites. J. Phys. Condes. Matter 2003, 15, 2867. [Google Scholar] [CrossRef]
- Liu, M.; Kitai, A.H.; Mascher, P. Point defects and luminescence centres in zinc oxide and zinc oxide doped with manganese. J. Lumin. 1992, 54, 35–42. [Google Scholar] [CrossRef]
- Hirshwald, W.; Bonasewicz, P.; Ernst, L.; Grade, M.; Hoffman, D.; Krebs, S.; Littbarski, R.; Neumann, G.; Grunze, M.; Kolb, D.; et al. Current Topics in Materials Science; Kaldis, E., Ed.; North Holland: Amsterdam, The Netherlands, 1981; Volume 7, p. 241. [Google Scholar]
- Riehl, N. Intrinsic defects and luminescence in II-VI-compounds. J. Lumin. 1981, 24, 335–342. [Google Scholar] [CrossRef]
- Zelikin, Y.M.; Zhukovskii, A.M. Yellow Luminescence of Zinc Oxide. Opt. Spektrosk. 1961, 11, 212–215. [Google Scholar]
- Alvi, N.H.; Ul Hasan, K.; Nur, O.; Willander, M. The origin of the red emission in n-ZnO nanotubes/p-GaN white light emitting diodes. Nanoscale Res. Lett. 2011, 6, 130. [Google Scholar] [CrossRef]
- Li, J.; Kuwabara, M. Preparation and luminescent properties of Eu-doped BaTiO3 thin films by sol–gel process. Sci. Technol. Adv. Mater. 2003, 4, 143–148. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).